Abstract
MDI 301 is a picolinic acid-substituted ester of 9-cis retinoic acid. It has been shown in the past that MDI 301 increases epidermal thickness, decreases matrix metalloproteinase (MMP) activity, and increases procollagen synthesis in organ-cultured human skin. Unlike all-trans retinoic acid (RA), MDI 301 does not induce expression of proinflammatory cytokines or induce expression of leukocyte adhesion molecules in human skin. In the present study we examined topical MDI 301 treatment for ability to improve the structure and function of skin in three models of skin damage in rodents and for ability to improve abrasion wound healing in these models. MDI 301 was applied daily to the skin of rats treated with the potent corticosteroid, clobetasol propionate, to the skin of diabetic rats (8 weeks posttreatment with streptozotocin) and to the skin of aged (14–16-month-old) rats. In all three models, subsequently induced abrasion wounds healed more rapidly in the retinoid-treated animals than in vehicle-treated controls. Immediately after complete wound closure, tissue from the wound site (as well as from a control site) was put into organ culture and maintained for 3 days. At the end of the incubation period, culture fluids were assessed for soluble type I collagen and for MMPs-2 and -9. In all three models, the level of type I collagen was increased and MMP levels were decreased by MDI 301. In all three models, skin irritation during the retinoid-treatment phase was virtually nonexistent.
Minor abrasions that occur in healthy skin are expected to heal without incident. Interventions are designed primarily to prevent infection and to provide support for the body’s own regenerative mechanisms. In contrast, wounds in chronically damaged and atrophic skin often go on to form nonhealing ulcers with devastating consequences. Diabetes alone is a contributing factor in up to 70% of the > 55,000 amputations that occur annually.1–4 Skin that has become atrophic as a consequence of the aging process also demonstrates impaired wound healing,5–7 as does skin that has been damaged as a result of extended corticosteroid use.8–10 The majority of wound-healing research is directed toward understanding the patho-physiology of impaired wound healing and identifying interventions that can mitigate the critical patho-physiological events.
Several past studies have demonstrated the efficacy of all-trans retinoic acid (RA) and its parent compound all-trans retinol (ROL, vitamin A) in wound healing. Although most studies have focused on wounds in the skin,11–17 retinoid efficacy has also been demonstrated in healing of wounds in other tissues (bone, cornea, respiratory tract, upper digestive system, and gut).18–25 While retinoids have the potential of improving skin structure and function, a consequence of topical retinoid use is skin irritation. 26 Irritated skin is characterized by redness, dryness, and flaking at the treated site. At the histological level, one sees a perivascular accumulation of mononuclear cells, with neutrophils and monocytes scattered throughout the dermis and occasional micro-abscesses in the dermis or epidermis. ROL tends to be less irritating than RA in most cases, but even with this agent, significant irritation is observed in many individuals.27 Likewise, skin irritation is also a complication with synthetic retinoidal agents currently on the market.28 Irritation is a major cause of non-compliance among retinoid users. In addition, excessive irritation may counteract the beneficial effects of retinoid use or actually predispose the tissue to abrasion wounding.
MDI 301 is a picolinic acid-substituted 9-cis retinoic acid ester. In a recent study, this agent was shown to increase epidermal proliferation, decrease matrix metalloproteinase (MMP) activity and increase procollagen synthesis in organ-cultured human skin.29 Unlike RA, MDI 301 did not induce expression of proinflammatory cytokines or lead to up-regulation of leukocyte adhesion molecules in the treated skin. When applied topically to the skin of hairless mice, essentially no skin irritation was observed.30 In the present study we examined MDI 301 for ability to improve healing of superficial abrasion wounds in three models of delayed wound healing in rodents. MDI 301 was applied daily to the skin of rats treated with the potent corticosteroid, clobetasol propionate (Temovate), to the skin of rats made diabetic by treatment with streptozotocin (STZ) or to the skin of aged rats. In all three models, subsequently induced abrasion wounds healed more rapidly in the MDI 301-treated animals than in vehicle-treated controls. In all three models, skin irritation during the retinoid-treatment phase was virtually nonexistent.
MATERIALS AND METHODS
MDI 301
MDI 301 is a 9-cis RA derivative in which the terminal carboxylic acid group has been replaced by a picolinic ester. MDI 301 was synthesized as described in the original patent (US Patent 5,837,728; Molecular Design International, Memphis, TN) and our previous report.30 For topical treatment, MDI 301 was dissolved in dimethyl sulfoxide (DMSO) at a 1.0% concentration and frozen at −80 °C protected from light. RA was purchased from Sigma Chemical Company (St. Louis, MO) and handled exactly as with MDI 301.
Hairless mice and hairless rats
Four- to 6-week-old male HRLS strain hairless mice were obtained from Taconic Farms Inc., Hudson, NY. Male CD hairless rats (250–300 g) were purchased from Charles River Laboratories, Portage, MI. Mice and rats were housed in temperature and light-controlled rooms and given water and feed ad libitum. All studies involving animals were approved by the University Committee on Use and Care of Animals.
Corticosteroid-induced skin damage
Temovate (0.05% solution of clobetasol propionate in cream base) was obtained from Glaxo Smith Kline (Philadelphia, PA). For 15 consecutive days, animals were treated with approximately 0.1mL (mice) or 0.5 mL (rats) of Temovate cream, applied to the back and flanks of the animals. At the end of the treatment period, mice were sacrificed and skin from the animals was used for histology or for organ culture as described below. In experiments with rats, the animals were subdivided into groups and used in wounding studies as described below.
Streptozotocin (STZ)-induced diabetes
Rats were rendered diabetic by a single intraperitoneal injection of STZ (70 mg/kg of body weight). For study inclusion, diabetic rats had a nonfasting blood glucose concentration of > 250 mg/dL in tail vein blood (measured by Glucometer; LifeScan Inc., Milpitas, CA) at 72 hours after STZ injection. Animals were individually housed with ad libitum access to water and suitable rat chow for the next 8 weeks. Weight and capillary glucose values were checked on a weekly basis until the end of the 8-week period. Diabetic rats were given small daily doses (0.5–1.0 U/day) of protamine zinc insulin (Aventis Pharmaceuticals, Kansas City, MO) as necessary to maintain blood glucose levels at approximately 300–350 mg/dL. At the end of the treatment period, animals were subdivided into groups and used in wounding studies as described below. Our previous studies have demonstrated that 8 weeks is sufficient for deficits in the skin to develop in STZ-treated animals.17
Aged skin model
Aged rats were purchased from Charles River Laboratories as retired breeders at 10–12 months of age and then aged for an additional 4 months in housing at the Unit for Laboratory animal Medicine (University of Michigan). At that time, the animals had reached a weight of approximately 600 g. Animals were then randomly assigned to groups and topically treated and used in abrasion wound studies as described below. Young (4–6-week-old) animals were used as controls. They were purchased immediately before use.
Skin irritation and epidermal thickness in hairless mice
Dilutions of the 1.0% solution of MDI 301 were prepared in DMSO. Once daily for 14 days, hairless mice were topically treated over the back and flank with 100 μL of the retinoid solutions. Some mice were treated with a 100 μL of a 0.1% solution of RA and others received 100 μL of DMSO alone. Each day during the retinoid-treatment phase, the animals were examined for signs of skin irritation. Irritation was characterized by redness, dryness (with small cracks developing in the skin), and epidermal flaking. On day 15, the animals were carefully evaluated for these features and given an overall “irritation score” of 1+ to 4+. A 1+ score indicated no difference between the retinoid-treated animals and the vehicle-treated controls; a score of 4+ indicated maximal irritation.
One day after the last treatment (i.e., on day 15), animals were sacrificed and skin samples from the treated site removed. One piece of skin from each animal was immediately fixed in 10% buffered formalin and used for histological evaluation. Epidermal thickness was assessed quantitatively in the skin samples following sectioning and staining with hematoxylin and eosin. A second piece of skin from each animal was cut into pieces approximately 2mm on a side. Four–five such tissue pieces were incubated for 3 days in 0.5mL of a culture medium consisting of growth factor-free, serum-free keratinocyte basal medium (Cambrex Inc., East Rutherford, NJ). Before use, the culture medium was supplemented with Ca2+ to a final concentration of 1.4mM. At the end of the 3-day incubation period, the organ culture fluid was collected and assayed for soluble type I collagen, MMPs-2 and -9 as described below.
Abrasion wound model
Abrasion wounding was performed in hairless rats as described previously.17 Briefly, under general anesthesia (ketamine/xylazine), paravertebral skin from the back and flanks was cleaned with 70% ethanol. A premeasured circular area, approximately 2 in. in diameter was scrubbed with a stiff-nylon bristle brush lightly wetted with acetone and concomitantly abraded with a piece of course sanding sponge. Abrasion was sufficient to remove the thin epidermis, the basement membrane and the upper most part of the subepithelial stroma. Oozing of fluid (with a small amount of blood) into the abraded area indicated that the appropriate degree of abrasion was achieved. The degree of injury approximates that commonly occurring after a minor scrape. Wounding was performed under sterile conditions in a laminar flow hood. Wound size was determined daily by measuring the X and Y-axis of the scabbed wound with calipers and calculating the area of the remaining scab. At the time of wound closure, animals were sacrificed and skin samples were removed and processed as described above for hairless mice. One piece of tissue from each site was fixed in 10% buffered formalin and used for histology. A second piece was incubated in organ culture for 3 days. At the end of the incubation period, the culture fluid was assayed for soluble type I collagen and MMPs as indicated below.
Soluble type I collagen
Culture fluids were assayed for type I collagen by Western blotting. Briefly, organ culture fluids representing equal quantity of protein were resolved using 8% sodium do-decyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions and transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk solution in Tris-buffered saline with 0.1% Tween (TTBS) for 1 hour at room temperature. Following this, they were incubated overnight with a rabbit antibody to rodent type I collagen (1: 10,000 dilution) (Abcam Inc., Cambridge, MA) in the same buffer at 4 °C. The membranes were then washed with TTBS and bound antibody was detected using the Phototope-HRP Western detection kit (Cell Signaling Technologies Inc., Danvers, MA). Images were scanned, digitized, and quantified using NIH image analysis software.
MMP production
Substrate embedded enzymography (zymography) was used to assess levels of latent and active MMPs-2 and -9 in organ culture fluids. As described previously,17 SDS-PAGE gels were prepared with the incorporation of gelatin (1 mg/mL) at the time of casting. After electrophoresis under nonreducing conditions to separate proteins and overnight incubation to allow for substrate digestion, zones of hydrolysis were identified as “holes” in the stained gels and quantified. Values for latent and active MMPs-2 and -9 bands were obtained following digitization.
Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA) followed by the Bonferroni posttest for selected pairs (Graph-Pad Prism version 4.00 for Windows, Graph-Pad Software, San Diego, CA). For experiments in which there were only two groups, the Student t-test was used to assess statistical significance of the differences. Data were considered significant at p < 0.05.
RESULTS
Topical treatment with MDI 301 induces epidermal hyperplasia but not irritation in hairless mice
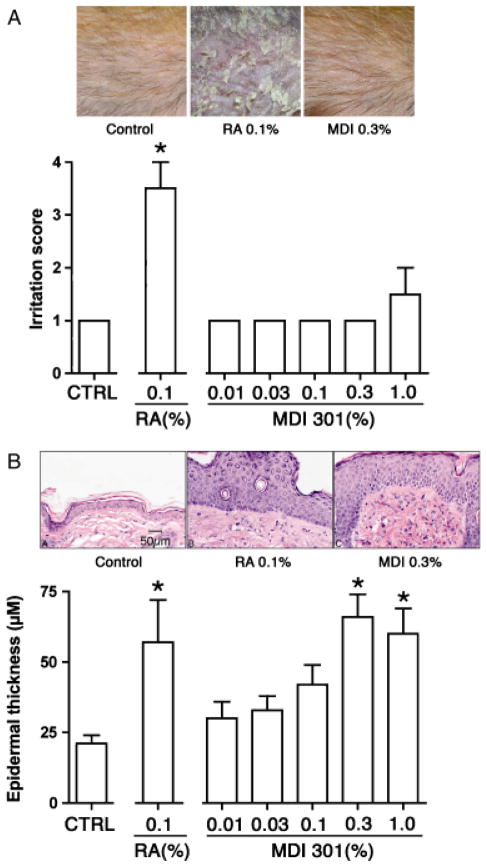
In the first series of experiments, hairless mice were treated topically with concentrations of MDI 301 ranging from 0.01 to 1.0%. Vehicle-treated mice and animals treated with 0.1% RA served as controls. One day after the last treatment (day 15), mice were evaluated grossly for signs of irritation. As shown in Figure 1A, concentrations as high as 0.3% MDI 301 produced no evidence of irritation while RA was (as expected) irritating at 0.1%. Additional mice were treated with MDI 301 at a concentration of 1.0%, and this concentration also induced minimal irritation (Figure 1A).
Figure 1.
Skin irritation and epidermal thickness in hairless mice treated with MDI 301. (A) Skin irritation. Values are means and standard deviations based on visual observations of three mice per treatment group (scored on a 1+ to 4+ scale). (Inset) Skin of mice treated for 7 days with vehicle alone (left), 0.1% RA (center), or 0.3% MDI 301 (right). (B) Epidermal thickening. Values are means and standard deviations based on measurements at four sites in histological sections of three mice per treatment group. (Inset) Histological appearance of mouse skin after treatment for 14 days with vehicle alone (left), 0.1% RA (center), or 0.3% MDI 301 (right) and sacrifice on day 15. Irritation scores and epidermal thickness values were evaluated by ANOVA followed by paired group comparisons. *Statistically significant difference from the control group at p < 0.05.
Mice were sacrificed after gross evaluation for irritation and tissue from treated sites evaluated histologically for epidermal hyperplasia (Figure 1B). Topical treatment of hairless mice with either retinoid induced a robust hyperplasia. With MDI 301, increased epidermal thickening could be seen at a concentration of 0.1% and was statistically significant at concentrations of 0.3% or higher. As expected, RA induced epidermal hyperplasia at 0.1%.
Topical treatment with MDI 301 stimulates collagen production in hairless mice
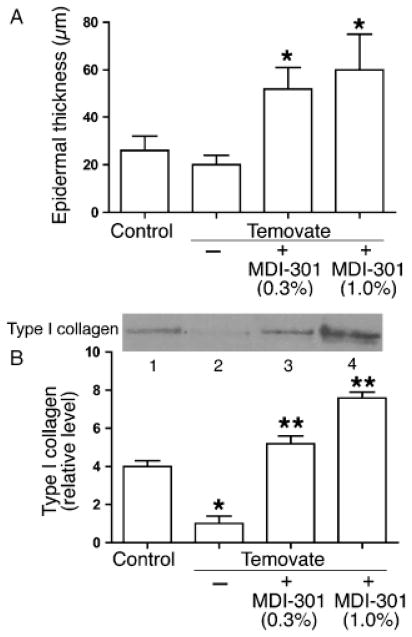
In the next experiments, hairless mice were treated daily for 15 days with Temovate and during the last 12 days with 0.3 or 1.0% MDI 301. As expected, the combination of Temovate and MDI 301 produced no evidence of skin irritation (not shown). At the end of the treatment period, skin samples from the treated site were obtained for histological evaluation. Additional skin samples were incubated in organ culture for 3 days and then analyzed for soluble type I collagen. As seen in Figure 2A, both concentrations of MDI 301 induced epidermal thickening in the Temovate-treated skin. Both concentrations also stimulated type I collagen production (Figure 2B). To establish that the ability of MDI 301 to induce epidermal thickening and collagen production (without irritation) was not unique to mice, a small cohort of CD hairless rats (three rats per group) were also treated with the combination of Temovate (15 days) and MDI-301 (0.3%) during the final 12 days. Similar to what was observed in hairless mice, epidermal thickening and collagen production were both stimulated by MDI 301 in Temovate-treated rats (not shown). Based on these consistent findings in control and Temovate-treated hairless mice and rats, we undertook subsequent wound healing experiments in hairless rats, using MDI 301 at a concentration of 0.3%.
Figure 2.
Epidermal thickness and soluble collagen elaboration in skin of control mice and mice treated with a combination of Temovate and MDI 301. (A) Epidermal thickness. Values are means and standard deviations based on measurements at four sites in histological sections of three mice per treatment group. (B) Soluble type I collagen. Values are means and standard deviations based on organ culture fluids from four animals per group. (Inset) Western blot examples of soluble collagen elaborated by organ-cultured skin from control mice (lane 1), mice treated with Temovate alone (lane 2), Temovate plus 0.3% MDI 301 (lane 3) or Temovate plus 1.0% MDI 301 (lane 4). Epidermal thickness values and soluble collagen values were evaluated by ANOVA followed by paired group comparisons. *Statistically significant difference from the control group at p < 0.05; ** Statistically significant difference from the Temovate alone group at p < 0.05.
Healing of superficial abrasion wounds in corticosteroid-treated skin and in diabetic skin: stimulation of wound healing by MDI 301
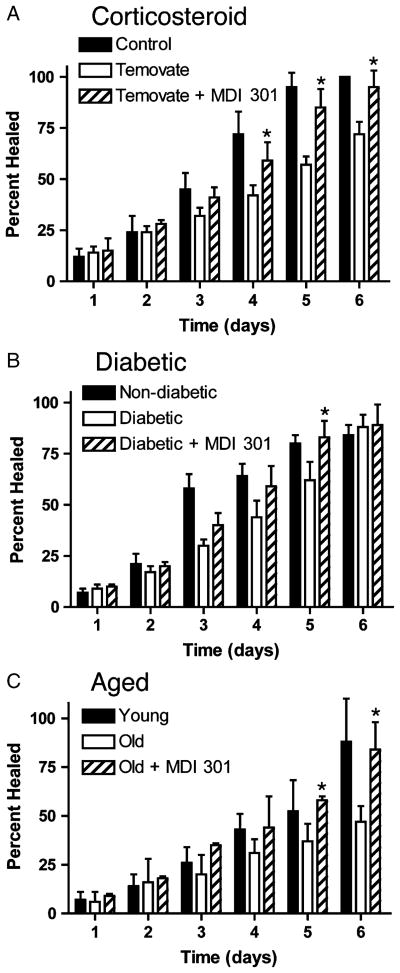
Skin atrophy was induced in hairless rats by treatment for 15 days with Temovate exactly as described above. Some of the animals were also treated with 0.3% MDI 301 once daily during the final 12 days. Others were treated with vehicle (DMSO) alone. At the end of the treatment phase, abrasion wounds were induced in the vehicle-treated and MDI 301-treated animals (as well as in hairless rats that had not been exposed to the steroid) and healing times assessed. The data from these studies are presented in Figure 3A. Wound closure was slower in the steroid-treated animals than in non-steroid–treated controls. Wound closure was significantly improved in those steroid-treated rats that also received MDI 301 as compared with steroid-treated rats that received vehicle alone (i.e., time to complete closure was reduced in the retinoid-treated animals). Differences could be seen by days 3 and 4, difference between vehicle-treated and MDI 301-treated animals was statistically significant (Figure 3A).
Figure 3.
(A) Abrasion wound healing in control hairless rats and hairless rats treated with Temovate plus vehicle or Temovate plus 0.3% MDI 301. Values represent the percentage of the wound that is closed and scab-free at each time point. Values are based on nine animals per group. Values from the three groups were evaluated statistically (ANOVA followed by paired group comparisons). * Statistically significant difference from the Temovate alone group at p < 0.05. (B) Abrasion wound healing in hairless rats in the STZ model of diabetes. Treatment groups include nondiabetic rats as well as diabetic rats treated with vehicle or with 0.3% MDI 301. Values represent the percentage of the wound that is closed and scab-free at each time point. Values are based on 9–11 animals per group. Values from the three groups were evaluated statistically (ANOVA followed by paired group comparisons). * Statistically significant difference from the diabetic alone group at p < 0.05. (C) Abrasion wound healing in hairless aged rats. Treatment groups include young rats as well as aged rats treated with vehicle or with 0.3% MDI 301. Values represent the percentage of the wound that is closed and scab-free at each time point. Values are based on three to five animals per group. Values from the three groups were evaluated statistically (ANOVA followed by paired group comparisons). * Statistically significant difference from the aged animal group at p < 0.05.
Figure 3B summarizes findings from studies in which superficial abrasion wounds were created in the skin of nondiabetic and STZ-diabetic hairless rats. For this study, rats were made diabetic with a single does of STZ. Beginning 8 weeks after STZ injection, the animals were treated for 14 days with vehicle alone or with MDI-301. Abrasion wounding was done at the end of the retinoid-treatment phase. Consistent with past results,17 wound closure was slower in the diabetic rats than in controls. It can be seen from Figure 3B that in diabetic rats pretreated for 14 days with 0.3% MDI 301, the average time to wound-closure time was reduced as compared with that seen in diabetic rats treated with vehicle alone. Differences between retinoid-treated and vehicle-treated rats were evident by day 3 and statistically significant by day 5.
Figure 3C compares healing of superficial skin wounds In young rats and aged rats treated for 14 days with 0.3% MDI 301 or with vehicle alone. The findings from this study are similar to findings from the other two models. Abrasion wound healing was significantly slower in skin of aged rats than in the skin of young controls. As in the other two models, topical pretreatment of the aged rats with MDI 301 substantially improved healing rates.
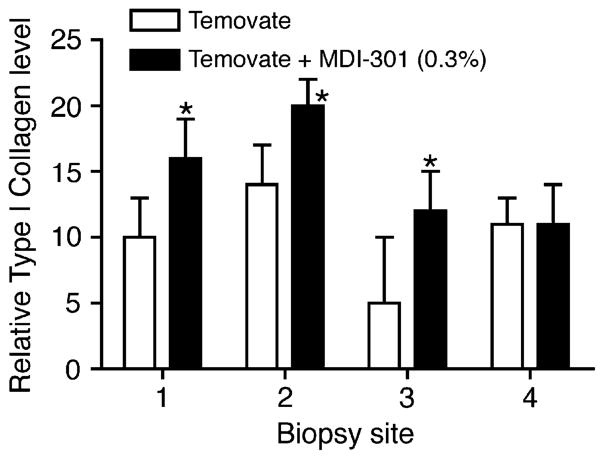
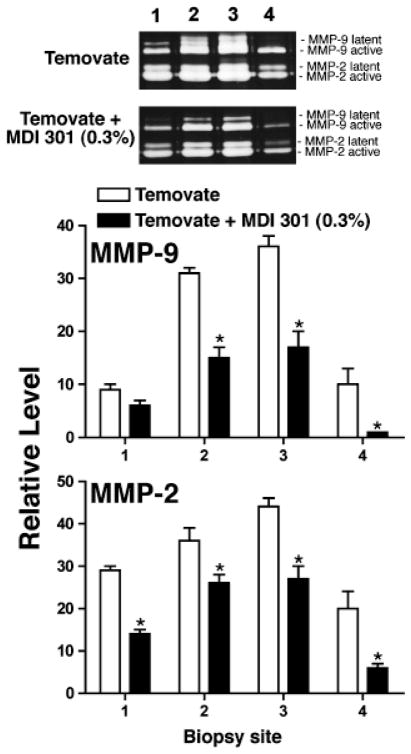
Type I collagen and MMP-2/MMP-9 were assessed in skin biopsies taken at the time of wound closure in all three models. With corticosteroid treated rats, skin biopsies were taken from four sites. Biopsy site one was from the area just beyond the initial wound margin; sites two and three were within the initial area encompassed by the abrasion wound and site four was from an area not exposed to Temovate or MDI 301 (and not abraded). Figure 4 compares levels of soluble type I collagen in organ culture fluids (corticosteroid-treated animals) and levels of the two MMPs are shown in Figure 5. Collagen levels were higher and MMP levels were lower in the culture fluids from biopsies of the retinoid-treated skin than in culture fluids from vehicle-treated animals. Table 1 summarizes the effects of MDI 301 on type I collagen and MMP levels in skin taken from control and treated diabetic rats and from control and treated aged rats. Consistent with what was seen in the corticosteroid model (Figures 4 and 5), organ culture fluids from MDI 301-treated skin had higher collagen levels and lower MMP levels than did culture fluid from vehicle-treated control skin.
Figure 4.
Soluble collagen (Western blotting) in organ culture fluid of skin from rats treated with Temovate plus vehicle or Temovate plus 0.3% MDI 301. Biopsy site 1 was from the area just beyond the initial wound margin; sites 2 and 3 were within the initial area encompassed by the abrasion wound and site 4 was from an area not exposed to Temovate or MDI 301 (and not abraded). Values are means and standard deviations based on organ cultures from nine rats per treatment group. Values were analyzed for statistical significance using the Student t-test, comparing each site separately. *Statistically significant difference from the Temovate alone group at p < 0.05.
Figure 5.
MMPs-2 and -9 (gelatin zymography) in organ culture fluid of skin from rats treated with Temovate plus vehicle or Temovate plus 0.3% MDI 301. Biopsy site 1 was from the area just beyond the initial wound margin; sites 2 and 3 were within the initial area encompassed by the abrasion wound and site 4 was from an area not exposed to Temovate or MDI 301 (and not abraded). Values are means and standard deviations based on organ cultures from nine rats per treatment group. Values were analyzed for statistical significance using the Student t-test, comparing each site separately. *Statistically significant difference from the Temovate alone group at p < 0.05. (Inset) Representative gelatin zymograms of organ culture fluid from rats treated with Temovate plus vehicle or Temovate plus MDI 301.
Table 1.
Soluble collagen and matrix metalloproteinase (MMP) levels in organ culture fluid from vehicle-treated and MDI 301-treated diabetic rats and aged rats
| Group | Soluble collagen (fold-change) | MMP-2 | MMP-9 |
|---|---|---|---|
| Diabetic model | |||
| Wound edge | |||
| Vehicle-treated | 1.2±0.3 | 1.8±0.2 | 3.1±0.4 |
| MDI 301-treated | 2.1±0.4* | 1.1±0.4* | 1.8±0.3* |
| Wound center | |||
| Vehicle-treated | 1.8±0.2 | 2.1±0.2 | 3.8±0.2 |
| MDI 301-treated | 2.5±0.6* | 1.3±0.3 | 2.0±0.4* |
| Aged model | |||
| Wound edge | |||
| Vehicle-treated | 1.3±0.2 | 1.7±0.2 | 2.8±0.2 |
| MDI 301-treated | 2.8±0.4* | 1.1±0.4 | 1.2±0.4* |
| Wound center | |||
| Vehicle-treated | 1.8±0.3 | 2.0±0.3 | 3.1±0.3 |
| MDI 301-treated | 3.0±0.5* | 1.3±0.3 | 1.4±0.2* |
Values for soluble collagen are based on Western blotting followed by digitization and densitometry. Values for MMP-2 and MMP-9 are based on gelatin zymography followed by digitization and densitometry. Values in each animal are compared with values obtained from organ cultures of skin from an untreated and unwounded site in the same animal, and expressed as a fold-change relative to the control site value. Values are means and standard deviations based on n=9 vehicle-treated and n=11MDI 301-treated animals in the diabetic model and n=3 vehicle-treated and n=5 MDI 301-treated animals in the aged skin model. Statistical significance was determined using the Student t-test.
Indicates p < 0.05 relative to vehicle-treated control.
DISCUSSION
In the present study, MDI 301, an experimental retinoid, was applied daily to the skin of rats treated with the potent corticosteroid, Temovate (clobetasol propionate), to the skin of diabetic rats (8 weeks posttreatment with STZ) and to the skin of aged rats (14–16 months old). In all three models, subsequently induced abrasion wounds healed more rapidly in the retinoid-treated animals than in vehicle-treated controls. In all three models, type I collagen levels increased and MMP levels decreased in response to MDI 301. Skin irritation during the retinoid-treatment phase was virtually nonexistent.
The biologically active retinoids have effects on the dermis that delay or prevent atrophy. Topical retinoid use induces the synthesis of types I and III procollagen31–33 and suppresses the major collagen-degrading enzymes in the skin.34,35 These retinoid effects in the skin reflect multiple mechanisms. Retinoids directly influence gene transcription and alter signaling cascades that regulate gene transcription. 36 In the case of MMP reduction, RA not only down-regulates enzyme production at the molecular level 34,35 but also up-regulates tissue inhibitor of metalloproteinases-1 (TIMP-1),37 the major MMP inhibitor in the skin.38 Along with these specific effects on collagen metabolism, retinoid treatment also stimulates proliferation of dermal fibroblasts, thus fostering additional collagen production. Although it is generally believed that improved wound healing in retinoid-treated skin reflects these dermal activities, it is important to keep in mind that retinoids are broad-acting molecules that influence the functioning of other elements in the skin, as well. Retinoid treatment increases keratinocyte proliferation and motility, 26,27,39 properties essential for rapid wound closure. Given the regenerative effects of retinoid use on both the dermal and epidermal component of the skin, it is not surprising that retinoids should be considered for use as wound-preventative or wound-healing agents. Several past studies have, in fact, demonstrated beneficial effects of retinoid use in wound healing. Although most of these studies have focused on wounds in the skin,11–17 retinoid efficacy has also been demonstrated in healing of wounds in other tissues—bone, cornea, respiratory tract, upper digestive system, and gut.18–25 Although retinoids have beneficial activities, skin irritation is commonly seen following topical application of RA.26–28 If skin irritation is too severe, retinoid use will simply be discontinued by most users. Equally important, excessive irritation may negate beneficial effects that would otherwise be seen, or actually increase sensitivity to wound formation.
In past studies, MDI 301 (a synthetic retinoid) has been demonstrated to increase epidermal thickness, induce collagen production and inhibit the major collagen-degrading enzymes in organ cultures of human skin.29 In these regards, MDI 301 and RA are similar. With the synthetic retinoid, however, surrogate markers of inflammation that are up-regulated by RA in human skin organ culture are not increased.29 Given this profile of activities, the use of MDI 301 in topical preparations should, in theory, have significant advantages over RA and other retinoids that are currently available as skin-repair agents. In the present study we demonstrate that MDI 301 does, in fact, have wound healing potential. Using three different models of delayed (impaired) skin wound healing in hairless rats, pretreatment of the rats for 12–14 days with MDI 301 before wounding was shown to decrease the time to wound closure as compared with vehicle-treated control animals. In all three models, biopsies from the wound site in MDI 301-treated animals (taken at the time of wound closure) produced higher levels of type I collagen and lower amounts of MMPs than did wound site biopsies from vehicle-treated animals. The ability of topical MDI 301 to improve healing of superficial wounds is similar to what we reported earlier with RA (diabetic model only).17 The ability of the synthetic retinoid to improve superficial wound healing in at-risk skin but without the irritation associated with RA and other currently available agents provides a strong rational for further development of this agent as a wound preventative.
Acknowledgments
This study was supported in part by NIH grants AR49621 and GM77724 from the USPHS.
References
- 1.NIH. NIH. Bethesda, MD: NIH; 1980. A report of the National Diabetes Advisory Board. [Google Scholar]
- 2.Diabetes in the United States: A strategy for prevention. US Department of Health and Human Services, National Center for Chronic Disease Prevention and Health Promotion, Division of Diabetes Translation; 1992. [Google Scholar]
- 3.Edmonds ME. Experience in a multidisciplinary diabetic foot clinic. In: Connor H, Boulton AJM, Ward JD, editors. The foot in diabetes: Proceedings of the First National Conference on the Diabetic Foot. Chicester, UK: John Wiley; 1987. pp. 121–34. [Google Scholar]
- 4.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care. 1990;13:13–21. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 5.Strigini L, Ryan T. Wound healing in elderly human skin. Clin Dermatol. 1996;14:197–206. doi: 10.1016/0738-081x(95)00155-9. [DOI] [PubMed] [Google Scholar]
- 6.Ashcroft G, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerentology. 2002;3:337–45. doi: 10.1023/a:1021399228395. [DOI] [PubMed] [Google Scholar]
- 7.Brem H, Tomic-Canic M, tarnovskaya A, Gill K, Ehrlich HP, Carasa M, Weinberger S, Baskin-Bey E, Entero H. Healing of elderly patients with diabetic foot ulcers, venous stasis ulcers and pressure ulcers. Surg Technol Int. 2003;11:161–7. [PubMed] [Google Scholar]
- 8.Anstead GM. Steroids, retinoids, and wound healing. Adv Wound Care. 1998;11:277–85. [PubMed] [Google Scholar]
- 9.Ehrlich HP, Hunt TK. Effect of cortisone and vitamin A on wound healing. Ann Surg. 1968;167:32. doi: 10.1097/00000658-196803000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wicke C, Halliday B, Allen D, Roche NS, Scheuenstuhl H, Spencer MM, Roberts AB, Hunt TK. Effects of steroids and retinoids on wound healing. Arch Surg. 2000;135:1265–70. doi: 10.1001/archsurg.135.11.1265. [DOI] [PubMed] [Google Scholar]
- 11.Seifter E, Rettura G, Padawer J, Stratford F, Kambosos D, Levenson SM. Impaired wound healing in streptozotocin diabetes. Ann Surg. 1981;194:42–50. doi: 10.1097/00000658-198107000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frosch PJ, Czarnetzki BM. Effect of retinoids on wound healing in diabetic rats. Arch Dermatol Res. 1989;281:424–6. doi: 10.1007/BF00455329. [DOI] [PubMed] [Google Scholar]
- 13.Popp C, Kligman AM, Stoudemayer TJ. Pretreatment of photoaged forearm skin with topical tretinoin accelerates healing of full-thickness wounds. Br J Dermatol. 1995;132:46–53. doi: 10.1111/j.1365-2133.1995.tb08623.x. [DOI] [PubMed] [Google Scholar]
- 14.Otley CC, Gayner SM, Ahmed I, Moore EJ, Roenigk RK, Sherris DA. Preoperative and postoperative topical tretinoin on high-tension excisional wounds and full-thickness skin grafts in a porcine model: a pilot study. Dermatol Surg. 1999;25:716–21. doi: 10.1046/j.1524-4725.1999.99005.x. [DOI] [PubMed] [Google Scholar]
- 15.Kitano Y, Yoshimura K, Uchida G, Sato K, Harii K. Pretreatment with topical all-trans retinoic acid is beneficial for wound healing in genetically diabetic mice. Arch Dermatol Res. 2001;293:512–21. doi: 10.1007/pl00007466. [DOI] [PubMed] [Google Scholar]
- 16.Basak PY, Eroglu E, Altuntas I, Agalar F, Basak K, Sutcu R. Comparison of the effects of tretinoin, adapalene and collagenase in an experimental model of wound healing. Eur J Dermatol. 2002;12:145–8. [PubMed] [Google Scholar]
- 17.Lateef H, Abatan OI, Aslam MN, Stevens MJ, Varani J. Pretreatment of diabetic rats with all-trans retinoic acid increases healing of subsequently-induced abrasion wounds. Diabetes. 2005;54:855–61. doi: 10.2337/diabetes.54.3.855. [DOI] [PubMed] [Google Scholar]
- 18.Hatchell DL, Ubels JL, Stekiel T, Hatchell MC. Corneal epithelial wound healing in normal and diabetic rabbits treated with tretinoin. Arch Ophthalmol. 1985;103:98–100. doi: 10.1001/archopht.1985.01050010104029. [DOI] [PubMed] [Google Scholar]
- 19.de Waard JW, Wobbes T, van der Linden CJ, Hendriks T. Retinol may promote fluorouracil-suppressed healing of experimental intestinal anastomoses. Arch Surg. 1995;130:959–65. doi: 10.1001/archsurg.1995.01430090045017. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald IM, Peters C, Chen MH. Effect of retinoic acid on wound healing of laser burns to porcine retinal pigment epithelium. Can J Ophthalmol. 1996;3:175–8. [PubMed] [Google Scholar]
- 21.Johansen S, Heegaard S, Prause JU, Rask-Pedersen E. The healing effect of all-trans retinoic acid on epithelial corneal abrasions in rabbits. Acta Ophthalmologica Scandinavica. 1998;76:401–4. doi: 10.1034/j.1600-0420.1998.760403.x. [DOI] [PubMed] [Google Scholar]
- 22.Sela J, Kaufman D, Shoshan S, Shani J. Retinoic acid enhances the effect of collagen on bone union, following induced non-union defect in guinea pig ulna. In am Res. 2000;49:679–83. doi: 10.1007/s000110050646. [DOI] [PubMed] [Google Scholar]
- 23.Maccabee MS, Trune DR, Hwang PH. Paranasal sinus mucosal regeneration: the effect of topical retinoic acid. Am J Rhinol. 2003;17:133–7. [PubMed] [Google Scholar]
- 24.Talas DU, Nayci A, Atis S, Comelekoglu U, Polat A, Bagdatoglu C, Renda N. The effects of corticosteroids and vitamin A on the healing of tracheal anastomoses. Int J Ped Otorhinolaryngol. 2003;67:109–16. doi: 10.1016/s0165-5876(02)00347-6. [DOI] [PubMed] [Google Scholar]
- 25.Yuen DE, Stratford AF. Vitamin A activation of transforming growth factor-beta1 enhances porcine ileum wound healing in vitro. Ped Res. 2004;55:935–9. doi: 10.1203/01.pdr.0000127023.22960.85. [DOI] [PubMed] [Google Scholar]
- 26.Griffiths CE, Kang S, Ellis CN, Kim KJ, Finkel LJ, Ortiz-Ferrer LC, White GM, Hamilton TA, Voorhees JJ. Two concentrations of topical tretinoin (retinoic acid) cause similar improvement of photoaging but different degrees of irritation. A double-blind, vehicle-controlled comparison of 0.1% and 0.025% tretinoin cream. Arch Dermatol. 1995;131:1037–44. [PubMed] [Google Scholar]
- 27.Kang S, Duell EA, Fisher GJ, Datta SC, Wang Z-Q, Reddy AP, Tavakkol A, Voorhees JJ. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid-binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J Invest Dermatol. 1995;105:549–56. doi: 10.1111/1523-1747.ep12323445. [DOI] [PubMed] [Google Scholar]
- 28.Phillips TJ, Gottlieb AB, Leyden JJ, Lowe NJ, Lew-Kaya DA, Sefton J, Walker PS, Gibson JR. Efficacy of 0.1% Tazarotene cream for the treatment of photodamage. Arch Dermatol. 2002;138:1486–93. doi: 10.1001/archderm.138.11.1486. [DOI] [PubMed] [Google Scholar]
- 29.Varani J, Fay K, Perone P. MDI-301: a non-irritating retinoid, induces changes in organ-cultured human skin that underlie repair. Arch Dermatol Res. 2007;298:439–48. doi: 10.1007/s00403-006-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varani J, Fligiel H, Zhang J, Aslam MN, Lu Y, Dehne LA, Keller ET. Separation of retinoid-induced epidermal and dermal thickening from skin irritation. Arch Dermatol Res. 2003;295:255–62. doi: 10.1007/s00403-003-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varani J, Warner RL, Phan SH, Datta SC, Fisher GJ, Voorhees JJ. Vitamin A antagonizes decreased cell growth, and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally-aged human skin. J Invest Dermatol. 2000;114:480–6. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- 32.Griffiths CEM, Russman G, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid) N Engl J Med. 1993;329:530–4. doi: 10.1056/NEJM199308193290803. [DOI] [PubMed] [Google Scholar]
- 33.Talwar HS, Griffiths CEM, Fisher GJ, Hamilton TA, Voorhees JJ. Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 1995;105:285–90. doi: 10.1111/1523-1747.ep12318471. [DOI] [PubMed] [Google Scholar]
- 34.Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. The molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature (London) 1996;379:335–8. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 35.Fisher GJ, Wang Z-Q, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. New Engl J Med. 1997;337:1419–28. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 36.Fisher GJ, Datta S, Wang ZQ, Li X-Y, Quan T, Chung JH, Kang S, Voorhees JJ. C-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest. 2000;106:663–70. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lateef H, Sevens M, Varani J. All-trans retinoic acid suppresses matrix metalloproteinase production/activation and increases collagen synthesis in diabetic skin in organ culture. Am J Pathol. 2004;165:167–74. doi: 10.1016/S0002-9440(10)63285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varani J, Hattori Y, Chi Y, Schmidt T, Perone P, Zeigler ME, Fader DJ, Johnson TJ. Elaboration of collagenolytic and gelatinolytic matrix metalloproteinases and their inhibitors by basal cell carcinomas of skin: comparison with normal skin. Br J Cancer. 2000;82:657–65. doi: 10.1054/bjoc.1999.0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varani J, Zeigler M, Dame MK, Kang S, Fisher GJ, Voorhees JJ, Stoll SW, Elder JT. Heparin-binding epidermal growth factor activation of keratinocyte ErbB receptors mediates epidermal hyperplasia, a prominent side-effect of retinoid therapy. J Invest Dermatol. 2001;117:1335–41. doi: 10.1046/j.0022-202x.2001.01564.x. [DOI] [PubMed] [Google Scholar]