Abstract
The biotransformation products of zearalenone, a Fusarium mycotoxin, were elucidated using the model plant Arabidopsis thaliana. After treatment of plant seedlings with 50 μM zearalenone, both the liquid media and the plant extracts were analysed by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). An array of 17 different metabolites, most prominently glucosides, malonylglucosides, di-hexose- and hexose–pentose disaccharides of zearalenone, and α- and β-zearalenol, were detected in the samples. Time courses for the different zearalenone metabolites were recorded and they give a closer insight into the metabolism kinetics. A scheme proposing the zearalenone metabolism in A. thaliana is given. The aspect of food safety regarding the (potential) occurrence of masked mycotoxins in agricultural commodities is discussed.
Keywords: Mycotoxins, conjugated mycotoxins, masked mycotoxins, zearalenone, metabolism, mass spectrometry, Arabidopsis thaliana
Introduction
Mycotoxins (reviewed in Hussein and Brasel 2001; Bennet and Klich 2003), which are poisonous secondary metabolites of moulds, can be released into food and feed after the growth of certain fungi on plant commodities. Living plants, however, are able to metabolize these xenobiotics (Wallnöfer et al. 1996; Cole and Edwards 2000). Humans and animals that consume parts of the contaminated plants (e.g. cereals, nuts, raisins, etc.) are, therefore, not only exposed to the native mycotoxins, but also to altered forms. Little is known about the occurrence, bioavailability and metabolism of these compounds.
Zearalenone (ZON) is a Fusarium mycotoxin with strong oestrogenic activity. The main concern about this substance is caused by its ability to bind to oestrogen receptors in mammalian cells (Bennet and Klich 2003). It was shown that ZON can be transformed to α-zearalenol (α-ZOL), β-zearalenol (β-ZOL) and ZON-4-glucoside (ZON-4-Glc) by plant enzymes in maize cell suspension cultures (Engelhardt et al. 1988). In addition, α-ZOL-4-glucoside (α-ZOL-4-Glc) and β-ZOL-4-glucoside (β-ZOL-4-Glc) were detected in such cultures (Engelhardt et al. 1999). A mini-survey of 24 wheat samples revealed that for 22 samples ZON contamination was above the lower limit of quantification and for ten of these samples (42%) ZON-4-Glc could also be quantified (Schneweis et al. 2002). The ZON-4-Glc fraction was in the range of approximately 10–20% of ZON content. The only plant metabolites of mycotoxins that have been subject to animal studies are ZON-4-Glc and ZON-4-sulfate (ZON-4-Sulf). ZON-4-Glc is completely cleaved to ZON during digestion in swine (Gareis et al. 1990; Gareis 1994), while ZON-4-Sulf is easily cleaved by acid and in rats (Plasencia and Mirocha 1991). Gareis coined the term ‘masked mycotoxins’ to emphasize substances that are not usually detected in routine analysis but which contribute to the total mycotoxin content. The picture further complicates when non-extractable bound residues are taken into account. Experiments with radiolabelled 14C-ZON in maize cell suspension cultures indicated that over 50% of the initial radioactivity was incorporated as insoluble ‘bound residue’ in the plant matrix, which still might be partly bioavailable (Engelhardt et al. 1999).
Arabidopsis thaliana, or mouse-ear cress, is a small flowering plant that is widely used as a model organism in plant biology. Arabidopsis is a member of the Brassicaceae family, which includes cultivated species such as cabbage and radish. Arabidopsis is not of major agronomic significance, but it offers important advantages for basic research in genetics and molecular biology (The Arabidopsis Information Resource (TAIR) 2005). The plants possess a small genome (about 125 Mb) that has been fully sequenced since 2000, a rapid life cycle and they are also easy to cultivate in a restricted space. It has been shown that Arabidopsis is susceptible to the cereal ear blight fungal pathogens Fusarium graminearum and Fusarium culmorum (Urban et al. 2002).
Due to the steady advancement of analytical instrumentation during time, nowadays very selective and sensitive techniques are available. Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) has become the state of the art in metabolism research (Levsen et al. 2005).
The present paper reports on an assay for monitoring the metabolic transformation of ZON in plants using Arabidopsis as model plant and a QTrap LC-MS/MS (Applied Biosystems, Foster City, CA, USA) instrument for the identification and quantification of the metabolites. After treatment of plant seedlings with ZON in liquid medium, both the medium and the plant extracts were analysed for metabolites. The multiple reaction monitoring (MRM) mode was chosen for analysis because of its high selectivity and sensitivity. Masses of potential analytes were calculated from known metabolic reactions. The identities of detectable metabolites were confirmed with enhanced product ion (MS/MS) and multiple mass spectrometry (MS3) scans.
Material and methods
Chemicals and materials
Murashige and Skoog basal salt mixture and MS-grade ammonium acetate were purchased from Sigma Chemical Co. (St Louis, MO, USA); agar and dimethyl sulfoxide (DMSO) were from Fluka (Buchs SG, Switzerland). Sucrose was obtained from Agrana (Vienna, Austria); 12% sodium hypochlorite solution was from Roth (Karlsruhe, Germany); solid potassium hydroxide was from VWR (Vienna, Austria). LC-grade methanol was purchased from J. T. Baker (Deventer, the Netherlands). Water for use as an LC mobile phase was purified successively by reverse osmosis and by a Milli-Q plus system from Millipore (Molsheim, France). ZON, α-ZOL, β-ZOL and zearalanone (ZAN) were purchased from biopure Referenzsubstanzen GmbH (Tulln, Austria). ZON-4-Glc was synthesized (Scharnhorst 2003) according to a modified protocol from Grabley et al. (1992). ZON-4-Sulf was isolated from F. graminearum-inoculated rice (Plasencia and Mirocha 1991). Authentic α-ZOL-4-Glc and β-ZOL-4-Glc were available from fermentation mixtures after treatment of transgenic yeast with α-ZOL or β-ZOL (Poppenberger et al. 2006).
Treatment of A. thaliana with zearalenone and sample preparation
Seeds of Arabidopsis, accession Columbia, were sterilized in aqueous sodium hypochlorite solution (5%) for 3 min, rinsed with sterile water twice, stratified for 48 h and germinated on Murashige and Skoog agar plates (Murashige and Skoog 1962) containing 4.5% (m/v) sucrose at 22°C under continuous light (approximately 70 μEm−2 s−1). After 10 days, approximately 40 seedlings per sample were transferred to 20 ml liquid Murashige and Skoog medium containing 2.5% (m/v) sucrose and shaken at 60 rpm for another 3 days for acclimatization. In each experiment the ZON concentration was adjusted to 50 μmol l−1 (15.9 mg l−1) by adding 100 μl of a 10 mM ZON stock solution (in 10% DMSO, 0.01N potassium hydroxide (KOH)) to 20 ml liquid medium. Seedlings were harvested at different time periods (0, 30 min, 2, 5, 12 and 24 h, n=3 per time point) after treatment with a single dose of ZON.
After harvest, the seedlings were weighed, rinsed with 20 ml water per sample and homogenized in 4 ml acetonitrile/water (75/25, v/v) using an Ultra-Turrax® T25 mixer (IKA, Staufen, Germany) at 24 000 rpm for 1 min. Extracts were centrifuged at 5300 rpm for 10 min to remove insoluble cell wall components, and then filtered through a paper filter (Roth, Karlsruhe, Germany). Each liquid medium was combined with the washing water obtained after rinsing of the seedlings and diluted 1:1 (v/v) with methanol to ensure that ZON is completely dissolved. Matrix blanks (both plant medium and plant extracts) to which no ZON was added were prepared accordingly. The samples were stored at 4°C in the dark until analysis by high-performance liquid chromatography (HPLC)-MS/MS. Quantification was performed using external calibration with mixed standards containing 10, 30, 100, 300 and 1000 μg l−1 of ZON, α-ZOL, β-ZOL, ZON-4-Sulf and ZON-4-Glc in mobile phase. Samples were diluted 1:10 (or 1:20 for time point 0) with mobile phase if the respective concentration exceeded 1000 μg l−1 and measured again. Recoveries for ZON, α-ZOL, β-ZOL, ZON-4-Sulf and ZON-4-Glc in the plant medium were elucidated by spiking diluted Murashige and Skoog medium (1:1 v/v with methanol) with 10, 30, 100, 300 and 1000 μg l−1 of the mentioned analytes before measurement with LC-MS/MS.
Liquid chromatography parameters
HPLC separation was achieved on a Thermo (Waltham, MA, USA) Aquasil C18 column (100 × 4.6 mm, 3 μm) at 25°C by applying a methanol/5 mM aqueous ammonium acetate linear gradient from 30 to 90% methanol. After an initial hold time of 0.5 min, 90% methanol was reached after 7.5 min and kept constant until 10 min. Afterwards the column was re-equilibrated with 30% methanol until the end of the run at 15 min. A total of 25 μl aliquots were injected into the HPLC-MS/MS. A flow rate of 0.75 ml min−1 was chosen.
Mass spectrometry parameters
ZON and its metabolites were detected and characterized with a 2000 QTrap MS/MS instrument equipped with a turbo ionspray (electrospray) source. This instrument uses a triple quadrupole ion path and is capable of performing all of the conventional tandem quadrupole modes as well as several high-sensitivity ion-trap mass spectrometer scans using the final quadrupole as a linear ion trap (enhanced modes). To monitor for assumed metabolites, the instrument was operated in MRM mode. MRM transitions (dwell time of 25 ms) were monitored in a single LC run to screen for numerous conjugates (Table I). MS settings were as follows: curtain gas 20 psi (137.9 kPa), source temperature 400°C, source gas 25 psi (172.4 kPa), auxiliary gas 65 psi (448.2 kPa), collision-induced dissociation (CID) gas 6 (arbitrary units, corresponding to the pressure in the collision cell), ionspray voltage −4200 V.
Table I.
Common conjugations after metabolic transformation with the according mass shift after H-substitution (changed after Levsen et al. 2005).
| Conjugation | Mass shift (amu) |
|---|---|
| Methylation | 14 |
| Acetylation | 42 |
| S-methylation | 46 |
| Glycine (Gly) | 57 |
| Sulfatation (Sulf) | 80 |
| Cysteine (Cys) | 119 |
| N-Ac-Cys | 161 |
| Glucose (Glc) | 162 |
| Cys-Gly | 176 |
| Glucuronic acid | 176 |
| N-Ac-Glc | 203 |
| Glc-Sulfate | 242 |
| γ-Glu-Cys | 248 |
| Malonyl-Glc | 248 |
| Glc-Xyl | 294 |
| Glutathione | 305 |
| Glc-Glc | 324 |
| Acetyl-Glc-Glc | 366 |
| Malonyl-Glc-Glc | 410 |
| Glc-Glc-Glc | 486 |
MRM intensities were optimized for standard substances, resulting in the following parameters: ZON m/z 317.1 → m/z 130.9 (declustering potential (DP) −51 V, collision energy (CE) −38 eV), α-ZOL and β-ZOL m/z 319.1 → m/z 129.9 (DP=−71 V, CE=−48 eV), ZAN m/z 319.1 → m/z 275.1 (DP=−56 V, CE=−16 eV), ZON-4-Sulf m/z 397.2 → m/z 317.1 (DP=−46 V, CE=−18 eV), ZON-4-Glc m/z 479.1 → m/z 317.1 (DP=−31 V, CE=−22 eV), α-ZOL-4-Glc and β-ZOL-4-Glc m/z 481.1 → m/z 319.1 (DP=−31 V, CE=−22 eV). For all other potential metabolites, ions with m/z ratios corresponding to [M – H]− of the assumed conjugates were allowed to pass the first quadrupole (Q1) for fragmentation in Q2. Q3 was mainly set to the m/z value of deprotonated ZON (m/z 317.1) or α-ZOL and β-ZOL (m/z 319.1), respectively. Default values of DP=−30 V and CE=−30 eV were used to monitor the ZON and ZOL conjugates. In the case of detection of the chromatographic peaks, MS/MS spectra were recorded in the enhanced product ion (EPI) mode (linear ion trap (LIT) fill time=50 ms, LIT scan speed = 1000 amu s−1). In addition, MS3 spectra were recorded for confirmation (data not shown).
Results and discussion
A. thaliana was treated with 50 μM ZON (about 15.9 mg l−1) for 0, 0.5, 2, 5, 12 and 24 h in triplicate. None of the screened analytes was detected in the matrix blanks (medium and plant extracts), which were treated in the same manner as the samples, but to which no ZON was added.
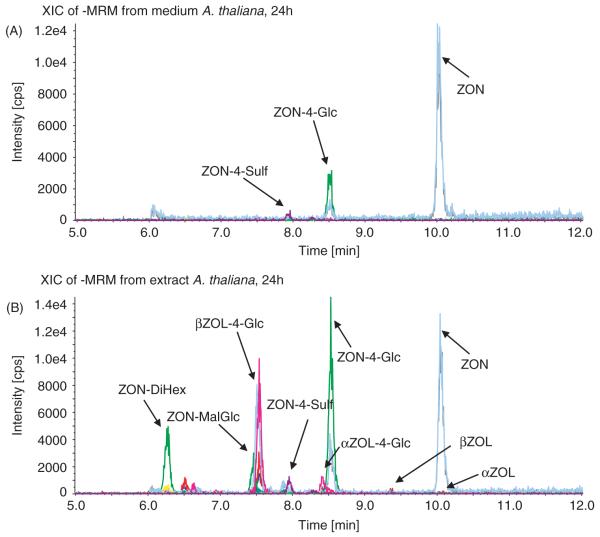
In the media, ZON and its metabolites ZON-4-Glc, ZON-4-Sulf, β-ZOL and, to a lesser extent, also α-ZOL were detected after treatment with ZON. For all of these metabolites standards were available that showed identical retention times, MS/MS and MS/MS/MS spectra (data not shown), and proved the identities of the detected metabolites. Spiking experiments at different concentrations in blank media revealed no significant matrix effects for the components mentioned above (data not shown). Recoveries for all analytes were close to 100% at all concentrations. A typical MRM chromatogram of the medium after treatment of Arabidopsis with ZON for 24 h is shown in Figure 1A.
Figure 1.
Total-ion current liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) multiple reaction monitoring (MRM) chromatograms of the growth medium (A) and cell extract (B) of a single Arabidopsis thaliana seedling (10 days old) that was exposed to 50 μM zearalenone for 24 h.
In the plant extracts, α-ZOL-Glc (at low levels) and β-ZOL-Glc were also unambiguously identified (besides ZON, α-ZOL, β-ZOL, ZON-4-Glc and ZON-4-Sulf), but their concentration are only given in relative values due to the lack of known concentrations of the authentic standards. Figure 1B shows an MRM chromatogram of a cell extract after 24-h ZON exposure to A. thaliana. Furthermore, the chromatographic peaks, corresponding to the MRM transitions of malonyl-glucosides (ZON-MalGlc, α-ZON-MalGlc, β-ZOL-MalGlc), di-hexose- (ZON-DiHex, α-ZOL-DiHex, β-ZOL-DiHex) and hexose–pentose disaccharides (ZON-HexPent, α-ZOL-HexPent, β-ZOL-HexPent) of ZON, α-ZOL and β-ZOL were detected, although often in low levels (especially for the α-ZOL derivatives). For β-ZOL a tri-hexose conjugate (β-ZOL-TriHex) was also measured. MS/MS (EPI) and MS3 scans confirmed the presence of ZON or the ZOLs, respectively, in all metabolites, but it has to be pointed out that no standards were available for the malonylglucosides and the di- or tri-saccharides. Further experiments (e.g. using LC-nuclear magnetic resonance (NMR) or authentic standards) are therefore required to characterize fully the molecular structures of these compounds.
Since xylose conjugates are usually found as conjugation products of xenobiotics in plants (Vostrowsky and Hirsch 2005), we suggest the hexose–pentose disaccharides that were detected for the first time in this study to be glucose–xylose conjugates. According to other detected plant metabolites (Cole and Edwards 2000; Levsen et al. 2005), the di- and tri-hexose conjugates are assumed to be di- and tri-glucosides, respectively.
In the present study, we could not verify the exact location of the conjugation site by LC-MS/MS, as the added moieties were first cleaved from the molecules after low-energy CID, which is in good accordance with other studies (Zaia 2004). It is, therefore, not certain whether the di-hexose conjugates are 2,4-di-hexosides (with one molecule of sugar (glucose) conjugated to each of the phenolic groups of ZON) or oligosaccharides. ZON-2,4-diglucoside has been shown to be a microbial transformation product of ZON (El-Sharkawy 1989). The detection of a tri-hexose conjugate of β-ZOL, but the absence of a ZON tri-hexoside derivative, suggests that the non-phenolic hydroxy group of β-ZOL could also be conjugated to a hexose in the found metabolite. Additional (enzymatic and NMR spectroscopy) experiments are planned to confirm the structures of the di-hexoside- and the hexose–pentose disaccharides.
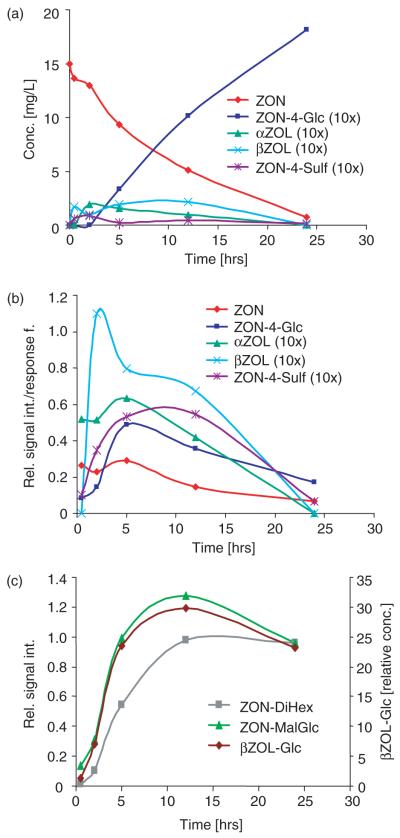
Time-course experiments with points at 0, 30 min, 2, 5, 12 and 24 h were performed as well. While after 0 min all of the added ZON was recovered in the medium, after 24 h almost all available ZON (>97%) was metabolized. Figure 2A summarizes the concentrations of the detected metabolites in the medium over time.
Figure 2.
(a) Concentrations of zearalenone (ZON), ZON-4-Glc, ZON-4-Sulf, α-ZOL and β-ZOL found in the growth medium, depending on the time of exposure of Arabidopsis thaliana to 50 μM ZON. (b) Relative signal intensities divided by the response factors of ZON, ZON-4-Glc, ZON-4-Sulf, α-ZOL and β-ZOL found in the cell extracts, depending on the time of exposure of A. thaliana to 50 μM ZON. (c) Relative signal intensities of ZON-DiHex, ZON-MalGlc and β-ZOL-Glc found in the cell extracts, depending on the time of exposure of A. thaliana to 50 μM ZON.
Phase I metabolism led to α-ZOL and β-ZOL, which were further conjugated very similarly to ZON in phase II metabolism. Intracellular (plant extract) concentrations (Figure 2B) of ZON, α-ZOL and β-ZOL, but also of ZON-4-Glc and ZON-4-Sulf, increased during the first few hours (often about 5 h) to reach a maximum, but then declined as the substances were further processed to ZON-DiHex, ZON-MalGlc and ZON-HexPent. It has to be pointed out that matrix effects were not investigated for plant extracts and the yields of the chosen extraction method are unclear. For this reason Figure 2B contains the signal intensities of ZON, α-ZOL, β-ZOL, ZON-4-Glc and ZON-4-Sulf divided by the response factors of the respective analytes as measured in standard solution.
Figure 2C shows the relative signal intensities of ZON-DiHex, ZON-MalGlc and β-ZOL-Glc as a function of the incubation time, which reached a maximum at about 12 h before the concentrations also declined. Additional time points at 36 and 48 h (data not shown) revealed that the concentration for these substances (as well as for ZON-HexPent and β-ZOL-DiHex) remained roughly constant between 24 and 36 h and then declined further.
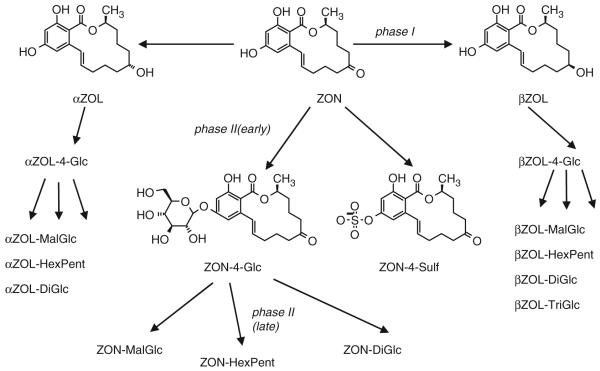
The variety of the identified ZON metabolites, in total 17 different substances, is summarized in Figure 3. The proposed pathway shows metabolic activation (phase I) and conjugation (phase II) processes. The later occurrence of malonylglucosides and disaccharides suggests that they derive from the respective monoglucosides, which is also in agreement with the metabolism of other xenobiotics in plants (Cole and Edwards 2000).
Figure 3.
Proposed biotransformation pathway of zearalenone in Arabidopsis thaliana, covering both phase I and II metabolism.
As part of their metabolism, plants are capable of transforming xenobiotics into a huge variety of conjugated forms. Nonetheless, usually only the parent mycotoxin is measured in the routine analysis of feed and food. It has been shown that some of these conjugates can be cleaved easily by the gut microflora of swines (Gareis et al. 1990), releasing the precursor toxin. The total available amount of mycotoxins in foodstuff, therefore, might currently be underestimated.
It has to be stressed that Arabidopsis is in no way an agricultural crop. In different plants the metabolism of a given xenobiotic might look very different. Still, the ease of use of Arabidopsis plants combined with its known genome sequence allows a more general consideration of the interactions that occur between plants and mycotoxins. Genes responsible for certain metabolic pathways can be identified and looked for in other, more economically important, plants. Also, a variety of (available) mutants of Arabidopsis can be compared in their metabolic behaviour towards various xenobiotics with the wild-type plants using LC-MS/MS. Regarding the aspect of food safety, the authors want to screen wheat and maize for the occurrence of ZON and other mycotoxin plant metabolites in the near future. In addition, the structural identities of the metabolites found will be confirmed by LC-NMR.
Conclusion
This work shows the great capability of plants to metabolize xenobiotic compounds. A method was used for the determination of ZON bio-transformation products in Arabidopsis. Seventeen different metabolites of ZON have been identified to occur in ZON-treated Arabidopsis plants. Known transformation products include ZON-4-Glc, ZON-4-Sulf, α-ZOL, β-ZOL, α-ZOL-Glc and β-ZOL-Glc. Di-hexosides, hexose-pentosides and malonylglucosides of ZON, α-ZOL and β-ZOL are described for the first time.
Acknowledgements
The authors thank Professor Gerhard Adam for scientific support. They also thank the Austrian Genome Research programme (GEN-AU), the Christian Doppler Society, the Austrian Science Fund (FWF Project No. P16410) and the Lower Austrian Government for funding.
References
- Bennet JW, Klich M. Mycotoxins. Clinical Microbiology Reviews. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DJ, Edwards R. Secondary metabolism of agrochemicals in plants. In: Roberts T, editor. Metabolism of agrochemicals in plants. Chichester; Wiley: 2000. pp. 107–154. [Google Scholar]
- El-Sharkawy SH. Microbial transformation of zearalenone III. Formation of 2,4-O-β-diglucoside. Acta Pharmaceutica Jugoslavica. 1989;39:303–310. [Google Scholar]
- Engelhardt G, Ruhland M, Wallnöfer PR. Metabolism of mycotoxins in plants. Advances in Food Sciences. 1999;21:71–78. [Google Scholar]
- Engelhardt G, Zill G, Wohner B, Wallnöfer PR. Transformation of the Fusarium mycotoxin zearalenone in maize cell suspension cultures. Naturwissenschaften. 1988;75:309–310. doi: 10.1007/BF00367324. [DOI] [PubMed] [Google Scholar]
- Gareis M. Maskierte Mykotoxine. Übersichten zur Tierernährung. 1994;22:104–113. [Google Scholar]
- Gareis M, Bauer J, Thiem J, Plank G, Grabley S, Gedek B. Cleavage of zearalenone glycoside, a ‘masked’ mycotoxin during digestion in swine. Journal of Veterinary Medicine B. 1990;37:236–240. doi: 10.1111/j.1439-0450.1990.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Grabley S, Gareis M, Böckers W, Thiem J. Glycosylation of mycotoxins. Synthesis. 1992;11:1078–1080. [Google Scholar]
- Hussein HS, Brasel JM. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology. 2001;167:101–134. doi: 10.1016/s0300-483x(01)00471-1. [DOI] [PubMed] [Google Scholar]
- Levsen K, Schiebel HM, Behnke B, Dötzer R, Dreher W, Elend M, Thiele H. Structure elucidation of phase II metabolites by tandem mass spectrometry. Journal of Chromatography A. 2005;1067:55–72. doi: 10.1016/j.chroma.2004.08.165. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Plasencia J, Mirocha CJ. Isolation and characterization of zearalenone sulfate. Applied and Environmental Microbiology. 1991;57:146–150. doi: 10.1128/aem.57.1.146-150.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenberger B, Berthiller F, Bachmann H, Lucyshyn D, Peterbauer C, Mitterbauer R, Schuhmacher R, Krska R, Glössl J, Adam G. Heterologous expression of Arabidopsis UDP-glucosyltransferases in Saccharomyces cerevisiae for production of zearalenone-4-O-glucoside. Applied and Environmental Microbiology. 2006;72:4404–4410. doi: 10.1128/AEM.02544-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharnhorst K. Nachweis von Zearalenon und dem Metaboliten Zearalenon-4-O-β-Glukosid in Mais und Maisprodukten. 2003. pp. 1–62. Universität für Bodenkultur Wien und Technische Universität Wien. Diploma thesis. [Google Scholar]
- Schneweis I, Meyer K, Engelhardt G, Bauer J. Occurrence of zearalenone-4-β-D-glucopyranoside in wheat. Journal of Agricultural and Food Chemistry. 2002;50:1736–1738. doi: 10.1021/jf010802t. [DOI] [PubMed] [Google Scholar]
- The Arabidopsis Information Resource (TAIR) [Accessed on 17 November 2005]. 2005. Available at: http://www.arabidopsis.org.
- Urban M, Daniels S, Mott E, Hammond-Kosack K. Arabidopsis is susceptible to the cereal ear blight fungal pathogens Fusarium graminearum and Fusarium culmorum. Plant Journal. 2002;32:961–973. doi: 10.1046/j.1365-313x.2002.01480.x. [DOI] [PubMed] [Google Scholar]
- Vostrowsky O, Hirsch A. [Accessed on 18 March 2005]. 2005. Available at: http://www.organik.uni-erlangen.de/vostrowsky/natstoff/04Kohlenhydrate.pdf.
- Wallnöfer PR, Preiß U, Ziegler W, Engelhardt G. Konjugatbildung organischer Schadstoffe in Pflanzen. Zeitschrift für Umweltchemie und Ökotoxikologie. 1996;8:43–46. [Google Scholar]
- Zaia J. Mass spectrometry of oligosaccharides. Mass Spectrometry Reviews. 2004;23:161–227. doi: 10.1002/mas.10073. [DOI] [PubMed] [Google Scholar]