Abstract
Activation of the host innate immune response after systemic administration of adenoviral vectors constitutes a principal impediment to successful clinical gene replacement therapies. Although helper-dependent adenoviruses (HDAds) lack all viral functional genes, systemic administration of a high dose of HDAd still elicits a potent innate immune response in host animals. Toll-like receptors (TLRs) are innate receptors that sense microbial products and trigger the maturation of antigen-presenting cells and cytokine production via MyD88-dependent signaling (except TLR3). Here we show that mice lacking MyD88 exhibit a dramatic reduction in proinflammatory cytokines after intravenous injection of a high dose of HDAd, and show significantly reduced induction of the adaptive immune response when compared with wild-type and TLR2-deficient mice. Importantly, MyD88–/– mice also show significantly higher and longer sustained transgene expression than do wild-type mice. Chromatin immunoprecipitation studies using wild-type and MyD88-deficient primary mouse embryonic fibroblasts showed significant MyD88-dependent transcriptional silencing of the HDAd-encoded transgenes. Our results demonstrate that MyD88 signaling, activated by systemic delivery of HDAd, initiates an innate immune response that suppresses transgene expression at the transcriptional level before initiation of the adaptive immune response.
Introduction
Host immune responses represent the foremost obstacle limiting the clinical translation of adenoviral gene replacement therapy. First-generation adenoviral vectors (FGAds) induce strong dose-related host innate and adaptive immune responses after systemic administration (Muruve et al., 1999). This is exacerbated by a nonlinear dose response (characterized by a threshold effect) that increases the dose required for therapeutic efficacy (McCormack et al., 2006). The adaptive immune response to FGAds is mediated primarily by an adenovirus-specific major histocompatibility complex class I-restricted CD8+ cytotoxic T cell response (cytotoxic T lymphocytes) directed against vector-transduced cells expressing viral genes and transgenes (Muruve, 2004). The host immune system targets antigenic epitopes associated with viral proteins expressed by FGAds. However, the development of helper-dependent adenoviral vectors (HDAds), devoid of all functional viral genes, has largely, but not entirely, overcome this cell-mediated host immune response. Several groups have now reported long-term persistence of vector, transgene expression, and minimal chronic toxicity in multiple small- and large-animal models treated with HDAd (Mian et al., 2004; Brunetti-Pierri et al., 2005, 2006, 2008, 2009; Toietta et al., 2005; McCormack et al., 2006; Cerullo et al., 2007b). Regrettably, after systemic administration of HDAd, a rapid host immune response (within 6 hr) is initiated similar to the response against FGAds. This acute response is characterized by infiltration of monocytes and activated neutrophils, with a concomitant increase in cytokines and chemokines (Muruve et al., 2004). Understanding the biology of the early innate host response to HDAd is essential for developing a strategy to improve the safety and efficacy of adenovirus-mediated gene therapy. The complexity of this response includes a multitude of redundant cellular and humoral factors the complexities and interactivities of which are only partially understood.
Toll-like receptors (TLRs) are crucial components of pathogen recognition and are emerging as significant players in adenovirus-induced acute toxicity (Basner-Tschakarjan et al., 2006; Cerullo et al., 2007a; Hartman et al., 2007; Hensley and Amalfitano, 2007; Hensley et al., 2007; Iacobelli-Martinez and Nemerow, 2007; Zhu et al., 2007). To date, 11 TLRs have been identified in mammals, but their individual roles have been only partially characterized and much remains to be elucidated, particularly their specificity for various specific pathogen motifs (Akira et al., 2006). The expression of some TLRs has been shown in vitro to be altered after exposure to adenovirus (Hartman et al., 2007). Previously we, and others, have found that TLR9, which localizes in late endosomes or lysosomes, is one of the major sensor molecules in adenoviral vector recognition both in vitro and in vivo (Cerullo et al., 2007a; Yamaguchi et al., 2007; Appledorn et al., 2008). TLR2, a cell surface receptor, has also been reported to recognize infection by an adenoviral vector and to activate extracellular signal-regulated kinase (ERK) signaling during the innate immune response (Appledorn et al., 2008). The myeloid differentiation primary response gene-88 (MyD88) is critical for signaling from all TLRs except TLR3 (Akira et al., 2006), and is expressed in a variety of human and murine tissues albeit at various levels (Bonnert et al., 1997). MyD88 associates with the Toll/interleukin-1 receptor (TIR) domain of TLRs, the interleukin (IL)-1 receptor-associated kinases IRAK1 and IRAK4, and tumor necrosis factor receptor-associated factor-6 (TRAF6). This interaction results in downstream activation of interferon regulatory factor-3 and/or −7 (IRF3 and/or IRF7), and of the IκB kinase (IKK)-α/β/γ and mitogen-activated protein kinase (MAPK) cascades, leading to NF-κB and AP-1 activation (Garcia-Sastre and Biron, 2006). These cascades are rapidly induced after stimulation with TLR ligands, leading to activation of the proinflammatory genes and type I interferons (IFNs). TLR/MyD88 signaling is crucial for the induction of innate (Nociari et al., 2007; Yamaguchi et al., 2007; Zhu et al., 2007) and adaptive immune (Hartman et al., 2007) responses to adenoviral vector. MyD88 signaling, activated by infection with adenovirus, has been reported to regulate the kinetics of NF-κB and MAPK activation (Nociari et al., 2007) and induces the expression of cytokines and chemokines (Hartman et al., 2007).
The objective of this study was to seek greater understanding of the role of MyD88 signaling in host innate and adaptive immune response after systemic delivery of HDAd. We also examined how MyD88 signaling affects transgene expression of HDAd at early (before induction of the adaptive immune response) and late (after induction of the adaptive immune response) time points. Here, we show that TLR/MyD88 signaling activated by infection with HDAd is critical for inducing a helper T cell type 1 (Th1) immune response and for epigenetic silencing of transgene expression. These observations suggest that pharmacological blockade of this pathway will not only attenuate the innate and adaptive immune responses but also result in increased transgene expression by HDAd gene transfer.
Materials and Methods
Adenoviral vectors
The HDAd HD28E4LacZ (serotype 5), containing the β-galactosidase transgene driven by the cytomegalovirus (CMV) promoter, was produced as described elsewhere (Zhu et al., 2007). Helper virus contamination of this viral preparation was assessed by Southern blot and PhosphorImager analysis and was estimated to be <0.05% as described elsewhere (Zhu et al., 2007).
Mice and injections
MyD88–/– mice were provided by S. Akira (Department of Host Defense, Research Institute for Microbial Diseases, Osaka University, Osaka, Japan) (Adachi et al., 1998), and TLR2–/– and C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). All the mice were housed under pathogen-free conditions; food and water were provided ad libitum. All the mice used in this experiment were males between 8 and 12 weeks of age. All experimental procedures were conducted in accordance with institutional guidelines for animal care and use. HDAd was diluted in sterile Ringer's solution (147 mM NaCl, 4 mM KCl, 1.13 mM CaCl2) prewarmed at 37°C and injected into the tail vein. The injections were performed with a total volume of 200 μl. Blood was collected retro-orbitally for analyses. Serum was frozen immediately and stored at −80°C until analysis. On sacrifice, the liver was harvested and kept on dry ice or at −80°C until analysis.
Cytokine analysis
Mouse IL-6 and monocyte chemoattractant protein (MCP)-1 in serum were assayed with a BD cytokine multiplex bead array system (BD Biosciences), and analyzed with a BD FACSArray instrument (BD Biosciences) according to the manufacturer's instructions. IL-12p40 was assayed with an immunoassay kit (BioSource International, Camarillo, CA) according to the manufacturer's instructions.
Quantitative real-time RT-PCR analysis of cytokine expression
Mice were injected with HDAdLacZ at 5 × 1012 viral particles (VP)/kg as described herein. The animals were killed at 0 hr (preinjection) or at 6 hr (postinjection), and total RNA was extracted from the liver of each animal, using TRIzol reagent (Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized from RNA samples, using SuperScript III with oligo(dT) priming (Invitrogen), and analyzed by SYBR green quantitative real-time PCR analysis (10 min at 95°C and then 45 cycles of 10 sec at 95°C, 7 sec at 60°C, and 30 sec at 72°C) with a Roche LightCycler 1.1 and Roche master mix (Roche, Indianapolis, IN) according to the manufacturer's protocol. The following primer sequences were designed and used for the analysis: 5′-GGAAATCGTGGAAATGAGAAA-3′ and 5′-GAATTGGATGGTCTTGGTCCTTAG-3′ for IL-6; and 5′-AAGCAGACCCTTACAGAGTGAAAA-3′ and 5′-ATGTGATGGGAGAACAGATTCCT-3′ for IL-12p40. To control for template variation among samples, the mRNA level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was determined with specific primers (5′-GCAAGAGAGGCCCTATCCCAA-3′ and 5′-CTCCCTAGGCCCCTCCTGTTATT-3′).
Time course of vector genome DNA and LacZ mRNA expression in mouse liver
Mice were injected with HDAdLacZ at 5 × 1012 VP/kg as described previously. At defined time points after injection, mice were killed by CO2 inhalation. Total DNA was extracted from liver, using a DNeasy blood and tissue kit (Qiagen, Valencia, CA), and total RNA was extracted with TRIzol reagent. RNA samples were treated with a TURBO DNA-free kit (Ambion, Austin, TX) and then reverse transcribed into complementary DNA (cDNA) with the SuperScript III first-strand cDNA synthesis system (Invitrogen). DNA and cDNA samples prepared from each liver were analyzed by quantitative real-time PCR analysis (10 min at 95°C and then 45 cycles of 10 sec at 95°C, 7 sec at 60°C, and 30 sec at 72°C), using the Roche LightCycler 1.1 and Roche master mix (Roche) and human stuffer gene-specific primers (5′-TCTGAATAATTTTGTGTTACTCATAGCGCG-3′ and 5′-CCCATAAGCTCCTTTTAACTTGTTAAAGTC-3′) and LacZ gene-specific primers (5′-atactgtcgtcgtcccctcaaact-3′ and 5′-cctccagataactgccgtcactc-3′). Vector copy numbers per microgram of total DNA were calculated by comparison with a standard curve generated by quantitative PCR of the original plasmid DNA of HDAdLacZ (pHDAd-LacZ).
Preparation of mouse embryonic fibroblasts
Embryonic day 14 (E14) embryos were dissected, minced, and soaked for 15 min in 3 ml of 0.25% trypsin–EDTA at 37°C, and Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) was added. Cells were dissociated by pipetting and filtered through a 70-μm mesh screen. The cell suspension was placed and cultured in DMEM supplemented with 10% FBS. The primary cultured fibroblasts were used for experiment within 10 passages.
Preparation of bone marrow-derived macrophages
Bone marrow cells were extracted from the femurs and tibias of mice, and red blood cells were removed with lysis solution (Qiagen). Bone marrow cells were cultured in DMEM supplemented with 10% FBS and 30% supernatant derived from confluent L929 cell cultures. On day 7, immature macrophages were collected. This procedure yields a pure population of macrophage colony-stimulating factor-dependent, adherent macrophages.
Quantitative PCR analysis and chromatin immunoprecipitation assay
Fibroblasts were seeded at subconfluency in 12-well plates. After 24 hr, fibroblasts were infected with HDAdLacZ at 1000 VP/cell. Total RNA and total cellular DNA were extracted at various time points with TRIzol reagent (Invitrogen) and a DNeasy blood and tissue kit (Qiagen). First-strand cDNA prepared from the RNA samples and DNA samples was subjected to quantitative real-time PCR analysis as described herein. A chromatin immunoprecipitation (ChIP) PCR assay was performed with a ChIP assay kit (Millipore, Temecula, CA) with anti-histone H3 (dimethylated at K9) (mAbcam 1220; Abcam, Cambridge, MA), anti-histone H3 (acetylated at K9) (ab10812; Abcam) antibodies as described previously (Suzuki et al., 2007, 2009). Briefly, chromatin was cross-linked with 1% formaldehyde. The cells were washed and resuspended in sodium dodecyl sulfate lysis buffer and then sonicated. The soluble chromatin was precleared by incubation with protein A–agarose–salmon sperm DNA slurry. Antibodies were added to the precleared supernatant and immunoprecipitated overnight at 4°C. After washing, antibody-bound histone–DNA complex was eluted, and histone–DNA cross-links were reversed by heating to 65°C for 4 hr. The immunoprecipitated DNA from each sample was subjected to quantitative PCR analysis with CMV promoter-specific primers (5′-GGGTGGTGACTCAATGGCCTTTAC-3′ and 5′-CCCACATTGACTTATATGCTTGCCAAC-3′).
Titering of antibodies to adenoviral particle and transgene product
ELISA-based titering experiments were conducted as previously described (Appledorn et al., 2008). Briefly, 5 × 108 VP/well or 2 μg of β-galactosidase protein/well (each diluted in phosphate-buffered saline [PBS]) was used to coat the wells of a 96-well plate overnight at 4°C. Plates were washed with PBS–Tween 20 (0.05%) (PBS-T) solution, and blocking buffer (3% bovine serum albumin [BSA] in PBS) was added to the wells, and incubated at room temperature for 2 hr. For titering of total IgG and IgG2b antibodies, plasma was diluted to 1:300 in sample dilution buffer (1% BSA in PBS), added to the wells, and incubated overnight at 4°C. Wells were washed with PBS-T and horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG and anti-mouse IgG2b were added at a 1:3000 dilution in PBS-T. Tetramethylbenzidine (Sigma-Aldrich, St. Louis, MO) substrate was added to each well, and the reaction was stopped with 2 N sulfuric acid. Plates were read at 450 nm in a microplate spectrophotometer.
IFN-γ secretion from splenocyte culture
As a measure of cell-mediated immune response to HDAd, we assessed the frequency of IFN-γ-producing T cells (Barcia et al., 2007). Seven days postinjection, spleens were isolated from each group of mice, and splenocytes (5 × 106 cells/well) were cultured in 24-well plates with RPMI supplemented with 50 μM 2-mercaptoethanol and 10% FBS. After 48 hr, medium was replaced with RPMI medium containing a 5 × 109 VP/well concentration of heat-inactivated HDAdLacZ (inactivated at 85°C for 15 min) and incubated at 37°C for 24 hr. The IFN-γ concentration in the medium of each sample was measured with the BD cytokine multiplex bead array system (BD Biosciences), and analyzed with a BD FACSArray instrument (BD Biosciences) according to the manufacturer's instructions.
Results
MyD88 is required for the innate immune response triggered by HDAd administration in vitro
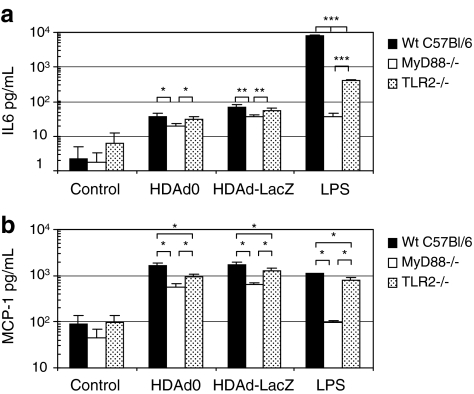
Previously, we found that TLR9 is a key sensor molecule responding to infection with helper-dependent adenoviral vectors (HDAd) in vitro and in vivo (Cerullo et al., 2007a). We sought to further characterize additional molecular components involved in the innate immune recognition of HDAd, specifically evaluating myeloid differentiating factor-88 (MyD88) via a genetically deficient mouse model. MyD88 signaling has been shown to be involved in the innate immune response to first-generation adenoviral vectors (FGAds) (Hartman et al., 2007; Yamaguchi et al., 2007; Appledorn et al., 2008) and it is critical for the signaling of all TLRs except TLR3. To determine whether TLR/MyD88 signaling is also activated in response to HDAd, we first evaluated the cytokine response from bone marrow-derived macrophages (BMDMs). BMDMs were isolated from wild-type C57BL/6 (wild-type), MyD88 knockout (MyD88–/–), and TLR2 knockout (TLR2–/–) mice after infection with HDAd0 and HDAdLacZ (Fig. 1). HDAd0 contains only the adenoviral packaging signal and a human genomic DNA stuffer sequence but no transgenes; HDAdLacZ contains a β-galactosidase expression cassette under the control of the CMV promoter (Cerullo et al., 2007a).
FIG. 1.
Role of bone marrow-derived macrophages (BMDMs) in helper-dependent adenovirus (HDAd)-induced innate immune responses. BMDMs were established from bone marrow cells isolated from each mouse as described in Materials and Methods. BMDMs (1 × 106) were exposed to HDAd0 or HDAdLacZ (5000 VP/cell) or lipopolysaccharide (LPS, 10 μg/ml) for 24 hr. (a) Interleukin (IL)-6 and (b) monocyte chemoattractant protein (MCP)-1 in the medium were measured with the BD cytokine multiplex bead array system and analyzed. Data are presented as means ± SD (n = 4). *p < 0.02, **p < 0.009, ***p < 0.005 for IL-6; *p < 0.001 for MCP-1. Wt, wild-type.
BMDMs infected with HDAdLacZ showed slightly higher IL-6 expression compared with cells infected with HDAd0. There was no difference in the expression level of MCP-1 after infection with HDAd0 or HDAdLacZ. These results suggest that the host sensing mechanism for induction of IL-6 may be different from that for MCP-1. MyD88–/– BMDMs showed a 50–70% reduction in the secretion of both IL-6 and MCP-1 compared with that of wild-type mice, indicating that MyD88 signaling is a central mediator of the innate immune response elicited by HDAd infection. Also, residual activation supports previously published data, suggesting the presence of MyD88-independent pathways in response to adenoviral infection (Hartman et al., 2007; Zhu et al., 2007). Although there was no significant difference in IL-6 expression between wild-type and TLR2–/– mouse-derived BMDMs, TLR2–/– BMDMs showed a 30–40% reduction in MCP-1 expression compared with wild-type cells. This finding further supports the notion that the IL-6 and MCP-1 responses may be differentially induced by recognition of viral particle and/or transgene sequence (products). Not surprisingly, these data show that HDAd similarly stimulates the host innate immune response in an MyD88-dependent and -independent fashion. In addition to these and previous observations of TLR9 signaling, TLR2 signaling is thus also revealed to be involved in the response to HDAd, as has been reported for FGAds (Appledorn et al., 2008).
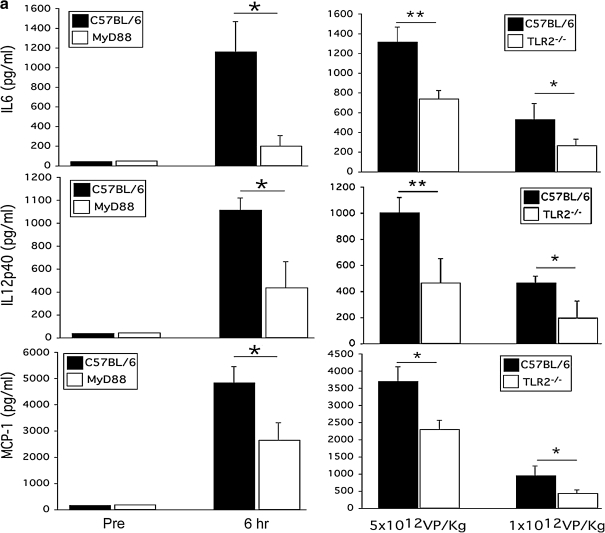
MyD88 contributes to the acute inflammatory response triggered by systemic HDAd administration in vivo
To investigate the sensing by TLR2 and the role of MyD88 in HDAd-induced systemic acute toxicity, MyD88–/– (Sato et al., 2004), TLR2–/–, and C57BL/6 wild-type mice (n = 5 each group) were injected via the tail vein with HDAdLacZ at 5 × 1012 VP/kg. Blood samples were collected 6 hr after injection for the evaluation of cytokines including IL-6 and IL-12, and MCP-1 (Fig. 2a). MyD88–/– mice showed a dramatic (greater than 80%) reduction in IL-6, and an approximately 60% reduction in IL-12. Reduction in MCP-1 was also observed, albeit to a significantly lesser extent. TLR2–/– mice (n = 5 per treatment group) injected with HDAdLacZ at either 5 × 1012 or 1 × 1012 VP/kg showed a dose-responsive reduction in proinflammatory cytokine levels compared with the control group. At the dose of 5 × 1012 VP/kg, the IL-6 level (6 hr postinjection) was approximately 50% lower in TLR2–/– versus C57BL/6 mice. Similarly, IL-12 and MCP-1 levels were 60 and 40% lower (respectively) in TLR2-deficient mice. Significantly, similar reductions were found in mice treated with the lower dose of vector (1 × 1012 VP/kg) (Fig. 2a). These results support our in vitro data and indicate that MyD88 signaling is essential for the full induction of a host innate immune response subsequent to systemic administration of HDAd. Furthermore, TLR2 is seen to be an additional sensor molecule upstream of MyD88 necessary to mediate the response to HDAd.
FIG. 2.
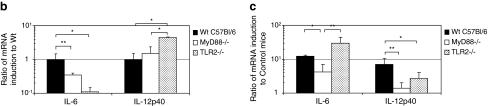
TLR2 and MyD88 signaling attenuates the innate immune response to HDAdLacZ in vivo. Wild-type C57BL/6, MyD88–/–, and TLR2–/– mice were injected with a high dose (5 × 1012 VP/kg) or low dose (1 × 1012 VP/kg) of HDAdLacZ via the tail vein. Serum samples were collected 6 hr postinjection. (a) Cytokine and chemokine levels were assayed. IL-6 and MCP-1 levels were measured with the BD cytokine multiplex bead array system. IL-12p40 levels were measured by enzyme-linked immunoassay. Data are presented as means ± SD (n = 5). *p < 0.002 for IL-6 of wild-type versus MyD88–/–, *p < 0.03 for IL-12p40 of wild-type versus MyD88–/–, *p < 0.04 for MCP-1 of wild-type versus MyD88–/–. *p < 0.03 and **p < 0.03 for IL-6 of wild-type versus TLR2–/–, *p < 0.05 and **p < 0.05 for IL-12p40 of wild-type versus TLR2–/–, *p < 0.03 for MCP-1 of wild-type versus TLR2–/–. (b) Basal mRNA levels of various cytokines were examined in the livers of untreated wild-type, MyD88–/–, and TLR2–/– mice. Data are presented as means ± SD (n = 4). *p < 0.001, **p < 0.01. (c) Induction of cytokine transcription in the liver after tail vein injection of HDAdLacZ (5 × 1012 VP/kg) was determined by RT-PCR 6 hr postinjection. Each value was calculated relative to that of untreated mice. Data are presented as means ± SD (n = 4). *p < 0.05, **p < 0.01.
Adenoviral vector localizes primarily in the liver after systemic administration (Manickan et al., 2006). To evaluate whether the expression of these cytokines in serum correlates with mRNA induction in liver, we assessed the mRNA expression of these cytokines in the liver of mice treated with HDAd. We first ascertained the baseline endogenous mRNA expression level of these cytokines (Fig. 2b). MyD88–/– mice showed slightly reduced levels of IL-6 compared with wild-type mice at baseline, whereas IL-12p40 was not significantly different among the genotypes. Although TLR2–/– mice exhibited a significant reduction in cytokine levels in serum compared with wild-type mice, IL-12p40 showed significantly higher expression at baseline. We evaluated the transcriptional induction of these cytokine signals in the liver 6 hr postinjection of a high dose of HDAdLacZ (Fig. 2c). IL-6 and IL-12p40 in the liver of MyD88–/– mice showed significantly lower levels compared with wild-type mice. These data suggest that liver, which is a major target of adenoviral vector after systemic administration (Manickan et al., 2006), contributes to the innate immune cytokine response. Although IL-6 protein levels in the serum of TLR2–/– mice showed a significant reduction compared with wild-type mice, induction of IL-6 mRNA in the liver of TLR2–/– mice exhibited a lesser degree, but still significantly higher induction than that in wild-type mice. Koizumi and colleagues reported that spleen, not liver, is the major site of cytokine and chemokine expression after systemic administration of adenoviral vectors (Koizumi et al., 2007). Our results suggest that the induction of cytokine mRNA in the liver transduced with HDAd may partially contribute to the expression of cytokines in the serum of mice.
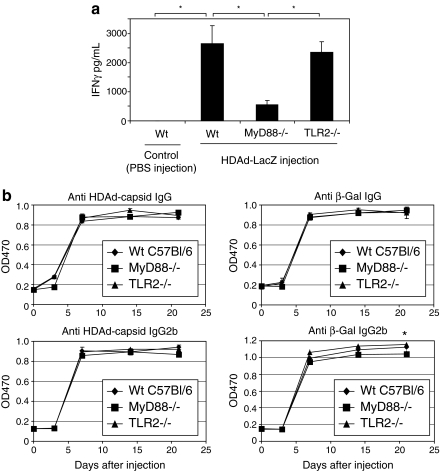
MyD88 contributes to the induction of a Th1 adaptive immune response to systemically administered HDAd
To investigate how the innate immune response induced via MyD88 signaling can affect the adaptive immune response to HDAd, we evaluated the IFN-γ secretion by splenocytes to determine the magnitude of a T cell response against adenoviral capsid proteins 7 days postinjection of a high dose of HDAdLacZ (Fig. 3a). Splenocytes were isolated from each group of mice 7 days after vector administration, at the apparent peak of the T cell response (Barcia et al., 2007). Heat-inactivated HDAd was used to stimulate the splenocytes in vitro against adenoviral capsid proteins. After 24 hr, sample medium was collected to determine the concentration of secreted IFN-γ. There was no induction of IFN-γ from splenocytes isolated from wild-type mice injected with PBS after stimulation with heat-inactivated HDAd, or from splenocytes isolated from MyD88–/– and TLR2–/– mice (data not shown). These results showed that any IFN-γ production detected from splenocytes of HDAdLacZ-treated mice were indeed adenoviral capsid specific. Interestingly, splenocytes isolated from MyD88–/– mice injected with HDAdLacZ exhibited an approximately 80% reduction in IFN-γ secretion compared with that isolated from wild-type mice injected with HDAdLacZ. These results correlated with the magnitude of induction of innate immune cytokines IL-6 and IL-12 in serum. Given that IL-6 and IL-12 can induce the development of the Th1 adaptive immune response (Guo et al., 2008), and IFN-γ is one of major cytokines secreted from Th1 lymphocytes (Niesner et al., 2008), loss of MyD88 signaling thus affects both the innate and adaptive immune responses to HDAd infection. Although TLR2–/– mice showed a significant reduction in innate cytokines compared with 6-hr postinjection wild-type mice (Fig. 2a), there was no difference in IFN-γ secretion by splenocytes in this assay. Abrogation of TLR2 signaling upstream of MyD88 may be insufficient to inhibit the innate immune-dependent Th1 immune response to HDAd because of redundancy at the sensor level, for example, by TLR9.
FIG. 3.
MyD88 signaling contributes to the adaptive immune response to HDAd. (a) Spleens from each group of mice (n = 5 for each group) were collected 7 day postinjection of HDAdLacZ (5 × 1012 VP/kg). After 48 hr, splenocytes were exposed to heat-inactivated adenoviral capsid protein for 24 hr. IFN-γ in the medium was measured with the BD cytokine multiplex bead array system and analyzed. Data are presented as means ± SD (n = 5). The experiments were repeated twice with similar results. *p < 0.0001. (b) Serum samples were collected at various time points (0, 3, 7, 14, and 21 days) from each group of mice (n = 5) after systemic administration of HDAdLacZ (5 × 1012 VP/kg). The development of antibodies to viral particles and transgene product (β-Gal) was evaluated by ELISA as described in Materials and Methods. Data are presented as means ± SD (n = 5). *p < 0.01.
We also evaluated the development of antibody to adenoviral particles and β-galactosidase (β-Gal) in the serum of mice at various time points (Fig. 3b). At subsequent time points, total IgG to viral particles and β-Gal was detected at similar levels in all groups of mice. These results demonstrated that although MyD88 signaling induced by HDAd infection might contribute to adaptive cellular immunity (Th1), it is not a major determinant of the adaptive humoral immune response (Th2), in our experimental context.
To better assess the effect of MyD88 signaling on the Th1 adaptive immune response, we also measured the development of IgG2b, which correlates more closely with a Th1 immune response. Although there was no difference in the development of IgG2b to viral particles in these mice, MyD88–/– mice exhibited a slight, but significant reduction in development of IgG2b to β-Gal compared with wild-type mice. Interestingly, TLR2–/– mice also showed a slight, but significantly higher titer of IgG2b to β-Gal compared with wild-type mice. These results suggested that the major process for development of antibody to adenovirus and LacZ occurs in an MyD88-independent manner. Together, our results show that MyD88 signaling affects the development of Th1-dependent antibody response to HDAd in vivo.
MyD88 signaling affects transgene expression of HDAd in the liver
To identify whether the innate and adaptive immune responses affect the level of transgene expression from HDAd, we evaluated β-Gal expression in the liver of MyD88–/–, TLR2–/–, and wild-type mice after systemic administration of HDAdLacZ (Fig. 4). We first performed 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) histochemistry of liver at two different time points (3 days and 3 weeks) (Fig. 4a). Although innate cytokine expression was significantly different among these mice, there was no difference in hepatocyte transduction 3 days postinjection at the dose used in our experiments. Surprisingly, the liver of MyD88–/– mice showed strong LacZ activity after 3 weeks, whereas that of wild-type and TLR2–/– mice showed poor LacZ activity (in wild-type mice) and undetectable levels (in TLR2–/– mice). To investigate the mechanism whereby transgene expression was lost, we first determined the vector genome and LacZ mRNA copy numbers in the liver of HDAdLacZ-injected mice at various time points (Fig. 4b and c). Interestingly, there was no significant difference in vector copy number in the liver of these mice 4 days postinjection. However, vector copy number in the liver of MyD88–/– mice injected with HDAdLacZ showed approximately 10-fold higher levels compared with wild-type mice at 3 weeks postinjection. By contrast, TLR2–/– mice had similarly decreased vector content compared with wild-type mice. These results correlate with the relative suppression of a Th1 immune response in MyD88–/– but not in wild-type or TLR2–/– mice. Hence, the functional decrease in Th1 response correlated with persistent vector content in MyD88–/– mice, presumably due to an attenuated cell-mediated adaptive immune response to HDAd and/or LacZ transgene. Our previous study of the LacZ transgene in the HDAd background demonstrated that LacZ was itself sufficient to stimulate a significant immune response (Mian et al., 2005).
FIG. 4.
Transgene expression in the liver of wild-type, MyD88–/–, and TLR2–/– mice. Each group of mice was injected with HDAdLacZ at 5 × 1012 VP/kg. (a) X-Gal staining of liver from wild-type, MyD88–/–, and TLR2–/– mice was performed 3 days and 3 weeks postinjection. The animals were killed 4 days and 3 weeks after injection, and the copy numbers of (b) vector genome DNA and (c) β-Gal mRNA transcripts in their livers were determined by real-time quantitative PCR. Data are presented as means ± SD (n = 4). *p < 0.001 for vector DNA copies; *p < 0.001 for β-Gal mRNA transcripts. Color images available online at www.liebertonline.com/hum.
Interestingly, although vector DNA levels were similar among all groups of mice 4 days postinjection, MyD88–/– mice showed an approximately 10-fold higher LacZ mRNA level compared with wild-type and TLR2–/– mice at this time point. MyD88–/– mice also exhibited 10-fold higher LacZ mRNA levels compared with wild-type mice and 100-fold higher levels compared with TLR2–/– mice at 3 weeks postinjection. Although the higher level of transgene expression in MyD88–/– mice could be attributable only to the relative increase in copy number at 3 weeks postinjection, it could also be due to differential epigenetic transcriptional silencing of the transgene 4 days postinjection.
MyD88 signaling regulates transgene expression at the transcriptional level in vitro
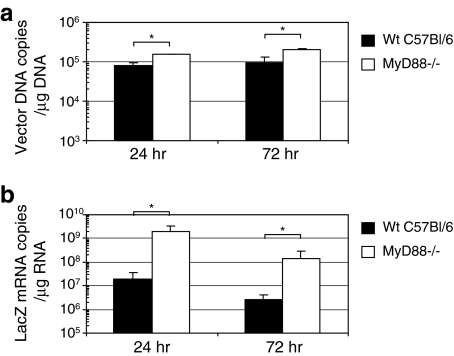
To test whether MyD88 signaling induced by infection with HDAd regulates transgene expression at the cellular level, we assessed transgene expression in primary mouse embryonic fibroblasts (MEFs) isolated from wild-type and MyD88–/– embryos. We isolated DNA and RNA from these HDAd-infected MEFs at various time points (24 and 72 hr) and measured vector and LacZ mRNA copy number in infected MEFs (Fig. 5a and b). HDAdLacZ-infected MyD88–/– MEFs showed 2- to 3-fold higher vector copy levels compared with transduced wild-type MEFs. Interestingly, the LacZ mRNA copy number in transduced MyD88–/– MEFs exhibited approximately 100-fold higher levels than that of wild-type MEFs. These results show that MyD88 signaling induced by infection with HDAd could regulate transgene expression of HDAd at either the transcriptional or posttranscriptional level.
FIG. 5.
Transgene expression in wild-type and MyD88–/– mouse embryonic fibroblasts (MEFs). MEFs derived from wild-type or MyD88–/– mice were infected with HDAdLacZ at 500 VP/cell. Cells were harvested at 24 and 72 hr and subjected to real-time quantitative PCR of (a) vector genome DNA as well as (b) β-Gal mRNA copy numbers. Data are presented as means ± SD (n = 4). *p < 0.01 for vector DNA copies. *p < 0.001 for β-Gal mRNA transcripts.
Differential chromatin modification of vector DNA in wild-type versus MyD88–/– MEFs
To determine whether MyD88 signaling could directly regulate transcription of vector transgenes by epigenetic modification of chromatin, chromatin immunoprecipitation (ChIP) PCR analysis was performed. DNA samples were harvested at various time points (24 and 72 hr after infection) from infected wild-type and MyD88-deficient MEFs, and immunoprecipitated with an antibody specific to histone H3, either dimethylated at Lys-9 (Met-K9-H3) or acetylated at Lys-9 (Ac-K9-H3). Met-K9-H3 is known to associate with transcriptionally inactive heterochromatic domains, whereas Ac-K9-H3 is known to associate with transcriptionally active euchromatic domains (Zhang and Reinberg, 2001). The immunoprecipitated DNA–chromatin complexes were evaluated by real-time PCR analysis using CMV promoter-specific primers (Fig. 6a). The ratio of Ac-K9-H3-associated DNA to Met-K9-H3-associated DNA was calculated for each sample and plotted in Fig. 6b. Ac-K9-H3-associated (transcriptionally active) vector DNA increased over time relative to Met-K9-H3-associated (transcriptionally inactive) vector DNA in MyD88-deficient fibroblasts. In contrast, the ratio of Ac-K9-H3 to Met-K9-H3 of wild-type fibroblasts was significantly lower compared with that of MyD88-deficient fibroblasts. These results indicate that vector DNA of HDAdLacZ attains a histone-modified chromatin structure and becomes transcriptionally inactivated in an MyD88-dependent mechanism in wild-type fibroblasts.
FIG. 6.
Histone modification in the CMV promoter region of infected MEFs. (a) Schematic structure of the HDAdLacZ vector genome and location of the primer pair used for quantification of chromatin immunoprecipitation (ChIP) data by real-time quantitative PCR. (b) MEFs derived from wild-type and MyD88 mice were infected with HDAdLacZ at 1000 VP/cell. Cells were harvested at 24 and 72 hr and ChIP experiments were performed as described in Materials and Methods. The ratio of Ac-K9-H3-associated vector DNA to Met-K9-H3-associated vector DNA was calculated. A higher ratio correlates with transcriptionally active loci. Data are presented as means ± SD (n = 5). *p < 0.01, **p < 0.0001.
Discussion
To confront the intrinsic toxicity of early-generation adenoviral vectors, helper-dependent adenovirus vectors, which are deleted of all viral coding sequences, were developed (Parks et al., 1996; Palmer and Ng, 2003). Elimination of all the viral coding genes decreased the adaptive response of the host immune system, thereby allowing long-term persistence of vector, although this effect may be prevented by an immune response against the transgene product. In several animal disease models, HDAd has demonstrated long-term transgene expression and reduced immunogenicity as compared with their first-generation vector counterparts in both small- and large-animal models (Brunetti-Pierri et al., 2005, 2008, 2009; McCormack et al., 2006; Cerullo et al., 2007b; Gau et al., 2009). However, our experience (as well as that of others) with HDAd has demonstrated an acute toxicity, similar to that of FGAds, shortly after in vivo delivery that continues to limit their clinical translation (Morral et al., 2002; Brunetti-Pierri et al., 2004). Although several studies of adenoviral vectors have begun to characterize the mechanisms of the innate immune activation responsible for this acute toxic response (Muruve, 2004; Muruve et al., 2004), the great redundancy and context dependence of the response still pose a significant obstacle. However, how these innate factors might regulate the adaptive immune response in the context of HDAd gene therapy is still unknown. The host innate immune response includes a multitude of cellular and humoral factors and no single pathway accounts for the overall response. By systematically identifying the specific host pathways that mediate the innate response, we may be able to develop combinatorial therapies that decrease overall toxicity and, hence, increase the maximal tolerated dose (MTD) for any specific intervention. Our previous studies with HDAd, and also with other FGAds, clearly demonstrated that TLR9 is one factor serving as a critical endosomal sensor molecule recognizing adenoviral vector genomes after systemic intravenous delivery (Cerullo et al., 2007a; Yamaguchi et al., 2007; Appledorn et al., 2008). Appledorn and colleagues reported that several adenovirus-induced innate immune responses are also dependent on surface TLR2 (Appledorn et al., 2008). In this study we characterized the role of MyD88, which is the key signal mediator of TLR signaling, in the immune response to HDAd both in vitro and in vivo. We also assessed whether the TLR2 response to FGAd was similar to that to HDAd. Moreover, we assessed the effect of MyD88 signaling on the adaptive immune response and on transgene expression after HDAd infection at early and late time points.
We showed that MyD88–/– mice exhibit a 50–70% reduction in proinflammatory cytokine production when challenged systemically with high doses of HDAdLacZ (similar to the reduction observed with FGAd). The data presented in this study along with our previous study on TLR9 (Cerullo et al., 2007a) suggest that TLR9 as well as other molecules, such as TLR2 in particular, converge to activate an MyD88 signaling pathway in response to HDAd. Similar to reports by others on FGAd (Appledorn et al., 2008), we found a significant involvement of TLR2 activation in response to HDAd vectors. Moreover, we demonstrated that the liver and presumably hepatocytes and Kupffer cells are a significant source of these cytokines (Liu et al., 2000). Importantly, loss of MyD88 was not able to completely abrogate activation of the proinflammatory cytokine cascade associated with the innate immune response, underscoring the contribution of TLR/MyD88-independent pathways. The innate immune recognition of adenoviral vector by plasmacytoid dendritic cells (pDCs) is mediated by TLR9 and is dependent on MyD88, whereas in non-pDCs (e.g., conventional DCs) such responses also depend on cytosolic sensing of adenoviral DNA (Zhu et al., 2007).
Interestingly, when MyD88–/– mice were injected with an HDAd vector expressing the highly immunogenic protein LacZ (HDAdLacZ), higher levels of vector and RNA were correlated with sustained transgene expression in MyD88–/– mice. This relative increase in vector copies was associated with a reduced MyD88-dependent Th1 adaptive immune response. Although our results suggest that TLR2 is a sensor molecule involved in the induction of the innate immune response to systemic administration of HDAd, TLR2–/– mice showed no difference in Th1 adaptive immune response and less LacZ expression in liver compared with wild-type mice. In contrast to TLR2–/– mice, TLR9–/– mice exhibited a significant reduction in both innate (Cerullo et al., 2007a) and Th1 adaptive immune responses (see Supplemental Fig. 1 at www.liebertonline.com/hum). As a result, TLR9–/– mice administered HDAd showed strong LacZ activity in liver compared with wild-type mice after 3 weeks (see Supplementary Fig. 2 at www.liebertonline.com/hum). These results suggest that TLR9-dependent activation of MyD88 may be a key signaling pathway for induction of the Th1 adaptive immune response to systemic administration of HDAd.
At the same time, no significant effect was noted on the Th2-dependent antibody response to either adenovirus or LacZ. These results indicate that MyD88 signaling may regulate the Th1 adaptive immune response to adenoviral and/or transgene product. Hence, downregulating the MyD88-dependent adaptive response might serve as an important translational target, especially given the outcome of AAV-based clinical trials in which an unexpected loss of coagulation factor IX expression was thought to be due to triggering of Th1-dependent CTL responses (Manno et al., 2006). Although HDAd-based gene therapy has facilitated sustained expression in animal models, similar to that seen with AAV, the potential of Th1 adaptive immune clearance of transgene product and transduced cells must be considered for clinical trials.
Another important finding of this study is that MyD88 signaling can mediate the transcriptional and/or posttranscriptional silencing of the HDAd-encoded transgene in cultured MEFs as well as in vivo in the liver of mice before induction of an adaptive immune response. The CMV promoter-encoding sequence of the vector DNA in wild-type fibroblasts showed a significantly lower ratio of transcriptionally active versus repressed chromatin marks, whereas MyD88-deficient fibroblasts showed a significantly higher ratio 72 hr after infection. These data indicate that the vector DNA can be sensed and transcriptionally inactivated through MyD88-dependent mechanisms. Heterochromatic modifications are often linked to DNA methylation (Bird and Wolffe, 1999), but Chen and colleagues reported that episomal transgene expression in liver transduced with plasmid-based vector is independent of CpG methylation (Chen et al., 2008). The vector DNA of HDAd exists episomally, similar to plasmid DNA vector after nuclear import. The epigenetic modification of vector DNA of HDAd may be independent of vector DNA methylation. The herpes simplex virus amplicon vector, also a DNA viral vector, is silenced at the transcriptional level by modification of vector DNA through activation of the IFN–STAT1 signaling pathway (Suzuki et al., 2007). This might represent a general mechanism for episomal viral DNAs including adenoviral vectors. It would be interesting to pursue whether MyD88 signaling also induced this epigenetic modification in a fashion dependent on IFN–STAT signaling.
In this study, we defined MyD88 signaling as a critical innate immune response pathway that is activated after intravenous administration of HDAd similar to the response seen with FGAd. However, MyD88-independent signaling also contributes to the overall response to vector (Zhu et al., 2007). For example, a cytoplasmic sensor molecule may also recognize infection by HDAd. AIM2 was reported as a cytoplasmic DNA sensor that can induce the innate immune response (Burckstummer et al., 2009). Further studies to assess infection with HDAd independent of MyD88 signaling will be necessary to fully define the molecular mechanisms that specify the innate and adaptive immune responses to HDAd in vivo. These data show interactions between HDAd and the host innate immune system similar to those reported for FGAd. Furthermore, they demonstrate how this interaction can affect the adaptive immune response in the context of HDAd. As HDAd is the likely vehicle for moving forward with adenovirus-based gene replacement therapy clinical trials for inherited disorders, it constitutes a crucial preclinical base for further improving the therapeutic index of these vectors.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (DK56787 and HL87836 to B. Lee; R00 HL088692 to N.B.-P.). The authors thank the Morphology Core Laboratory of the Gulf Coast Digestive Disease Center (P30 DK56338) and Dorene M. Rudman for assistance in performing enzyme histochemistry.
Author Disclosure Statement
No competing financial interests exist.
References
- Adachi O. Kawai T. Takeda K. Matsumoto M. Tsutsui H. Sakagami M. Nakanishi K. Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Akira S. Uematsu S. Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Appledorn D.M. Patial S. McBride A. Godbehere S. Van Rooijen N. Parameswaran N. Amalfitano A. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol. 2008;181:2134–2144. doi: 10.4049/jimmunol.181.3.2134. [DOI] [PubMed] [Google Scholar]
- Barcia C. Jimenez-Dalmaroni M. Kroeger K.M. Puntel M. Rapaport A.J. Larocque D. King G.D. Johnson S.A. Liu C. Xiong W. Candolfi M. Mondkar S. Ng P. Palmer D. Castro M.G. Lowenstein P.R. One-year expression from high-capacity adenoviral vectors in the brains of animals with pre-existing anti-adenoviral immunity: Clinical implications. Mol. Ther. 2007;15:2154–2163. doi: 10.1038/sj.mt.6300305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basner-Tschakarjan E. Gaffal E. O'Keeffe M. Tormo D. Limmer A. Wagner H. Hochrein H. Tuting T. Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-α production. J. Gene Med. 2006;8:1300–1306. doi: 10.1002/jgm.964. [DOI] [PubMed] [Google Scholar]
- Bird A.P. Wolffe A.P. Methylation-induced repression: Belts, braces, and chromatin. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- Bonnert T.P. Garka K.E. Parnet P. Sonoda G. Testa J.R. Sims J.E. The cloning and characterization of human MyD88: A member of an IL-1 receptor related family. FEBS Lett. 1997;402:81–84. doi: 10.1016/s0014-5793(96)01506-2. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N. Palmer D.J. Beaudet A.L. Carey K.D. Finegold M. Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum. Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N. Nichols T.C. McCorquodale S. Merricks E. Palmer D.J. Beaudet A.L. Ng P. Sustained phenotypic correction of canine hemophilia B after systemic administration of helper-dependent adenoviral vector. Hum. Gene Ther. 2005;16:811–820. doi: 10.1089/hum.2005.16.811. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N. Ng T. Iannitti D.A. Palmer D.J. Beaudet A.L. Finegold M.J. Carey K.D. Cioffi W.G. Ng P. Improved hepatic transduction, reduced systemic vector dissemination, and long-term transgene expression by delivering helper-dependent adenoviral vectors into the surgically isolated liver of nonhuman primates. Hum. Gene Ther. 2006;17:391–404. doi: 10.1089/hum.2006.17.391. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N. Clarke C. Mane V. Palmer D.J. Lanpher B. Sun Q. O'Brien W. Lee B. Phenotypic correction of ornithine transcarbamylase deficiency using low dose helper-dependent adenoviral vectors. J. Gene Med. 2008;10:890–896. doi: 10.1002/jgm.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N. Stapleton G.E. Law M. Breinholt J. Palmer D.J. Zuo Y. Grove N.C. Finegold M.J. Rice K. Beaudet A.L. Mullins C.E. Ng P. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol. Ther. 2009;17:327–333. doi: 10.1038/mt.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckstummer T. Baumann C. Bluml S. Dixit E. Durnberger G. Jahn H. Planyavsky M. Bilban M. Colinge J. Bennett K.L. Superti-Furga G. An orthogonal proteomic–genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- Cerullo V. Seiler M.P. Mane V. Brunetti-Pierri N. Clarke C. Bertin T.K. Rodgers J.R. Lee B. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol. Ther. 2007a;15: 378–385. doi: 10.1038/sj.mt.6300031. [DOI] [PubMed] [Google Scholar]
- Cerullo V. Seiler M.P. Mane V. Cela R. Clarke C. Kaufman R.J. Pipe S.W. Lee B. Correction of murine hemophilia A and immunological differences of factor VIII variants delivered by helper-dependent adenoviral vectors. Mol. Ther. 2007b;15:2080–2087. doi: 10.1038/sj.mt.6300308. [DOI] [PubMed] [Google Scholar]
- Chen Z.Y. Riu E. He C.Y. Xu H. Kay M.A. Silencing of episomal transgene expression in liver by plasmid bacterial backbone DNA is independent of CpG methylation. Mol. Ther. 2008;16:548–556. doi: 10.1038/sj.mt.6300399. [DOI] [PubMed] [Google Scholar]
- Garcia-Sastre A. Biron C.A. Type 1 interferons and the virus–host relationship: A lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- Gau C.L. Rosenblatt R.A. Cerullo V. Lay F.D. Dow A.C. Livesay J. Brunetti-Pierri N. Lee B. Cederbaum S.D. Grody W.W. Lipshutz G.S. Short-term correction of arginase deficiency in a neonatal murine model with a helper-dependent adenoviral vector. Mol. Ther. 2009;17:1155–1163. doi: 10.1038/mt.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B. Chang E.Y. Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J. Clin. Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman Z.C. Kiang A. Everett R.S. Serra D. Yang X.Y. Clay T.M. Amalfitano A. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J. Virol. 2007;81:1796–1812. doi: 10.1128/JVI.01936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley S.E. Amalfitano A. Toll-like receptors impact on safety and efficacy of gene transfer vectors. Mol. Ther. 2007;15:1417–22. doi: 10.1038/sj.mt.6300217. [DOI] [PubMed] [Google Scholar]
- Hensley S.E. Cun A.S. Giles-Davis W. Li Y. Xiang Z. Lasaro M.O. Williams B.R. Silverman R.H. Ertl H.C. Type I interferon inhibits antibody responses induced by a chimpanzee adenovirus vector. Mol. Ther. 2007;15:393–403. doi: 10.1038/sj.mt.6300024. [DOI] [PubMed] [Google Scholar]
- Iacobelli-Martinez M. Nemerow G.R. Preferential activation of Toll-like receptor nine by CD46-utilizing adenoviruses. J. Virol. 2007;81:1305–1312. doi: 10.1128/JVI.01926-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi N. Yamaguchi T. Kawabata K. Sakurai F. Sasaki T. Watanabe Y. Hayakawa T. Mizuguchi H. Fiber-modified adenovirus vectors decrease liver toxicity through reduced IL-6 production. J. Immunol. 2007;178:1767–1773. doi: 10.4049/jimmunol.178.3.1767. [DOI] [PubMed] [Google Scholar]
- Liu S. Salyapongse A.N. Geller D.A. Vodovotz Y. Billiar T.R. Hepatocyte Toll-like receptor 2 expression in vivo and in vitro: Role of cytokines in induction of rat TLR2 gene expression by lipopolysaccharide. Shock. 2000;14:361–365. doi: 10.1097/00024382-200014030-00021. [DOI] [PubMed] [Google Scholar]
- Manickan E. Smith J.S. Tian J. Eggerman T.L. Lozier J.N. Muller J. Byrnes A.P. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol. Ther. 2006;13:108–117. doi: 10.1016/j.ymthe.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Manno C.S. Pierce G.F. Arruda V.R. Glader B. Ragni M. Rasko J.J. Ozelo M.C. Hoots K. Blatt P. Konkle B. Dake M. Kaye R. Razavi M. Zajko A. Zehnder J. Rustagi P.K. Nakai H. Chew A. Leonard D. Wright J.F. Lessard R.R. Sommer J.M. Tigges M. Sabatino D. Luk A. Jiang H. Mingozzi F. Couto L. Ertl H.C. High K.A. Kay M.A. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- McCormack W.M., Jr. Seiler M.P. Bertin T.K. Ubhayakar K. Palmer D.J. Ng P. Nichols T.C. Lee B. Helper-dependent adenoviral gene therapy mediates long-term correction of the clotting defect in the canine hemophilia A model. J. Thromb. Haemost. 2006;4:1218–1225. doi: 10.1111/j.1538-7836.2006.01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian A. McCormack W.M., Jr. Mane V. Kleppe S. Ng P. Finegold M. O'Brien W.E. Rodgers J.R. Beaudet A.L. Lee B. Long-term correction of ornithine transcarbamylase deficiency by WPRE-mediated overexpression using a helper-dependent adenovirus. Mol. Ther. 2004;10:492–499. doi: 10.1016/j.ymthe.2004.05.036. [DOI] [PubMed] [Google Scholar]
- Mian A. Guenther M. Finegold M. Ng P. Rodgers J. Lee B. Toxicity and adaptive immune response to intracellular transgenes delivered by helper-dependent vs. first generation adenoviral vectors. Mol. Genet. Metab. 2005;84:278–288. doi: 10.1016/j.ymgme.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Morral N. O'Neal W.K. Rice K. Leland M.M. Piedra P.A. Aguilar-Cordova E. Carey K.D. Beaudet A.L. Langston C. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum. Gene Ther. 2002;13:143–154. doi: 10.1089/10430340152712692. [DOI] [PubMed] [Google Scholar]
- Muruve D.A. The innate immune response to adenovirus vectors. Hum. Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- Muruve D.A. Barnes M.J. Stillman I.E. Libermann T.A. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- Muruve D.A. Cotter M.J. Zaiss A.K. White L.R. Liu Q. Chan T. Clark S.A. Ross P.J. Meulenbroek R.A. Maelandsmo G.M. Parks R.J. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J. Virol. 2004;78:5966–5972. doi: 10.1128/JVI.78.11.5966-5972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesner U. Albrecht I. Janke M. Doebis C. Loddenkemper C. Lexberg M.H. Eulenburg K. Kreher S. Koeck J. Baumgrass R. Bonhagen K. Kamradt T. Enghard P. Humrich J.Y. Rutz S. Schulze-Topphoff U. Aktas O. Bartfeld S. Radbruch H. Hegazy A.N. Löhning M. Baumgart D.C. Duchmann R. Rudwaleit M. Häupl T. Gitelman I. Krenn V. Gruen J. Sieper J. Zeitz M. Wiedenmann B. Zipp F. Hamann A. Janitz M. Scheffold A. Burmester G.R. Chang H.D. Radbruch A. Autoregulation of Th1-mediated inflammation by twist1. J. Exp. Med. 2008;205:1889–1901. doi: 10.1084/jem.20072468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nociari M. Ocheretina O. Schoggins J.W. Falck-Pedersen E. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J. Virol. 2007;81:4145–4157. doi: 10.1128/JVI.02685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D. Ng P. Improved system for helper-dependent adenoviral vector production. Mol. Ther. 2003;8:846–852. doi: 10.1016/j.ymthe.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Parks R.J. Chen L. Anton M. Sankar U. Rudnicki M.A. Graham F.L. A helper-dependent adenovirus vector system: Removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N. Takahashi N. Suda K. Nakamura M. Yamaki M. Ninomiya T. Kobayashi Y. Takada H. Shibata K. Yamamoto M. Takeda K. Akira S. Noguchi T. Udagawa N. MyD88 but not TRIF is essential for osteoclastogenesis induced by lipopolysaccharide, diacyl lipopeptide, and IL-1α. J. Exp. Med. 2004;200:601–611. doi: 10.1084/jem.20040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M. Chiocca E.A. Saeki Y. Early STAT1 activation after systemic delivery of HSV amplicon vectors suppresses transcription of the vector-encoded transgene. Mol. Ther. 2007;15:2017–2026. doi: 10.1038/sj.mt.6300273. [DOI] [PubMed] [Google Scholar]
- Suzuki M. Kasai K. Ohtsuki A. Godlewski J. Nowicki M.O. Chiocca E.A. Saeki Y. ICP0 inhibits the decrease of HSV amplicon-mediated transgene expression. Mol. Ther. 2009;17:707–715. doi: 10.1038/mt.2008.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toietta G. Mane V.P. Norona W.S. Finegold M.J. Ng P. McDonagh A.F. Beaudet A.L. Lee B. Lifelong elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector. Proc. Natl. Acad. Sci. U.S.A. 2005;102:3930–3935. doi: 10.1073/pnas.0500930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T. Kawabata K. Koizumi N. Sakurai F. Nakashima K. Sakurai H. Sasaki T. Okada N. Yamanishi K. Mizuguchi H. Role of MyD88 and TLR9 in the innate immune response elicited by serotype 5 adenoviral vectors. Hum. Gene Ther. 2007;18:753–762. doi: 10.1089/hum.2007.016. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Reinberg D. Transcription regulation by histone methylation: Interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15:2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- Zhu J. Huang X. Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J. Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.