Abstract
Oncolytic virotherapy can be achieved in two ways: (1) by exploiting an innate ability of certain viruses to selectively replicate in tumor tissues, and (2) by using viruses to deliver toxic or immunostimulatory genes to tumors. Vesicular stomatitis virus (VSV) selectively replicates in tumors lacking adequate type I interferon response. The efficacy of oncolytic virotherapy using VSV against B16 melanomas in C57BL/6 mice is dependent on CD8+ T and natural killer cells. Because immunotherapies that prime specific CD8+ T cells against melanocyte/melanoma antigens can generate significant therapeutic responses, we hypothesized that engineering VSV to express the potent T cell costimulatory molecule CD40 ligand (VSV-CD40L) would enhance virotherapy with concomitant priming of melanoma-specific T cells. However, we observed no difference in antitumor efficacy between the parental VSV-GFP and VSV-CD40L. In contrast, intratumoral injection of a replication-defective adenovirus expressing CD40L (Ad-CD40L) consistently produced significantly greater therapy than either replication-competent VSV-GFP or VSV-CD40L. The Ad-CD40L-mediated tumor regressions were associated with specific T cell responses against tumor-associated antigens (TAAs), which took several days to develop, whereas VSV-CD40L rapidly induced high levels of T cell activation without specificity for TAAs. These data suggest that the high levels of VSV-associated immunogenicity distracted immune responses away from priming of tumor-specific T cells, even in the presence of potent costimulatory signals. In contrast, a replication-defective Ad-CD40L allowed significant priming of T cells directed against TAAs. These observations suggest that an efficiently primed antitumor T cell response can produce similar, if not better, therapy against an established melanoma compared with intratumoral injection of a replication-competent oncolytic virus.
Introduction
Infection of normal cells with vesicular stomatitis virus (VSV), a negative-strand rhabdovirus, induces type I interferon responses (IFN-α/β), thereby blocking viral replication and extinguishing infection. In contrast, many tumor cells have defects in their IFN response (Balachandran and Barber, 2004; Lichty et al., 2004; Barber, 2005) allowing free-ranging infection and lysis of tumors (Stojdl et al., 2000; Balachandran et al., 2001; Bell et al., 2003). Therefore, VSV has been proposed as an antitumor agent with high levels of tumor specificity and has been shown to be a potent oncolytic agent against a variety of human and rodent tumors in both immunodeficient and immunocompetent models (Stojdl et al., 2000; Balachandran et al., 2001; Fernandez et al., 2002; Bell et al., 2003; Obuchi et al., 2003; Porosnicu et al., 2003; Balachandran and Barber, 2004; Lichty et al., 2004; Ebert et al., 2005; Freeman et al., 2006; Bergman et al., 2007; Diaz et al., 2007). Indeed, we have shown that delivery of oncolytic VSV into B16ova melanomas growing in immune-competent C57BL/6 mice led to tumor regression and was curative in a proportion of animals (Diaz et al., 2007; Kottke et al., 2008; Qiao et al., 2008). Direct intratumoral injection of VSV resulted in recruitment of multiple immune effectors; of these CD8+ T cells and natural killer (NK) cells were critical for the antitumor therapy (Diaz et al., 2007). We also observed that oncolytic VSV virotherapy primed specific T cell responses against tumor-associated antigens (TAAs) expressed within the B16ova tumors only when either the virus itself was engineered to express a TAA (Diaz et al., 2007), or when the precursor frequency of antigen-specific T cells specific for a TAA was artificially increased before virotherapy by adoptive transfer of T lymphocytes (Kottke et al., 2008; Qiao et al., 2008).
Immunotherapy strategies directed at inducing heat shock protein-mediated killing of normal melanocytes lead to the priming of CD8+ T cell responses specific for melanocyte/melanoma-associated antigens; these CD8+ T cells directly mediate regression and cures of small established B16ova melanomas (Daniels et al., 2004; Sanchez-Perez et al., 2006). Furthermore, additional coexpression of the potent T cell-costimulatory molecule CD40 ligand (CD40L) increases the frequency of melanocyte/melanoma-specific T cells, induces long lasting antitumor memory responses, overcomes suppressive regulatory T cell responses, and allows for the treatment of significantly larger established B16ova tumors (Daniels et al., 2004; Sanchez-Perez et al., 2006).
CD40L (CD154, gp39), a membrane glycoprotein that is a member of the tumor necrosis factor (TNF) superfamily, is transiently expressed on the surface of activated CD4+ T cells (Banchereau et al., 1994; Van Kooten and Banchereau, 2000). CD40L interacts with its receptor CD40—expressed on B cells and antigen-presenting cells (APCs)—to potentiate both humoral and adaptive cell-mediated immune responses. CD40L has been shown to induce effective antitumor immunity after the administration of irradiated tumor vaccines (Mackey et al., 1997, 1998) and expression of CD40L on either mouse tumors, or intratumorally implanted dendritic cells, confers durable protection against subsequent tumor challenges, and generates potent antimelanoma cytolytic T cell responses (Kikuchi and Crystal, 1999; Kikuchi et al., 2000; Suna et al., 2000; Schmitz et al., 2001).
Given that VSV virotherapy in immunocompetent mice is itself an immunotherapy dependent on CD8+ T cells, as well as our experience that CD40L is a potent costimulator of anti-B16ova CD8+ T cell responses, we hypothesized that engineering VSV to express CD40L (VSV-CD40L) would further enhance VSV virotherapy by priming synergistic melanoma-specific T cell responses. In particular, we reasoned that the replication-competent, oncolytic VSV platform would allow for high levels of expression of the transgene through the infected tumor and that this would combine with virus-mediated tumor cell death to create a highly inflammatory tumor microenvironment. This combination of tumor cell death, in the presence of high levels of CD40L expression, would be predicted to lead to high levels of antigen release in an inflammatory milieu. This “danger-filled” microenvironment would be ideally suited to cross-priming of TAA to naive T cells by APCs. The generation of increased levels of T cells specific for TAAs would then provide further therapeutic effect against established B16ova tumors, whereas a simple intratumoral injection of oncolytic VSV was insufficient to induce complete tumor regressions.
Here, we show that expression of potent immune stimulator CD40L in the VSV platform added no improvement over VSV-GFP in the B16ova tumor model. Both viruses induced rapid T cell activation that was not, however, associated with specific T cell responses against TAAs expressed in B16ova tumor cells. We further compared the efficacy of intratumoral injections of this immunogenic, replication-competent VSV-CD40L with a much less immunogenic, replication-defective adenovirus expressing CD40L. Interestingly, Ad-CD40L consistently generated significantly greater therapy than either replication-competent VSV-CD40L or VSV-GFP, and was associated with specific T cell responses against TAAs. Overall, these data suggest that the choice of a vector platform is critical when considering the therapeutic goal of gene/immuno/virotherapy (i.e., direct tumor cell lysis vs. immune priming) and that an appropriately primed immune response directed against TAAs can be at least as effective, if not more so, as direct virus-mediated tumor cell destruction mediated by an oncolytic virus such as VSV.
Materials and Methods
Cell lines
Murine B16ova melanoma cells (H2-Kb) were derived from B16 cells by transduction with a cDNA encoding the chicken ovalbumin gene (Linardakis et al., 2002). Cell lines were grown in Dulbecco's modified Eagle's medium (DMEM) with glucose (4.5 g/liter) and l-glutamine without sodium pyruvate (Mediatech, Herndon, VA) supplemented with 10% (v/v) fetal bovine serum (Life Technologies, Carlsbad, CA). All cell lines were monitored routinely and found to be free of Mycoplasma infection (Diaz et al., 2007).
Viruses
Adenoviral vectors
Ad-GFP is a replication-defective adenovirus serotype 5 (Ad5) vector (ΔE1 and ΔE3) in which the green fluorescent protein (GFP)-encoding gene is driven by a cytomegalovirus (CMV) promoter at the E1 region (Hingorani et al., 2008). Ad-CD40L is also a replication-deficient Ad5 (ΔE1 and ΔE3) expressing murine CD40L (Ad-CD40L) that is driven by the CMV promoter. Ad-CD40L was created with an AdMax kit (Microbix Biosystems, Toronto, ON, Canada). Specifically, cDNA from the open reading frame of the mouse CD40L gene was cloned into the pDC515 shuttle plasmid and cotransfected into 293A cells along with the Ad.flp plasmid. The Flp-mediated recombination of both plasmids in 293A cells allowed for the generation of Ad-CD40L (Thanarajasingam, 2006). Large-scale production of Ad-CD40L was done in 293A cells and later fractionated by cesium chloride gradient centrifugation. Further purification was performed by passage through a disposable PD-10 desalting column (GE Healthcare Bio-Sciences, Uppsala, Sweden).
VSV (replication competent)
VSV-GFP (Indiana serotype) was a gift from G. Barber (University of Miami School of Medicine, Miami, FL) and was described previously (Fernandez et al., 2002). VSV-CD40L was constructed by polymerase chain reaction (PCR) amplification of the mouse CD40L gene from pCR2.1-CD40L, and subsequently this PCR product was digested with the restriction enzymes XhoI and NheI and ligated into the plasmid pVSV-XN2 (genomic plasmid of VSV Indiana serotype and a kind gift from J. Rose, Yale University, New Haven, CT) to yield pVSV-CD40L. Recombinant VSV-CD40L and the parental VSV-XN2 were recovered by the method described previously (Lawson et al., 1995). Bulk amplification of plaque-purified VSV was performed by infecting BHK-21 cells (multiplicity of infection [MOI], 0.01) for 24 hr. Filtered supernatants were harvested and subjected to two rounds of 10% sucrose (10%, w/v) in 1 × phosphate-buffered saline (PBS) (Mediatech, Inc., Herndon, VA) cushion centrifugation at 27,000 rpm for 1 hr at 4°C. The pelleted virus was resuspended in 1 × PBS, aliquoted, and kept at −80°C. VSV was titrated in BHK-21 cells, using a standard plaque assay (Diaz et al., 2007).
In vitro studies
Viral titer determination
Cultures of either BHK-21 or B16ova melanoma cells were grown overnight in 6-well plates (750,000 cells per well). Cells were washed once with 1 × PBS and infected with recombinant VSV (MOI of 1 unless otherwise indicated) in plain DMEM for 1 hr at 37°C in a humidified 5% CO2 incubator. Virus was siphoned out and replaced with regular culture medium. Supernatants were harvested at various time points, clarified, filtered, serially diluted in plain DMEM, and titrated in BHK-21 cells, using a standard plaque assay (Diaz et al., 2007).
Viability assays
Overnight cultures of BHK-21 or B16ova melanoma cells (1 × 104 cells per 50 μl of medium per well) in 96-well plates (three replicate wells per sample) were infected with 50 μl of VSV (MOI, 1.0) and incubated at 37°C in a humidified 5% CO2 incubator. At the indicated time points, cell viability was assessed with a cell proliferation kit I (MTT; Roche Diagnostics, Mannheim, Germany) according to the manufacturer's recommended protocol.
Test for CD40L functionality
Bone marrow-derived dendritic cells (BMDCs) were isolated from the bone marrow (femur and tibia) of C57BL/6 mice as described previously (Inaba et al., 1992; Crittenden et al., 2003). Briefly, bone marrow was flushed with 1 × PBS supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin–streptomycin solution (Mediatech, Herndon, VA) to obtain single-cell suspensions. Bone marrow cells were washed with PBS, treated with ammonium chloride–potassium (ACK) lysis buffer, and incubated in 1 ml of RPMI 1640 containing antibodies (10 μg/ml) to MHC class II (I-Ab), Mac-3, CD8a (HO2.2), B220, CD3e, and Gr-1 (all BD Pharmingen; BD Biosciences, San Jose, CA) on a rotary shaker at 4°C for 30 min. Bone marrow cells were then washed and suspended in baby rabbit complement (Accurate Chemical and Scientific, Westbury, NY), diluted 15-fold (v/v) per 1 × 107 cells/ml of RPMI culture medium, at 37°C for 45 min. Cells were washed and plated at a concentration of 2 × 106 cells/ml in BMDC culture medium (RPMI 1640 supplemented with 10% [v/v] FBS, 1% [v/v] penicillin–streptomycin, granulocyte-macrophage colony-stimulating factor [GM-CSF, 10 ng/ml], and interleukin [IL]-4 [1 ng/ml]) and incubated at 37°C in a humidified 5% CO2 incubator. One milliliter of BMDC culture medium was added to the cultured bone marrow cells on days 3 and 5. On day 6, immature BMDCs were harvested and cocultured with VSV-infected B16ova cells. Specifically, overnight monolayer cultures of B16ova melanoma cells were infected with VSV (MOI, 0.1) for 12 hr and cocultured with immature BMDCs for a total of 48 hr. Cells were then scraped, washed, and stained with fluorochrome-conjugated antibodies: anti-CD40L–PE (phycoerythrin), anti-CD11c–APC (allophycocyanin), anti-I-Ab–FITC (fluorescein isothiocyanate), anti-CD40–FITC, and anti-CD86–FITC (all from BD Biosciences) in 1 × PBS containing 0.1% bovine serum albumin (BSA) and 0.01% sodium azide (FACS buffer) for 30 min at 4°C. Cells were then subjected to flow cytometry and data were analyzed with CellQuest software (BD Biosciences).
In vivo studies
Survival studies
All mouse in vivo protocols were approved by the Mayo Foundation Institutional Animal Care and Use Committee (Rochester, MN). Female C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME) at 6–8 weeks of age. To establish subcutaneous tumors, 5 × 105 B16ova cells suspended in 100 μl of 1 × PBS were injected into the right flanks of mice. Viral injections (5 × 108 plaque-forming units [PFU] suspended in 50 μl of 1 × PBS) were performed intratumorally at the indicated time points after tumor seeding. Immune cell depletions were performed by intraperitoneal injections (0.1 mg per mouse) of anti-CD8 (Lyt 2.43), produced by the Monoclonal Antibody Core Facility (Mayo Clinic, Rochester, MN) and IgG control (ChromPure rat IgG; Jackson ImmunoResearch, West Grove, PA) on day 4 after tumor implantation and then weekly thereafter. Flow cytometric analyses of spleens and lymph nodes confirmed subset-specific depletions (Diaz et al., 2007).
Viral titer determination
Established subcutaneous B16ova melanoma tumors were intratumorally injected with a single dose of 5 × 108 PFU of VSV. At the indicated time points, mice were killed and tumors were harvested and placed in 2-ml cryotubes and immediately snap-frozen in liquid nitrogen. To determine the viral titers, tumors were homogenized in 1 ml of 1 × PBS and the supernatants were clarified, serially diluted in plain DMEM, and titrated in BHK-21 cells according to a standard plaque assay (Diaz et al., 2007).
Histopathology of tumor sections
Tumors were harvested, fixed in 10% neutral buffered formalin (Fisher Scientific, Kalamazoo, MI), and then paraffin embedded and sectioned. Hematoxylin and eosin (H&E)-stained sections were prepared and analyzed by a blinded pathologist on the basis of the degree of tissue necrosis and immune infiltration (Diaz et al., 2007).
Enzyme-linked immunosorbent assay analysis for IFN-γ secretion
Spleens or tumor-draining lymph nodes were harvested from mice at the indicated times. One million cells were plated (unless otherwise indicated) in 24-well plates and incubated at 37°C with the indicated peptides, that is, H-2Kb-restricted peptides TRP-2180–188 SVYDFFVWL, ova SIINFEKL, and VSV N protein-derived RGYVYQGL, which were synthesized at the Mayo Foundation core facility (all peptide concentrations were 5 μg/ml). Cell-free supernatants were collected after 48 hr and tested by enzyme-linked immunosorbent assay (ELISA) for IFN-γ (OptEIA mouse IFN-γ ELISA set; BD Biosciences).
Flow cytometry
For analysis of phenotype, 1 × 106 cells were washed in 1 × PBS containing 0.1% BSA and 0.01% sodium azide (FACS buffer), resuspended in 50 μl of FACS buffer, and exposed to fluorochrome-conjugated primary antibodies (anti-CD40L–PE, anti-I-Ab–PE, anti-CD45–PerCP [peridinin chlorophyll protein], anti-CD8–FITC, and their corresponding isotype controls from BD Biosciences), for 30 min at 4°C. Cells were then washed and resuspended in 500 μl of PBS containing 4% formaldehyde (Diaz et al., 2007). For tetramer analysis, the iTAG MHC class I murine tetramer SA–PE H2Kb-OVA (Beckman Coulter, Fullerton, CA) and anti-CD8–FITC were used to stain mouse splenocytes according to the manufacturer's specifications. Cells were subjected to flow cytometry (FACScan; BD Biosciences) and data were analyzed with CellQuest software (BD Biosciences).
Statistics
Survival data from the animal studies were analyzed by log-rank test, using GraphPad Prism 4 (GraphPad Software, La Jolla, CA). Immunological assays and other in vitro experiments were analyzed with JMP software (SAS Institute, Cary, NC). Statistical significance was determined at the level of p < 0.05 (Diaz et al., 2007).
Results
In vitro characterization of VSV-CD40L
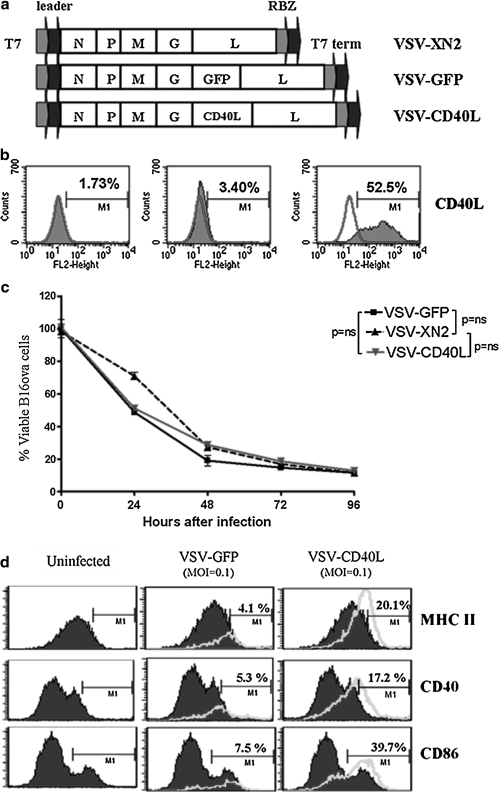
VSV-GFP and VSV-CD40L were derived from the replication-competent VSV-XN2 backbone by cloning the cDNA for either GFP or murine CD40L between the glycoprotein and large polymerase genes (Fig. 1a). VSV-CD40L-infected B16ova cells expressed high levels of CD40L on the cell surface 12 hr after infection (Fig. 1b) and VSV-CD40L had cytotoxic activity against B16ova tumor cells in vitro similar to those of VSV-XN2 and VSV-GFP (Fig. 1c). B16ova cells infected with VSV-CD40L induced maturation of bone marrow-derived dendritic cells (BMDCs), as shown by upregulation of CD40, CD86, and MHC class II molecules, at significantly higher levels than B16ova cells infected by VSV-GFP (Fig. 1d). Taken together, these data show that replication-competent VSV-CD40L retains the cytolytic properties of the parental VSV and expresses CD40L at high levels and with biological activity.
FIG. 1.
Vesicular stomatitis virus encoding mouse CD40 ligand (VSV-CD40L). (a) Schematic diagram of VSV genome (Indiana serotype) in cDNA form containing sequences for T7 RNA polymerase leader and terminator and hepatitis virus delta ribozyme (RBZ). VSV-XN2 served as the backbone for recombinant VSVs by inserting the transgenes (GFP or CD40L) between the glycoprotein (G) and large polymerase protein (L) genes. N, nucleoprotein; P, phosphoprotein; M, matrix. (b) Twelve hours after infection with VSV-CD40L or VSV-XN2 (MOI, 0.001), infected B16ova cells were collected and stained with anti-CD40L and analyzed by flow cytometry. The graph is representative of triplicate samples. (c) B16ova melanoma cells (5 × 103) were infected with VSV (MOI, 1.0). The proportion of viable cells was measured by MTT assay at the indicated time points postinfection relative to the uninfected control. Values are averages of three samples (±SEM) and representative of two independent experiments. (d) Twelve hours after infection with VSV (MOI, 0.1), B16ova melanoma cells were cocultured with immature bone marrow-derived dendritic cells (BMDCs) for another 48 hr. Cells were then collected and stained for DC maturation markers. Shaded histogram, DCs only; unshaded histograms, DCs cocultured with VSV-infected B16ova cells. Data shown are representative of two independent experiments. ns, not significant.
Intratumoral virotherapy with VSV-CD40L against subcutaneous B16ova tumors
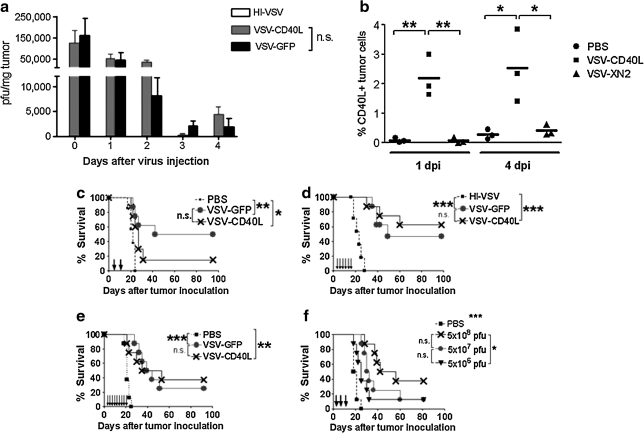
VSV-CD40L and VSV-GFP were detectable at similar titers for up to 4 days after a single intratumoral injection into subcutaneous B16ova tumors (Fig. 2a), but no virus was detectable 10 days postinjection (data not shown). However, similar to our observation with VSV-GFP (Galivo et al., 2010), we did not observe any rise in intratumoral viral titer of VSV-CD40L that would indicate progressive viral replication. Consistent with these data, significant levels of CD40L expression were observed within injected tumors up to 4 days postinfection (Fig. 2b). After direct injection of viruses into 7-day established subcutaneous B16ova tumors, using two (Fig. 2c), six (Fig. 2d), or nine injections (Fig. 2e), both viruses generated significant survival advantages compared with treatment with PBS, with a fraction of mice having complete tumor regression (two doses of VSV-CD40L with respect to PBS, p = 0.0299; six doses, p = 0.0001; nine doses, p = 0.0018). However, in none of these experiments did treatment with VSV-CD40L generate significantly improved survival over treatment with VSV-GFP (Fig. 2c–e) (two doses, p = 0.1468; six doses, p = 0.5269; nine doses, p = 0.8224). Similarly, with three intratumoral injections, there was no difference in survival of mice treated with 5 × 108 PFU of VSV-CD40L or VSV-XN2 (data not shown). Reduction of the injected titer of VSV-CD40L by 1 log did not generate a significant loss of therapy compared with that observed with injection of 5 × 108 PFU, although a reduction of 2 logs of injected dose generated significantly poorer therapy compared with a dose of 5 × 108 PFU/injection (Fig. 2f). We also constructed several additional VSVs, expressing transgenes that we believed would enhance adaptive T cell responses to tumor-associated antigens, based on our previous experience with the B16ova model, including heat shock protein-70 (hsp70) (Daniels et al., 2004; Qiao et al., 2006) and CCL21 (Thanarajasingam et al., 2007). However, similar to our experience with VSV-CD40L, neither VSV-hsp70 nor VSV-CCL21 generated significantly improved therapy compared with VSV-GFP when given two, six, or even nine times directly into B16ova tumors (data not shown). Collectively, these data show that intratumoral injection of VSV expressing biologically active CD40L, or at least two other immune stimulatory genes, provided no greater therapy than similar titers of VSV-GFP.
FIG. 2.
Intratumoral administration of VSV-CD40L into established subcutaneous B16ova tumors in C57BL/6 mice. (a) C57BL/6 mice (n = 3 mice per group per time point) bearing 7-day-old B16ova tumors were given one intratumoral injection of VSV at a dose of 5 × 108 PFU. Tumors were collected and snap-frozen in liquid nitrogen at various times postinfection, and titrated for VSV by virus plaque assay. (b) Similar to (a), VSV-injected B16ova tumors (three mice per group) were harvested 1 and 4 days after injection, disrupted to obtain single-cell suspensions, stained with PE-conjugated anti-CD40L, and subjected to flow cytometric analysis. dpi, days postinjection. (c–e) B16ova melanoma tumors were established subcutaneously in C57BL/6 mice 7 days before intratumoral viral administration. Either VSV-GFP or VSV-CD40L (5 × 108 PFU) was injected two times (c), six times (d), and nine times (e). Tumor growth and overall survival were monitored (n = 8 mice per group). (f) Dose–response survival curve using VSV-CD40L. Three intratumoral injections of recombinant VSVs were administered into subcutaneous B16ova tumors at various doses: 5 × 108, 5 × 107, and 5 × 106 PFU given every other day. n.s., not significant. *p < 0.05, **p < 0.01, ***p < 0.001.
Potent immunotherapeutic activity by a replication-defective adenoviral vector expressing CD40L against B16ova
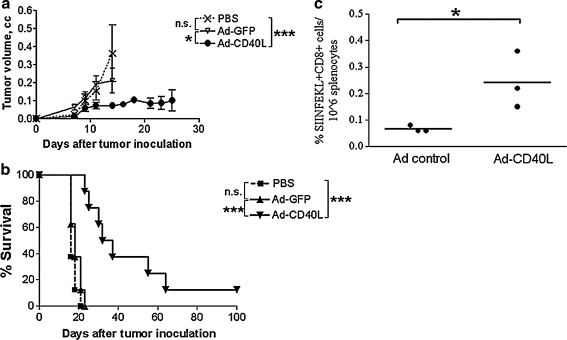
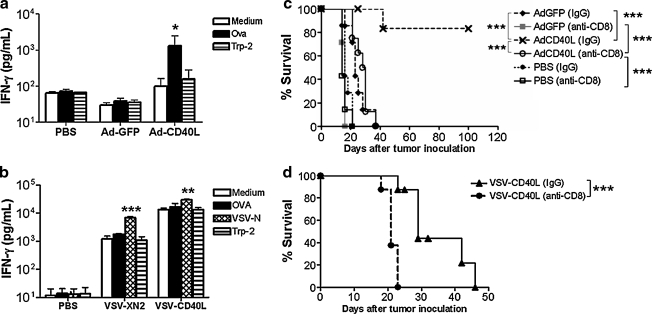
Because of our own experience with CD40L in the context of inflammatory killing of normal melanocytes as a vaccine against B16ova (Daniels et al., 2004; Sanchez-Perez et al., 2006), and because of other reports of the efficacy of CD40L as an effective immunotherapeutic agent against tumors (Kikuchi and Crystal, 1999; Kikuchi et al., 2000; Suna et al., 2000; Schmitz et al., 2001), we investigated whether intratumoral expression of CD40L in a nononcolytic platform would have therapeutic value in our B16ova model. In this respect, expression of CD40L from a replication-defective, E1-deleted adenoviral vector was highly effective at delaying tumor growth when given two times (data not shown) or six times (Fig. 3a). These results were highly correlated with increased survival of tumor-bearing mice after two intratumoral (Fig. 3b) or six intratumoral injections (data not shown). Therapeutic efficacy was associated with the induction of T cell responses specific for the tumor-associated OVA antigen (Fig. 3c), consistent with the observation that regression of B16ova tumors started several days after the first intratumoral injection of Ad-CD40L (Fig. 3a and b).
FIG. 3.
Replication-defective adenoviral vector expressing CD40L. (a) Growth curve of established subcutaneous B16ova tumors in immunocompetent mice (n = 8 mice per group) after six intratumoral treatments with Ad-GFP and Ad-CD40L (1 × 109 PFU per dose). (b) Kaplan–Meier plot of immunocompetent mice harboring subcutaneous B16ova tumors after treatment consisting of two intratumoral administrations of either Ad-GFP (given on days 7 and 8) or Ad-CD40L (given on days 7 and 8). Both adenovectors were given at a dose of 1 × 109 PFU. (c) Spleens from mice with regressing AdCD40L-treated tumors were harvested 15 days after tumor challenge, along with those of control-treated mice. The splenocytes were stained with MHC class I murine tetramer SA–PE H2Kb-OVA and anti-CD8–FITC and subjected to flow cytometry. *p < 0.05, ***p < 0.001.
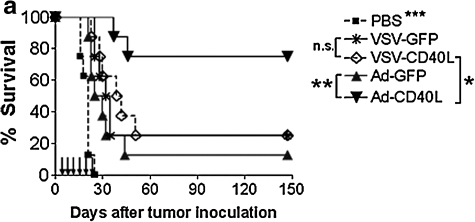
Given that intratumoral expression of CD40L can mediate therapy in our B16ova model, we then compared the relative efficacies of the two different vector platforms: replicating, oncolytic VSV versus nonreplicating, nononcolytic adenovirus. For these experiments, we used six local injections of VSV or adenovirus for comparison of efficacy. As described previously, intratumoral injections of VSV-GFP and VSV-CD40L induced significant extension of survival times compared with control-treated mice, although there was no difference between the two VSV vectors (Fig. 4a). In contrast, treatment with Ad-CD40L cured 75% of the mice in the experiment shown in Fig. 4a and was significantly more effective compared with both control Ad-GFP (p = 0.0028) and, significantly, either the replication-competent VSV-GFP (p = 0.017) or VSV-CD40L (p = 0.041) (Fig. 4a). Interestingly, the levels of intratumoral CD40L expression measured 1 and 4 days postinfection were comparable in tumors treated with Ad-CD40L and VSV-CD40L (data not shown).
FIG. 4.
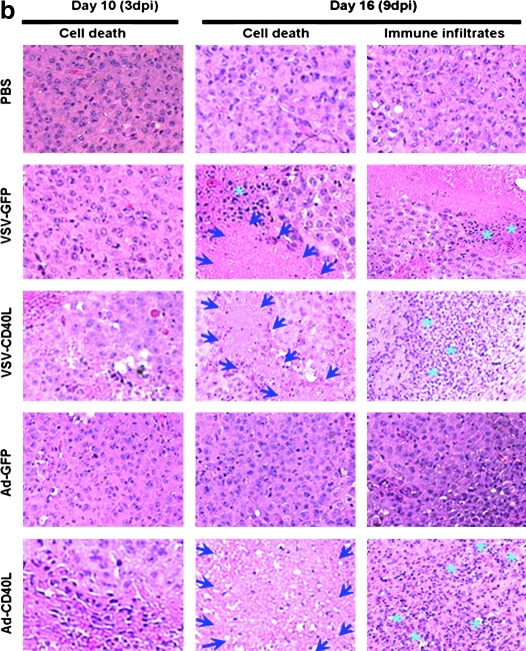
Comparative study between replicating VSV-CD40L and replication-defective Ad-CD40L as intratumoral virotherapeutic agents against B16ova tumors. (a) Kaplan–Meier plot of C57BL/6 mice (n = 8 per group) bearing scB16ova tumors treated with six intratumoral injections of VSV (5 × 108 PFU of VSV-GFP or VSV-CD40L) and adenovirus (1 × 109 PFU of Ad-GFP or Ad-CD40L) given 1 day apart, three times per week for 2 weeks. *p < 0.05, **p < 0.01, ***p < 0.001. (b) Histologic and immune effects of intratumoral virus injections on B16ova melanoma tumors. Subcutaneous B16ova tumors were treated with three intratumoral injections of virus on days 7, 8, and 9 after tumor inoculation. Tumors were harvested on days 10 and 16, fixed in 10% neutral buffered formalin, and processed for H&E staining. The degree of cell death (areas bounded by arrows) and immune infiltration (asterisks) were scored independently by a blinded pathologist. Photos are representative of three tumors per treatment group.
At early time points after intratumoral injection (3 days after the first injection), tumors treated with either VSV-CD40L or Ad-CD40L contained areas of moderate to severe necrosis, whereas tumors treated with PBS, VSV-GFP, or Ad-GFP were scored as containing only minimal necrosis (Fig. 4b). At later time points after injection, however, differences between tumors were more evident. Thus, 9 days after the first intratumoral injection, Ad-CD40L-treated tumors contained moderate to severe immune cell infiltration, composed primarily of lymphocytes. In contrast, VSV-treated tumors contained significantly lower levels of immune infiltration (Fig. 4b). Tumors treated with either PBS or Ad-GFP were scored lowest, with only slight immune infiltration (Fig. 4b). The extent of immune infiltration also correlated well with the degree of necrosis recorded for different treatments. Thus, at late times after treatment, Ad-CD40L-treated tumors consistently contained severe necrosis (>75% necrotic areas) compared with intermediate scores (20–75% necrotic areas) for both VSV-treated groups and only minimal necrosis (<10% necrotic areas) for tumors treated with Ad-GFP (Fig. 4b). In the absence of CD40L, Ad-GFP induced similar histology to PBS injection, unlike that of the more immunogenic VSV. In agreement with these histological data, RPA and RT-PCR analyses confirmed that intratumoral injection of VSV involved a rapid induction of multiple inflammatory cytokines within the tumor environment that were not induced by either Ad-GFP or Ad-CD40L, and that all these cytokines had returned to baseline levels by 3 days after viral injection (data not shown and Galivo et al., 2010). Altogether, these data indicate that intratumoral injection of VSV vectors (±CD40L) induced a rapidly developing proinflammatory intratumoral environment, associated with necrosis and infiltration by immune cells, that resolved within a few days; however, injection of Ad-CD40L was correlated with relatively low levels of acute inflammatory reactivity within the tumor but led, over longer periods of time, to increasing levels of tumor destruction as judged by increasing necrosis, immune infiltration, and tumor regression.
T cell activation by VSV and adenoviral vectors
Consistent with the results of Fig. 3b, mice treated intratumorally with Ad-CD40L contained splenocytes that secreted IFN-γ specifically in response to stimulation with the immunodominant epitope of the ovalbumin tumor-associated antigen (Fig. 5a). In contrast, splenocytes from mice treated with either VSV-GFP or VSV-CD40L secreted significantly higher baseline levels of IFN-γ compared with the PBS control group (Fig. 5b). This higher level of baseline T cell activity was consistently enhanced specifically only in response to stimulation by a VSV-specific epitope but not by tumor-associated epitopes derived from OVA or TRP-2 (Fig. 5b).
FIG. 5.
Role of T lymphocytes in Ad-CD40L-mediated antitumor effects against B16ova tumors. (a and b) In animals treated with intratumoral (a) adenovirus or (b) VSV, spleens were harvested on day 16 after tumor challenge, dissociated into single-cell suspensions (1 × 105 cells per well), and cultured in vitro for 48 hr in the presence of the indicated peptides. Supernatants were harvested and assayed for IFN-γ, using ELISA. Values represent averages of three spleens per group, each done in triplicate wells (means ± SEM). (c) Kaplan–Meier plot of CD8+ T cell-depleted C57BL/6 mice (n = 7 mice per group) after six intratumoral injections of adenovectors. Depleting antibodies against CD8+ T cells or control antibody (IgG isotype control) was given intraperitoneally starting on day 4 after tumor implantation and weekly thereafter. Virus was injected intratumorally starting on day 7 and given every other day for 2 weeks. (d) Kaplan–Meier plot of subcutaneous B16ova melanoma-bearing C57BL/6 mice (n = 8 mice per group) treated with intratumoral VSV-CD40L in the presence or absence of anti-CD8 depleting antibody. *p < 0.05, **p < 0.01, ***p < 0.001.
To investigate further the role of these T cell responses, we repeated the therapeutic studies in mice depleted of immune subsets. In contrast to the significant numbers of cures after treatment of subcutaneous B16ova tumors in C57BL/6 mice with Ad-CD40L (Figs. 3a and b, 4a, and 5c), no tumors were cured when these experiments were repeated in mice depleted of CD8+ T cells (p = 0.0003 in CD8+ T cell-depleted mice compared with nondepleted mice) (Fig. 5c). However, a significant survival benefit was still retained in CD8-depleted mice treated with Ad-CD40L compared with CD8-depleted mice treated with either PBS (p = 0.0003) or Ad-GFP (p =0.0002) (Fig. 5c). Additional depletion studies showed that NK cells are also important for the therapy produced by Ad-CD40L, although significantly less so than contributed by CD8+ T cells (data not shown). Consistent with our previously published observations (Diaz et al., 2007), depletion of CD8+ T cells significantly reduced the therapy associated with intratumoral injection of either VSV-GFP (data not shown) or VSV-CD40L (p = 0.0003 compared with treatment in nondepleted mice) (Fig. 5d).
Taken together these data indicate that CD8+ T cells are critical mediators of antitumor therapy for the treatment of subcutaneous B16ova tumors with both VSV-CD40L and Ad-CD40L. However, the antitumor mechanisms mediated by these CD8+ T cells appear to differ significantly. Thus, intratumoral treatment with VSV, irrespective of whether the additional CD40L transgene is expressed, induced generalized T cell activation but with specificity only for viral antigens and not for tumor-associated antigens. Treatment with Ad-CD40L, however, generated clearly detectable tumor antigen-specific T cell responses.
Discussion
Our data are consistent with the hypothesis that the therapeutic and immunological consequences of intratumoral delivery/expression of the same immunostimulatory gene will be different depending on the viral platform that is used for transgene expression. Moreover, these data have significant implications for how different antitumor gene/viro-therapies need to be matched carefully to the vector by which the genes are delivered to tumors.
The overriding rationale for the use of oncolytic viruses in cancer therapy is based on the notion that these viruses will initiate a spreading infection through the tumor, leading to high levels of tumor cell lysis and direct killing. In contrast, gene-immunotherapies for cancer depend on the assumption that appropriately primed immune cell responses will be able to clear tumors through indirect, immune cell-based effector mechanisms. In this study, our initial hypothesis was that it would be possible to combine these two approaches by achieving high levels of CD40L expression through infected tumors by using a highly lytic replication-competent virus. The resulting combination of tumor cell death (oncolysis), in the presence of high levels of CD40L, might lead to cross-priming of TAAs to naive T cells by APCs, resulting in amplification of CD4+/CD8+ T cells specific for TAAs. In turn, our hypothesis was that these T cell responses would provide further supportive therapy against established B16ova tumors, for which a simple intratumoral injection of oncolytic VSV was insufficient to induce complete tumor regression.
We show here that coexpression of CD40L from replication-competent VSV added no increased therapeutic benefit against established subcutaneous B16ova tumors in immune-competent mice. Intratumoral injection of either VSV-GFP or VSV-CD40L provided identical therapeutic benefits in this model, associated with rapidly induced inflammatory reactivity within the tumors characterized by intratumoral necrosis and infiltration (Fig. 4) and expression of proinflammatory cytokines within hours of vector injection (Galivo et al., 2010). These antitumor effects were associated with increased baseline T cell activation in the spleen, but without detectable specificity for tumor-associated antigens (Fig. 5b). The mechanisms by which VSV induces this hyperactivation of T cells in vivo are currently under investigation. We have shown that intratumoral injection of VSV leads to rapid trafficking of the virus into the tumor-draining lymph nodes. Once there, we believe that VSV leads to rapid T cell activation through direct activation of Toll-like receptors on the T cell surface, a hypothesis that is being tested with mice deficient in various TLR and signaling pathways. Also, these activated CD8+ T cells were critical mediators of antitumor therapy as their prior depletion largely abolished antitumor therapy induced by VSV intratumoral treatment. This response, characterized by generalized T cell activation, resulted in specific immunization against viral proteins as seen by increased T cell priming against virus-specific epitopes (Fig. 5b). However, these multiple, and highly immunogenic, viral targets are likely to be significantly more immunodominant than most of the tumor-associated antigens encoded within the immunosuppressive tumor microenvironment. Significantly, in these studies, we measured responses to the model OVA antigen expressed in B16ova tumors against which no immunological tolerance is in place in C57BL/6 mice. In contrast to our previous report, wherein direct injections of VSV-GFP into B16ova tumors yielded high frequencies of tumor-infiltrating CD8+ T cells reactive against both viral and OVA antigens (Diaz et al., 2007), we were still unable to detect any priming of OVA-specific T cell responses in the spleens, despite the immunologically foreign nature of this TAA, after treatment with either VSV-GFP or VSV-CD40L (Fig. 5). These data suggest that the immunogenicity of the TAA was not the only significant issue in determining whether VSV-mediated oncolysis can be associated with priming of T cell responses against TAAs. In this respect, we have previously shown that T cell responses against tumor-associated antigens can be primed in vivo after direct intratumoral injection of VSV if the virus itself encodes the TAA (Diaz et al., 2007). We believe that these effects are, in part, mediated by direct trafficking of the injected virus to the lymph nodes where priming against virally encoded antigens was efficient (R.M. Diaz, F. Galivo, P. Wongthida, and R.G. Vile, unpublished observations). We have also previously shown that VSV-mediated therapy of B16ova tumors is dependent on both NK and CD8+ T cells (Diaz et al., 2007). Our data here shed further light on these findings. At short periods of time after VSV intratumoral injection (up to 96 hr), we have observed activation of both NK and CD8+ T cells, associated with high levels of IFN-γ production from splenocytes in treated mice (Wongthida et al., 2010). In contrast, at later time points (>7 days), IFN-γ production is contributed predominantly by T cells with a hyperactivated phenotype (Fig. 5b). We could never detect a significant increase in the levels of IFN-γ secretion from these T cells stimulated by a tumor-associated antigen (OVA or TRP-2) compared with stimulation with either an irrelevant antigen or even just medium alone (Fig. 5b). However, we cannot exclude the possibility that a small, tumor antigen-specific T cell response may exist in the spleens of VSV-treated mice but is obscured by the high background of IFN-γ production. Finally, our depletion data (Diaz et al., 2007) indicate that VSV-mediated therapy of B16ova tumors in the C57BL/6 model is dependent on both of these NK and CD8+ T cell activation events in vivo.
Despite the oncolytic properties of VSV, which are likely to provide high levels of tumor antigen release, VSV is a poor platform for the subsequent priming of antitumor T cell responses because of the high immunogenicity of the viral proteins and the route of delivery of these proteins to the antigen-presenting machinery in the lymph nodes. For these reasons, inclusion of a costimulatory molecule such as CD40L to the VSV platform adds no additional benefits in terms of priming antitumor T cell responses because it may be that immune stimulation was already largely maximal because of the immunogenicity of this virus in vivo. Therefore, the therapeutic mechanisms of VSV-CD40L are not different from those of VSV-GFP, both of which rely on the oncolytic activity of the virus, and the innate immune response to it, without the benefit of further recruitment of appropriately primed antitumor T cell responses. Indeed, we have reported that intratumoral injection of VSV leads to an acute, innate immune response to this highly immunogenic virus that is dependent on viral gene expression but not on viral replication, antibody, or adaptive cellular responses (Galivo et al., 2010).
In contrast to the comparison between VSV-GFP and VSV-CD40L, the addition of CD40L to a replication-defective adenoviral vector significantly enhances its therapeutic efficacy relative to the Ad-GFP control vector. Indeed, Ad-CD40L leads to high proportions of mice being cured of established subcutaneous B16ova tumors. Therapy is associated with intratumoral destruction (necrosis, immune infiltration [Fig. 4], and tumor regression [Figs. 3 and 4]), which take several days to develop, consistent with its dependence on the priming of CD8+ T cell responses (Fig. 5), which are specific for antigens expressed within the tumor (Figs. 3 and 5). In contrast to VSV, the adenovirus backbone (as represented by Ad-GFP) induced a histologic profile and splenocyte reactivity similar to those of PBS. These data suggest that one important difference is the inherent immunogenicity of the vector platforms. Replication-defective adenovirus is poorly immunogenic, as evidenced by the relative lack of intratumoral inflammation after local administration of Ad-GFP, whereas the more immunogenic VSV-GFP induced a local innate proinflammatory reaction followed by leukocytic infiltration within the injected tumor. The relatively weak immunogenicity associated with the replication-defective adenoviral vector platform allows for priming of T cell responses against tumor-associated antigens because of the lack of more immunogenic, immune-distracting antigenic targets within the tumor at the site of CD40L expression.
Interestingly, when we compare the therapeutic efficacies of the VSV-CD40L and Ad-CD40L vectors, we consistently observe better tumor cure rates when using the replication-defective, immune-stimulating Ad-CD40L compared with the replication-competent, highly immunogenic, oncolytic VSV-CD40L. There are many potentially confounding pitfalls inherent in comparing different vector types, including how to control for factors such as titer, timing of doses, ratios of infectious and noninfectious particles, and the tumor model being used. In our studies, we used the same number of injections of both viruses, at similar doses, which correspond to the maximal achievable dose of Ad-CD40L and the maximal tolerated dose of VSV-CD40L or VSV-GFP. It may be that the performance of either vector would be increased by changing the timing and/or frequency of injections and we are currently investigating these variables. Nonetheless, under the conditions that we used here, it is clear that the replication-defective, less immunogenic adenoviral vector platform expressing CD40L is significantly more effective as an antitumor immunotherapeutic agent than a strongly immunogenic, replication-competent VSV-CD40L. These results indicate that, at least in this model, an appropriately primed T cell response against tumor-associated antigens can outperform the therapeutic effects induced by a potently oncolytic virus such as VSV. However, our results do suggest that there are still opportunities to combine the direct oncolytic activity of replicating viruses with tumor selectivity, such as VSV, with additional priming of antitumor immune responses to achieve additive or synergistic therapeutic benefits—including the use of combinations of oncolytic viruses with replication-defective vectors encoding immune stimulatory genes such as CD40L.
In summary, we have shown that a highly immunogenic, replication-competent virus may not be an effective platform for the additional expression of immune stimulatory genes designed to prime tumor specific T cell responses. In contrast, a poorly immunogenic, replication-defective vector, despite its lower potential for transgene delivery/expression and amplification, can facilitate the generation of specific, and highly effective, T cell-mediated antitumor immunotherapy that, in this particular model, outperformed that induced by oncolytic VSV. These data suggest that careful consideration should be given to matching the choice of delivery vector with the therapeutic effector mechanisms (direct tumor cell lysis, antitumor immunity, or both) to achieve the optimal in vivo therapeutic response.
Acknowledgments
The authors thank Toni Higgins for expert secretarial assistance. This work was supported by the Richard Schulze Family Foundation, the Mayo Foundation, and NIH grants CA107082-02, CA130878-01, and CA132736.
Author Disclosure Statement
No competing financial interests exist.
References
- Balachandran S. Barber G.N. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell. 2004;5:51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Balachandran S. Porosnicu M. Barber G.N. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or Myc function and involves the induction of apoptosis. J. Virol. 2001;75:3474–3479. doi: 10.1128/JVI.75.7.3474-3479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J. Bazan F. Blanchard D. Brière F. Galizzi J.P. Van Kooten C. Liu Y.J. Rousset F. Saeland S. The CD40 antigen and its ligand. Annu. Rev. Immunol. 1994;12:881–926. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Barber G.N. VSV-tumor selective replication and protein translation. Oncogene. 2005;24:7710–7719. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- Bell J.C. Lichty B. Stojdl D. Getting oncolytic virus therapies off the ground. Cancer Cell. 2003;4:7–11. doi: 10.1016/s1535-6108(03)00170-3. [DOI] [PubMed] [Google Scholar]
- Bergman I. Griffin J.A. Gao Y. Whitaker-Dowling P. Treatment of implanted mammary tumors with recombinant vesicular stomatitis virus targeted to Her2/neu. Int. J. Cancer. 2007;121:425–430. doi: 10.1002/ijc.22680. [DOI] [PubMed] [Google Scholar]
- Crittenden M. Gough M. Harrington K. Olivier K. Thompson J. Vile R.G. Expression of inflammatory chemokines combined with local tumor destruction enhances tumor regression and long-term immunity. Cancer Res. 2003;63:5505–5512. [PubMed] [Google Scholar]
- Daniels G.A. Sanchez-Perez L. Diaz R.M. Kottke T. Thompson J. Lai M. Gough M. Karim M. Bushell A. Chong H. Melcher A. Harrington K. Vile R.G. A simple method to cure established tumors by inflammatory killing of normal cells. Nat. Biotechnol. 2004;22:1125–1132. doi: 10.1038/nbt1007. [DOI] [PubMed] [Google Scholar]
- Diaz R.M. Galivo F. Kottke T. Wongthida P. Qiao J. Thompson J. Valdes M. Barber G. Vile R.G. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- Ebert O. Harbaran S. Shinozaki K. Woo S.L.C. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immune-competent mice. Cancer Gene Ther. 2005;12:350–358. doi: 10.1038/sj.cgt.7700794. [DOI] [PubMed] [Google Scholar]
- Fernandez M. Porosnicu M. Markovic D. Barber G.N. Genetically engineered vesicular stomatitis virus in gene therapy: Application for treatment of malignant disease. J. Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A.I. Zakay-Rones Z. Gomori J.M. Linetsky E. Rasooly L. Greenbaum E. Rozenman-Yair S. Panet A. Libson E. Irving C.S. Galun E. Siegal T. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther. 2006;13:221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Galivo Diaz R.M. Wongthida P. Thompson J. Kottke T. Barber G. Melcher A. Vile R.G. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther. 2010;17:158–170. doi: 10.1038/gt.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani M. White C.L. Zaidi S. Merron A. Peerlinck I. Gore M.E. Nutting C.M. Pandha H.S. Melcher A.A. Vile R.G. Vassaux G. Harrington K.J. Radiation-mediated up-regulation of gene expression from replication-defective adenoviral vectors: Implications for sodium iodide symporter gene therapy. Clin. Cancer Res. 2008;14:4915–4924. doi: 10.1158/1078-0432.CCR-07-4049. [DOI] [PubMed] [Google Scholar]
- Inaba K. Inaba M. Romani N. Aya H. Deguchi M. Ikehara S. Muramatsu S. Steinman R. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T. Crystal R.G. Anti-tumor immunity induced by in vivo adenovirus vector-mediated expression of CD40 ligand in tumor cells. Hum. Gene Ther. 1999;10:1375–1387. doi: 10.1089/10430349950018049. [DOI] [PubMed] [Google Scholar]
- Kikuchi T. Miyazawa N. Moore M.A.S. Crystal R.G. Tumor regression induced by intratumor administration of adenovirus vector expressing CD40 ligand and naive dendritic cells. Cancer Res. 2000;60:6391–6395. [PubMed] [Google Scholar]
- Kottke T. Diaz R.M. Kaluza K. Pulido J. Galivo F. Wongthida P. Thompson J. Willmon C. Barber G.N. Chester J. Selby P. Strome S. Harrington K. Melcher A. Vile R.G. Use of biological therapy to enhance both virotherapy and adoptive T-cell therapy for cancer. Mol. Ther. 2008;16:1910–1918. doi: 10.1038/mt.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson N.D. Stillman E.A. Whitt M.A. Rose J.K. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. U.S.A. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichty B.D. Power A.T. Stojdl D.F. Bell J.C. Vesicular stomatitis virus: Re-inventing the bullet. Trends Mol. Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Linardakis E. Bateman A. Phan V. Ahmed A. Gough M. Olivier K. Kennedy R. Errington F. Harrington K.J. Melcher A. Vile R. Enhancing the efficacy of a weak allogeneic melanoma vaccine by viral fusogenic membrane glycoprotein-mediated tumor cell-tumor cell fusion. Cancer Res. 2002;62:5495–5504. [PubMed] [Google Scholar]
- Mackey M.F. Gunn J.R. Ting P.P. Kikutani H. Dranoff G. Noelle R.J. Barth R.J., Jr. Protective immunity induced by tumor vaccines requires interaction between CD40 and its ligand, CD154. Cancer Res. 1997;57:2569–2574. [PubMed] [Google Scholar]
- Mackey M.F. Gunn J.R. Maliszewski C. Kikutani H. Noelle R.J. Barth R.J., Jr. Cutting edge: Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J. Immunol. 1998;161:2094–2098. [PubMed] [Google Scholar]
- Obuchi M. Fernandez M. Barber G.N. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J. Virol. 2003;77:8843–8856. doi: 10.1128/JVI.77.16.8843-8856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porosnicu M. Mian A. Barber G.N. The oncolytic effect of recombinant vesicular stomatitis virus is enhanced by expression of the fusion cytosine deaminase/uracil phosphoribosyltransferase suicide gene. Cancer Res. 2003;63:8366–8376. [PubMed] [Google Scholar]
- Qiao J. Moreno J. Sanchez-Perez L. Kottke T. Thompson J. Caruso M. Diaz R.M. Vile R. VSV-G pseudotyped, MuLV-based, semi-replication-competent retrovirus for cancer treatment. Gene Ther. 2006;13:1457–1470. doi: 10.1038/sj.gt.3302782. [DOI] [PubMed] [Google Scholar]
- Qiao J. Kottke T. Willmon C. Galivo F. Wongthida P. Diaz R.M. Thompson J. Ryno P. Barber G.N. Chester J. Selby P. Harrington K. Melcher A. Vile R.G. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat. Med. 2008;14:37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- Sanchez-Perez L. Kottke T. Daniels G.A. Diaz R.M. Thompson J. Pulido J. Melcher A. Vile R.G. Killing of normal melanocytes, combined with heat shock protein 70 and CD40L expression, cures large established melanomas. J. Immunol. 2006;177:4168–4177. doi: 10.4049/jimmunol.177.6.4168. [DOI] [PubMed] [Google Scholar]
- Schmitz V. Barajas M. Wang L. Peng D. Duarte M. Prieto J. Qian C. Adenovirus-mediated CD40 ligand gene therapy in a rat model of orthotopic hepatocellular carcinoma. Hepatology. 2001;34:72–81. doi: 10.1053/jhep.2001.25757. [DOI] [PubMed] [Google Scholar]
- Stojdl D.F. Abraham N. Knowles S. Marius R. Brasey A. Lichty B.D. Brown E.G. Sonenberg N. Bell J.C. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J. Virol. 2000;74:9580–9585. doi: 10.1128/jvi.74.20.9580-9585.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suna Y. Penga D. Lecanda J. Schmitz V. Barajas M. Qian C. Prieto J. In vivo gene transfer of CD40 ligand into colon cancer cells induces local production of cytokines and chemokines, tumor eradication and protective antitumor immunity. Gene Ther. 2000;7:1467–1476. doi: 10.1038/sj.gt.3301264. [DOI] [PubMed] [Google Scholar]
- Thanarajasingam U. Immunology. Mayo Graduate School; Rochester, MN: 2006. Manipulating immune cell recruitment, activation and function for melanoma therapy; p. 202. [Google Scholar]
- Thanarajasingam U. Sanz L. Diaz R. Qiao J. Sanchez-Perez L. Kottke T. Thompson J. Chester J. Vile R.G. Delivery of CCL21 to metastatic disease improves the efficacy of adoptive T-cell therapy. Cancer Res. 2007;67:300–308. doi: 10.1158/0008-5472.CAN-06-1017. [DOI] [PubMed] [Google Scholar]
- Van Kooten C. Banchereau J. CD40–CD40 ligand. J. Leukoc. Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- Wongthida P. Diaz R.M. Galivo F. Kottke T. Thompson J. Pulido J. Pavelko K. Pease L. Melcher A. Vile R.G. The type III interferon IL-28 mediates antitumor efficacy of oncolytic VSV in a fully immune competent model. Cancer Res. 2010. (in press). [DOI] [PMC free article] [PubMed]