Abstract
Many African-Americans carry an amyloidogenic transthyretin mutation (TTR V122I), with a high risk for cardiac TTR amyloid deposition after age 65. We wished to determine the allele frequency and its clinical penetrance in community-dwelling African-Americans.
Study subjects
5000 consenting African-Americans, ages 41 to 93, in two community studies of cardiovascular risk (CHS and ARIC).
Methods
genotyping of banked DNA for TTR V122I allele status; review of cardiovascular and demographic parameters in CHS and ARIC databases with statistical comparisons of the frequency of congestive heart failure, survival and occurrence of features of cardiac amyloidosis, in carriers of the amyloidogenic allele and controls.
Results
119 (3.23%) of 3712 ARIC and 17 (2.12%) of 805 CHS African-Americans carried TTR V122I. After age 65 (CHS) the frequency of congestive heart failure (38% vs 15%, RR 2.62, p = 0.04) and mortality (76% vs 53 %, RR 1.46, p =0.08) were higher in V122I allele carriers than in age, gender and ethnically matched controls. In ARIC (all subjects younger than 65) there were no differences between carriers and non-carriers in mortality, frequency of congestive heart failure or findings consistent with cardiac amyloidosis.
Conclusions
Heterozygosity for the amyloidogenic TTR V122I mutation is relatively common in community dwelling African-Americans. Before 65 the allele has no discernible impact on cardiac function or mortality. After age 70carriers show a higher frequency of congestive failure and greater mortality with more echocardiographic evidence suggestive of cardiac amyloidosis, findings consistent with age dependent clinical penetrance of this autosomal dominant gene.
Introduction
Cardiac amyloidosis is a serious form of cardiomyopathy with a uniformly fatal outcome. Cardiac amyloid may be derived from immunoglobulin L-chains (AL), but in the elderly the precursor of the deposited fibril is frequently the wild type homotetrameric serum protein transthyretin (TTR) (Senile Systemic Amyloidosis) or a subset of TTR mutations (Familial Amyloid Cardiomyopathy) in which cardiac deposition is dominant (1).
Elderly African-Americans are subject to an autosomal dominant form of cardiac amyloidosis in which isoleucine is substituted for valine at TTR position 122 (TTR V122I) (2;3). There are an estimated 1–1.5 million carriers of the allele in the U.S. with eleven per cent in the age group at immediate risk. The allele renders the TTR homotetramer kinetically unstable enhancing the rate of dissociation of the monomer, which misfolds, aggregates and deposits in the myocardium (4). It is rare in individuals of non-African descent (5).
A large post-mortem study, encompassing the results of 52,000 autopsies performed from 1949 to 1982, did not find cardiac TTR amyloid deposition prior to age 60. The frequency increased with each decade thereafter and was higher in African-Americans than in Caucasian-Americans or Hispanics of Mexican origin (1)’ (6). All TTR V122I allele carriers had some TTR amyloid deposition in their hearts after age 65. However there was insufficient information available to assess its clinical impact. Hence the penetrance of the allele, i.e. the association of heart failure, arrhythmia or echocardiographic abnormalities consistent with cardiac amyloidosis was not established.
In a more clinical analysis African-Americans under 65 with NYHA stage III and IV heart failure, participating in BEST, the frequency of the amyloidogenic allele was the same as in the general African-American population (3.5%) but in those over 65 ten percent were allele carriers, suggesting that elderly African-Americans with severe congestive heart failure were more likely to have the allele than those without heart failure or those with similar degrees of heart failure under age 65 (7). A recent report from a major amyloidosis clinical research center indicated that approximately half the African-Americans with congestive heart failure and a biopsy confirmed diagnosis of cardiac amyloidosis carry the amyloidogenic allele (8).
In order to determine whether clinical heart disease was increased among the allele carriers in a less ascertainment-biased manner we elected to compare the frequency of congestive heart failure, arrhythmias, echocardiographic features of cardiac amyloidosis and mortality in carriers and non-carriers of the amyloidogenic allele in two community dwelling African-American populations. In one (CHS) all participants were over 65, while in ARIC all subjects were younger than 65. We hypothesized that if TTR V122I cardiac amyloidosis behaves as an autosomal dominant disease with age dependent penetrance ARIC allele carriers would have no greater frequency of clinical features associated with cardiac amyloidosis than individuals carrying the wild type allele, while CHS TTRV122I positive subjects might display more frequent congestive heart failure, higher mortality and more clinical evidence of cardiac amyloidosis.
Materials and Methods
"The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents."
Study Objectives
1. To determine the carrier frequency of the TTR V122I allele in community-dwelling African-Americans above (CHS) and below (ARIC) age 65.
2. To establish whether carriers of the amyloidogenic allele displayed an increased frequency of congestive heart failure and decreased survival.
3. To ask if TTRV122I carriers showed more echocardiographic and electrocardiographic features consistent with cardiac amyloidosis than non-carriers.
Populations Studied
CHS is an observational study that has subjects in four geographic centers with participants selected to represent a community demographic sample relatively free of ascertainment bias, except for age (9). From 1989 to 1990, 5201 subjects of all races were recruited and examined; an additional 687 self-identified African-Americans were added between 1992 and 1993. CHS ultimately enrolled 931 African-Americans, primarily from Forsyth County, North Carolina. The CHS population ranged in age from 65 to 93 at the time of enrollment. All had detailed medical histories recorded, complete physical examinations, electrocardiography and echocardiography performed according to standardized protocols (10; 11).
ARIC was designed to analyze cardiac risk in a community-based population (15,792 subjects) 45 to 64 years old in 4 communities (Jackson, MS, Forsyth County, NC, Washington County, MD and the northwestern suburbs of Minneapolis, MN) (12). The African-American cohort (4266 participants) was recruited primarily in Jackson, MS (approximately 90%), with smaller groups of subjects from the other sites. Baseline studies were performed between 1986 and 1989 (13). Echocardiography, according to a prescribed standard protocol, was carried out in Jackson at visit 3 between 1993 and 1995 (14; 15).
Ethnicity and Genotyping
The ethnicity of the participants in both studies was established by self-identification. Both studies had central stored plasma and DNA collections obtained from consenting participants (16–24).
Genotyping was performed on genomic DNA obtained from consenting participants using two nested polymerase chain amplification assays (25) (26). Each sample was assayed at least twice, some as many as four times by at least two investigators.
Clinical Analyses
The clinical data were acquired prospectively independent of the genotyping results. After genotyping was completed we analyzed the clinical, electro and echocardiographic data provided by the CHS and ARIC Coordinating Centers (11; 12). We compared the clinical, electrocardiographic and echocardiographic features and the frequency of potentially interacting conditions, including hypertension and diabetes, of the TTR V122I allele carriers with homozygous wild type subjects. Evidence from an independent study indicated that smoking had no effect on outcome or presentation so smoking history was not included as a variable in the analysis.
We utilized CHS and ARIC determinations of the occurrence of incident congestive heart failure (CHF), which involved adjudication by expert panel review of the history, physical findings and chest x-ray reports (CHS). Self-report was confirmed by the presence of fatigue, dyspnea, orthopnea and paroxysmal nocturnal dyspnea in the medical record, the documentation of physical findings including edema, pulmonary rales, gallop rhythm, displaced LV apical impulse, and confirmation by chest x-ray. Ingestion of medications prescribed for heart failure including a diuretic and digoxin and/or a vasodilator supported the diagnosis (27).
Ages at death and causes of death (although no autopsy results) were available in both databases.
The details of acquisition of the electrocardiographic and echocardiographic data in both studies have been reported (10;11;13;15). We compared features included in the database that have been associated with clinically significant cardiac amyloid deposition in carriers and non-carriers of the amyloidogenic allele (28–30).
Statistical Analyses
We used non-parametric Mann-Whitney statistics to compare echocardiographic measurements in allele carriers and controls in both the ARIC and CHS cohorts (Instat Prism).
The earlier autopsy analysis suggested that the appearance of cardiac pathology and symptoms were likely to be age related and that deaths in the TTR V122I cohort might peak in the eighth decade while those associated with wild type TTR continued to increase through the ninth decade. To accommodate this possibility we constructed a synthetic case control study in which a subset of allele negative CHS participants more closely comparable to the identified allele carriers in age (with birth dates within one year) were analyzed as controls for the V122I positive subjects. It was not possible to perform a formal matched case control analysis since there were many more females than males (71 females and 14 male allele negative controls compared with 11 female and 6 male allele carriers), and the proportion of allele positive and allele negative individuals in the 1 year age blocks also varied, although all were over age 65, thus in the age group at risk. We randomly chose approximately five times as many non-carrier individuals (88) that were comparable to the allele carriers (17) in age (being born in the same years) and gender (as a group but without individual matching) and ethnicity.
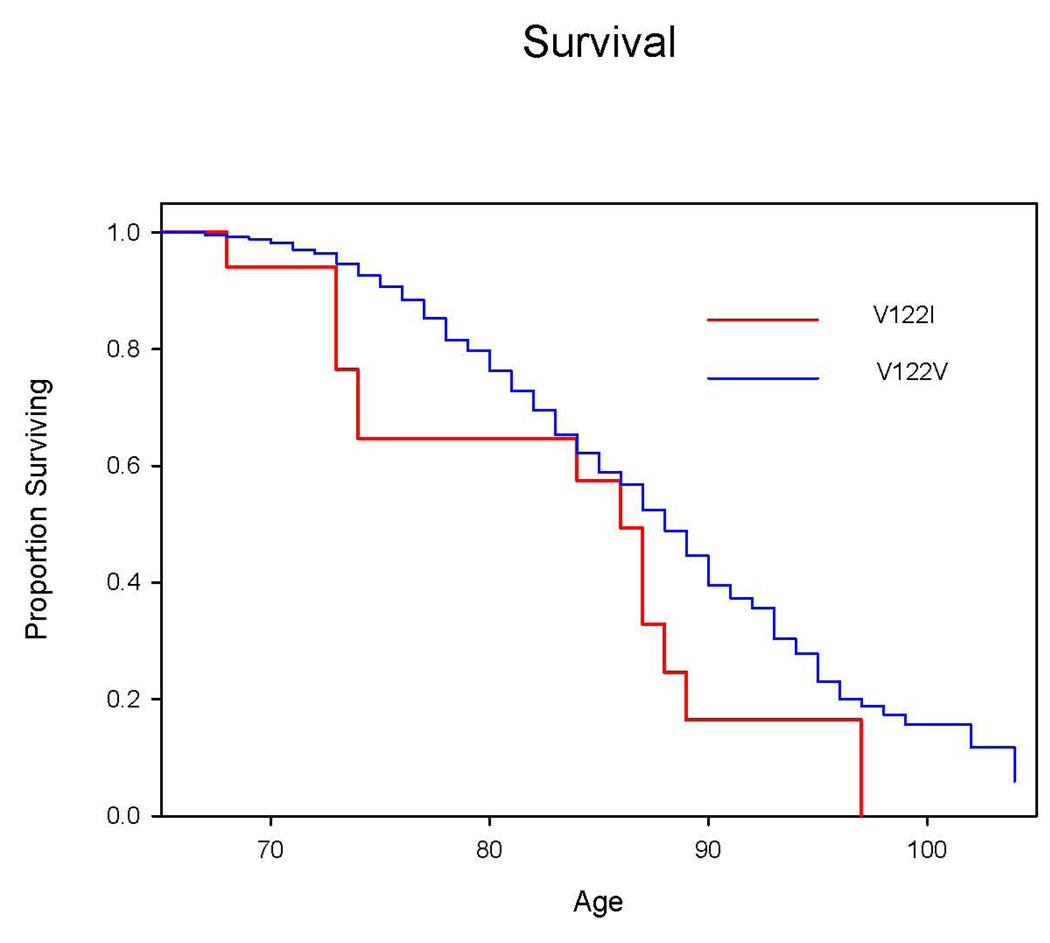
The potential mortality effect of the TTR V122I allele was assessed by a variety of methods. We compared the number of surviving individuals to the number that were dead at the time of analysis using Fisher’s exact test (Table 2). Kaplan Meier plots were constructed for the TTR V122I (17 subjects) and TTR V122V (788 subjects) subsets comprising the entire African-American CHS study population (Figure 1). We utilized logrank tests and Cox regression, with age and gender as stratification factors (covariates).
Table 2.
Clinical Features of African-American Participants in CHS
| Parameter | A.Fib. | CHF | CHF>70 | Death @ 15 yrs |
|---|---|---|---|---|
| All Consenting CHS Participants | ||||
| V122I | 0/11 | 5/17 | 5/13 | 13/17 |
| V122V | 9/591 | 172/788 | 79/538 | 414/788 |
| RR | 0 | 1.35 | 2.62 | 1.46 |
| 95% CI | 0.64 – 2.86 | 1.28 – 5.37 | 1.11 – 1.91 | |
| P | 0.55 | 0.04 | 0.08 | |
| Age and Gender Comparable CHS Participants | ||||
| V122I | 0/11 | 5/17 | 5/13 | 13/17 |
| V122V | 1/79 | 19/88 | 16/67 | 36/88 |
| RR | 0 | 1.36 | 1.75 | 1.87 |
| 95%CI | 0.59 – 3.15 | 0.79 – 3.86 | 1.30 – 2.69 | |
| P | 0.5 | 0.29 | 0.009 | |
N.A. = not available; RR = relative risk, the risk of the particular clinical feature in the V122I allele carriers relative to the corresponding risk in the V122V controls ; CI = confidence interval.
by logrank
Figure 1.
Results
Genotyping
DNA samples were successfully genotyped from all 805 consenting African-American participants in CHS (Table 1). Seventeen (11 females and 6 males) carried the amyloidogenic V122I TTR allele. The prevalence was 2.12%, with an allele frequency of 0.011. No homozygotes were identified.
Table 1.
TTR Genotypes of Consenting Self-identified African-American Subjects in ARIC and CHS
| Population (Age) | AA (#) | DNA (#) | V122I | V122V | No data | V122I (%) | Allele frequency |
|---|---|---|---|---|---|---|---|
| CHS (>65) | 921 | 805 | 17 | 788 | 0 | 2.11 | 0.012 |
| ARIC total (41–64) | 4422 | 4139 | 119 | 3593 | 427 (10.3%) | 3.23 | 0.016 |
| ARIC Jackson | 3648 | 3592 | 111 | 3105 | 376 (10.5%) | 3.45 | 0.017 |
| ARIC Forsyth | 497 | 494 | 7 | 440 | 47 (9.5%) | 1.56 | 0.007 |
| ARIC MN | 21 | 20 | 1 | 16 | 3 (15.0%) | 5.9 | 0.029 |
| ARIC MD | 34 | 34 | 0 | 32 | 2 (5.9)% | <3.2 | <0.016 |
Of the 4266 ARIC African-Americans, we obtained clear genotyping results from 3712 (83%). Of the 17% without results, 7% had no DNA available. The remaining non-informative samples (10%) did not amplify well under the conditions of either of our assays or gave equivocal results that could not be resolved, presumably related to the quality of available DNA, hence were dropped from the analysis. We identified 117 heterozygotes and 2 homozygotes for an overall prevalence of 3.23% (allele frequency 0.0167). In the Jackson cohort (3592 successfully genotyped) the prevalence was 3.45%. In the 529 non-Jackson subjects, primarily from Forsyth County North Carolina, the prevalence was 1.56% (P = 0.04) (see Table 1).
Electrocardiograms
The frequency of incident atrial fibrillation was low and virtually identical in CHS (0/11 vs 11/653, P = 1) and ARIC African-American carriers and non-carriers. Low voltage across the precordium was not recorded for any allele carrier.
Congestive Heart Failure
In ARIC the reported incidences of orthopnea (12% vs 16% P = 0.3), paroxysmal nocturnal dyspnea (15% vs 13% P = 0.74) and peripheral edema, representing signs of congestive heart failure were similar in subjects positive and negative for the amyloidogenic allele.
In CHS the relative risk (RR) for the incidence of congestive heart failure in the total study population and the post-hoc matched subset was increased in the V122I carriers, 2.62 [95% CI 1.28 – 5.37] and 1.75 [95%CI 0.79 – 3.86] (respectively) with the difference in the analysis of the entire CHS population being statistically significant (P = 0.04) (Table 2). TTR V122I carriers represented 2.8 percent of African-American individuals with congestive heart failure in all of CHS, but almost 7 percent in the similar age and gender analysis.
Echocardiography
Only ten of the seventeen CHS allele carriers had interpretable echocardiograms, severely limiting our statistical power to clinically determine whether cardiac amyloidosis was responsible. Nonetheless when we examined features associated with cardiac amyloidosis we found that both the interventricular septum and posterior ventricular wall were thicker in allele carriers than controls (Table 3). The differences were more significant in the more closely age matched group than in the entire cohort. While the tendency toward a lower E/A was seen in the TTR V122I carriers in both analyses it did not reach statistical significance in either set. In the total CHS study population there was no difference in left ventricular mass (LVM). In the smaller age and gender comparable group LVM was greater in the allele carriers but the difference was not statistically significant.
Table 3.
Echocardiographic Measurements TTR V122I vs TTR V122V Carriers in CHS and ARIC
| Parameter | V122V | V122I | |||||
|---|---|---|---|---|---|---|---|
| 25% | Median | 75% | 25% | Median | 75% | P | |
| CHS All Consenting African-Americans | |||||||
| LVM | 116 | 144 | 179 | 121 | 151 | 175 | 0.80 |
| E/A | 0.67 | 0.79 | 0.92 | 0.65 | 0.77 | 0.87 | 0.40 |
| IVSs | 1.34 | 1.49 | 1.64 | 1.42 | 1.67 | 1.96 | 0.10 |
| IVSd | 0.81 | 0.92 | 1.04 | 0.89 | 0.96 | 1.11 | 0.26 |
| LVWs | 1.35 | 1.49 | 1.65 | 1.43 | 1.79 | 2.02 | 0.06 |
| LVWd | 0.80 | 0.89 | 1.0 | 0.89 | 0.96 | 1.12 | 0.14 |
| CHS Age and Gender Comparable African-Americans | |||||||
| LVM | 105 | 128 | 165 | 121 | 151 | 175 | 0.35 |
| E/A | 0.69 | 0.78 | 0.90 | 0.65 | 0.77 | 0.87 | 0.47 |
| IVSs | 1.33 | 1.47 | 1.62 | 1.42 | 1.67 | 1.96 | 0.09 |
| IVSd | 0.81 | 0.89 | 1.05 | 0.89 | 0.96 | 1.11 | 0.13 |
| LVWs | 1.32 | 1.47 | 1.67 | 1.43 | 1.79 | 2.02 | 0.06 |
| LVWd | 0.79 | 0.89 | 1.01 | 0.78 | 0.96 | 1.12 | 0.07 |
| ARIC All Consenting African-Americans | |||||||
| LVM | 195 | 242 | 246 | 208 | 246 | 305 | 0.43 |
| E/A | 0.83 | 1.00 | 1.2 | 0.91 | 1.04 | 1.2 | 0.11 |
| IVSs | 1.38 | 1.54 | 1.75 | 1.34 | 1.50 | 1.79 | 0.78 |
| IVSd | 1.0 | 1.13 | 1.28 | 1.01 | 1.14 | 1.26 | 0.74 |
| LVWs | 1.58 | 1.74 | 1.95 | 1.57 | 1.67 | 1.89 | 0.47 |
| LVWd | 1.02 | 1.13 | 1.27 | 1.05 | 1.13 | 1.27 | 0.77 |
All comparisons were done by Mann-Whitney non-parametric analysis. The comparisons showing statistical trends are signified by the bold font in the P-value column.
In ARIC the results of M-mode and 2D echocardiograms were available for more than 1500 of the African-Americans, all in Jackson, thus providing a statistically more robust sample than available from CHS. The 1500 included 50–60 allele carriers. There were no differences between carriers of the amyloidogenic and wild type alleles (Table 3). Representative ARIC data are shown for comparison between the TTR V122I and TTR V122V subjects. They cannot be compared with the CHS echocardiographic data, since the echocardiograms were obtained by different observers using different protocols.
Mortality
Approximately 15 years after enrollment in the study a larger proportion of the CHS V122I carriers than V122V subjects had died. Those individuals constituted a larger fraction of the total study deaths than of the initial study subjects, i.e. 3.2% of all deaths vs. 2.1% of all participants at entry. In the total CHS population the relative risk (RR) for death was 1.95 (95%CI 0.92 – 4.14) (allele carriers/controls), while in the age and gender comparable analysis the RR was 2.59 (95%CI 1.01 – 6.63) (Table 2). The distribution of the recorded causes of death did not differ between groups.
A Kaplan-Meier plot of overall survival for the TTR V122V and V122I groups with survival information as of June 2008 shows that the two survival curves barely overlap (Figure 1). The difference between the two curves achieves statistical significance: χ2 = 5.39, p=0.02 via stratified logrank analysis with stratification for gender; or, p=0.03 via Cox regression, with gender as a covariate (itself significant at p<0.001). Hence TTR V122I is associated with higher mortality in the CHS population.
In ARIC there was no difference in mortality between the V122I and V122V carriers. 24% of the carriers of the amyloidogenic allele and 26% of the allele negatives had died (P=0.64).
The frequencies of diabetes mellitus (18% of allele negatives vs. 27% allele positives on oral hypoglycemic agents or insulin) and hypertension (7.5% of allele negatives vs. 9% of allele positives had systolic blood pressures over 145 and diastolic readings over 90) were not significantly different between the allele and non-allele carriers in CHS. The sample size was not large enough to determine if either diabetes or hypertension had an impact on the frequency of congestive heart failure. There did not appear to be any effect on mortality.
Discussion
CHS and ARIC are population based studies of cardiovascular risk in the community with substantial African-American cohorts. Combined they included subjects 41 to over 100 years old. The frequency of the TTR V122I allele in African-American participants in CHS (0.011) was lower than in ARIC (0.016) with the difference approaching statistical significance (p = 0.10). The analysis of the survival data from CHS shows that TTR V122I is associated with reduced survival compared to the wild type allele (Figure 1). The absence of any effect in ARIC African-American participants who have not yet reached the age at risk, i.e. 65, supports a causative relation. If the amyloidogenic allele had an impact on survival throughout life one would have expected to see increased mortality in the allele carriers in both ARIC and CHS. A significant proportion of the ARIC cohort has since passed age 65. Among the males Kaplan Meier plots indicate a similar mortality effect of the amyloidogenic allele as seen in the CHS study. However among the female carriers no such effect was seen. The latter could reflect the general effect of gender on mortality.
It is possible that the lower prevalence of the TTR V122I allele in CHS could also reflect genetic stratification, such that the CHS African-Americans differ from other African-American populations with higher allele frequencies. The degree of admixture in the CHS population is approximately 24% (31), while that in ARIC is approximately 18% (Kao, L, personal communication), a 25% difference, which is insufficient to account for the TTR V122I prevalence difference, (i.e. 3.23% vs 2.12%; 3.23–2.12 = 1.11/2.12 = 52%). It is likely that the difference in prevalence reflects a combination of age, i.e. survival effect, and stratification.
There is a higher risk of heart failure after age 70 (5 years after the appearance of anatomic cardiac amyloid deposition at autopsy) in the allele carriers than in controls (6). The TTR V122I carriers make up almost 7 percent of the individuals with heart failure over age 70 in the case control cohort, a proportion similar to the 10 percent allele frequency in the over 60 age group with heart failure studied in BEST (7).
Analysis of the original CHS echocardiographic data (Table 3) showed that features associated with cardiac amyloidosis are more common in the allele carriers than in the controls. The relatively small number of carriers limited our power to identify such differences yet the Mann-Whitney analysis of the data from the comparisons between allele carriers and the age, gender and ethnically similar allele negative individuals suggested that even in a grossly under-powered study statistical trends support the association of the TTR V122I allele with findings consistent with cardiac amyloidosis in the elderly, particularly in the absence of similar trends in a much larger population that has not yet reached the age at risk.
There did not appear to be any interaction with hypertension or diabetes since their frequencies were similar in both allele and non-allele CHS carriers. Further when we compared the frequency of diabetes and hypertension, systolic, diastolic or both, between TTR V122V and TTR V122I carriers there were no significant differences in the proportion of diabetic or hypertensive participants in each cohort that had died. A similar comparison of surviving subjects carrying the two alleles showed that the only significant difference was a higher proportion of individuals with elevated systolic blood pressure among the living V122I carriers. It is noteworthy that within a study population that might be expected to show similar abnormalities in cardiac function as a result of other cardiovascular pathology, the presence of the amyloidogenic TTR allele still appears to be discriminant in the at risk age cohort.
The CHS analysis was limited by missing data and technology that while adequate was not equal to what is presently available to perform similar analyses (32;33). Endomyocardial biopsies or autopsy specimens were not available in CHS hence we could not anatomically confirm that all the allele carriers had cardiac amyloidosis, as was convincingly shown in the earlier autopsy study (6). Nonetheless in the aggregate in community-dwelling African-Americans over age 65 the TTR V122I allele is associated with a higher incidence of congestive heart failure, a greater chance of death and the suggestion of more echocardiographic features of cardiac amyloidosis. The same associations were not seen in a similar population examined before age 65 reinforcing the notion that heterozygosity for the amyloidogenic allele produces disease only after age 65.
If the clinical penetrance of the allele is high starting in the seventh decade genetic testing at age 60 might be useful in identifying individuals at risk before the appearance of clinical and perhaps anatomic disease so that they could be evaluated echocardiographically or by cardiac magnetic resonance imaging. Such testing would have two purposes. In the event of the subsequent development of congestive heart failure it would suggest avoiding potentially toxic therapies such as digoxin, calcium channel blockers and perhaps ACE inhibitors (34–37). In the longer term it would identify individuals who might benefit from treatment with compounds capable of aborting the process of TTR amyloidogenesis which, when available, could be used for both prophylaxis and therapy (38).
Acknowledgments
The authors thank the staff and participants of ARIC and CHS for their important contributions.
Sources of Support: AGRO1 19259 (JB,CT); The W.M. Keck Foundation (JB), CHS is supported by NHLBI contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the NHLBI, with additional funds from the NINDS. ARIC is carried out as a collaborative study supported by NHLBI contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A full list of principal CHS investigators can be found at http://www.chs-nhlbi.org/pi.htm and ARIC principal investigators at http://www.cscc.unc.edu/aric/.
References
- 1.Buck FS, Koss MN, Sherrod AE, Wu A, Takahashi M. Ethnic distribution of amyloidosis: an autopsy study. Mod Pathol. 1989;2:372–377. [PubMed] [Google Scholar]
- 2.Gorevic PD, Prelli FC, Wright J, Pras M, Frangione B. Systemic Senile Amyloidosis. Identification of a new prealbumin (transthyretin) variant in cardiac tissue: immunologic and biochemical similarity to one form of familial amyloidotic polyneuropathy. J Clin Invest. 1989;83:836–843. doi: 10.1172/JCI113966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobson DR, Gorevic PD, Buxbaum JN. A homozygous transthyretin variant associated with senile systemic amyloidosis: evidence for a late-onset disease of genetic etiology. Am J Hum Genet. 1990;47(1):127–136. [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang X, Buxbaum JN, Kelly JW. The V122I cardiomyopathy variant of transthyretin increases the velocity of rate-limiting tetramer dissociation, resulting in accelerated amyloidosis. Proc Natl Acad Sci U S A. 2001;98(26):14943–14948. doi: 10.1073/pnas.261419998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asl KH, Nakamura M, Yamashita T, Benson MD. Cardiac amyloidosis associated with the transthyretin Ile122 mutation in a Caucasian family. Amyloid. 2001;8(4):263–269. doi: 10.3109/13506120108993823. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson DR, Pastore RD, Yaghoubian R, Kane I, Gallo G, Buck FS, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336(7):466–473. doi: 10.1056/NEJM199702133360703. [DOI] [PubMed] [Google Scholar]
- 7.Buxbaum J, Jacobson DR, Tagoe C, Alexander A, Kitzman DW, Greenberg B, et al. Transthyretin V122I in African Americans with congestive heart failure. J Am Coll Cardiol. 2006;47(8):1724–1725. doi: 10.1016/j.jacc.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 8.Connors LH, Prokaeva T, Lim A, Theberge R, Falk RH, Doros G, et al. Cardiac amyloidosis in African Americans: comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J. 2009;158(4):607–614. doi: 10.1016/j.ahj.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 10.Furberg CD, Manolio TA, Psaty BM, Bild DE, Borhani NO, Newman A, et al. Major electrocardiographic abnormalities in persons aged 65 years and older (the Cardiovascular Health Study). Cardiovascular Health Study Collaborative Research Group. Am J Cardiol. 1992;69(16):1329–1335. doi: 10.1016/0002-9149(92)91231-r. [DOI] [PubMed] [Google Scholar]
- 11.Gardin JM, Wong ND, Bommer W, Klopfenstein HS, Smith VE, Tabatznik B, et al. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5(1):63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 12.Investigators ARIC. The atherosclerosis risk in communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13.Vitelli L, Crow R, Shahar E, Hutchinson R, Rautaharju P, Folsom AR. Electrocardiographic Findings in a Healthy Biracial Population. Am J Cardiol. 1998;81:453–459. doi: 10.1016/s0002-9149(97)00937-5. [DOI] [PubMed] [Google Scholar]
- 14.Fox ER. The prognostic value of the mitral diastolic filling velocity ratio for all-cause mortality and cardiovascular morbidity in African Americans: the Atherosclerotic Risks in Communities (ARIC) study. Am Heart J. 2006;152(4):749–755. doi: 10.1016/j.ahj.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Skelton TN, Andrew ME, Arnett DK, Burchfiel CM, Garrison RJ, Samdarshi TE, et al. Echocardiographic left ventricular mass in African-Americans: the Jackson cohort of the Atherosclerosis Risk in Communities Study. Echocardiography. 2003;20(2):111–120. doi: 10.1046/j.1540-8175.2003.03000.x. [DOI] [PubMed] [Google Scholar]
- 16.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin JM, Kitzman DW. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: The Cardiovacular Health Study. J Am Coll Cardiol. 2001;37:1042–1048. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 17.Drazner MH, Rame JM, Marion P, Gottdiener JS, Kitzman DW, Gardin JM, et al. Increased Left Ventricular Mass is a risk factor for the development of a depressed left ventricular ejection fraction: The Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–2215. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 18.Gardin JM, McClelland R, Kitzman DW, Lima JAC, Bommer WE, Klopfenstein HS, et al. M-mode echocardiographic predictors of six-to-seven year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort: The Cardiovascular Health Study. Am J Cardiol. 2001;87:1051–1057. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]
- 19.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 20.Gottdiener JS, McClelland R, Marshall RJ, Shemanski L, Furberg C, Kitzman DW, et al. Outcome of congestive heart failure in elderly persons: Influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 21.Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left Atrial Volume, Geometry, and Function in Systolic and Diastolic Heart Failure of Persons >=65 Years of Age (The Cardiovascular Health Study) Am J Cardiol. 2006;97:83–89. doi: 10.1016/j.amjcard.2005.07.126. [DOI] [PubMed] [Google Scholar]
- 22.Kitzman DW, Gardin JM, Gottdiener JS, Arnold AM, Boineau R, Aurigemma GP, et al. Importance of heart failure with preserved systolic function in patients > or = 65 Years of Age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 23.Liao L, Anstrom KJ, Gottdiener JS, Pappas PA, Whellan DJ, Kitzman DW, Aurigemma GP, et al. Long-term costs and resource use in elderly participants with congestive heart failure in the Cardiovascular Health Study. Am Heart J. 2007;153:245–252. doi: 10.1016/j.ahj.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Mathew ST, gottdiener JS, Kitzman DW, Aurigemma GP. Congestive heart failure in the elderly: the Cardiovascular Health Study. Am J Geriatr Cardiol. 2004;13:61–68. doi: 10.1111/j.1076-7460.2004.02121.x. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson DR. A specific test for transthyretin 122 (Val-lle) based on PCR-primer introduced restriction analysis (PCR-PIRA): confirmation of the gene frequency in Blacks. Am J Hum Genet. 1992;50:195–198. [PMC free article] [PubMed] [Google Scholar]
- 26.Alexander A, Subramanian N, Buxbaum JN, Jacobson DR. Drop-In, Drop-Out Allele-Specific PCR: A Highly Sensitive, Single-Tube Method. Mol Biotechnol. 2004;28(3):171–174. doi: 10.1385/MB:28:3:171. [DOI] [PubMed] [Google Scholar]
- 27.Psaty BM, Kuller LH, Bild DE, Burke GL, Kittner SJ, Mittelmark MB, et al. Methods of Assessing Prevalent Cardiovascular Disease in the Cardiovascular Disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 28.Koyama J, Ray-Sequin PA, Falk RH. Prognostic significance of ultrasound myocardial tissue characterization in patients with cardiac amyloidosis. Circulation. 2002;106(5):556–561. doi: 10.1161/01.cir.0000023530.86718.b0. [DOI] [PubMed] [Google Scholar]
- 29.Falk RH, Plehn JF, Deering T, Shick ECJ, Bosnay P, Rubinow A, et al. Sensitivity and specificity of the echocardiographic features of cardiac amyloidosis. Am J Cardiol. 1987;59:418–422. doi: 10.1016/0002-9149(87)90948-9. [DOI] [PubMed] [Google Scholar]
- 30.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112(13):2047–2060. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- 31.Reiner AP, Ziv E, Lind DL, Nievergelt CM, Schork NJ, Cummings SR, et al. Population Structure, Admixture, and Aging-Related Phenotypes in African American Adults: The Cardiovascular Health Study. Am J Hum Genet. 2005;76(3):463–477. doi: 10.1086/428654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koyama J, Ray-Sequin PA, Davidoff r, Falk RH. Usefulness of pulsed tissue Doppler imaging for evaluating systolic and diastolic left ventricular function in patients with AL (primary) amyloidosis. Am J Cardiol. 2002;89(9):1067–1071. doi: 10.1016/s0002-9149(02)02277-4. [DOI] [PubMed] [Google Scholar]
- 33.Koyama J, Ray-Sequin PA, Falk RH. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation. 2003;107(19):2446–2452. doi: 10.1161/01.CIR.0000068313.67758.4F. [DOI] [PubMed] [Google Scholar]
- 34.Gertz MA, Skinner M, Connors LG, Falk RH, Cohen AS, Kyle RA. Selective binding of nifedipine to amyloid fibrils. Am J Cardiol. 1985;55:1646. doi: 10.1016/0002-9149(85)90996-8. [DOI] [PubMed] [Google Scholar]
- 35.Gertz MA, Falk RH, Skinner M, Cohen AS, Kyle RA. Worsening of congestive heart failure in amyloid heart disease treated by calcium channel-blocking agents. Am J Cardiol. 1985;55:1845. doi: 10.1016/0002-9149(85)90995-6. [DOI] [PubMed] [Google Scholar]
- 36.Rubinow A, Skinner M, Cohen AS. Digoxin sensitivity in amyloid cardiomyopathy. Circulation. 1981;63:1285–1288. doi: 10.1161/01.cir.63.6.1285. [DOI] [PubMed] [Google Scholar]
- 37.Parikh S, de Lemos J. Current therapeutic strategies in cardiac amyloidosis. Curr Treat Options Cardiovasc Med. 2005;7(6):443–448. doi: 10.1007/s11936-005-0029-8. [DOI] [PubMed] [Google Scholar]
- 38.Adamski-Werner SL, Palaninathan SK, Sacchettini JC, Kelly JW. Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis. J Med Chem. 2004;47(2):355–374. doi: 10.1021/jm030347n. [DOI] [PubMed] [Google Scholar]