Abstract
The easy accessibility of skin makes it an excellent target for gene transfer protocols. To take full advantage of skin as a target for gene transfer, it is important to establish an efficient and reproducible delivery system. Electroporation is a strong candidate to meet this delivery criterion. Electroporation of the skin is a simple, direct, in vivo method to deliver genes for therapy. Previously, delivery to the skin was performed by means of applicators with relatively large distances between electrodes, resulting in significant muscle stimulation and pain. These applicators also had limitations in controlling the directionality of the applied field. To resolve this issue, a system consisting of an array of electrodes that decreased the distance between them and that were independently addressable for directional control of the field was developed. This new multielectrode array (MEA) was compared with an established electrode. In a rat model, comparable reporter expression was seen after delivery with each electrode. Delivery was also evaluated in a guinea pig model to determine the potential of this approach in an animal model with skin thickness and structure similar to human skin. The results clearly showed that effective delivery was related to both the electrode and the parameters chosen. With the MEA, the muscle twitching associated with application of electric fields was notably reduced compared with conventional electrode systems. This is important, as it will facilitate the translation of electroporation-mediated gene delivery to skin for clinical use with DNA vaccines or for therapies for cancer or protein deficiencies.
Introduction
The skin is an attractive target for gene medicine, particularly for applications pertaining to cutaneous diseases, vaccines, and some metabolic disorders. It is easily accessible for both delivery and monitoring. As with any gene transfer approach, it is essential to develop a reliable delivery system. Electroporation is an effective means of delivering plasmid DNA to many tissues in vivo (Heller and Heller, 2006). Several studies have shown that electroporation efficiently delivers plasmid DNA to the skin, increasing local and serum expression levels compared with injection alone (Titomirov et al., 1991; Glasspool-Malone et al., 2000; Dujardin et al., 2001; Heller et al., 2001, 2007, 2008; Maruyama et al., 2001; Chesnoy and Huang, 2002; Babiuk et al., 2003; Medi et al., 2005; Pavselj and Preat, 2005). Skin electroporation delivery has been successfully performed in rodent, porcine, and nonhuman primate model systems (Glasspool-Malone et al., 2000).
Electroporation of skin is a simple delivery method for prophylactic or therapeutic gene therapy applications. A key component of delivery is the electrode applicator used to apply the electric fields. Current electrode systems are cumbersome and typically induce significant muscle twitching and discomfort. This is related to the distance between the electrodes and the applied voltage. The current designs are also limited with respect to expandability, and the number of addressable electrode pairs that are contained within the electrode applicator is also limited, which impacts the directional control of the fields.
This brief report describes an approach that could overcome these limitations. A multielectrode array (MEA) applicator (Fig. 1) was developed. With the MEA, the applied voltage is minimized by maintaining a short electrode distance. By reducing the distance between the electrodes, the area of tissue affected by each electric pulse and the depth of penetration of the field are reduced as well. This diminishes or eliminates the muscle twitching and sensation (discomfort) associated with the application of the pulsed electric fields. In addition, the electrodes within the applicator are independently addressable for directional control of the field. Finally, the treatment area can eventually be expanded by the addition of rows of electrodes to the array. In this way, the treatment area can be increased without increasing the applied voltage.
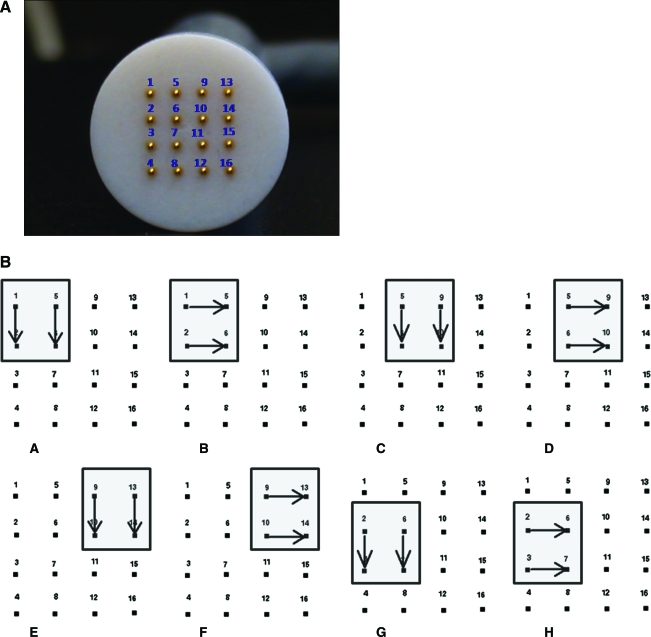
FIG. 1.
Multielectrode array (MEA). (A) The novel multielectrode array was constructed by embedding 16 gold-plated posts, approximately 0.3 mm in diameter, into a flat Teflon disk. The electrodes were embedded so that their circular ends protruded approximately 300 μm from the disk end. The disk was placed in a handle for ease of use. Electrodes are numbered 1–16 to facilitate description of the pulsing sequence explained in Materials and Methods. (B) Illustration depicting the firing sequence. Arrows indicate the active electrodes and four pulses were applied each time. Direction and/or active electrodes change for each set of four pulses. Following sequence H the pattern continues according to the same pattern until all electrodes have been fired, which includes nine 2-mm squares. Color images available online at www.liebertonline.com/hum.
Materials and Methods
Plasmid
gWizLuc was commercially prepared (Aldevron, Fargo, ND). Endotoxin levels were <0.1 EU/μg plasmid.
Animals
Female Hartley guinea pigs (250–300 g) or male Sprague Dawley rats (200–250 g) were used in this study. All animals were anesthetized in an induction chamber charged with 3% isoflurane in O2 and then fitted with a standard rodent mask and kept under general anesthesia during treatment.
Electroporation procedure
Animals received an intradermal injection of gWizLuc (2 mg/ml) in sterile injectable saline via the abdomen (rat) or flank (guinea pig) followed immediately by administration of electric pulses. For those groups receiving electric pulses with the 4PE, the electrode was placed around the injection site and two sets of four pulses in perpendicular directions were administered as previously described (Heller et al., 2007, 2008). The 4PE consists of four stainless steel plates (3.5 mm wide) that are insulated except for 2–3 mm at the bottom of each inside face. The four plates are situated around a nonconductive “stopper” that orients the plates to form a 6 × 6 mm square. This allows the 4PE to be placed around a 6-mm-diameter area formed after the 50-μl injection. In addition, this orientation allows pulses to be applied in two electric field orientations at a 90° angle. For those groups receiving electric pulses with the MEA, the applicator was placed over the injection site and pulses were applied via groups of four electrodes. The firing sequence covered a series of 2 × 2 mm squares and was fired as follows (Fig. 1B): 1 and 5 to 2 and 6; 1 and 2 to 5 and 6; 5 and 9 to 6 and 10; 5 and 6 to 9 and 10; 9 and 13 to 10 and 14; 9 and 10 to 13 and 14; 2 and 6 to 3 and 7; 2 and 3 to 6 and 7, and so on. The electric pulses were generated with a high-voltage power supply (HV Rack, 1/4C24-P250; Ultravolt, Ronkonkoma, NY). The applied voltage was controlled by the high-voltage power supply, whereas pulse width, pulse frequency, and pulsing sequence were controlled by a customized program using LabVIEW 8.2.1 (National Instruments, Austin, TX). The applied voltages were varied as described.
Luciferase reporter assay
At the indicated time points after plasmid delivery, luciferase activity was quantified as previously described (Heller et al., 2000). Activity was expressed as total nanograms of luciferase per tissue sample. Statistical analysis was performed by Kruskal–Wallis nonparametric analysis of variance. Group differences were compared by Dunn's multiple comparisons test.
Histological analysis
Forty-eight hours after treatment, guinea pigs were killed and the 6-mm-diameter treated area was removed. Each sample was fixed and four sections were stained with hematoxylin and eosin and then examined histologically for damage. Samples were graded for percent damage, using a schema including surface damage (burning and/or necrosis) and subepidermal necrosis (Heller et al., 2007). The total amount of damage (surface and subepidermal area affected) was determined as a percentage of the total treatment area.
Results and Discussion
Delivery of plasmid DNA to the skin by electroporation was previously evaluated in both mouse and rat skin, using a specially constructed electrode (4PE; see Materials and Methods) (Heller et al., 2007, 2008). Skin delivery has been performed with caliper electrodes as well. Although cutaneous delivery was successful and high levels of expression were achieved, these electrode designs have some drawbacks. First, the field is developed across a small set of anodes and cathodes that are kept a specific distance apart. With the exception of relatively small surface areas, these designs prevent optimal interaction of the applicator with the target tissue. Second, the nature of these electrode designs produces excessive distance between the electrode pairs, which requires additional voltage to create the desired field strength. Thus, the administration of electric pulses results in significant muscle stimulation. This stimulation has been associated with pain or discomfort in clinical studies (Zhang and Rabussay, 2002; Wong et al., 2006; Zupanic et al., 2007; Daud et al., 2008; Bodles-Brakhop et al., 2009; Wallace et al., 2009). This becomes an important issue, particularly if it is necessary to treat a large area to obtain therapeutic levels of a delivered transgene. To reduce the sensation associated with the administration of electric pulses through the electrodes, another electrode configuration was developed and tested. The MEA (Fig. 1) contained 16 electrodes configured in 4 rows of 4 electrodes with a 2-mm electrode separation. The electrodes within the MEA are independently addressable and thus can be administered in specific pulsing patterns.
Intradermal delivery of a plasmid encoding luciferase was used to evaluate the effectiveness of this new design in a Sprague Dawley rat model (Fig. 2). Several electroporation parameters, based on previous results with the 4PE, were tested and the levels of expression compared with delivery with the 4PE (four pulses applied in two perpendicular directions for a total of eight pulses). The pulsing pattern of the MEA was similar to the 4PE, with separate pulses applied in perpendicular directions between each group of four electrodes. Four pulses were administered between each of the anode–cathode pairs (two perpendicular directions for a total of eight pulses) in a series of 2-mm squares as described in Materials and Methods. The MEA contains a total of nine 2-mm squares and therefore a complete MEA sequence included 72 pulses.
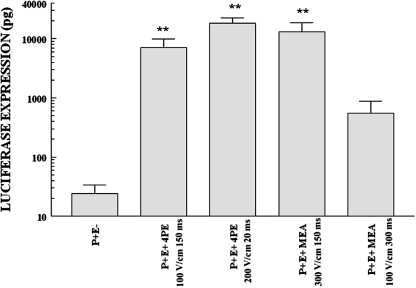
FIG. 2.
Comparison of MEA and 4PE in Sprague Dawley rats. Intradermal injection of gWizLuc plasmid (50 μl, 2 mg/ml) was performed via the abdomen. Electroporation was performed with either the 4PE or MEA under the specified conditions. Columns and error bars represent the mean of the means and standard error of the means for three replicate experiments of four samples each. **p < 0.01 compared with injection alone. P+, injection with gWizLuc; E−, no electroporation; E+, electroporation administered.
These results show that both electrode configurations can effectively deliver this reporter plasmid. All electroporation conditions tested with both electrodes showed significantly higher expression than injection of plasmid without electroporation. In addition, both the 4PE pulsing conditions and the 300 V/cm, 150 msec pulsing conditions with the MEA were significantly higher than the levels obtained with the MEA at 100 V/cm and 300 msec. These results suggest that the applied electric field strength is an important consideration, because comparable expression was obtained with increased field strength, but not with increased pulse duration. Although it was necessary to increase the field strength to achieve comparable expression patterns with the MEA, muscle stimulation during pulse administration was greatly reduced or eliminated with the MEA applicator when compared with the pronounced muscle contractions seen with the 4PE and caliper electrodes. Although this is not testable in animals, this suggests that the potential discomfort associated with delivery using this electrode design would be reduced or eliminated.
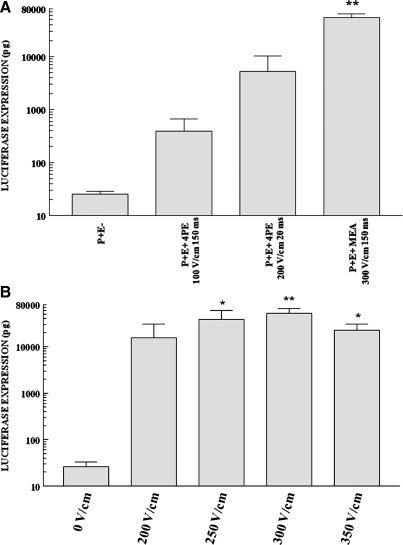
To better simulate delivery to skin similar in thickness to human skin, a second series of experiments to test the MEA was performed with guinea pigs. Expression in guinea pig skin, using parameters of 300 V/cm and 150 msec (Fig. 3A), was higher than that observed in rat skin (p < 0.01) when using the MEA and the same pulsing conditions. Interestingly, expression levels obtained with the 4PE and the same parameters as with rat skin were greatly reduced. Expression levels obtained with the MEA were significantly higher (p < 0.01) than those obtained with the 4PE or injection only (Fig. 3A). As was seen in the rat studies, there was a greatly reduced level of muscle twitching with the MEA compared with the 4PE. The effect of varying the field strength with the MEA was also evaluated. Delivery of plasmid with applied field strengths of 250, 300, and 350 V/cm at a pulse width of 150 msec resulted in significantly higher expression than injection of plasmid without electroporation (Fig. 3B). Expression levels did not significantly differ between these three groups. Delivery of plasmid with an applied field strength of 200 V/cm at a pulse width of 150 msec did not result in significantly higher expression than injection of plasmid without electroporation.
FIG. 3.
Delivery of gWizLuc to Hartley guinea pig skin with MEA and 4PE. Intradermal injection of gWizLuc plasmid (50 μl, 2 mg/ml) was performed via the flank. (A) Electroporation was performed with the MEA or 4PE at the specified field strengths and pulse widths. Columns and error bars represent the mean of the means and standard error of the means for three replicate experiments. **p < 0.01, comparing the MEA with injection alone or 4PE. P+, injection with gWizLuc; E−, no electroporation; E+, electroporation administered. (B) Electroporation was performed with the MEA at the specified field strengths at a pulse width of 150 msec. Columns and error bars represent the mean of the means and standard error of the means. For 250 and 300 V/cm there were eight replicate experiments; for 200 V/cm there were four replicate experiments; and for 350 V/cm there were three replicate experiments of four samples each. *p < 0.05; **p < 0.01 when compared with injection alone (eight replicate experiments).
In addition to expression levels, it was also critical to determine whether delivery with the MEA caused damage to the skin. The delivery was repeated using injection of plasmid alone, injection of plasmid with electroporation at 300 V/cm and 150 msec, or injection of saline without electroporation (four samples for each group). Guinea pigs were killed 48 hr after the procedure and the treated area was removed for histological evaluation. Minimal to no damage was seen in the guinea pigs receiving plasmid and saline injection. Less than 5% damage was seen in the electroporated samples. These results are similar to what was reported previously when delivery was performed to mouse skin (Heller et al., 2007).
Electroporation is a powerful tool for use in nonviral gene therapy. Efficient and effective delivery has been accomplished preclinically to a variety of tissues and for multiple therapeutic applications (Heller and Heller, 2006; Bodles-Brakhop et al., 2009). The first clinical trials using this delivery approach for gene therapy have been initiated. The results from the first trial have been reported and demonstrate the effectiveness of this approach in the delivery of the immune modulator interleukin-12 to melanomas (Daud et al., 2008). The results presented here show that effective delivery of plasmid DNA can be accomplished with a multielectrode array. This array was designed to maintain the distance between electrodes independent of the actual delivery area, minimizing the applied voltage needed for delivery. Although a higher applied field at the same applied voltage was needed compared with a standard electrode, the muscle twitching associated with application of electric fields was observed to be drastically reduced. These improvements to the electrode applicator will facilitate the translation of electroporation-mediated gene delivery to the clinic for use with DNA vaccines or for therapies for cancer or protein deficiencies.
Acknowledgments
This research was supported in part by research grants from the National Institutes of Health (R21 DK055588 and R01 EB005441) and by the Center for Molecular Delivery at the University of South Florida. Genetronics donated the pulse generator used with the 4PE.
Author Disclosure Statement
With respect to duality of interest, Drs. Heller, Gilbert, and Jaroszeski are coinventors on patents that cover the technology used in the work reported in this brief report. The patents have been licensed to RMR Technologies and sublicensed to Inovio Biomedical. Drs. Heller, Gilbert, and Jaroszeski have ownership interest in RMR Technologies and own stock and stock options in Inovio.
References
- Babiuk S. Baca-Estrada M.E. Foldvari M. Baizer L. Stout R. Storms M. Rabussay D. Widera G. Babiuk L. Needle-free topical electroporation improves gene expression from plasmids administered in porcine skin. Mol. Ther. 2003;8:992–998. doi: 10.1016/j.ymthe.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Bodles-Brakhop A.M. Heller R. Draghia-Akli R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: Current clinical developments. Mol. Ther. 2009;17:585–592. doi: 10.1038/mt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnoy S. Huang L. Enhanced cutaneous gene delivery following intradermal injection of naked DNA in a high ionic strength solution. Mol. Ther. 2002;5:57–62. doi: 10.1006/mthe.2001.0511. [DOI] [PubMed] [Google Scholar]
- Daud A.I. DeConti R.C. Andrews S. Urbas P. Riker A.I. Sondak V.K. Munster P.N. Sullivan D.M. Ugen K.E. Messina J.L. Heller R. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J. Clin. Oncol. 2008;26:5896–5903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin N. Van Deŕ Smissen P. Préat V. Topical gene transfer into rat skin using electroporation. Pharm. Res. 2001;18:61–66. doi: 10.1023/a:1011026726938. [DOI] [PubMed] [Google Scholar]
- Glasspool-Malone J. Somiari S. Drabick J.J. Malone R.W. Efficient nonviral cutaneous transfection. Mol. Ther. 2000;2:140–146. doi: 10.1006/mthe.2000.0107. [DOI] [PubMed] [Google Scholar]
- Heller L. Jaroszeski M.J. Coppola D. Pottinger C. Gilbert R. Heller R. Electrically mediated plasmid DNA delivery to hepatocellular carcinomas in vivo. Gene Ther. 2000;7:826–829. doi: 10.1038/sj.gt.3301173. [DOI] [PubMed] [Google Scholar]
- Heller L.C. Heller R. In vivo electroporation for gene therapy. Hum. Gene Ther. 2006;17:890–897. doi: 10.1089/hum.2006.17.890. [DOI] [PubMed] [Google Scholar]
- Heller L.C. Jaroszeski M.J. Coppola D. McCray A.N. Hickey J. Heller R. Optimization of cutaneous electrically mediated plasmid DNA delivery using novel electrode. Gene Ther. 2007;14:275–280. doi: 10.1038/sj.gt.3302867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller L.C. Jaroszeski M.J. Coppola D. Heller R. Comparison of electrically mediated and liposome-complexed plasmid DNA delivery to the skin. Genet. Vaccines Ther. 2008;6:16–23. doi: 10.1186/1479-0556-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller R. Schultz J. Lucas M.L. Jaroszeski M.J. Heller L.C. Gilbert R.A. Moelling K. Nicolau C. Intradermal delivery of interleukin-12 plasmid DNA by in vivo electroporation. DNA Cell Biol. 2001;20:21–26. doi: 10.1089/10445490150504666. [DOI] [PubMed] [Google Scholar]
- Maruyama H. Ataka K. Higuchi N. Sakamoto F. Gejyo F. Miyazaki J. Skin targeted gene transfer using in vivo electroporation. Gene Ther. 2001;8:1808–1812. doi: 10.1038/sj.gt.3301604. [DOI] [PubMed] [Google Scholar]
- Medi B.M. Hoselton S. Marepalli R.B. Singh J. Skin targeted DNA vaccine delivery using electroporation in rabbits. I. Efficacy. Int. J. Pharm. 2005;294:53–63. doi: 10.1016/j.ijpharm.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Pavselj N. Preat V. DNA electrotransfer into the skin using a combination of one high- and one low-voltage pulse. J. Control. Release. 2005;106:407–415. doi: 10.1016/j.jconrel.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Titomirov A.V. Sukharev S. Kistanova E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim. Biophys. Acta. 1991;1088:131–134. doi: 10.1016/0167-4781(91)90162-f. [DOI] [PubMed] [Google Scholar]
- Wallace M. Evans B. Woods S. Mogg R. Zhang L. Finnefrock A.C. Rabussay D. Fons M. Mallee J. Mehrotra D. Schödel F. Musey L. Tolerability of two sequential electroporation treatments using MedPulser DNA delivery system (DDS) in healthy adults. Mol. Ther. 2009;17:922–928. doi: 10.1038/mt.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T.W. Chen C.H. Huang C.C. Lin C.D. Hui S.W. Painless electroporation with a new needle-free microelectrode array to enhance transdermal drug delivery. J. Control. Release. 2006;110:557–565. doi: 10.1016/j.jconrel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Zhang L. Rabussay D.P. Clinical evaluation of safety and human tolerance of electrical sensation induced by electric fields with non-invasive electrodes. Bioelectrochemistry. 2002;56:233–236. doi: 10.1016/s1567-5394(02)00057-9. [DOI] [PubMed] [Google Scholar]
- Zupanic A. Ribaric S. Miklavcic D. Increasing the repetition frequency of electric pulse delivery reduces unpleasant sensations that occur in electrochemotherapy. Neoplasma. 2007;54:246–250. [PubMed] [Google Scholar]