Abstract
Objective/Background
The National Institute of Mental Health (NIMH) Research Units on Pediatric Psychopharmacology (RUPP) Autism Network found an effect size of d = 1.2 in favor of risperidone on the main outcome measure in an 8-week double-blind, placebo-controlled trial for irritability in autistic disorder. This paper explores moderators and mediators of this effect.
Method
Intention-to-treat (ITT) analyses were conducted with suspected moderators and mediators entered into the regression equations. MacArthur Foundation Network subgroup guidelines were followed in the evaluation of the results.
Results
Only baseline severity moderated treatment response: Higher severity showed greater improvement for risperidone but not for placebo. Weight gain mediated treatment response negatively: Those who gained more weight improved less with risperidone and more with placebo. Compliance correlated with outcome for risperidone but not placebo. Higher dose correlated with worse outcome for placebo, but not risperidone. Of nonspecific predictors, parent education, family income, and low baseline prolactin positively predicted outcome; anxiety, bipolar symptoms, oppositional-defiant symptoms, stereotypy, and hyperactivity negatively predicted outcome. Risperidone moderated the effect of change in 5′-nucleotidase, a marker of zinc status, for which decrease was associated with improvement only with risperidone, not with placebo.
Conclusion
The benefit–risk ratio of risperidone is better with greater symptom severity. Risperidone can be individually titrated to optimal dosage for excellent response in the majority of children. Weight gain is not necessary for risperidone benefit and may even detract from it. Socioeconomic advantage, low prolactin, and absence of co-morbid problems nonspecifically predict better outcome. Mineral interactions with risperidone deserve further study.
Introduction
The National Institute of Mental Health (NIMH) Research Units on Pediatric Psychopharmacology (RUPP) Autism Network reported the intention-to-treat (ITT) analyses of an 8-week, double-blind, placebo-controlled trial of risperidone for irritability (aggression, self-injury, and severe tantrums) in autistic disorder (autism) (RUPP Autism Network 2002). The effect size d was 1.2 in favor of risperidone on the main outcome measure, the Irritability subscale of the Aberrant Behavior Checklist (ABC) (Aman et al. 1985). This paper explores possible moderators, mediators, and other predictors of that effect.
Moderators can be patient, family, or other contextual characteristics that predict the differential effects of treatment choice and thus suggest a way to match patients to treatments. The seminal Baron and Kenny (1986) guidelines for defining moderators specified only that an interaction between the suspected moderator and independent variable (in this case, treatment) occur. However, the MacArthur Foundation Network subgroup (Kraemer et al. 2002; Kraemer et al. 2008) introduced widely accepted modifications to this definition. The MacArthur guidelines state that to be considered a moderator, a variable must: (1) Have temporal precedence, (2) be independent from treatment, and (3) interact significantly with treatment in the model of analysis. These more stringent guidelines were adopted here as requirements for moderation.
A mediator is a postrandomization variable that is associated with treatment and may help to explain the mechanism through which the independent variable is affecting the dependent variable. Theoretically, the treatment variable affects the mediator, which, in turn, affects the outcome variable (Holmbeck 1997). Given a factorial model, the MacArthur guidelines for mediation require: (1) The temporal precedence of treatment, (2) an association between the mediator and treatment (in this case, point-biserial correlation), and (3) a main effect of the mediator or an interaction between the mediator and treatment. Although this definition does not require a significant effect of treatment on outcome, the absence of such treatment effect would be unusual if the definition is met.
Moderator and mediator analyses in this paper were mainly exploratory, with few a priori hypotheses. However, we did expect that better treatment compliance (measured via tablet count and medication diary) and dose would be related to the effectiveness of risperidone.
Methods
The design, assessment and ITT results of the RUPP Autism Network risperidone study have been detailed elsewhere (McDougle et al. 2000; Arnold et al. 2000; Scahill et al. 2001; RUPP Autism Network 2002). Briefly, it was a double-blind comparison of risperidone (n = 49) versus placebo (n = 52) for 8 weeks, with a weight-based, flexible clinical drug titration for the first 4 weeks. Participants were children and adolescents ages 5–17 (mean 8.8 years) with Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association 1994) autistic disorder and severe irritability. Primary ITT analyses showed highly significant effects of risperidone on both of the primary outcome measures: The ABC Irritability subscale (57% decrease vs. 14% decrease) and the Clinical Global Impressions–Improvement (CGI-I) (75.5% vs. 11.5% with CGI-I less than 3). The effect size (Cohen d) on the ABC Irritability subscale was 1.2 at 8 weeks.
Using the primary outcome measure of the ABC Irritability subscale score, we explored the effects of possible moderators and mediators. Casting a wide net, we included demographic characteristics, diagnostic measures, symptom severity, and exploratory laboratory analyses (prolactin, leptin, and mineral assays). Mineral assays were included because of reports of mineral abnormalities in autism, especially zinc and copper and their ratio (Faber et al. 2009) and such related proteins as ferritin and ceruloplasmin (Chauhan et al. 2004) and because prolactin, known to be increased by risperidone, has been suspected of increasing ceruloplasmin (DiSilvestro 1986). First, potential moderators (Table 1) and mediators (Table 2) were entered into respective correlation matrices to check for collinearity. By predetermination, any correlation of 0.5 or stronger would result in combining the variables or discarding one. Most correlations were well below 0.2 and nonsignificant. The exceptions were parental education and income, which were correlated with each other (r = 0.40, p < 0.0001), and the Autism Diagnostic Interview–Revised (ADI-R) (Lord et al. 1994) Stereotypy subscale, which correlated both with the Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS) (Scahill et al. 1997) at 0.22 (p < 0.03) and with the ADI-R Communication Impairment subscale at 0.27 (p < 0.01). Because all correlations were below r = 0.5, all variables were considered in the analysis.
Table 1.
Potential Moderators of Response to Risperidone
| Moderator | na | Mean (SD) | Range | Median Cut Point |
|---|---|---|---|---|
| Age (in years) | 101 | 8.8 (2.7) | 5.1–16.9 | 8.2 |
| Sex | 101 | 1.2 (0.4) | M–F | |
| IQ | 91 | 48.4 (24.4) | 9–111 | 48 |
| Income | 99 | 4.4 (2.2) | 1–7 | $50K |
| Parent Education Level | 101 | College degree/not | ||
| Ethnicity | 101 | Caucasian/not | ||
| ADI-R | ||||
| Social Impairment | 101 | 26.2 (3.4) | 14–33 | 27 |
| Communication impairment | 101 | 17.3 (17.0) | 7–25 | 17 |
| Stereotypy | 100 | 7.8 (2.7) | 1–12 | 8 |
| CY-BOCS | 97 | 15.3 (3.4) | 3–20 | 16 |
| ABC (BL) | ||||
| Irritability | 101 | 25.8 (7.3) | 3–44b | 25 |
| Stereotypic behaviors | 101 | 9.8 (4.7) | 1–21 | 10 |
| Hyperactivity | 100 | 32.1 (9.0) | 11–48 | 33 |
| CGI Severity | 101 | 5.1 (0.70) | 4–7 | 5 |
| CSI (BL) | ||||
| Inattention | 101 | 17.1 (6.0) | 1–27 | 18 |
| Hyperactivity | 101 | 16.7 (6.1) | 2–27 | 17 |
| Conduct | 101 | 4.3 (4.5) | 0–16 | 3 |
| Oppositional | 101 | 10.0 (5.6) | 1–22 | 10 |
| Enuresis | 101 | 1.9 (2.1) | 0–6 | 1 |
| Encopresis | 101 | 0.8 (1.1) | 0–3 | 0 |
| Anxiety | 101 | 14.4 (8.0) | 0–42 | 13 |
| Anorexia | 98 | 0.8 (1.6) | 0–7 | 0 |
| Bulimia | 99 | 1.1 (1.7) | 0–9 | 0 |
| Depression | 97 | 3.1 (4.0) | 0–24 | 2 |
| Bipolar disorder | 98 | 7.3 (5.9) | 0–24 | 6 |
| Leptin (ng/mL) | 100 | 5.6 (5.7) | 1.0–36.7 | 3.4 |
| Prolactin (ng/mL) | 95 | 10.2 (8.90) | 2.3–39.6 | 6 |
| Weight (kg) | 96 | 35.2 (18.7) | 16.1–35.2 | 30 |
| Ferritin (ng/mL) | 60 | 25.0 (21.6) | 0.5–116.8 | 20.9 |
| Ceruloplasmin (U/L) | 55 | 162.3 (82.7) | 34.0–393.0 | 150.0 |
| CeruloplasminRID (mg/L) | 57 | 334.0 (157.3) | 97.0–875.0 | 328.0 |
| Serum zinc (μg/dL) | 60 | 162.7 (58.1) | 48.1–291.9 | 164.5 |
| Copper (μg/dL) | 56 | 81.8 (18.6) | 36.8–116.3 | 85.7 |
| 5′-Nucleotidase (U/L) | 49 | 5.9 (1.3) | 3.7–9.2 | 5.8 |
| Serum Iron (μg/dL) | 39 | 108.0 (73.7) | 15.0–390.0 | 95.0 |
n = 101.
Includes an outlier who entered the study by mistake. The range was otherwise 18–44.
Abbreviations: IQ = Intelligence quotient; ADI-R = Autism Diagnostic Interview–Revised; CYBOCS = Children's Yale-Brown Obsessive Compulsive Scale; ABC = Aberrant Behavior Checklist; CGI = Clinical Global Impressions; CSI = Child Symptom Inventory; BL = baseline; RID = radial immunodiffusion.
Table 2.
Potential Mediators of Response to Risperidone
| |
Placebo |
Risperidone |
||||||
|---|---|---|---|---|---|---|---|---|
| Potential mediator | na | Mean (SD) | Range | Median | n | Mean (SD) | Range | Median |
| Dose | 51 | 2.4 (0.4) | 0.6–2.4 | 1.7 | 49 | 1.7 (0.4) | 0.4–2.3 | 1.3 |
| % Compliance | 51 | 93.6 (15.0) | 6.0–100.0 | 99.0 | 49 | 98.5 (1.80) | 93.0–100.0 | 99.0 |
| % Changeb | % Changeb | |||||||
| Weight change (kg) | 50 | 2.0 (4.0) | −9.0–11.0 | 2.0 | 49 | 11.0 (10.0) | −5.0–38.0 | 10.0 |
| Leptin change (ng/mL) | 40 | 35.0 (55.0) | −34.0–187.0 | 22.0 | 41 | 88.0 (125.0) | −36.0–669.0 | 71.0 |
| Prolactin change (ng/mL) | 33 | 24.0 (79.0) | −70.0–265.0 | 5.0 | 41 | 470.0 (360.0) | −58.0–1508.0 | 413.0 |
| Ferritin (ng/ml) change | 15 | −12.0 (52.0) | −73.0–106.0 | −21.0 | 20 | −2.0 (78.0) | −80.0–296.0 | −19.0 |
| Ceruloplasmin (U/L) change | 13 | −3.0 (49.0) | −89.0–88.0 | −7.6 | 19 | −6.0 (53.0) | −79.0–154.0 | −9.0 |
| CeruloplasminRID (mg/L) change | 16 | −0.5 (35.0) | −42.0–73.0 | −11.0 | 17 | −0.6 (44.0) | −60.0–73.0 | 0.0 |
| Serum zinc (μg/dL) change | 14 | −10.0 (26.0) | −66.0–45.0 | −10.0 | 19 | −7.0 (22.0) | −45.0–35.0 | −8.0 |
| Serum copper (μg/dL) change | 15 | −3.6 (19.0) | −42.0–23.0 | −1.4 | 18 | 3.7 (15.0) | −25.0–28.0 | 5.7 |
| 5′-Nucleotidase (U/L) change | 10 | −5.0 (21.0) | −42.0–22.0 | −2.0 | 13 | 2.0 (16.0) | −26.0–32.0 | 3.4 |
| Serum iron (μg/dL) change | 5 | 89.0 (251.0) | −46.0–533.0 | −38.0 | 10 | 11.0 (125.0) | −61.0–360.0 | 25.0 |
N = 101.
Percent change reflects the difference between endpoint (week 8) and baseline values divided by baseline values.
The suspected moderators and mediators were centered according to the guidelines recommended by Kraemer and Blasey (2004). The ordinal moderators were centered around their respective medians, while the binary moderators (and treatment group) were set to −0.5 or 0.5. As change scores, mediators were all considered to be deviations from zero. Centering the data allows the effects to be evaluated at the level of the independent variable that is representative of the group and helps to diminish the effects of multicollinearity (Kraemer and Blasey 2004).
Some variables presented with special considerations for data analysis. For potential mediators, change scores were calculated as percent change (absolute change divided by baseline value) for ease of comparison. For medication-related variables, dose and compliance, different methods were used. Dose, considered to be the prescribed dosage of risperidone, was to be maintained during the final 4 weeks of treatment at the child's optimal dose. This optimal dose was analyzed as both absolute mg/day and mg/kg per day. Compliance was 100% less the percent of noncompliance, which was calculated as the excess number of tablets returned beyond what should have been returned if all doses were taken, divided by the number that should have been taken. In 31 instances of missing tablet returns, the medication diary kept by the parent was consulted to determine missed doses. Both tablet count and diary reports were converted to percents of missed versus prescribed tablets/doses, and compliance was reported as 100% less this value. The percents determined by the two methods had about the same distribution, and where tablet count was not available, the diary report was used.
With each of these potential moderators and mediators, we reran the original ITT analysis of the primary dimensional outcome variable, the ABC Irritability subscale score, with the suspected moderator or mediator entered into the model. Where possible, variables were used in continuous form for more power in the test of significance (exceptions included gender, education level, and ethnicity), but dichotomous splits were made for visual examination (see Tables 1 and 2 for split points). Because of randomization, all suspected moderators were independent from and temporally preceded the randomly assigned treatment, so the remaining criterion was a significant three-way interaction of moderator, treatment, and time. Suspected mediators all followed treatment. Therefore, the criteria of a significant association between mediator and treatment (judged by point-biserial correlation) and significant three-way interaction of mediator, treatment, and time or a main effect of the mediator were used for mediation effects. For mediators and moderators, significance was set at p = 0.01 rather than 0.05 as partial correction for the large number of comparisons, a compromise between the risks of Type 1 and Type 2 errors. For two variables expected to be associated with the effectiveness of risperidone, dose and compliance, alpha was set at 0.05 to detect an effect that is consistently shown in the literature. These variables are not technically considered mediators because they are not conceptually distinct from the medication itself, but it is useful to analyze them as mediators.
Results
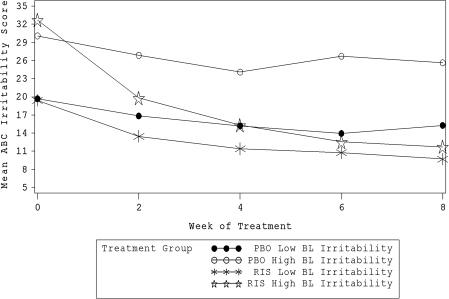
Results from the moderator analysis, including the test of the three-way interaction of time, treatment, and moderator and its statistical significance, are presented in Table 3. Mean changes in ABC Irritability subscale score by treatment and moderator group (using median dichotomous split; Table 1) are also included for visual inspection of effects. Only the baseline ABC Irritability subscale score was a significant moderator of response to risperidone (χ2 = 15.09, p = 0.0001). High baseline ABC Irritability subscale score severity was associated with greater risperidone improvement compared to placebo than was low initial severity (Fig. 1). Within the low initial severity group, the effect size d between placebo and risperidone improvement was 0.71. Within the high initial severity group, however, d = 1.78.
Table 3.
Moderator Analyses of 8-Week ABC Irritability Subscale Score Ratings: Mean Decrease in ABC Irritability Subscale Score from Baseline
| Moderator | Placebo | Risperidone | Placebo | Risperidone | Interactiona |
|---|---|---|---|---|---|
| Sex | Male | Female | χ2 = 2.21c | ||
| Meanb (SD) | 5.17 (7.43) | 15.25 (10.34) | 0.83 (8.98) | 18.33 (7.48) | p = 0.14 |
| n |
36 |
36 |
6 |
9 |
Pool var = 78.61 |
| Age | >8.15 Years | <8.15 Years | χ2 = 0.16 | ||
| Mean (SD) | 2.87 (8.10) | 14.61 (10.81) | 6.05 (7.34) | 16.70 (9.24) | p = 0.69 |
| n |
23 |
18 |
19 |
27 |
Pool var = 79.75 |
| Education | University Degree | <University Degree | χ2 = 1.61 | ||
| Mean (SD) | 3.70 (7.00) | 13.00 (7.87) | 4.86 (8.66) | 18.61 (10.87) | p = 0.20 |
| n |
20 |
22 |
22 |
23 |
Pool var = 77.18 |
| Ethnicity | Non-Caucasian | Caucasian | χ2 = 0.01c | ||
| Mean (SD) | 4.67 (10.53) | 15.50 (8.82) | 4.11 (6.10) | 16.03 (10.39) | p = 0.91 |
| n |
15 |
14 |
27 |
31 |
Pool var = 81.56 |
| High | Low | ||||
| Income | χ2 = 0.09 | ||||
| Mean (SD) | 5.20 (5.01) | 15.00 (10.43) | 4.48 (8.87) | 16.32 (8.98) | p = 0.76 |
| n |
20 |
25 |
21 |
19 |
Pool var = 75.47 |
| IQ | χ2 = 1.05 | ||||
| Mean (SD) | 2.47 (5.25) | 15.00 (10.25) | 6.74 (8.04) | 16.61 (10.22) | p = 0.30 |
| n |
17 |
23 |
23 |
18 |
Pool var = 75.30 |
| Weight | χ2 = 0.08 | ||||
| Mean (SD) | 5.42 (8.98) | 15.24 (10.21) | 2.83 (5.92) | 16.08 (9.38) | p = 0.78 |
| n |
24 |
17 |
18 |
24 |
Pool var = 77.75 |
| CGI Illness severity | χ2 = 0.10 | ||||
| Mean (SD) | 4.31 (7.30) | 14.23 (8.34) | 4.31 (8.20) | 16.53 (10.43) | p = 0.76 |
| n |
13 |
13 |
29 |
32 |
Pool var = 81.07 |
| ADI-R Social impairment | χ2 = 0.43 | ||||
| Mean (SD) | 4.94 (10.46) | 16.35 (10.76) | 3.88 (5.62) | 15.57 (9.52) | p = 0.51 |
| n |
17 |
17 |
25 |
28 |
Pool var = 82.02 |
| ADI-R Communication impairment | χ2 = 1.01 | ||||
| Mean (SD) | 2.78 (5.53) | 14.35 (10.18) | 5.46 (9.16) | 17.08 (9.58) | p = 0.31 |
| n |
18 |
20 |
24 |
25 |
Pool var = 79.78 |
| ADI-R Stereotypy | χ2 = 0.14 | ||||
| Mean (SD) | 2.47 (6.03) | 13.29 (11.56) | 6.00 (9.09) | 17.43 (8.47) | p = 0.71 |
| n |
19 |
17 |
22 |
28 |
Pool var = 78.84 |
| CY-BOCS | χ2 = 0.08 | ||||
| Mean (SD) | 4.39 (7.22) | 13.75 (10.46) | 4.06 (9.22) | 17.30 (8.61) | p = 0.78 |
| n |
23 |
20 |
17 |
23 |
Pool var = 78.69 |
| ABC Irritability | χ2 = 15.24 | ||||
| Mean (SD) | 5.65 (8.52) | 21.21 (8.99) | 3.09 (7.14) | 9.76 (6.81) | p = 0.00 |
| n |
20 |
24 |
22 |
21 |
Pool var = 63.07 |
| ABC Stereotypy | χ2 = 0.00 | ||||
| Mean (SD) | 3.32 (8.39) | 15.70 (11.91) | 5.13 (7.45) | 16.05 (7.34) | p = 0.95 |
| n |
19 |
23 |
23 |
22 |
Pool var = 81.21 |
| ABC Hyperactivity | χ2 = 2.44 | ||||
| Mean (SD) | 5.31 (7.97) | 18.80 (9.59) | 2.69 (7.59) | 13.52 (9.57) | p = 0.12 |
| n |
26 |
25 |
16 |
20 |
Pool var = 77.10 |
| CSI Inattention | χ2 = 3.03 | ||||
| Mean (SD) | 3.33 (6.79) | 17.68 (9.08) | 5.61 (9.10) | 13.60 (10.49) | p = 0.08 |
| n |
24 |
25 |
18 |
20 |
Pool var = 78.77 |
| CSI Hyperactivity | χ2 = 0.63 | ||||
| Mean (SD) | 5.31 (7.97) | 18.20 (8.53) | 2.69 (7.59) | 12.96 (10.76) | p = 0.43 |
| n |
25 |
25 |
17 |
20 |
Pool var = 77.02 |
| CSI Conduct | χ2 = 3.42 | ||||
| Mean (SD) | 3.65 (8.89) | 18.44 (8.82) | 5.11 (6.51) | 12.65 (10.30) | p = 0.06 |
| n |
23 |
25 |
19 |
20 |
Pool var = 76.92 |
| CSI Oppositional | χ2 = 0.13 | ||||
| Mean (SD) | 4.33 (7.08) | 15.72 (8.39) | 4.28 (8.97) | 16.05 (11.62) | p = 0.71 |
| n |
24 |
25 |
18 |
20 |
Pool var = 81.63 |
| CSI Enuresis | χ2 = 3.06 | ||||
| Mean (SD) | 4.48 (8.75) | 16.93 (9.42) | 4.00 (6.15) | 13.94 (10.57) | p = 0.08 |
| n |
27 |
29 |
15 |
16 |
Pool var = 80.49 |
| CSI Encopresis | χ2 = 2.89 | ||||
| Mean (SD) | 4.26 (9.82) | 19.05 (8.88) | 4.43 (5.97) | 12.83 (9.92) | p = 0.09 |
| n |
19 |
22 |
23 |
23 |
Pool var = 76.39 |
| CSI Anxiety | χ2 = 0.24 | ||||
| Mean (SD) | 6.43 (7.77) | 17.90 (7.82) | 1.74 (7.31) | 14.08 (11.17) | p = 0.62 |
| n |
23 |
21 |
19 |
24 |
Pool var = 76.90 |
| CSI Anorexia | χ2 = 0.10 | ||||
| Mean (SD) | 1.18 (8.27) | 12.64 (10.96) | 5.67 (7.51) | 17.97 (9.05) | p = 0.75 |
| n |
11 |
14 |
30 |
29 |
Pool var = 77.18 |
| CSI Bulimia | χ2 = 0.52 | ||||
| Mean (SD) | 4.00 (9.52) | 17.53 (9.28) | 4.52 (6.67) | 15.38 (10.39) | p = 0.47 |
| n |
17 |
17 |
25 |
26 |
Pool var = 81.41 |
| CSI Depression | χ2 = 3.72 | ||||
| Mean (SD) | 2.59 (8.67) | 17.00 (9.73) | 4.83 (7.11) | 15.57 (10.23) | p = 0.05 |
| n |
17 |
20 |
23 |
23 |
Pool var = 81.21 |
| CSI Bipolar disorder | χ2 = 0.02 | ||||
| Mean (SD) | 4.65 (6.31) | 16.20 (10.53) | 5.13 (8.69) | 16.31 (8.71) | p = 0.90 |
| n |
26 |
30 |
15 |
13 |
Pool var = 77.23 |
| Leptin | χ2 = 0.24 | ||||
| Mean (SD) | 3.42 (8.23) | 14.45 (8.21) | 5.95 (8.10) | 16.57 (10.79) | p = 0.63 |
| n |
19 |
20 |
19 |
23 |
Pool var = 81.07 |
| Prolactin | χ2 = 3.79 | ||||
| Mean (SD) | 4.85 (5.53) | 12.48 (9.69) | 4.32 (10.25) | 19.10 (8.58) | p = 0.05 |
| n |
20 |
21 |
19 |
21 |
Pool var = 75.62 |
| Ferritin | χ2 = 0.15 | ||||
| Mean (SD) | 2.11 (5.43) | 12.82 (9.01) | 8.17 (8.42) | 15.78 (10.16) | p = 0.69 |
| n |
18 |
11 |
12 |
18 |
Pool var = 69.96 |
| Ceruloplasmin | χ2 = 0.04 | ||||
| Mean (SD) | 5.73 (7.41) | 15.00 (7.07) | 3.92 (7.38) | 15.87 (10.09) | p = 0.83 |
| n |
15 |
12 |
12 |
15 |
Pool var = 66.86 |
| CeruloplasminRID | χ2 = 4.87 | ||||
| Mean (SD) | 3.94 (8.12) | 18.55 (5.79) | 5.75 (6.37) | 10.81 (9.01) | p = 0.03 |
| n |
17 |
11 |
12 |
16 |
Pool var = 58.74 |
| Serum zinc | χ2 = 0.19 | ||||
| Mean (SD) | 4.69 (6.55) | 15.65 (9.38) | 4.93 (8.38) | 12.36 (9.91) | p = 0.66 |
| n |
13 |
17 |
15 |
14 |
Pool var = 76.04 |
| Copper | χ2 = 0.21 | ||||
| Mean (SD) | 2.71 (5.31) | 15.36 (8.21) | 4.64 (7.10) | 13.56 (9.98) | p = 0.65 |
| n |
17 |
11 |
11 |
16 |
Pool var = 61.24 |
| 5′-Nucleotidase | χ2 = 0.00 | ||||
| Mean (SD) | 5.43 (6.31) | 14.10 (9.96) | 1.80 (5.80) | 14.29 (9.89) | p = 0.96 |
| n |
14 |
10 |
10 |
14 |
Pool var = 67.84 |
| Serum iron | χ2 = 3.07 | ||||
| Mean (SD) | 2.25 (6.41) | 15.11 (9.58) | 5.57 (9.57) | 12.09 (10.45) | p = 0.08 |
| n | 12 | 9 | 7 | 11 | Pool var = 80.79 |
Three-way interaction between Time, Moderator, and Treatment. χ2 is for the Wald Statistic associated with the coefficient of the interaction term in the GLM.
Mean decrease in Irritability.
Denotes categorical analysis.
Abbreviations: ABC = Aberrant Behavior Checklist; SD = standard deviation; IQ = Intelligence quotient; CGI = Clinical Global Impressions; ADI-R = Autism Diagnostic Interview–Revised; CY-BOCS = Children's Yale-Brown Obsessive Compulsive Scale; CSI = Child Symptom Inventory; RID = radial immunodiffusion.
FIG. 1.
Baseline ABC Irritability subscale score as a moderator of response to risperidone. ABC = Abberrant Behavior Checklist, PBO = placebo, RIS = risperidone, BL = baseline. Lines represent mean ABC Irritability Subscale score at each week by treatment and moderator subgroups.
Although no other variables were found to be significant moderators of response to risperidone, several variables had a significant main effect on outcome without a significant three-way interaction with time and treatment (Table 4). These are considered nonspecific predictors of outcome (Kraemer et al. 2002) because they were predictive of response in both treatment groups, but not associated with a differential response to treatment.
Table 4.
Nonspecific Predictors of Treatment Outcome
| Variable | χ2(p) | |
|---|---|---|
| Positive association | ||
| (Predicts good outcome) | Higher parent education level | 8.04 (0.005) |
| Higher income | 9.62 (0.002) | |
| Lower prolactin | 23.18 (0.000) | |
| Negative association (predicts worse outcome) | ||
| CSI | Anxiety | 11.20 (0.001) |
| Bipolar disorder | 6.79 (0.009) | |
| Oppositional-defiant D/O | 9.75 (0.002) | |
| ABC | High hyperactivity | 30.52 (0.000) |
| High stereotypic behavior | 7.00 (0.008) |
Abbreviations: CSI = Child Symptom Inventory; ABC = Aberrant Behavior Checklist.
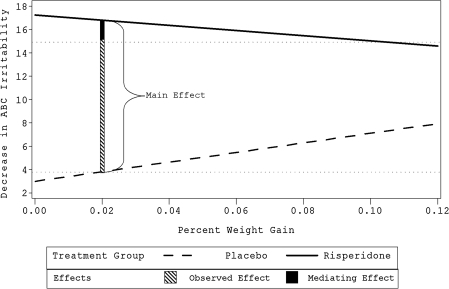
Results from the mediator analysis are presented in Table 5. Weight gain was the only significant mediator of response to risperidone. Weight gain had a main effect on outcome (χ2 = 19.34, p = 0.0001) and was correlated with treatment (point-biserial r = 0.57, p = 0.0001). To explore the mediating effect of weight gain, regression equations for outcome on weight gain were calculated for each treatment group. Figure 2 shows the “observed effect,” which is the difference between groups on average ABC Irritability Subscale score improvement at end point (11.2). It also illustrates the “main effect,” or the improvement predicted by the regression lines if the influence of weight gain were removed. Some weight gain was developmentally appropriate, so we examined the effect at the mean placebo weight gain (2% of baseline weight), which was the best estimate of the amount of weight the children would have gained were they not assigned to the study drug. In this case, the “main effect” of 13.0 was greater than the “observed effect,” indicating that the mediating effect was negative; i.e., greater weight gain was associated with less irritability subscale improvement in the risperidone group, albeit with more improvement in the placebo group.
Table 5.
Mediator Analyses of 8-Week ABC Irritability Ratings: Mean Decrease from Baseline in ABC Irritability
| |
Low |
High |
|
||
|---|---|---|---|---|---|
| Variable | Placebo | Risperidone | Placebo | Risperidone | Interactiona |
| Dose | 6.55 (10.35) | 14.0 (11.0) | 2.35 (7.18) | 15.8 (9.31) | χ2 (p) = 8.19 (0.004)b |
| n | 18 | 25 | 17 | 24 | Pool var = 93.86 |
| Compliance | 4.93 (7.37) | 11.76 (10.25) | 2.63 (9.07) | 18.04 (9.48) | χ2 (p) = 7.39 (0.007)b |
| n | 27 | 25 | 24 | 24 | Pool var = 82.22 |
| Weight % gain | 1.76 (7.1) | 16.21 (11.01) | 5.60 (8.85) | 13.68 (9.32) | χ2 (p) = 19.34 (0.000)b,c |
| n | 25 | 24 | 25 | 25 | Pool var = 83.81 |
| Leptin % change | 8.64 (8.95) | 16.95 (11.47) | 2.50 (8.04) | 15.05 (8.07) | χ2 (p) = 10.66 (0.001)c |
| n | 14 | 19 | 14 | 20 | Pool var = 87.10 |
| Prolactin % change | 8.36 (7.79) | 14.39 (10.56) | 2.79 (9.36) | 17.85 (9.05) | χ2 (p) = 1.82 (0.18) |
| n | 14 | 18 | 14 | 20 | Pool var = 86.77 |
| Ferritin % change | 3.63 (4.34) | 18.30 (7.77) | 3.00 (7.57) | 11.30 (12.07) | χ2 (p) = 4.46 (0.035) |
| n | 8 | 10 | 7 | 10 | Pool var = 75.17 |
| Ceruloplasmin % change | 4.00 (7.05) | 16.70 (9.70) | 2.00 (5.33) | 13.11 (8.49) | χ2 (p) = 0.04 (0.85) |
| n | 7 | 10 | 6 | 9 | Pool var = 66.56 |
| CeruloplasminRID % change | 3.88 (7.20) | 17.33 (7.87) | 1.50 (5.24) | 7.80 (9.20) | χ2 (p) = 3.35 (0.067) |
| n | 8 | 12 | 8 | 5 | Pool var = 54.31 |
| Serum zinc % change | 3.57 (5.09) | 14.10 (10.19) | 3.86 (6.96) | 13.00 (10.31) | χ2 (p) = 0.00 (0.99) |
| n | 7 | 10 | 7 | 9 | Pool var = 73.93 |
| Serum copper % change | 3.00 (4.28) | 16.11 (8.16) | 3.63 (7.23) | 13.56 (10.25) | χ2 (p) = 0.19 (0.67) |
| n | 7 | 8 | 8 | 9 | Pool var = 63.66 |
| 5′-Nucleotidase % change | 0.80 (3.63) | 14.71 (12.83) | 3.80 (4.27) | 13.00 (7.77) | χ2 (p) = 17.35 (0.000) |
| n | 5 | 7 | 5 | 6 | Pool var = 74.48 |
| Serum iron % change | −1.67 (2.08) | 16.00 (8.15) | 5.00 (4.24) | 13.40 (12.30) | χ2 (p) = 1.42 (0.23) |
| n | 3 | 5 | 2 | 5 | Pool var = 81.59 |
Three-way interaction between Time, Mediator, and Treatment. χ2 is for the Wald statistic associated with the coefficient of the interaction term in the GLM.
Variable is significantly correlated with treatment.
Chi-squared for main effect of variable on outcome.
Abbreviations: ABC = Abberrant Behavior Checklist; RID = radial immunodiffusion.
FIG. 2.
Weight gain (percent of baseline kg) as mediator of response to risperidone. ABC = Aberrant Behavior Checklist. Lines represent regression of outcome on weight gain. Main effect is expected effect when weight gain is unaffected by treatment, and is drawn at the mean placebo weight gain. Observed effect is the difference between average placebo and risperidone response on the outcome measure. The two horizontal dotted ghost lines at about 4 and 15 represent mean improvement of respective treatment groups.
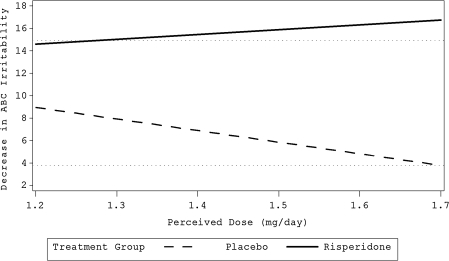
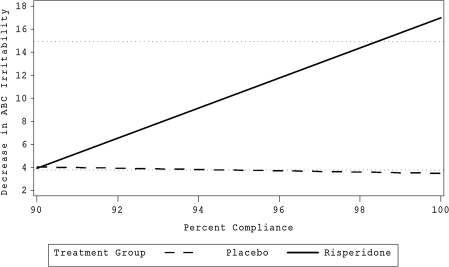
The two medication-related variables, dose and compliance, were significant predictors of the effectiveness of risperidone. Dose had a strong and significant point-biserial correlation with treatment (r = 0.47, p = 0.0009); children taking risperidone were likely to receive lower doses than children randomized to placebo. Dose was also part of a three-way interaction with time and treatment (χ2 = 8.19, p = 0.0042). Figure 3 illustrates the relationship of dose to outcome. Compliance was also significantly (although weakly) correlated with treatment (r = 0.22, p = 0.028) and part of a significant three-way interaction (χ2 = 7.39, p = 0.006). For children in the risperidone group, but not in the placebo group, better compliance was associated with greater improvement (Fig. 4).
FIG. 3.
Relationship between optimal perceived dose (mg/day) and outcome. Horizontal dotted ghost lines at 4 and 15 represent mean improvement of respective treatment groups. Bolded lines represent the regression of ABC Irritability subscale score on the perceived dose of risperidone.
FIG. 4.
Relationship between percent compliance and outcome. Horizontal dotted ghost lines at 4 and 15 represent mean improvement of respective treatment groups. Bolded lines represent the regression of ABC Irritability subscale score on the percent of compliance with the treatment prescription.
Some variables measuring change during treatment had either main or interactive effects, but were not correlated with treatment (and therefore were not mediators). Percent change in serum 5′-nucleotidase, a measure of body zinc status (Bales et al. 1994), was part of a significant three-way interaction with time and treatment (χ2 = 17.35, p < 0.0001). The 5′-nucleotidase change was independent of treatment assignment, but in the presence of risperidone, decrease of 5′-nucleotidase was associated with greater improvement than increase was. This relationship did not hold for placebo, which tended the opposite direction. Therefore, as explained by Kraemer et al. (2002), the association of improvement with degree of serum 5′-nucleotidase decrease was itself moderated by risperidone.
Discussion
Overall, the finding of few moderators of risperidone response is scientifically disappointing but clinically encouraging, although it should be noted that the possibility of Type II errors (false negatives) can not be ruled out. These results suggest that risperidone is effective for a wide range of children with autism and irritability, aggression, self-injury, and other disruptive behaviors. Only one variable was found to be a significant moderator of response to risperidone: Initial ABC Irritability subscale score. The fact that high initial severity on the ABC Irritability subscale at baseline is related to greater improvement from baseline than low initial severity is intuitively reasonable. The high-severity risperidone group had more room to improve and thus could show more improvement. Regression to the mean cannot account completely for the greater improvement because overall declines were virtually identical for both high and low initial-irritability placebo groups (parallel slopes). Possibly a better explanation could be the behavioral pharmacology phenomenon of rate dependency (Sahakian and Robbins 1977), in which treatment tends to “normalize” the target symptom regardless of initial severity, similar to aspirin’s reducing any fever to the same normal temperature. Inspection of the graph in Fig. 1 confirms that regardless of initial severity, the outcome at 8 weeks is very similar. The practical clinical conclusion, again not revolutionary, is that the risk/benefit ratio of risperidone is more favorable for those with greatest severity.
Due to missing blood specimens, the sample sizes of the blood mineral data were considerably smaller than those for other variables, with consequent loss of power and increased risk of false-negative errors. Therefore the mineral marginal trends, such as ceruloplasmin radial immunodiffusion (RID) moderation (p = 0.027; see Table 3), are worth exploring in a larger sample, although no conclusions can be drawn from this study.
The nonspecific predictors of outcome (Table 4) were numerous. Most were variables that predicted poorer response, regardless of the treatment to which the child was randomized. It seems intuitive that a child with various psychological and behavioral symptoms beyond those required for study entry, like those on CSI and ABC subscales referenced in Table 4, would experience poorer outcome than a child without such a burden. Parent income and education were positively associated with outcome, which is consistent with more family resources being available to help the child. Inspection of the raw data in Table 3 suggests that the general prediction of response by baseline prolactin level may have actually been a moderator that lacked sufficient power to show statistical significance (the significance level of the three-way interaction was 0.053, a possible false negative). Lower baseline prolactin predicted more improvement (reduction by 19.1. vs. 12.5) in the risperidone group, but not in the placebo group, which actually showed a nominal tendency the other direction (4.32 vs. 4.85). Low baseline prolactin predicted better outcome only in the risperidone group, and this effect was strong enough to carry the whole sample with a significance level (0.000) that would withstand severe correction for multiple tests. Thus, the association of lower baseline prolactin with better outcome could be compatible with a hypothesis that those with lower prolactin might benefit more from, or be able to tolerate higher doses of, a drug that raises prolactin. However, in this sample, baseline prolactin and risperidone dose did not correlate, casting doubt on the latter (tolerability) hypothesis. The hypothesis of greater benefit from the same dose when starting with low prolactin would be partially supported by correlation of improvement with prolactin increase, but the modest tendency in this direction was not significant.
Weight gain was the only significant mediator of response to risperidone. It is well known that risperidone and other antipsychotics are commonly associated with weight gain (Allison et al. 1999), and, in this sample, active medication and weight gain were significantly correlated. The mediating effect of weight gain, however, was a negative one for risperidone. In another analysis including the placebo nonresponders from this study who received an open trial of risperidone as well as those originally assigned to risperidone (total n = 72 receiving risperidone), McCracken et al. (2009) found that weight gain (in kg) was significantly negatively correlated with improvement in ABC Irritability subscale score (r = −0.23, p = 0.048). We cannot infer that weight gain worsened outcome, because in the placebo group it was positively associated with improvement. One might suspect that the finding could be an artifact of those with less favorable clinical outcomes having their dose pushed higher than those with more favorable outcomes; if side effects, including weight gain, increased with dose, this could cause an association of poorer outcome with weight gain. However, this explanation would not be compatible with the dose analysis, in which those taking higher doses of risperidone had at least as good outcomes as those taking lower doses (Fig. 3). In any event, the finding does suggest that amending risperidone treatment in some way to prevent weight gain should not interfere with clinical benefit and might even increase the effectiveness. One way to explore this might be a randomized controlled trial of risperidone alone versus risperidone plus diet and exercise or versus risperidone plus metformin to prevent weight gain. Metformin, an antidiabetic drug, has been reported to be safe and effective to combat weight gain of antipsychotics in adults (Wu et al. 2008) and children (Klein et al. 2006). If the treatment including weight gain prevention produced better symptom outcomes than risperidone alone, then weight gain would be a negative mediator of some clinical value, and the behavioral effect would add to the physical health value of preventing excessive weight gain.
The association of dose with outcome may have been an artifact of the study titration combined with the method with which we analyzed the data (following Kraemer et al. 2002). Both treatment groups were included in the analysis. In this study, dose was titrated against clinical effect and side effects in both treatment groups. It is not unexpected that higher doses were associated with worse outcome for the placebo group relative to lower doses, while dose did not significantly affect response to risperidone, which was titrated to an individually optimal dose. These findings are consistent with a physician prescribing increasing levels of placebo in the face of unimproved behavior and no side effects. Indeed, the mean and median doses were higher for placebo than for risperidone (2.4 and 1.7 mg vs, 1.7 and 1.3 mg, respectively). Although interesting, this finding is probably not clinically relevant to treatment with risperidone other than suggesting that it is possible to titrate individually to a consistent level of symptom control.
Compliance was related to outcome as predicted. Overall, compliance levels were high, a result of relatively tight monitoring. Within the risperidone group, good compliance was associated with better outcome than was noncompliance, but this association did not hold for the placebo group. Ostensibly, those participants who were able to comply better with dosing were able to reap the benefits of risperidone treatment. Of course, one might argue that they were able to comply because they were already better, but this argument would be incompatible with not finding such an effect in the placebo group. It is interesting that the regression lines shown in Fig. 4 predict that at a compliance level of about 90%, the response to risperidone would essentially equal the average placebo response. This finding reinforces the common knowledge that compliance is important.
The enzyme 5′-nucleotidase requires zinc for activity, so it has been studied for indication of zinc status in people without liver problems (which could elevate its levels) (Bales et al. 1994; Prasad 1994; Blostein-Fujii et al. 1997). Although the relationship of zinc status to 5′-nucleotidase activities in growing children has not been directly studied, it has been studied in other contexts. Plasma or serum 5′-nucleotidase activities have reflected moderate changes in zinc status in diabetic adult women (Blostein-Fujii et al. 1997), in elderly men and women (Bales et al. 1994), in young adult women (Zhang W, DiSilvestro RA, unpublished results), and in growing rats (DiSilvestro RA, unpublished results). Results have to be interpreted in the light of children tending to have lower 5′-nucleotidase serum activities than adults (Belfield and Goldberg 1971). In this sample, serum 5′-nucleotidase change was itself moderated by risperidone; if a child experienced a decrease in serum 5′-nucleotidase while taking risperidone (but not placebo), then (s)he was likely to experience greater improvement in irritability than those with 5′-nucleotidase increase. This suggests that increases in body zinc status are associated with less improvement with risperidone. However, this suggestive association is qualified by the absence of a parallel relationship between plasma zinc level and ABC Irritability subscale score improvement. Nevertheless, the finding was of such high significance (p = 0.0001) that it would withstand severe correction for multiple testing and deserves exploration in future studies.
This study has several limitations, including missing data for the mineral assays, which depended on frozen leftover serum from other blood tests. There is risk of both Type I error from multiple tests and Type II error from insufficient power from subdividing the sample. One might argue that the alpha of 0.01 was not adequate correction for multiple tests, but we felt it struck a reasonable balance between the two error possibilities in these exploratory analyses. As it happened, most of the significant findings had p values that would have withstood several-fold greater Bonferroni correction, so this may be a moot point. The marginal trends at 0.01< p < 0.05 (e.g., ceruloplasmin moderation and ferritin mediation) could conceivably be false negatives, but could also just be chance variations. The only way to tell is to repeat the analyses in a new (and larger) sample.
Conclusion
More severely irritable patients with autism benefit more from risperidone because all patients tend to improve to the same symptomatic level; this makes the risk/benefit ratio more favorable for more severe cases. Prevention of weight gain from risperidone treatment may benefit psychological as well as physical health by improving risperidone response. The 5′-nucleotidase finding, if upheld by replication, offers interesting hypotheses, including augmentation of the risperidone effect by changing body levels of zinc or other minerals. Possibly dietary changes made possible by risperidone-induced appetite increases may play a role. We will soon be exploring this issue in a newly completed study of risperidone in which food-frequency questionnaires were collected at key points. Meanwhile, it seems reasonable for clinicians to recommend daily intake (RDI) multivitamin/mineral supplements (not megadoses) for idiosyncratically unbalanced diets, encourage a better balance, and suggest that parents use the increased appetite from risperidone to enforce a better balanced diet. Other common-sense clinical principles confirmed by the findings reported here include the importance of compliance.
Footnotes
This research was supported by contracts from the National Institute of Mental Health (N01MH70009, to Dr. Scahill; N01MH70010, to Dr. McCracken; N01MH70001, to Dr. McDougle; and N01MG80011, to Dr. Aman), General Clinical Research Center grants from the National Institutes of Health (M01 RR00750, to Indiana University; M01 RR00052, to Johns Hopkins University; M01 RR0034, to Ohio State University; and M01 RR06022, to Yale University), and a grant from the Korczak Foundation (to Dr. Scahill). Study medications were donated by Janssen Pharmaceutica.
Disclosures
Dr. Arnold has received research funding from Celgene, Shire, Noven, Lilly, Targacept, Sigma Tau, Neuropharm, and Novartis; has consulted for Abbott, Neuropharm, Novartis, Noven, Shire, Sigma Tau, and Organon; and has been on speaker's bureau for Abbott, Shire, McNeil, and Novartis. Dr. Disilvestro consults for Albion Laboratories. Dr. McDougle has received research funding from Bristol-Myers Squibb Co., has consulted to Bristol-Myers Squibb Co., F. Hoffman-LaRoche Ltd., and Forest Research Institute, and has been on speaker's bureau for Bristol-Myers Squibb Co. Dr. McCracken has received research funding from Bristol-Myers Squibb, Eli Lilly, McNeil, Pfizer, Shire, Johnson & Johnson, and Aspect; consulted for Abbott, Eli Lilly, Janssen, Johnson & Johnson, McNeil, Novartis, Wyeth, Shire, and Sanofi-Aventis; and has been on speaker's bureau for UCB and Eli Lilly. Dr. Aman has received research funding from Bristol-Myers Squibb Co. and Johnson and Johnson and has consulted for Bristol-Myers Squibb Co. Dr. Scahill has served as an advisor or consultant for Janssen, Pfizer, and Bristol-Myers Squibb. Dr. Posey has lectured for Janssen and has had financial affiliations with Bristol-Myers Squibb Co., Eli Lilly, Forest, and Shire. The other authors have no financial ties or conflicts of interest to disclose.
References
- Allison D. Mentore J. Heo M. Chandler L. Cappelleri J. Infante M. Weiden P. Antipsychotic-induced weight gain: A comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- Aman MG. Singh NN. Stewart AQ. Field CJ. The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985;89:485–491. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic, Statistical Manual of Mental of Disorders. 4th. Washington (DC): American Psychiatric Association; 1994. (DSM-IV) [Google Scholar]
- Arnold LE. Aman MG. Martin A. Collier-Crespin A. Vitiello B. Tierney E. Asarnow R. Bell-Bradshaw F. Freeman BJ. Gates-Ulanet P. Klin A. McCracken JT. McDougle CJ. McGough JJ. Posey DJ. Scahill L. Swiezy NB. Ritz L. Volkmar F. Assessment in multisite randomized clinical trials (RCTs) of patients with autistic disorder: The Autism RUPP network. J Autism Dev Disord. 2000;30:99–111. doi: 10.1023/a:1005451304303. [DOI] [PubMed] [Google Scholar]
- Bales C. DiSilvestro R. Currie K. Plaisted C. Joung H. Galanos A. Lin P. Marginal zinc deficiency in older adults: Responsiveness of zinc status indicators. J Am Coll Nutr. 1994;13:455–462. doi: 10.1080/07315724.1994.10718434. [DOI] [PubMed] [Google Scholar]
- Baron R. Kenny D. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Belfield A. Goldberg DM. Normal ranges and diagnostic value of serum 5′-nucleotidase and alkaline phosphatase activities in infancy. Arch Dis Child. 1971;46:842–846. doi: 10.1136/adc.46.250.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blostein-Fujii A. DiSilvestro RA. Frid D. Katz C. Malarkey W. Short term zinc supplementation in type II diabetic women: Effects on plasma 5′-nucleotidase activities, insulin-like growth factor levels & lipoprotein oxidation rates in vitro. Am J Clin Nutr. 1997;66:639–642. doi: 10.1093/ajcn/66.3.639. [DOI] [PubMed] [Google Scholar]
- Chauhan A. Chauhan V. Brown W. Cohen I. Oxidative stress in autism: Increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin—the antioxidant proteins. Life Sci. 2004;75:2539–2549. doi: 10.1016/j.lfs.2004.04.038. [DOI] [PubMed] [Google Scholar]
- DiSilvestro RA. Plasma levels of immunoreactive ceruloplasmin and other acute phase proteins during lactation. Proc Soc Exp Biol Med. 1986;183:257–261. doi: 10.3181/00379727-183-42415. [DOI] [PubMed] [Google Scholar]
- Faber S. Zinn G. Kern J. Kingston H. The plasma zinc/serum copper ratio as biomarker in children with autism spectrum disorders. Biomarkers. 2009;14(3):171–180. doi: 10.1080/13547500902783747. [DOI] [PubMed] [Google Scholar]
- Holmbeck G. Toward terminological, conceptual, and statistical clarity in the study of mediators and moderators: Examples from the child-clinical and pediatric psychology literatures. J Consult Clin Psych. 1997;65:599–610. doi: 10.1037//0022-006x.65.4.599. [DOI] [PubMed] [Google Scholar]
- Klein D. Cottingham E. Sorter M. Barton B. Morrison J. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163:2072–2079. doi: 10.1176/ajp.2006.163.12.2072. [DOI] [PubMed] [Google Scholar]
- Kraemer H. Blasey C. Centring in regression analyses: A strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13:141–151. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer H. Wilson G. Fairburn C. Agras W. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- Kraemer H. Kiernan M. Essex M. Kupfer D. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychol. 2008;27:S101–S108. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C. Rutter M. LeCouteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- McCracken J. Aman M. McDougle C. Scahill L. Tierney E. Arnold L. Posey D. Whelan F. Chuang S. Ritz L. Vitiello B. Research Units on Pediatric Psychopharmacology Autism Network: Dopamine genes, weight in children with autism treated with risperidone. Submitted for publication.
- McDougle C. Scahill L. McCracken J. Aman M. Tierney E. Arnold L. Freeman B. Martin A. McGough J. Cronin P. Posey D. Riddle M. Ritz L. Swiezy N. Vitiello B. Volkmar F. Votolato N. Walson P. Research Units on Pediatric Psychopharmacology (RUPP) Autism Network: Background and rationale for an initial controlled study of risperidone. Child Adolesc Psychiatr Clin N Am. 2000;9:201–224. [PubMed] [Google Scholar]
- Prasad AS. The Biochemistry of Zinc. Norwell (Massachusetts): Kluwer Academic Publishers; 1994. [Google Scholar]
- RUPP (Research Units on Pediatric Psychopharmacology) Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ. Robbins TW. Are the effects of psychomotor stimulant drugs on hyperactive children really paradoxical? Med Hypotheses. 1977;3:154–158. doi: 10.1016/0306-9877(77)90065-2. [DOI] [PubMed] [Google Scholar]
- Scahill L. Riddle M. McSwiggin-Hardin M. Ort S. King R. Goodman W. Cicchetti D. Leckman J. Children's Yale-Brown Obsessive Compulsive Scale: Reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Scahill L. McCracken J. McDougle C. Aman M. Arnold LE. Tierney E. Cronin P. Davies M. Ghuman J. Gonzalez N. Koenig K. Lindsay R. Martin A. McGough J. Posey D. Swiezy N. Volkmar F. Ritz L. Vitiello B. Methodological issues in designing a multisite trial of risperidone in children and adolescents with autism. J Child Adolesc Psychopharm. 2001;11:377–388. doi: 10.1089/104454601317261555. [DOI] [PubMed] [Google Scholar]
- Wu R. Zhao JP. Jin H. Shao P. Fang MS. Guo XF. He YQ. Liu YJ. Chen JD. Li LH. Lifestyle intervention, metformin for treatment of antipsychotic-induced weight gain: A randomized controlled trial. J Am Med Assoc. 2008;299:185–193. doi: 10.1001/jama.2007.56-b. [DOI] [PubMed] [Google Scholar]