Abstract
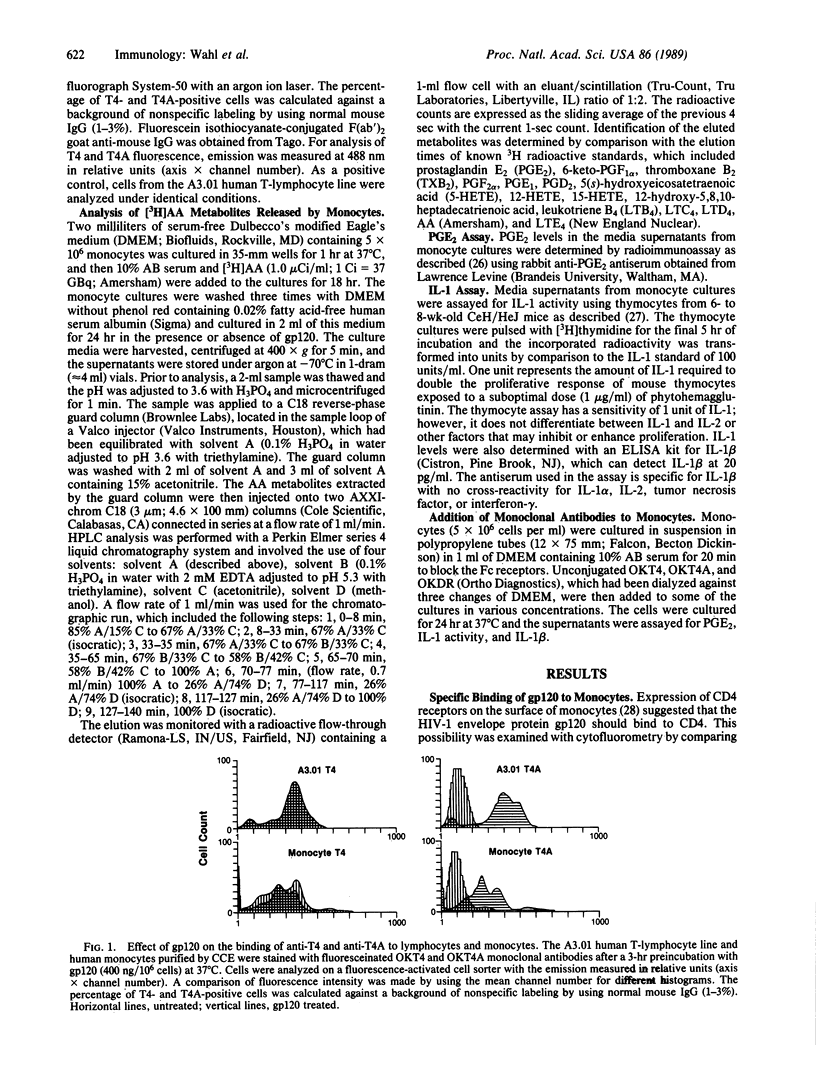
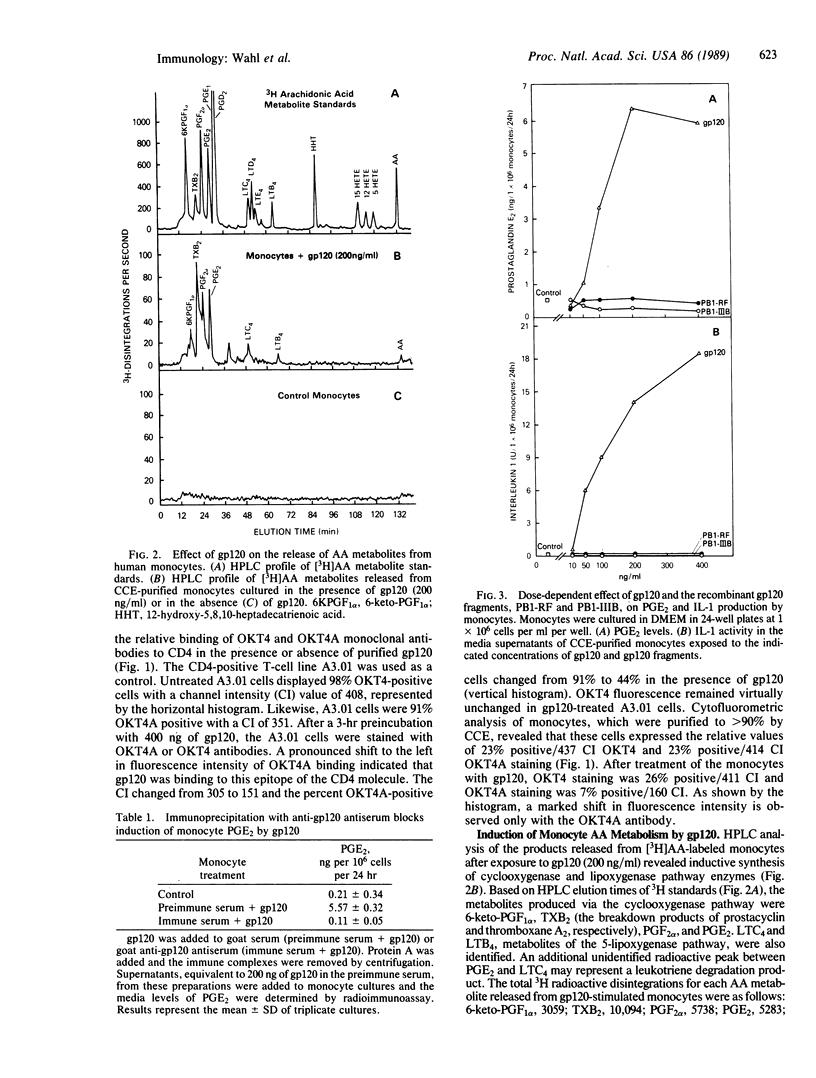
This study reports on the direct effect of the envelope glycoprotein (gp120) of the human immunodeficiency virus type 1 (HIV-1) on human monocyte function. Addition of preparations of purified gp120 from the HIV-1 to human monocytes resulted in the production of interleukin 1 (IL-1) and arachidonic acid metabolites from the cyclooxygenase and lipoxygenase pathways. Quantification of prostaglandin E2 (PGE2) and IL-1 revealed an increase in both mediators with 50 ng of gp120 per ml and an increase of 12- and 30- to 40-fold with 200-400 ng of gp120 per ml, respectively. Unlike native gp120, the recombinant nonglycosylated gp120 fragments PB1-RF and PB1-IIIB, as well as one of the core structural proteins of HIV-1, p24, did not increase arachidonic acid metabolism or IL-1 activity. Cytofluorometric analysis revealed that gp120 blocked the binding of OKT4A to the CD4 on monocytes, whereas OKT4 binding was unaffected. Involvement of the CD4 in signal transduction was further demonstrated by the ability of OKT4 and OKT4A monoclonal antibodies to increase monocyte PGE2, IL-1 activity, and nanogram amounts of IL-1 beta.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atluru D., Goodwin J. S. Control of polyclonal immunoglobulin production from human lymphocytes by leukotrienes; leukotriene B4 induces an OKT8(+), radiosensitive suppressor cell from resting, human OKT8(-) T cells. J Clin Invest. 1984 Oct;74(4):1444–1450. doi: 10.1172/JCI111556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copelan E. A., Rinehart J. J., Lewis M., Mathes L., Olsen R., Sagone A. The mechanism of retrovirus suppression of human T cell proliferation in vitro. J Immunol. 1983 Oct;131(4):2017–2020. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gelderblom H. R., Hausmann E. H., Ozel M., Pauli G., Koch M. A. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987 Jan;156(1):171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Gelderblom H. R., Reupke H., Pauli G. Loss of envelope antigens of HTLV-III/LAV, a factor in AIDS pathogenesis? Lancet. 1985 Nov 2;2(8462):1016–1017. doi: 10.1016/s0140-6736(85)90570-7. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983 Oct;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Poiesz B., Ruscetti F. W., Gallo R. C. Immunological properties of a type C retrovirus isolated from cultured human T-lymphoma cells and comparison to other mammalian retroviruses. J Virol. 1981 Jun;38(3):906–915. doi: 10.1128/jvi.38.3.906-915.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Robert-Guroff M., Miyoshi I., Golde D., Gallo R. C. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982 Nov 5;218(4572):571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Krakauer T., Mizel D., Oppenheim J. J. Independent and synergistic thymocyte proliferative activities of PMA and IL 1. J Immunol. 1982 Sep;129(3):939–941. [PubMed] [Google Scholar]
- LEVIN J., BANG F. B. THE ROLE OF ENDOTOXIN IN THE EXTRACELLULAR COAGULATION OF LIMULUS BLOOD. Bull Johns Hopkins Hosp. 1964 Sep;115:265–274. [PubMed] [Google Scholar]
- Lifson J., Coutré S., Huang E., Engleman E. Role of envelope glycoprotein carbohydrate in human immunodeficiency virus (HIV) infectivity and virus-induced cell fusion. J Exp Med. 1986 Dec 1;164(6):2101–2106. doi: 10.1084/jem.164.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin K., Nygren A., Arthur L. O., Robey W. G., Morein B., Ramstedt U., Gidlund M., Wigzell H. A specific assay measuring binding of 125I-Gp 120 from HIV to T4+/CD4+ cells. J Immunol Methods. 1987 Feb 26;97(1):93–100. doi: 10.1016/0022-1759(87)90110-4. [DOI] [PubMed] [Google Scholar]
- Mann D. L., Lasane F., Popovic M., Arthur L. O., Robey W. G., Blattner W. A., Newman M. J. HTLV-III large envelope protein (gp120) suppresses PHA-induced lymphocyte blastogenesis. J Immunol. 1987 Apr 15;138(8):2640–2644. [PubMed] [Google Scholar]
- Mathes L. E., Olsen R. G., Hebebrand L. C., Hoover E. A., Schaller J. P. Abrogation of lymphocyte blastogenesis by a feline leukaemia virus protein. Nature. 1978 Aug 17;274(5672):687–689. doi: 10.1038/274687a0. [DOI] [PubMed] [Google Scholar]
- Mathes L. E., Olsen R. G., Hebebrand L. C., Hoover E. A., Schaller J. P., Adams P. W., Nichols W. S. Immunosuppressive properties of a virion polypeptide, a 15,000-dalton protein, from feline leukemia virus. Cancer Res. 1979 Mar;39(3):950–955. [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- Mitsuya H., Guo H. G., Cossman J., Megson M., Reitz M. S., Jr, Broder S. Functional properties of antigen-specific T cells infected by human T-cell leukemia-lymphoma virus (HTLV-I). Science. 1984 Sep 28;225(4669):1484–1486. doi: 10.1126/science.6206569. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Guo H. G., Megson M., Trainor C., Reitz M. S., Jr, Broder S. Transformation and cytopathogenic effect in an immune human T-cell clone infected by HTLV-I. Science. 1984 Mar 23;223(4642):1293–1296. doi: 10.1126/science.6322299. [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., Cross G. D., Callaway C. S., McDougal J. S. In vitro infection of human monocytes with human T lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV). J Immunol. 1986 Jul 1;137(1):323–329. [PubMed] [Google Scholar]
- Payan D. G., Goetzl E. J. Specific suppression of human T lymphocyte function by leukotriene B4. J Immunol. 1983 Aug;131(2):551–553. [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Reitz M. S., Kalyanaraman V. S., Gallo R. C. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sézary T-cell leukaemia. Nature. 1981 Nov 19;294(5838):268–271. doi: 10.1038/294268a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Flomenberg N., Volkman D. J., Mann D., Fauci A. S., Dupont B., Gallo R. C. Alteration of T-cell functions by infection with HTLV-I or HTLV-II. Science. 1984 Oct 26;226(4673):459–462. doi: 10.1126/science.6093248. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Matthews T. J., Robey W. G., Lynn D. L., Robert-Guroff M., Mueller W. T., Langlois A. J., Ghrayeb J., Petteway S. R., Jr, Weinhold K. J. HTLV-III/LAV-neutralizing antibodies to an E. coli-produced fragment of the virus envelope. Science. 1986 Dec 12;234(4782):1392–1395. doi: 10.1126/science.2431482. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Robey W. G., Arthur L. O., Matthews T. J., Langlois A., Copeland T. D., Lerche N. W., Oroszlan S., Bolognesi D. P., Gilden R. V., Fischinger P. J. Prospect for prevention of human immunodeficiency virus infection: purified 120-kDa envelope glycoprotein induces neutralizing antibody. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7023–7027. doi: 10.1073/pnas.83.18.7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola-Pleszczynski M., Borgeat P., Sirois P. Leukotriene B4 induces human suppressor lymphocytes. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1531–1537. doi: 10.1016/s0006-291x(82)80081-8. [DOI] [PubMed] [Google Scholar]
- Rola-Pleszczynski M. Differential effects of leukotriene B4 on T4+ and T8+ lymphocyte phenotype and immunoregulatory functions. J Immunol. 1985 Aug;135(2):1357–1360. [PubMed] [Google Scholar]
- Sattentau Q. J., Dalgleish A. G., Weiss R. A., Beverley P. C. Epitopes of the CD4 antigen and HIV infection. Science. 1986 Nov 28;234(4780):1120–1123. doi: 10.1126/science.2430333. [DOI] [PubMed] [Google Scholar]
- Schneider J., Kaaden O., Copeland T. D., Oroszlan S., Hunsmann G. Shedding and interspecies type sero-reactivity of the envelope glycopolypeptide gp120 of the human immunodeficiency virus. J Gen Virol. 1986 Nov;67(Pt 11):2533–2538. doi: 10.1099/0022-1317-67-11-2533. [DOI] [PubMed] [Google Scholar]
- Wahl L. M., Katona I. M., Wilder R. L., Winter C. C., Haraoui B., Scher I., Wahl S. M. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). I. Characterization of B-lymphocyte-, T-lymphocyte-, and monocyte-enriched fractions by flow cytometric analysis. Cell Immunol. 1984 May;85(2):373–383. doi: 10.1016/0008-8749(84)90251-x. [DOI] [PubMed] [Google Scholar]
- Wainberg M. A., Spira B., Boushira M., Margolese R. G. Inhibition by human T-lymphotropic virus (HTLV-I) of T-lymphocyte mitogenesis: failure of exogenous T-cell growth factor to restore responsiveness to lectin. Immunology. 1985 Jan;54(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Wood G. S., Warner N. L., Warnke R. A. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983 Jul;131(1):212–216. [PubMed] [Google Scholar]



