Abstract
We compared the predictive value of cerebral perfusion as measured by arterial-spin labeling magnetic resonance imaging (ASL-MRI) with MRI-derived hippocampal volume for determining future cognitive and functional decline and subsequent conversion from mild cognitive impairment to dementia. Forty-eight mild cognitive impairment subjects received structural and ASL-MRI scans at baseline and clinical and neuropsychologic assessments annually. Thirteen subjects became demented during the period of longitudinal observation (2.7 ± 1.0 y). Cox regression analyses suggest that baseline hippocampal volume [relative risk (RR) = 0.99, P = 0.004], baseline right inferior parietal (RR = 0.64, P = 0.01) and right middle frontal (RR = 0.73, P = 0.01) perfusion were associated with conversion to dementia. Results from linear mixed effects modeling suggest that baseline perfusion from the right precuneus predicted subsequent declines in Clinical Dementia Rating Sum of Boxes (P = 0.002), Functional Activates Questionnaire (P = 0.01), and selective attention (ie, Stroop switching, P = 0.009) whereas baseline perfusion from the right middle frontal cortex predicted subsequent episodic memory decline (ie, total recognition discriminability score from the California Verbal Learning Test, P = 0.03). These results suggest that hypoperfusion as detected by ASL-MRI can predict subsequent clinical, functional, and cognitive decline and may be useful for identifying candidates for future Alzheimer disease treatment trials.
Keywords: mild cognitive impairment, dementia, cognitive and functional decline, ASL perfusion MRI, hippocampal volume
Mild cognitive impairment (MCI) is considered to be the transition between normal aging and Alzheimer disease (AD), the most prevalent dementing disorder in older adults. Studies with fluorodeoxyglucose (FDG) positron emission tomography (PET), which measures glucose metabolism, and technetium-99m hexamethylpropyleneamineoxime single photon emission computed tomography (SPECT), which measures cerebral blood flow, have reported reduced metabolism and perfusion in the medial temporal lobes and posterior cingulate gyri in AD and MCI.1–3 To date, relatively few FDG PET and SPECT studies of MCI have addressed prediction of future decline and the handful of studies that have addressed this issue have reported discrepant findings. Using region of interest (ROI) based-techniques, some authors have reported that reduced perfusion/glucose metabolism in the prefrontal and parietal cortices,4 temporoparietal cortex,5 precunei,6 posterior cingulate gyrus,7,8 and hippocampus,6,9–11 are sensitive early markers of progression to AD. Using principal component analysis, Borroni et al12 described a specific pattern of hypoperfusion in converters that involved the parietal and temporal lobes, precuneus and posterior cingulate cortex. Caroli et al13 recently reported that compared with control subjects, amnestic MCI who converted to AD had hypoperfusion in the parahippocampal and inferior temporal cortices whereas amnestic MCI patients who did not convert to dementia had hypoperfusion in the retrosplenial cortex.
To the extent that regional metabolism and perfusion are coupled, arterial spin-labeling magnetic resonance imaging (ASL-MRI), which labels arterial blood water as an endogenous diffusible tracer for perfusion, may be able to detect functional deficiencies in a way similar to FDG PET and SPECT.14 In support of this, ASL-MRI studies of AD and MCI patients have reported a similar pattern of regional hypoperfusion to that described in previous PET and SPECT studies.15–17 Moreover, ASL-MRI offers several advantages over PET and SPECT: it is noninvasive and free of exposure to ionizing radiation, intravenous contrast agents, and radioactive isotopes; it can be performed with most magnetic resonance scanners in 10 to 15 min; and because labeled water is cleared after a few seconds it can be rapidly repeated. An additional advantage is that perfusion and structural images can be acquired at the same imaging session. Thus, the first goal of this study was to find ASL perfusion MRI correlates of conversion to dementia in patients with MCI.
Because several studies have shown that hippocampal atrophy may serve as a predictor for progression from MCI to AD,18,19 the second goal of this study is to compare the predictive value of MRI-derived hippocampal volume (HV) with the predictive value of baseline perfusion for determining the risk of converting to dementia. The final goal of this study was to examine the value of baseline perfusion and HV for predicting future cognitive and functional decline. Numerous studies have reported impaired recognition memory in patients with probable AD20–23 and MCI24. Furthermore, in one of the pioneering studies on the concept of MCI,25 recognition memory was 1 of the 4 predictors of decline in subjects with MCI, with a sensitivity of 85.7% and a specificity of 100%. Therefore, we examined the predictive value of baseline perfusion and HVs for determining future decline in the California Verbal Learning Test (CVLT26) total recognition discriminability score. Deficits in selective attention, particularly with regard to response inhibition and task switching, have also been noted in patients with early AD27 and MCI.28,29 `Therefore, we examined the predictive value of baseline HV and perfusion for determining future decline in the Delis Kaplan Executive Function System30 Stroop test. Functional decline has clinical importance in planning the patient care. Therefore, we examined the ability of baseline HV and perfusion to predict functional decline. We focused on change in the Functional Activities Questionnaire (FAQ31), which measures an individual's ability to perform complex, higher order activities such as writing checks and assembling tax records, and change in the Clinical Dementia Rating Scale Sum of Boxes score (CDR-SB). Several authors have reported that the increased CDR-SB scores are associated with a higher probability of converting to dementia.32–34
METHODS
Participants
Forty-eight subjects recruited through flyers and referrals from local memory clinics (ie, Memory Disorders Clinic at the San Francisco Veterans Affairs Medical Center, the Memory and Aging Center at the University of California, San Francisco, and the Memory Disorders Clinic at the California Pacific Medical Center) were examined for this study. All participants provided written informed consent according to procedures approved by institutional review boards of the University of California San Francisco and the San Francisco VA Medical Center.
All participants had a Clinical Dementia Rating (CDR35) of 0.5 at baseline. To capture the broadest range of MCI, we operationally defined these individuals as having MCI, as others have previously done.36–38 Furthermore, we did not require that subjects perform below specific cutoffs on psychometric testing because we were interested in including individuals at the mildest end of the MCI spectrum (ie, those with CDR sum of boxes scores 0.5 to 1).
Clinical nurses from the referring memory clinic administered the CDR using the semistructured interview protocol developed by John Morris and colleagues at the Washington University School of Medicine. All of the clinical nurses had completed the Brief Training and Reliability Protocol offered by the Washington University Alzheimer's Disease Research Center. The Brief Training and Reliability Protocol includes an introduction to the CDR by Dr Morris, 3 videotaped patient interviews for training purposes, and 6 videotaped interviews for reliability certification. Successful completion of the 6 reliability tapes is achieved with agreement with a “gold standard” on at least 5 out of the 6 tapes. Prior research has shown that physicians and nonphysician health professionals demonstrate good reliability in administering the CDR after appropriate training.39,40
Procedures
The study procedures included a blood draw for genetic analysis, structural, and ASL-MRI scans at baseline. The subjects also received a medical evaluation (ie, medical history, physical, and neurologic examination) and neuropsychologic testing at baseline and each annual follow-up visit. The neuropsychologic tests assessed general cognitive ability [ie, Mini-Mental State Examination, (MMSE,41)] episodic memory (ie, CVLT-II26), and selective attention (ie, Delis Kaplan Executive Function System, Delis Kaplan Executive Function System, Stroop test, and switching condition). Depression was assessed with the self-reported, 30-item Geriatric Depression Scale (GDS)42 and independent activities of daily living were quantified with the FAQ.31
Structural MRI Acquisition and Analysis
All imaging was preformed on a 1.5 Tesla MR system (Siemens Vision System, Germany), using a standard head coil. Structural MRI included the following: 2D FLASH MRI along 3 orthogonal directions to obtain scout views of the brain for initial positioning of MRI slices (total acquisition time: 1 min). A double spin echo sequence to obtain proton density and T2-weighted MRIs, TR/TE1/ TE2 = 5000/20/85 ms, 51 contiguous axial slices (3 mm) covering the entire brain and angulated 2 to 10 degrees from the anterior to the posterior commissure line; 1.0 × 1.25 mm2 inplane resolution (total acquisition time: 12 min). Volumetric T1-weighted gradient echo MRI (MPRAGE) of entire brain, TR/TE/TI = 11/4/850 ms, 12 degree flip angle sequence with a spatially isotropic 3 resolution of 1.0 mm3.
ASL-perfusion Image Acquisition and Analysis
ASL-MRI were acquired with the Double Inversions with Proximal Labeling of Both Tag and Control Images43 method with a single-shot gradient-echo planar imaging sequence (TR 2500 ms, TE 15 ms, TI2 = 1500 ms, field of view 260 mm, matrix 128 × 128 mm, covering 7 slices above the anterior to the posterior commissure line, slice thickness 8 mm, slice gap 2 mm). A single shot gradient-echo planar imaging scan (TR 2500 ms, TE 15 ms, field of view 260 mm, 128 × 128 mm, 24 slices, slice thickness 5 mm, slice gap 2 mm) covering the whole head was also obtained to facilitate coregistration of perfusion and structural images.
ASL-perfusion data was preprocessed using Statistical Parametric Mapping (SPM2; Wellcome Department of Cognitive Neurology, London, UK) for Matlab (The Mathworks, Inc, Natick, MA). After image coregistration, a perfusion image was calculated by subtracting the labeled from unlabeled ASL-MR images. Perfusion intensity was adjusted for instrumental variability by correcting for receiver gain and coil loading. Each subject's perfusion image was corrected for partial volume effects and the confounding effect of atrophy-induced hypoperfusion44 using each subject's T1 image, segmented with Expectation-Maximization Segmentation (EMS).45 Next, a virtual white matter (WM) perfusion image was created by extracting the mean perfusion of the centrum semiovale and then multiplying this by the WM map (from segmentation), smoothed to the resolution of the perfusion image. This virtual WM perfusion image was then subtracted from the original perfusion image. Then, a gray matter (GM) map, smoothed to the resolution of the perfusion image, was multiplied by the perfusion image, corrected for WM perfusion, to obtain a perfusion image for GM only. Finally, to correct for GM atrophy effects, the GM masked perfusion image was divided by the smoothed GM map, producing a perfusion image per unit gray matter. The final perfusion images were normalized to a study specific T1 template, created using the EMS segmented GM, WM, and cerebral spinal fluid maps, and smoothed with a 10 mm Gaussian kernel.
HV
Boundaries of the hippocampus were defined using the semiautomated, atlas based, fluid transformation warping software by surgical navigation technologies described in previous publications.46,47 A covariance approach was used to adjust HV by total intracranial volume (ICV).48 Total ICV was calculated from the total volumes of the GM, WM, and cerebral spinal fluid masks from the EMS segmentation. Adjusted HV (aHV) was obtained with the following formula: aHV = raw HV-β(ICV-mean ICV), where β reflects the regression coefficient when raw HV is regressed against ICV and mean ICV reflects the group mean. Because we had no a prior hypotheses about laterality and because volumes of the right and left hippocampus were highly intercorrelated, we combined volumes from both hemispheres for data reduction purposes.
Statistical Analyses
We initially used the “single subject: conditions and covariates” SPM2 model to assess differences in baseline perfusion between converters and nonconverters on voxelby-voxel basis. Age and whole brain perfusion were entered as confounding variables. The significance threshold of the t statistics was set at 0.001, uncorrected for multiple comparisons, and the extent threshold was set at 10 voxels. Although a direct comparison between converters and nonconverters is not entirely appropriate analytically because the length of follow-up varied among patients, we nevertheless used this method as a way of obtaining ROIs on which to focus further analyses.
To accommodate the variable follow-up periods among the subjects, we extracted raw perfusion values from the ROIs identified in the voxel-wise SPM analysis, using the MarsBaR49 (http://marsbar.sourceforge.net/) toolbox in SPM2, and submitted these values to a survival analysis to evaluate the relationship between baseline perfusion and subsequent conversion to dementia. We used the Statistical Package for the Social Sciences version 16.0 to make estimates of relative risk (RR) with Cox regression models. Each predictor variable was evaluated univariately and all tests were 2-sided. Survival time was calculated as the interval from the initial baseline scan to the diagnosis of dementia for MCI subjects. For MCI subjects who remained nondemented, survival time was censored at the date of the last clinic visit. Because of sample size limitations, confidence intervals are not reported for estimates of risk. To compare the predictive value of the ASL-MRI measured perfusion with MRI-derived HVs; we entered baseline HVs and the raw ASL-perfusion MRI values extracted from the ROIs identified in the SPM analysis to a Cox regression analysis. Next, we used the forward stepwise selection method to build regression models. The likelihood-ratio test based on partial likelihood estimates was used for variable removal. Default P values for variable removal in the stepwise models were less than 0.10.
We quantified cognitive and functional decline by fitting linear mixed-effects models using restricted maximum likelihood (NLME package50 within R, http://www.r-project.org). Random effects for both intercept and slope were included in the model to accommodate the between subject variability in cognitive/functional decline. CVLT total recognition discriminability, Stroop switching, FAQ, and CDR-SB scores from each timepoint were entered as the dependent variable in 4 separate models. Because age, female sex, and depression are all established risk factors for cognitive and functional impairment in community-dwelling elderly individuals51 and because cross-sectional and prospective studies of nondemented elders have noted associations between low global cognitive scores and functional dependence52 and subsequent cognitive decline,53 our goal was to determine the predictive value of baseline perfusion and HVs beyond that which can be explained by demographic, baseline cognition, clinical, and genetic risk factors alone. Therefore we entered age, sex, years of education, apolipoprotein (APOE) ε4 status, baseline MMSE and GDS scores as covariates in all of the models. We also entered subsequent clinical status (ie, converted to dementia or remained nondemented) as a covariate in the models. The independent variables were time from baseline examination, imaging variable of interest (ie, baseline perfusion from the 4 ROIs, and baseline aHV) and time by imaging variable interactions.
We first examined the influence of baseline scores on subsequent decline by testing for a correlation between the intercept and slope random effects. We did this by comparing 2 models—1 that allowed for and 1 that disallowed for a correlation between intercept and slope through a likelihood ratio test. Next, we used a χ2 test to determine whether the uncorrelated model was an adequate simplification of the correlated model. If it was, we concluded that the baseline scores did not have significant explanatory power to predict future decline and we used the simpler, uncorrelated model. If the uncorrelated model was not an adequate simplification of the correlated model, then we concluded that the baseline scores did have significant explanatory power to predict future decline and we used a model that incorporated this correlation. To determine whether baseline perfusion or baseline aHV added further explanatory power, we fitted linear mixed-effects models with the imaging variable of interest as a covariate. We concluded that baseline perfusion or baseline aHV provided further explanatory power in cases where the time by imaging variable interaction was significant, as detected by conditional t-test in the linear mixed-effects model framework.
RESULTS
During the period of longitudinal observation (2.7 ± 1.0 y), 13 MCI subjects became demented (10 probable AD, 2 mixed dementia, and 1 vascular dementia). Thirty-five subjects remain nondemented. Table 1 summarizes the baseline demographic and clinical characteristics of the participants.
TABLE 1.
Demographic, Clinical, and Genetic Characteristics of Study Group
| Characteristic | All | Converted to Dementia | Remain Nondemented |
|---|---|---|---|
| N (% female) | 51 (33%) | 13 (23%) | 35 (13%) |
| Age (year), mean (SD) | 76.3 (7.2) | 77.1 (5.0) | 76.0 (7.8) |
| Education (year), mean (SD) | 16.5 (2.8) | 16.7 (2.9) | 16.5 (2.8) |
| Baseline GDS, mean (SD) | 6.3 (4.6) | 5.2 (4.7) | 6.8 (4.5) |
| Baseline MMSE, mean (SD) | 28.2 (1.7) | 27.5 (1.8) | 28.5 (1.7) |
| Follow-up time (year), mean (SD) | 2.7 (1.0) | 3.1 (1.1) | 2.5 (1.0) |
| Number with 2 timepoints | 7 | 2 | 5 |
| Number with 3 timepoints | 24 | 6 | 18 |
| Number with 4 timepoints | 10 | 3 | 7 |
| Number with 5 timepoints | 7 | 2 | 5 |
| Frequency of APOE ε4 allele carriers | 46% | 38% | 49% |
APOE indicates apolipoprotein E; GDS, Geriatric Depression Scale; MMSE, mini-mental state examination
Cerebral Perfusion Correlates of Conversion to Dementia
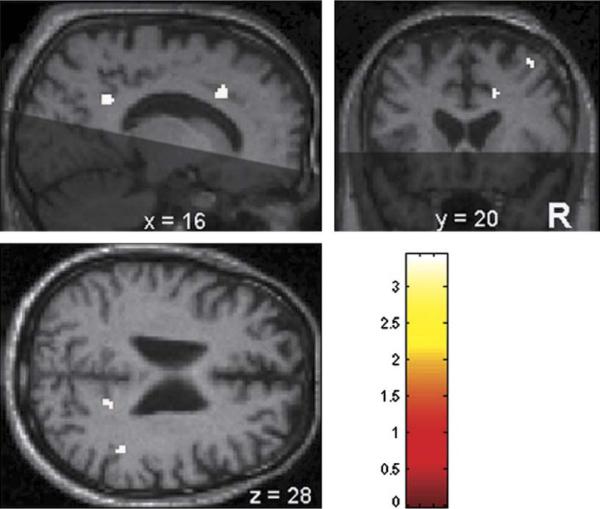
Figure 1 and Table 2 show results of the direct, voxel-wise comparison of the baseline ASL-MRI perfusion images of converters and nonconverters. Relative to subjects who remain nondemented, subjects who converted to dementia had hypoperfusion in the right precuneus, right inferior parietal cortex, right middle cingulum, and right middle frontal cortex.
FIGURE 1.
Statistical parametric maps showing regions of hypoperfusion in the 13 mild cognitive impairment subjects who converted to dementia relative to the 35 mild cognitive impairment subjects who remain nondemented. Converters had hypoperfusion in the right precuneus/posterior cingulum (shown in the sagittal and axial slices), right middle cingulum (shown in the sagittal and coronal slices), right middle frontal cortex (shown in the coronal slice), and right inferior parietal cortex (shown in the axial slice). The shaded areas in the sagittal and coronal slices represent regions not covered by our implementation of arterial-spin labeling magnetic resonance imaging.
TABLE 2.
Areas of Hypoperfusion in Subjects who Converted to Dementia Relative to Those who Remain Nondemented
| Brain Region | x | y | z | t Statistic | z Value |
|---|---|---|---|---|---|
| Right precuneus | 16 | −50 | 28 | 3.43 | 3.26 |
| Right inferior parietal | 44 | −42 | 28 | 3.41 | 3.23 |
| Right middle cingulum | 16 | 20 | 32 | 3.40 | 3.23 |
| Right middle frontal | 36 | 20 | 50 | 3.36 | 3.19 |
Voxel coordinates represent the peak voxel in local maxima, coordinates are expressed in Montreal Neurological Institute stereotactic space. P = 0.001, uncorrected, extent threshold = 10 voxels.
Table 3 shows that raw perfusion values extracted from the right precuneus, right inferior parietal cortex, right middle cingulum, and right middle frontal cortex were statistically significant predictor variables in univariate analyses of the risk of conversion.
TABLE 3.
Risk of Conversion: Univariate Analyses Region of Interest Relative Risk P Value
| Region of Interest | Relative risk | P |
|---|---|---|
| Right precuneus | 0.56 | 0.002 |
| Right inferior parietal cortex | 0.76 | 0.003 |
| Right middle cingulated | 0.64 | 0.009 |
| Right middle frontal cortex | 0.69 | 0.001 |
When we entered raw perfusion values from the 4 ROIs along with baseline HVs into a Cox Regression analysis, the forward variable selection method constructed a final model where baseline HV (RR = 0.99, P = 0.004), baseline perfusion from the right inferior parietal (RR = 0.64, P = 0.014), and middle frontal (RR = 0.73, P = 0.011) cortices were significant predictors of conversion to dementia.
Predictors of Episodic Memory Decline
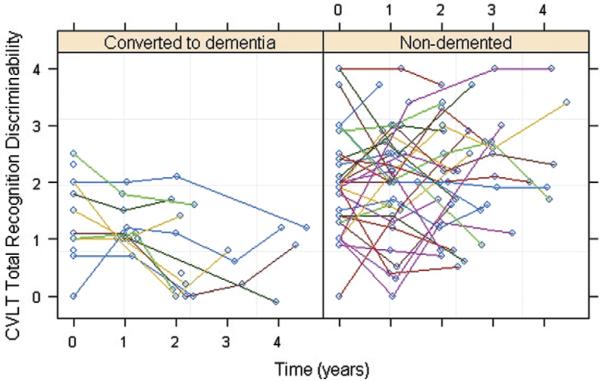
Figure 2 shows a scatter plot of change in the CVLT total recognition discriminability scores over time in the subjects. When we examined the influence of baseline CVLT total recognition discriminability scores on subsequent total recognition discriminability decline, there was no significant difference between the uncorrelated and correlated models. This suggested that baseline total recognition discriminability scores did not have significant explanatory power for determining the future decline. Thus, we used the simpler, uncorrelated model. This revealed that baseline perfusion from the right middle frontal cortex ROI significantly (P = 0.03) predicted decline in CVLT total recognition discriminability scores beyond time, age, sex, education, APOE ε4 and group status, and baseline MMSE and GDS scores. Time (P = 0.0003) and group status (P = 0.004) significantly contributed to the model fit whereas the other covariates did not.
FIGURE 2.
Scatter plots of the subjects' California Verbal Learning Test total recognition discriminability scores as a function of time.
Predictors of Selective Attention Decline
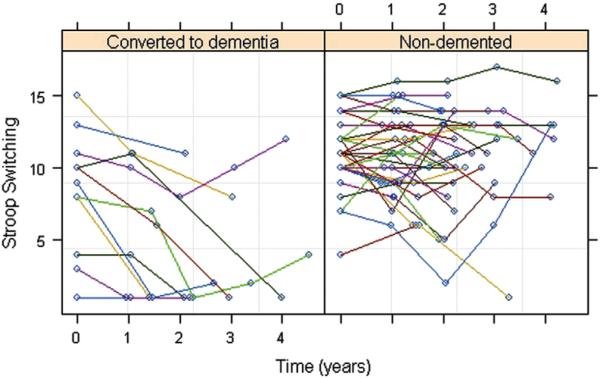
Figure 3 shows a scatter plot of change in the Stroop-switching, scaled scores over time. In examining the influence of baseline Stroop-switching scores on subsequent Stroop-switching decline, we found no significant difference between the uncorrelated and correlated models. Using the simpler, uncorrelated model, we found that baseline per-fusion from the right precuneus ROI (P = 0.009) and right inferior parietal (P = 0.004) ROIs significantly predicted decline in Stroop-switching scores beyond time, age, sex, education, APOE ε4 and group status, and baseline MMSE and GDS scores. Time (P<0.003), education (P<0.04), and group status (P<0.02) significantly contributed to the model fit whereas the other covariates did not.
FIGURE 3.
Scatter plots of the subjects' Delis Kaplan Executive Function System-Stroop switching (scaled) scores as a function of time.
Predictors of CDR-SB Decline
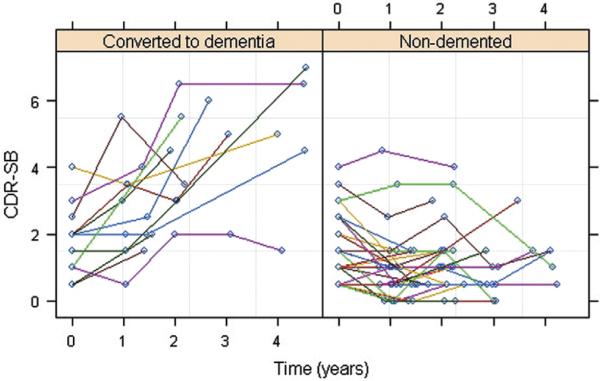
Figure 4 shows a scatter plot of change in the CDR-SB scores over time. In examining the influence of baseline CDR-SB scores on subsequent CDR-SB decline, we found no significant difference between the uncorrelated and correlated models. Using the simpler, uncorrelated model, we found that baseline perfusion from the right precuneus ROI (P = 0.002), right inferior parietal cortex (P = 0.008), and right middle cingulum (P = 0.046) significantly predicted decline in CDR-SB beyond time, age, sex, education, APOE e4 and group status, and baseline MMSE and GDS scores. Time (P<0.01), age (P<0.05), baseline GDS (P<0.01) and MMSE (P<0.05) scores, and group status (P<0.001) significantly contributed to the model fit whereas the other covariates did not.
FIGURE 4.
Scatter plots of the subjects' Clinical Dementia Rating Scale sum of boxes scores as a function of time.
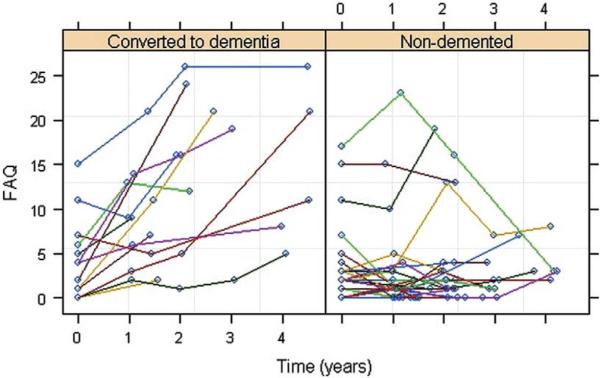
Predictors of FAQ Decline
Figure 5 shows a scatter plot of change in the FAQ scores over time. In examining the influence of baseline FAQ scores on subsequent FAQ decline, we found a significant difference between the uncorrelated and correlated models (p = 0.009), suggesting that baseline FAQ scores had significant explanatory power for determining future decline. Using a model that incorporated this correlation, we found that baseline perfusion from the right precuneus ROI (P = 0.01) significantly predicted FAQ decline beyond time, age, sex, education, APOE e4 and group status, and baseline MMSE and GDS scores. Time (P = 0.03), baseline GDS (P = 0.03) and MMSE (P = 0.04) scores, and group status (P = 0.005) significantly contributed to the model fit whereas the other covariates did not.
FIGURE 5.
Scatter plots of the subjects' Functional Activates Questionnaire scores as a function of time.
DISCUSSION
The first aim of this study was to find ASL perfusion MRI correlates of conversion to dementia. An initial voxel-wise analysis of the baseline perfusion data revealed regions of hypoperfusion in the right precuneus, right inferior parietal cortex, right middle cingulum, and right middle frontal cortex in MCI subjects who later became demented compared with MCI subjects who remained nondemented. Although these findings did not survive corrections for multiple comparisons, they are generally consistent with what has previously been reported in the FTD-PET and SPECT literature.4–6,54 Moreover, when we extracted raw perfusion values from these regions and submitted them to univariate survival analyses, the Cox regression models indicated that baseline perfusion from all 4 ROIs were statistically significant predictors of the risk of conversion.
Our next aim was to compare the predictive value of baseline ASL-MRI measured perfusion with the predictive value of baseline HVs. When we entered HVs and baseline perfusion from the 4 ROIs identified from the voxel-wise SPM analysis into a Cox regression analysis, a forward stepwise selection method selected baseline HVs, baseline perfusion from the right inferior parietal and right middle frontal cortices as significant predictor variables. The finding that baseline HV-predicted progression to dementia is consistent with previous reports.18,19 The finding that right inferior parietal and middle frontal perfusion were also significant predictors is line with previous reports of reduced regional cerebral blood flow in the frontal, prefrontal, and parietal cortices of converters relative to nonconverters.4 Other investigators have also reported reduced regional glucose metabolic rate in the right inferior parietal cortex in MCI subjects who converted with AD compared to nonconverters.5,54
A third objective of the study was to examine the predictive value of ASL-perfusion MRI and MRI-derived HVs for determining future cognitive and functional decline. Our results indicate that baseline right middle frontal perfusion predicted subsequent decline the CVLT total recognition discriminability index. This is in line with lesion studies that have reported recognition memory impairments in patients with right frontal lobe damage55,56 and with the vast functional neuroimaging literature that have implicated the right frontal lobe in memory retrieval.57 It is interesting to note that baseline HV did not predict subsequent recognition discriminability decline. However, some investigators have suggested that hippocampal atrophy may not be a good a surrogate for memory loss.58,59 Baseline perfusion from the right inferior parietal cortex predicted subsequent selective attention decline (ie, Stroop switching), consistent with the results of functional neuroimaging studies that have linked inferior parietal activity to switching tasks60,61 and right inferior parietal activity with Stroop interference.62 Baseline perfusion from the right precuneus also predicted Stroop-switching decline. The precuneus is a polymodal sensory brain region that has been proposed to be involved in “high-level integration between posterior association processes and anterior executive functions”63 such as attentional set-shifting,64 which is required for successful performance of the Stroop-switching task.
Given that functional losses are a diagnostic criterion for AD by Diagnostic and Statistical Manual of Mental Disorders-IV65 and that baseline perfusion from the right precuneus, inferior parietal cortex and right middle cingulum were all associated with increased risk of converting to dementia, it is not surprising that baseline perfusion from these regions also predicted declines in functional measures (ie, CDR-SB and FAQ). Moreover, subsequent clinical status (eg, converted to dementia or remained nondemented) significantly contributed to the model fit in all cases. This suggests that pathologic processes may be a strong component underlying the decline. However, it is diffcult to completely parcel out pathologic from nonpathologic decline as some of the nondemented MCI subjects may eventually convert to dementia.
This study has several limitations. Firstly, our implementation of ASL-MRI did not cover more inferior regions of the brain such as the medial temporal cortex, where histologic studies suggest AD pathology begins66 and where several investigators have noted perfusion/metabolic abnormalities in AD converters relative to nonconverters.6,9,11,13 Secondly, our ASL-MRI measurements of perfusion were based on a simple model of water perfusion in which an instantaneous exchange of water from intravascular to extravascular space was assumed. Moreover, the computations of perfusion did not include variable arterial transit times and variations in the T1 relaxation of the water labels. To the extent that these factions were systematically different between MCI patients who converted to dementia and those who did not, the measurements may have over-estimated or under-estimated cerebral perfusion. Thirdly, the make-up of the MCI group was heterogeneous. Consequently, the converters manifested different types of dementia at follow-up (ie, AD, mixed, and vascular dementia). Finally, our results may not generalize well to the broader population because study participants were drawn from memory clinic referrals and individuals who volunteered to participate in longitudinal studies of aging and dementia. These limitations notwithstanding, our results suggest that hypoperfusion as detected by ASL-MRI can predict subsequent clinical, functional, and cognitive decline and therefore may be useful for identifying candidates for future AD treatment trials.
Acknowledgments
Supported by NIH NIA R01 AG010897.
REFERENCES
- 1.Minoshima S, Frey KA, Koeppe RA, et al. A diagnostic approach in Alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36:1238–1248. [PubMed] [Google Scholar]
- 2.Kogure D, Matsuda H, Ohnishi T, et al. Longitudinal evaluation of early Alzheimer's disease using brain perfusion SPECT. J Nucl Med. 2000;41:1155–1162. [PubMed] [Google Scholar]
- 3.Johnson KA, Albert MS. Perfusion abnormalities in prodromal AD. Neurobiol Aging. 2000;21:289–292. doi: 10.1016/s0197-4580(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 4.Encinas M, De Juan R, Marcos A, et al. Regional cerebral blood flow assessed with 99mTc-ECD SPET as a marker of progression of mild cognitive impairment to Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2003;30:1473–1480. doi: 10.1007/s00259-003-1277-z. [DOI] [PubMed] [Google Scholar]
- 5.Chetelat G, Desgranges B, de la Sayette V, et al. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer's disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 6.Hirao K, Ohnishi T, Hirata Y, et al. The prediction of rapid conversion to Alzheimer's disease in mild cognitive impairment using regional cerebral blood flow SPECT. Neuroimage. 2005;28:1014–1021. doi: 10.1016/j.neuroimage.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 7.Minoshima S, Giordani B, Berent S, et al. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 8.Huang C, Wahlund LO, Svensson L, et al. Cingulate cortex hypoperfusion predicts Alzheimer's disease in mild cognitive impairment. BMC Neurol. 2002;2:9–14. doi: 10.1186/1471-2377-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishiwata A, Sakayori O, Minoshima S, et al. Preclinical evidence of Alzheimer changes in progressive mild cognitive impairment: a qualitative and quantitative SPECT study. Acta Neurol Scand Suppl. 2006;114:91–96. doi: 10.1111/j.1600-0404.2006.00661.x. [DOI] [PubMed] [Google Scholar]
- 10.Anchisi D, Borroni B, Franceschi M, et al. Heterogeneity of brain glucose metabolism in mild cognitive impairment and clinical progression to Alzheimer disease. Arch Neurol. 2005;62:1728–1733. doi: 10.1001/archneur.62.11.1728. [DOI] [PubMed] [Google Scholar]
- 11.Drzezga A, Lautenschlager N, Siebner H, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30:1104–1113. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- 12.Borroni B, Anchisi D, Paghera B, et al. Combined 99mTc-ECD SPECT and neuropsychological studies in MCI for the assessment of conversion to AD. Neurobiol Aging. 2006;27:24–31. doi: 10.1016/j.neurobiolaging.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Caroli A, Testa C, Geroldi C, et al. Cerebral perfusion correlates of conversion to Alzheimer's disease in amnestic mild cognitive impairment. J Neurol. 2007;12:1698–1707. doi: 10.1007/s00415-007-0631-7. [DOI] [PubMed] [Google Scholar]
- 14.Jueptner M, Weiller C. Review: does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage. 1995;2:148–156. doi: 10.1006/nimg.1995.1017. [DOI] [PubMed] [Google Scholar]
- 15.Detre JA, Alsop DC. Perfusion magnetic resonance imaging with continuous arterial spin labeling: methods and clinical applications in the central nervous system. Eur J Radiol. 1999;30:115–124. doi: 10.1016/s0720-048x(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 16.Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer's disease by spin-labeled magnetic resonance imaging. Ann Neurol. 2000;47:93–100. [PubMed] [Google Scholar]
- 17.Johnson NA, Jahng GH, Weiner MW, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jack CR, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grundman M, Sencakova D, Jack CR, Jr, et al. Brain MRI hippocampal volume and prediction of clinical status in a mild cognitive impairment trial. J Mol Neurosci. 2002;19:23–27. doi: 10.1007/s12031-002-0006-6. [DOI] [PubMed] [Google Scholar]
- 20.Sagar HJ, Sullivan EV, Gabrieli JD, et al. Temporal ordering and short-term memory deficits in Parkinson's disease. Brain. 1988;111:525–539. doi: 10.1093/brain/111.3.525. [DOI] [PubMed] [Google Scholar]
- 21.Tierney MC, Black SE, Szalai JP, et al. Recognition memory and verbal fluency differentiate probable Alzheimer disease from subcortical ischemic vascular dementia. Arch Neurol. 2001;58:1654–1659. doi: 10.1001/archneur.58.10.1654. [DOI] [PubMed] [Google Scholar]
- 22.Greene JD, Baddeley AD, Hodges JR. Analysis of the episodic memory deficit in early Alzheimer's disease: evidence from the doors and people test. Neuropsychologia. 1996;34:537–551. doi: 10.1016/0028-3932(95)00151-4. [DOI] [PubMed] [Google Scholar]
- 23.Backman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer's disease. Brain. 2001;124:96–102. doi: 10.1093/brain/124.1.96. [DOI] [PubMed] [Google Scholar]
- 24.Barbeau E, Didic M, Tramoni E, et al. Evaluation of visual recognition memory in MCI patients. Neurology. 2004;62:1317–1322. doi: 10.1212/01.wnl.0000120548.24298.db. [DOI] [PubMed] [Google Scholar]
- 25.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41:1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 26.Delis DC, Kramer JH, Kaplan E, et al. California Verbal Learning Test. 2nd ed. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- 27.Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer's disease. A critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- 28.Traykov L, Raoux N, Latour F, et al. Executive functions deficit in mild cognitive impairment. Cogn Behav Neurol. 2007;20:219–224. doi: 10.1097/WNN.0b013e31815e6254. [DOI] [PubMed] [Google Scholar]
- 29.Belleville S, Bherer L, Lepage E, et al. Task switching capacities in persons with Alzheimer's disease and mild cognitive impairment. Neuropsychologia. 2008;46:2225–2233. doi: 10.1016/j.neuropsychologia.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 31.Pfeffer RI, Kurosaki TT, Hurrah CH, Jr, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 32.Daly E, Zaitchik D, Copeland M, et al. Predicting conversion to Alzheimer disease using standardized clinical information. Arch Neurol. 2000;57:675–680. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- 33.Lynch CA, Walsh C, Blanco A, et al. The clinical dementia rating sum of box score in mild dementia. Dement Geriatr Cogn Disord. 2006;21:40–43. doi: 10.1159/000089218. [DOI] [PubMed] [Google Scholar]
- 34.Dickerson BC, Sperling RA, Hyman BT, et al. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch Gen Psychiatry. 2007;64:1443–1450. doi: 10.1001/archpsyc.64.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 36.DeCarli C, Mungas D, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller SL, Fenstermacher E, Bates J, et al. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry. 2008;79:630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickerson BC, Salat DH, Bates JF, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rockwood K, Strang D, MacKnight C, et al. Interrater reliability of the Clinical Dementia Rating in a multicenter trial. J Am Geriatr Soc. 2000;48:558–559. doi: 10.1111/j.1532-5415.2000.tb05004.x. [DOI] [PubMed] [Google Scholar]
- 40.Schafer KA, Tractenberg RE, Sano M, et al. Reliability of monitoring the clinical dementia rating in multicenter clinical trials. Alzheimer Dis Assoc Disord. 2004;18:219–222. [PMC free article] [PubMed] [Google Scholar]
- 41.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 42.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of Geriatric Depression Rating Scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 43.Jahng GH, Zhu XP, Matson GB, et al. Improved perfusion-weighted MRI by a novel double inversion with proximal labeling of both tagged and control acquisitions. Magn Reson Med. 2003;49:307–314. doi: 10.1002/mrm.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller-Gartner HW, Links JM, Prince JL, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab. 1992;12:571–583. doi: 10.1038/jcbfm.1992.81. [DOI] [PubMed] [Google Scholar]
- 45.Van Leemput K, Maes F, Vandermeulen D, et al. Automated model-based tissue classification of MR images of the brain. IEEE Trans Med Imaging. 1999;18:897–908. doi: 10.1109/42.811270. [DOI] [PubMed] [Google Scholar]
- 46.Haller JW, Banerjee A, Christensen GE, et al. Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology. 1997;202:504–510. doi: 10.1148/radiology.202.2.9015081. [DOI] [PubMed] [Google Scholar]
- 47.Hsu YY, Schuff N, Du AT, et al. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging. 2002;16:305–310. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathalon DH, Sullivan EV, Rawles JM, Pfefferbaum A. Correction for head size in brain-imaging measurements. Psychiatry Res. 1993;50:121–139. doi: 10.1016/0925-4927(93)90016-b. [DOI] [PubMed] [Google Scholar]
- 49.Tzurio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 50.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-Plus. Springer-Verlag; New York: 2000. [Google Scholar]
- 51.Stuck AE, Walthert JM, Nikolaus T, et al. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48:445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 52.Aguero-Torres H, Thomas VS, Winblad B, et al. The impact of somatic and cognitive disorders on the functional status of the elderly. J Clin Epidemiol. 2002;55:1007–1012. doi: 10.1016/s0895-4356(02)00461-4. [DOI] [PubMed] [Google Scholar]
- 53.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64:1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- 54.Mosconi L, Perani D, Sorbi S, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004;63:2332–2340. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- 55.Curran T, Schacter DL, Norman KA, et al. False recognition after a right frontal lobe infarction: memory for general and specific information. Neuropsychologia. 1997;35:1035–1049. doi: 10.1016/s0028-3932(97)00029-8. [DOI] [PubMed] [Google Scholar]
- 56.McDonald CR, Bauer RM, Filoteo JV, et al. Episodic memory in patients with focal frontal lobe lesions. Cortex. 2006;42:1080–1092. doi: 10.1016/s0010-9452(08)70220-x. [DOI] [PubMed] [Google Scholar]
- 57.Tulving E, Kapur S, Craik FIM, et al. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marquis S, Moore MM, Howieson DB, et al. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002;59:601–606. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- 59.Ylikoski R, Salonen O, Mantyla R, et al. Hippocampal and temporal lobe atrophy and age-related decline in memory. Acta Neurol Scand Suppl. 2000;101:273–278. doi: 10.1034/j.1600-0404.2000.101004273.x. [DOI] [PubMed] [Google Scholar]
- 60.Garavan H, Ross TJ, Li SJ, et al. A parametric manipulation of central executive functioning. Cereb Cortex. 2000;10:585–592. doi: 10.1093/cercor/10.6.585. [DOI] [PubMed] [Google Scholar]
- 61.Kubler A, Murphy K, Kaufman J, et al. Co-ordination within and between verbal and visuospatial working memory: network modulation and anterior frontal recruitment. Neuro-Image. 2003;20:1298–1308. doi: 10.1016/S1053-8119(03)00400-2. [DOI] [PubMed] [Google Scholar]
- 62.Liu X, Wang H, Corbly CR, et al. The involvement of the inferior parietal cortex in the numerical Stroop effect and the distance effect in a two-digit number comparison task. J Cogn Neurosci. 2006;18:1518–1530. doi: 10.1162/jocn.2006.18.9.1518. [DOI] [PubMed] [Google Scholar]
- 63.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 64.Nagahama Y, Okada T, Katsumi Y, et al. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. Neuro-image. 1999;10:193–199. doi: 10.1006/nimg.1999.0451. [DOI] [PubMed] [Google Scholar]
- 65.American Psychological Association . Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 66.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]