Abstract
Vγ2Vδ2+ T cells play a role in antimicrobial responses. It is unknown whether adaptive Vγ2Vδ2+ T cell responses during active mycobacterial coinfection of human immunodeficiency virus–infected humans can be generated during effective antiretroviral treatment. Here, simian immunodeficiency virus (SIV)mac-infected macaques previously exposed to bacille Calmette-Guérin (BCG) were reinfected with BCG, were treated either with tenofovir or tenofovir plus indinavir, and were assessed for the development of Vγ2Vδ2+ T cell responses during active BCG coinfection. A restored capacity of Vγ2Vδ2T+ cells to undergo major expansions and pulmonary migration during active BCG coinfection was detected after simultaneous BCG reinfection and treatment with tenofovir of the SIVmac-infected macaques. Interestingly, a restored expansion of Vγ2Vδ2+ T cells in the SIVmac/BCG-coinfected macaques was detectable, even though antiretroviral treatment was initiated 1 month after BCG reinfection. Importantly, the restored expansion of Vγ2Vδ2+ T cells coincided with increases in numbers of purified protein derivative–specific interferon-γ–producing CD4+ T cells and increases in the magnitude of their proliferative responses. In contrast, the SIVmac-infected control macaques exhibited diminished responses of Vγ2Vδ2+ T cells and mycobacterium-specific CD4+ T cells during active BCG coinfection. Our results suggest that the development of adaptive immune responses of phosphoantigen-specific Vγ2Vδ2+ T cells during active mycobacterium/HIV coinfection requires control of viral infection and immune competence of peptide-specific CD4+ T cells.
Vγ2Vδ2+ T cells play a role in antimicrobial immunity [1–5]. Vγ2Vδ2+ T cells exist only in primates and, in humans, constitute 60%–95% of the circulating γδ T cells [6]. Vγ2Vδ2+ T cells in humans and in nonhuman primates have specificity for nonpeptide phosphoantigens derived from microbial pathogens [2]. It has also been shown that both human and macaque Vγ2Vδ2+ T cells undergo major clonal expansions during infections [2, 3]. In vitro studies have shown that human Vγ2Vδ2+ T cells can produce antimicrobial cytokines and cytotoxic granules and mediate bactericidal activity on intracellular Mycobacterium tuberculosis organisms [1, 7]. In vivo studies in nonhuman primates have demonstrated that macaque Vγ2Vδ2+ T cells can mount adaptive (memory) immune responses during M. bovis bacille Calmette-Guérin (BCG) and M. tuberculosis infections [3, 8]. The rapid recall expansion of pulmonary Vγ2Vδ2+ T cells following aerosol challenge with M. tuberculosis is associated with a reduction of M. tuberculosis burdens and immune protection against fatal tuberculosis in BCG-vaccinated monkeys [3]. It is therefore important to further characterize immune responses and antimicrobial function of phosphoantigen-specific Vγ2Vδ2+ T cells.
Studies of humans have indicated that Vγ2Vδ2+ T cells are particularly susceptible to functional impairment or deletion during HIV-1 infection [6, 9]. A decline in numbers of Vγ2Vδ2+ T cells, including the subset that are CD45−CD27−, has been reported in HIV-1–infected individuals [9–15]. Moreover, in vitro studies have shown that HIV-1 infection can inhibit the ability of Vγ2Vδ2+ T cells to proliferate or to produce Th1 cytokines after stimulation with M. tuberculosis or nonpeptide antigens [6, 15–18]. It remains unknown whether active mycobacterial coinfection of HIV-1–infected humans can induce adaptive immune responses of Vγ2Vδ2+ T cells. Although in vitro the effector function of Vγ2Vδ2+ T cells can be improved by highly active antiretroviral therapy (HAART) [16, 17], there is no in vivo evidence showing that immune reconstitution by HAART can efficiently restore a defect of Vγ2Vδ2+ T cells and ensure the development of adaptive Vγ2Vδ2+ T cell responses during active mycobacterial coinfection.
We have recently demonstrated that, although healthy simian immunodeficiency virus (SIV)–negative macaques develop primary and memory Vγ2Vδ2+ T cell responses after BCG or M. tuberculosis infections, SIVmac-infected macaques cannot mount sound adaptive Vγ2Vδ2+ T cell immune responses during active BCG coinfection [3, 8, 19]. The absence of adaptive Vγ2Vδ2+ T cell responses in SIVmac/BCG-coinfected macaques is associated with profound CD4+ T cell deficiency and subsequent development of an SIVmac-related tuberculosis-like disease [19]. These findings suggest that control of SIV disease by effective antiretroviral treatment may facilitate the restoration of adaptive immune responses of Vγ2Vδ2+ T cells during active BCG coinfection of SIVmac-infected macaques. To test this possibility, we examined the extent to which the immune responses of Vγ2Vδ2+ T cells can be restored by antiretroviral treatment during active BCG coinfection of SIVmac-infected macaques. We also explored the immune mechanisms underlying the restoration of Vγ2Vδ2+ T cell responses after antiretroviral treatment of SIVmac/BCG-coinfected macaques.
MATERIALS AND METHODS
Macaques and virus
Ten rhesus (Macaca mulatta) and 2 pigtailed (M. nemestrina) macaques, 2–8 years of age, were used in the present study. These animals were maintained in accordance with the guidelines of the Committee on Animals of Harvard Medical School and the Guide for the Care and Use of Laboratory Animals [20]. For SIVmac infection, the macaques were inoculated intravenously with 106 TCID50 of SIVmac251, as described elsewhere [21].
M. bovis BCG coinfection
M. bovis BCG (Pasteur strain) was used for BCG coinfection, as described elsewhere [21]. To facilitate the evaluation of the restoration of Vγ2Vδ2+ T cell responses by antiretroviral treatment, SIVmac-infected macaques exposed to BCG prior to viral infection were included in the present study. These macaques did not show any clinical evidence of active BCG infection or BCG disease when they were infected with SIVmac251. These macaques had a 2–10-month course of SIVmac infection before they were reinfected with BCG and were assessed for the restoration of Vγ2Vδ2+ T cell responses after antiretroviral treatment.
Antiretroviral treatment of SIVmac/BCG-coinfected macaques
Two clinical settings were designed to evaluate the effect that antiretroviral drugs have on the restoration of Vγ2Vδ2+ T cell responses in SIVmac/BCG-coinfected macaques. In the first clinical setting, treatment with ((R)-9-(2-phosphonomethoxy)propyl) adenine (tenofovir; formerly termed “PMPA”) and BCG reinfection were done simultaneously. Tenofovir was subcutaneously injected daily for 1 month at a dose of 25 mg/kg, as described elsewhere [22]. In the second setting, antiretroviral treatment was initiated 1 month after BCG reinfection and lasted for 2.5 months. The antiretroviral drug regimen was composed of tenofovir and the protease inhibitor indinavir (Merck) [22]. Indinavir was administered subcutaneously twice daily at a dose of 20 mg/kg. We saw no evidence indicating that antiretroviral treatment alone could induce similar expansion of Vγ2Vδ2+ T cells in the 3 SIV-infected macaques that were not infected with BCG.
Immune flow-cytometry analyses of γδ T cell receptor (TCR) repertoire and CD4+ T cells
The γδ TCR repertoire of the macaques was analyzed by use of whole-blood staining, as described elsewhere [19]. Anti-human γδ monoclonal antibodies that cross-react with the corresponding macaque γδ T cells were used [19].
Proliferation assays
Conventional proliferation assays were conducted as described elsewhere [8]. In brief, fractionated peripheral-blood lymphocytes (PBLs; 1 × 105 cells/well) were cultured in triplicate in 96-well plates (Costar) in the presence of BCG purified protein derivative (PPD; 25 µg/mL), concanavalin A (ConA; 5 µg/mL), or medium alone. Five days later, cells were pulsed with [3H]-thymidine at 1.0 µCi/well, and uptake was measured 8 h later by use of a 1450 Microbeta scintillation counter (Wallac). The proliferation index was defined as the ratio of the mean counts per minute of PPD- or ConA-stimulated wells relative to the mean counts per minute of control wells (medium alone). A proliferation index of ≥3 was considered to be positive.
Enzyme-linked immunospot (ELISPOT) assays
To measure the numbers and interferon (IFN)–γ–production capacity of mycobacteria-specific T cells, PBLs were assessed for their specific recognition of PPD antigens by ELISPOT assay [8]. To estimate the relative number of PPD-specific IFN-γ–producing CD4+ T cells, PBLs were depleted of CD8+ T cells by use of immunomagnetic beads, with <5% of CD8+ T cells detected in the depleted PBLs, as described elsewhere [23]. The PBLs depleted of CD8+ T cells were used for ELISPOT assays. In these experiments, 96-well nitrocellulose-backed plates were coated with 1 µg anti–human IFN-γ (B27) by overnight incubation at room temperature. Cells (2 × 105) were plated in duplicate in each well with or without BCG antigens. Eighteen hours after culture, the plates were washed and were incubated for 2 h at room temperature with 500 ng of biotinylated anti–IFNγ. Spots were developed by adding horseradish peroxidase–streptavidin and freshly prepared substrate. The numbers of ELISPOT-forming cells produced in response to BCG antigens were calculated by subtracting the values of negative controls (medium alone). Data were expressed as numbers of PPD-specific IFN-γ–producing CD4+ T cells/106 CD8+-depleted PBLs.
Quantitative measurement of SIV RNA in plasma
Both real-time quantitative polymerase chain reaction (PCR) and quantitative competitive (QC)–PCR were used, as described elsewhere [19], to measure levels of SIV RNA in plasma. In brief, viral RNA in plasma was extracted by use of the RNA Extraction Kit (Qiagen), according to the manufacturer’s instructions, and were measured for expression levels by real-time quantitative PCR, as described elsewhere [19, 23]. The primers used for quantitation of SIVmac gag expression were as follows: forward primer, 5′-TGTCAAAAAATACTTTCGGTCTTAGC-3′; reverse primer, 5′-GATGACGCAGACAGTATTATAAAGGC-3′; and TaqMan probe, 5′-6FAM-CCATTAGTGCCAACAGGCTCAGAAAATTTAAAA-MGBNFQ-3′. The real-time quantitative PCR was performed in ABI 7700 according to the instructions from the manufacturer, as described elsewhere [3]. For QC-PCR, the extracted RNA was aliquoted into 6 different tubes, each of which contained defined copies of SIVmac gag competitor RNA. The RNA mixtures were reverse transcribed to cDNA and were competitively amplified by a 35-cycle PCR with a pair of SIVmac gag–specific primers [19]. The amplified PCR products (both wild-type and competitor) were separated on 2% agarose gels and were measured for their densities in a GS 700 Imaging Densitometer (Bio-Rad). Quantitation was achieved by data analysis using Molecular Analyst software (Bio-Rad). The intra- and interassay coefficient of variation using this protocol was <20%. The sensitivity of both the real-time quantitative PCR and the QC-PCR was 4 × 102 RNA copies in 1 mL of plasma.
Cloning and sequencing of Vδ2 TCR
Molecular cloning and sequencing of Vδ2 TCR were done by use of a PCR-based technique, as described elsewhere [3]. cDNA was synthesized by use of mRNA derived from PBLs obtained before and after BCG reinfection during antiretroviral treatment. The samples included those collected at the time when marked expansions of Vγ2Vδ2+ T cells were identified in the infected macaques. The cDNA was then amplified by a 30-cycle PCR with a pair of Vδ2- and Cδ-specific primers that bear EcoRI and XbaI restriction sites, respectively. The sequences for the primers were as follows: Vδ2, 5′-GCGCGAATTCAACGGATGGTTTGGTATGAGG-3′; and Cδ, 5′-GCGCTCTAGATATATCAACTGGTACAGG-3′. The PCR products were gel purified, were digested with EcoRI and XbaI, and were ligated into the plasmid SP65 (Promega) for cloning and sequencing. Up to 20 cDNA clones were sequenced and analyzed for each sample.
Statistical analysis
As described elsewhere [19], Student’s t test and nonparametric test were used to examine whether any differences in numbers of Vγ2Vδ2+ T cells and CD4+ T cells, SIV RNA in plasma, or BCG loads identified after BCG coinfection were statistically significant.
RESULTS
Detection of a restored expansion of Vγ2Vδ2+ T cells in SIVmac-infected macaques after simultaneous BCG reinfection and tenofovir treatment
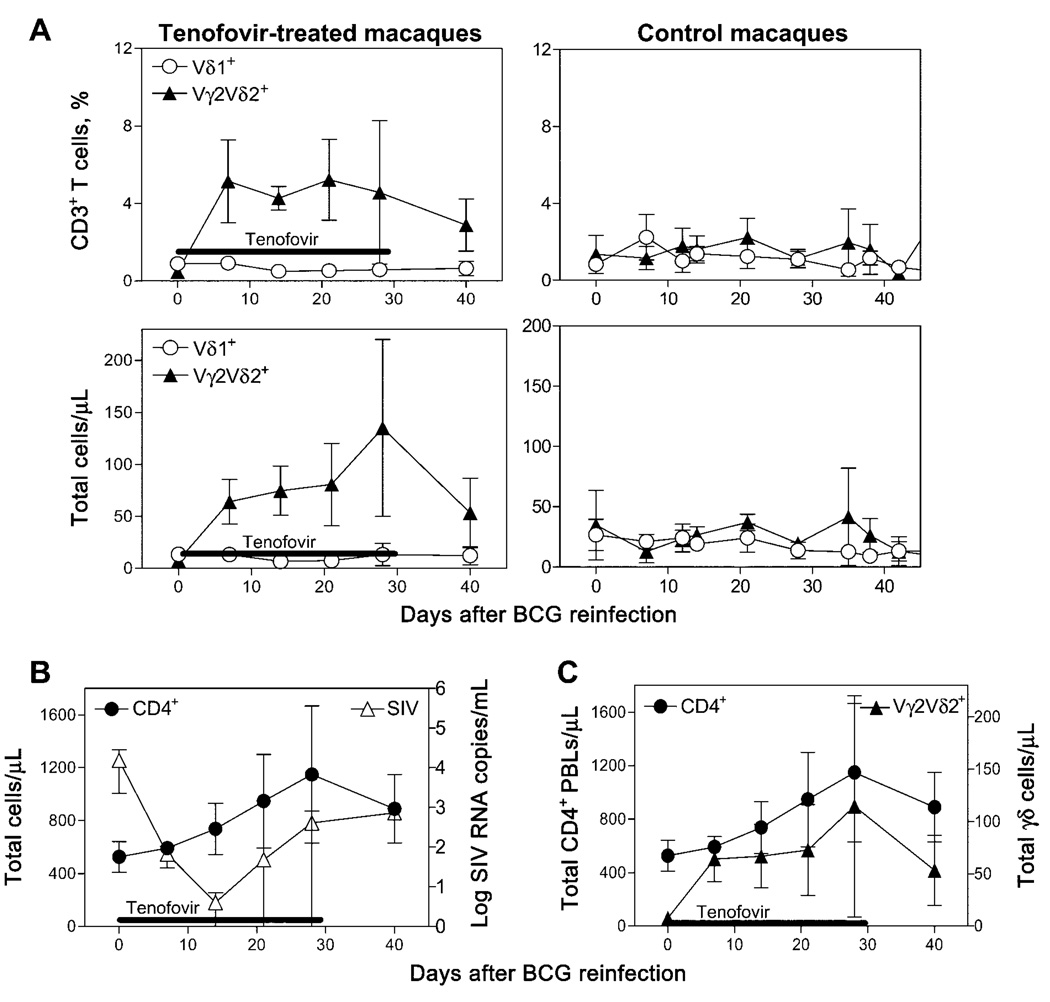
To determine whether Vγ2Vδ2+ T cell responses during active mycobacterial coinfection of HIV-infected individuals can be generated during an effective antiretroviral treatment, SIVmac-infected macaques that had been exposed to BCG prior to SIV infection were reinfected with BCG and were treated with the antiretroviral drug tenofovir. The immune responses of Vγ2Vδ2+ T cells were assessed in these macaques after simultaneous BCG reinfection and tenofovir treatment. A rapid recall expansion of Vγ2Vδ2+ T cells was identified in the PBLs of these SIVmac/BCG-coinfected macaques (figure 1A) after SIV viremia was significantly reduced by tenofovir treatment (figure 1B). Up to 15-fold increases in mean numbers of Vγ2Vδ2+ T cells were seen in PBLs as early as 7 days after coinfection of the 3 macaques after simultaneous BCG reinfection and tenofovir treatment (figure 1A). Importantly, the recall expansions of Vγ2Vδ2+ T cells after simultaneous BCG reinfection and tenofovir treatment occurred coincident with the marked increases in CD4+ T cell counts in the SIVmac/BCG-coinfected macaques (figure 1C). In contrast, SIVmac-infected control macaques that did not receive tenofovir showed only subtle expansions of Vγ2Vδ2+ T cells after BCG reinfection (figure 1A). These results therefore demonstrate that control of SIVmac infection by tenofovir treatment can rapidly restore a recall immune response of Vγ2Vδ2+ T cells during active BCG reinfection of SIVmac-infected macaques.
Figure 1.
Simultaneous bacille Calmette-Guérin (BCG) reinfection and treatment with tenofovir of simian immunodeficiency virus (SIV)mac-infected macaques resulted in the development of recall expansions of Vγ2Vδ2+ T cells in the blood. All data shown are the means for 3 macaques, with error bars indicating SEs. A, Shown are the increases in absolute nos. and percentages of Vγ2Vδ2+ T cells in the blood of 3 tenofovir-treated macaques (left) and 3 SIVmac-infected control macaques (right) after BCG reinfection. P < .05 (nonparametric test) for data 1–4 weeks after antiretroviral treatment, for comparison between treated and control macaques. B, Tenofovir treatment induced a decline in levels of plasma SIV RNA and an increase in CD4+ T cell counts in 3 SIVmac-infected macaques. Plasma SIV RNA were measured by use of real-time quantitative polymerase chain reaction [3]. The lower level 2 weeks after BCG reinfection was detected by use of concentrated samples. C, Restored expansion of Vγ2Vδ2+ T cells during simultaneous BCG reinfection and antiretroviral treatment coincided with the increase in nos. of circulating CD4+ T cells. PBLs, peripheral-blood lymphocytes.
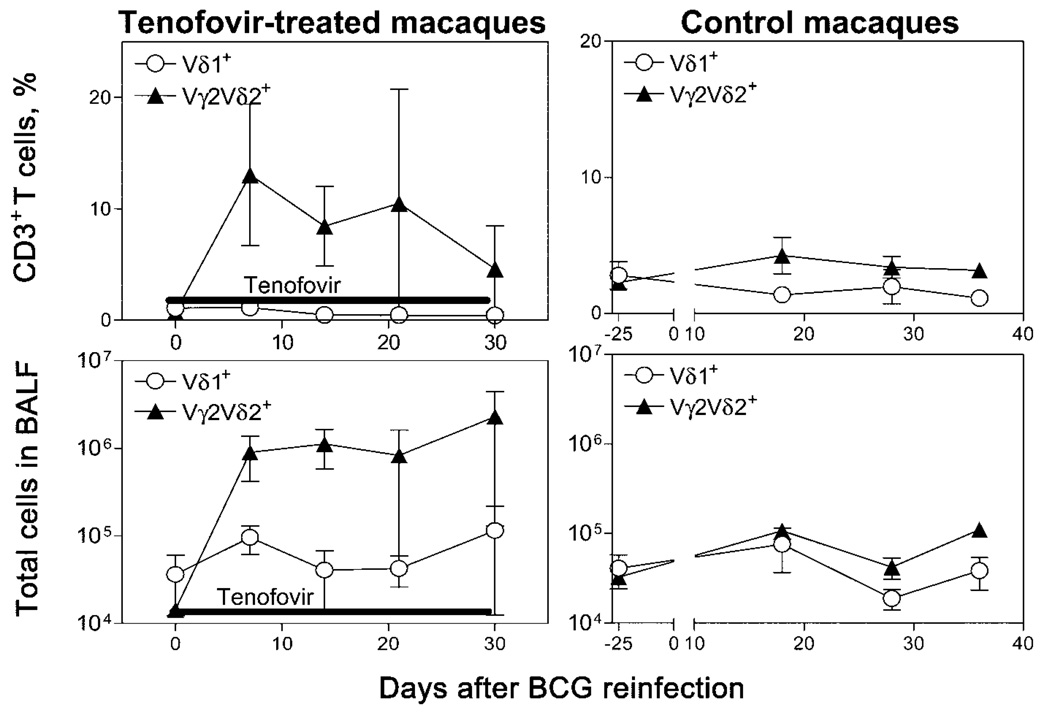
Restoration during tenofovir treatment of the capacity of Vγ2Vδ2+ T cells to migrate to the lung after BCG reinfection
We have recently demonstrated that Vγ2Vδ2+ T cells are able to undergo pulmonary migration during adaptive immune responses to systemic BCG infection [2, 3, 8]; the capacity of Vγ2Vδ2+ T cells to migrate to pulmonary compartments was inhibited in SIVmac-infected macaques [19]. Given the potential role that might be played by Vγ2Vδ2+ T cells in pulmonary immunity, it was important to determine whether the depressed capacity of Vγ2Vδ2+ T cells to migrate to the lung can be restored by effective antiretroviral treatment. Thus, cells in bronchoalveolar lavage fluid (BALF) were assessed for changes in numbers of Vγ2Vδ2+ T cells after simultaneous BCG reinfection and treatment with tenofovir of the SIVmac-infected macaques. Increases in absolute numbers and percentages of Vγ2Vδ2+ T cells in BALF were demonstrated after BCG reinfection in the tenofovir-treated macaques (figure 2). A 1–2-log increase in mean numbers of Vγ2Vδ2+ T cells was detected in BALF after simultaneous BCG reinfection and treatment with tenofovir of the 3 SIVmac-infected macaques (figure 2). In contrast, the SIVmac-infected control macaques not treated with antiretrovirals did not exhibit marked increases in numbers of Vγ2Vδ2+ T cells in the pulmonary compartment after BCG reinfection (figure 2). These results therefore demonstrate that control of SIVmac infection by antiretroviral treatment can facilitate a restoration of the capacity of Vγ2Vδ2+ T cells to migrate to the pulmonary compartment during adaptive immune responses to active BCG reinfection.
Figure 2.
Antiretroviral treatment after bacille Calmette-Guérin (BCG) reinfection of simian immunodeficiency virus (SIV)mac-infected macaques resulted in a restored capacity of Vγ2Vδ2+ T cells to undergo pulmonary migration. Shown are the absolute nos. and percentages of Vγ2Vδ2+ T cells after BCG reinfection in the bronchoalveolar lavage fluid (BALF) of 3 tenofovir-treated macaques and 3 SIVmac-infected control macaques. Data from day −25 were used as pretime points for the control macaques.
Restoration, by delayed antiretroviral treatment initiated 1 month after BCG reinfection, of an inhibited expansion of Vγ2Vδ2+ T cells in SIVmac-infected macaques
Because recall immune responses of Vγ2Vδ2+ T cells could last for months after BCG reinfection [3], we sought to determine whether an established suppression of Vγ2Vδ2+ T cell responses during active BCG coinfection could be reversed by a delayed antiretroviral treatment after the second BCG inoculation. To this end, SIVmac-infected macaques were reinfected with BCG and, 1 month later, were treated with antiretroviral drugs. Interestingly, although the SIVmac-infected macaques exhibited down-regulation of Vγ2Vδ2+ T cells for the initial month after BCG reinfection, control of SIV infection by antiretroviral treatment initiated 1 month after BCG inoculation was associated with a detectable expansion of Vγ2Vδ2+ T cells in PBLs of the SIVmac/BCG-coinfected macaques (figure 3A and 3B). As observed in the setting of simultaneous BCG reinfection and tenofovir treatment, the restoration of a late-phase Vγ2Vδ2+ T cell response in the SIVmac/BCG-coinfected macaques was associated with an increase in numbers of circulating CD4+ T cells after the delayed antiretroviral treatment (figure 3C). These results provide additional evidence that immune reconstitution after antiretroviral treatment is able to restore immune responses of Vγ2Vδ2+ T cells during active mycobacterial coinfection of SIVmac-infected macaques.
Figure 3.
Delayed antiretroviral treatment after bacille Calmette-Guérin (BCG) reinfection was able to restore expansion of Vγ2Vδ2+ T cells in simian immunodeficiency virus (SIV)mac-infected macaques. A, Antiretroviral treatment initiated 1 month after BCG reinfection resulted in increases in absolute nos. and percentages of Vγ2Vδ2+ T cells in 3 SIVmac-infected macaques. Before SIVmac infection and BCG reinfection, these macaques had received their first inoculations of BCG and exhibited typical primary expansions of Vγ2Vδ2+ T cells, as described elsewhere [2, 3]. B, Antiretroviral treatment led to a decline in SIVmac loads and an increase in CD4+ T cell counts. Plasma SIV RNA was measured by use of quantitative competitive polymerase chain reaction [19]. C, Increased CD4+ T cell counts coincided with the restored expansions of Vγ2Vδ2+ T cells. The antiretroviral regimen used for these macaques consisted of tenofovir and indinavir.
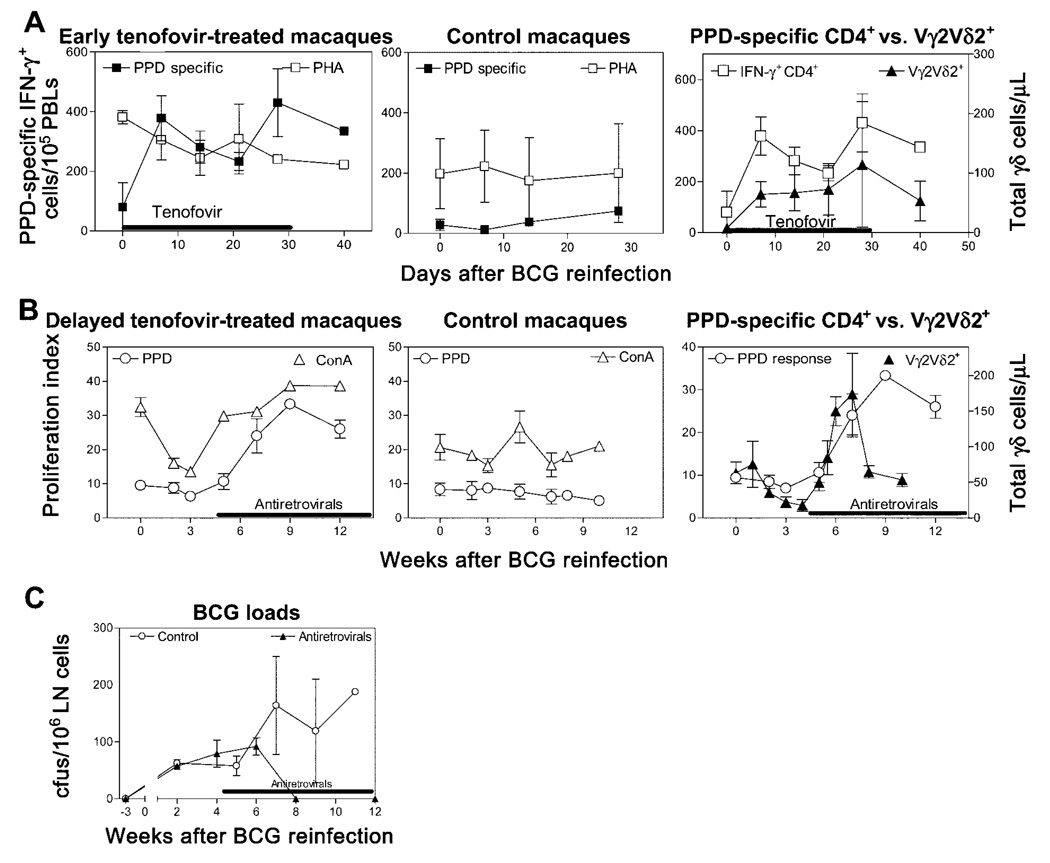
Restoration of Vγ2Vδ2+ T cell responses coincidental with (1) increases in numbers of mycobacteria-specific IFN-γ–producing CD4+ T cells and (2) increases in the magnitude of their proliferative responses
To explore immune mechanisms underlying the restoration of Vγ2Vδ2+ T cell expansion in vivo, we investigated the development of antigen-specific CD4+ T cell responses during antiretroviral treatment of SIVmac/BCG-coinfected macaques. After simultaneous BCG reinfection and tenofovir treatment, there was a marked increase in numbers of mycobacteria-specific IFN-γ–producing CD4+ T cells in the PBLs of the SIVmac/BCG-coinfected macaques (figure 4A). The emergence of mycobacterium-specific IFN-γ–producing CD4+ T cells was coincident with the in vivo expansion of CD4+ PBLs after treatment with tenofovir of the SIVmac/BCG-coinfected macaques (figures 1B and 4A). Importantly, the increased numbers of mycobacterium-specific IFN-γ–producing CD4+ T cells coincided with a recall expansion of Vγ2Vδ2+ T cells in the SIVmac/BCG-coinfected macaques (figure 4A). The restored expansion of Vγ2Vδ2+ T cells after delayed treatment with tenofovir of the SIVmac/BCG-coinfected macaques consistently coincided with the reconstitution of BCG-driven proliferative CD4+ T cell responses (figure 4B). The restored immune responses of Vγ2Vδ2+ T cells and CD4+ T cells after tenofovir treatment were associated with a decline of BCG bacterial organisms in lymph node cells of the SIVmac/BCG-coinfected macaques (figure 4C). These treated SIVmac/BCG-coinfected macaques survived BCG coinfection for up to 12 months of follow-up; in contrast, the SIVmac/BCG-coinfected macaques not treated with antiretrovirals did not have potent Vγ2Vδ2+ T cell and CD4+ T cell immune responses and subsequently developed a fatal SIVmac-related tuberculosis-like disease characterized clinically by diarrhea and weight loss and pathologically by disseminated granulomas within 4 months of BCG reinfection. These results suggest that the immune competence of CD4+ T cells during antiretroviral treatment of SIVmac/BCG-coinfected macaques is important for the restoration of their Vγ2Vδ2+ T cell responses.
Figure 4.
The immune competence of mycobacterium-specific CD4+ T cells during antiretroviral treatment and bacille Calmette-Guérin (BCG) reinfection coincided with the restored expansion of phosphoantigen-specific Vγ2Vδ2+ T cells. A, BCG reinfection induced a marked increase in nos. of purified protein derivative (PPD)–specific interferon (IFN)-γ–producing CD4+ T cells in 3 tenofovir-treated macaques but not in 3 simian immunodeficiency virus (SIV)mac-infected control macaques. Such an increase was coincident with the recall expansion of Vγ2Vδ2+ T cells. B, Potent proliferative responses of PPD-specific CD4+ T cells after BCG reinfection and delayed antiretroviral treatment coincided with the restored expansion of Vγ2Vδ2+ T cells in 3 SIVmac-infected macaques. In healthy SIV-negative macaques, BCG reinfection induced enhanced proliferative responses 1 week after the second BCG inoculation [8, 20]. Experiments were conducted with CD8-depleted peripheral-blood lymphocytes (PBLs) collected prospectively from the macaques. C, Restored immune responses of Vγ2Vδ2+ T cells and CD4+ T cells after antiretroviral treatment were associated with declines in nos. of BCG organisms in the lymph nodes (LN) of 3 SIVmac/BCG-coinfected macaques. Data were collected from the same macaques shown in panel B. ConA, concanavalin A; PHA, phytohemagglutinin.
Restoration of immune responses of Vγ2Vδ2+ T cells during antiretroviral treatment of SIVmac-infected macaques on a background of a polyclonal γδ TCR repertoire
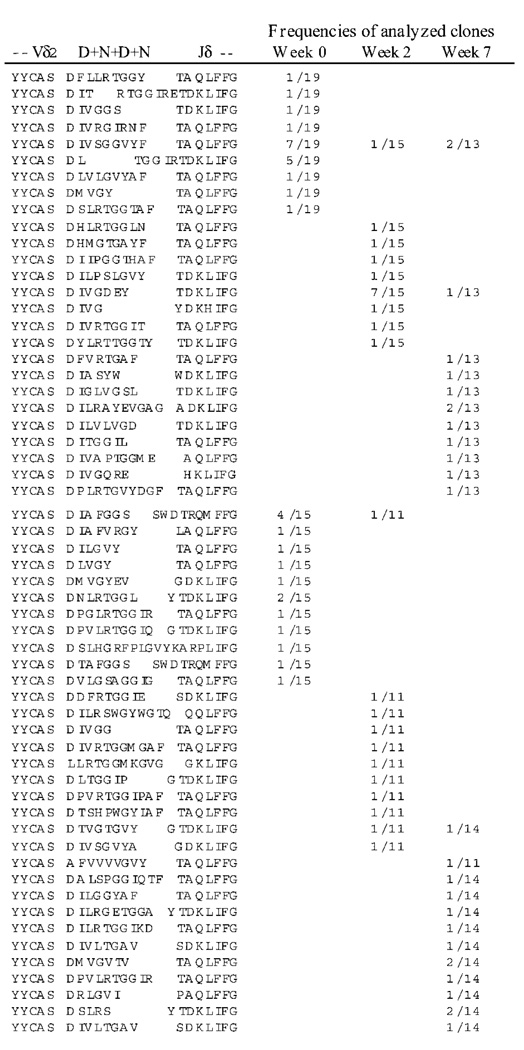
Given that HIV and SIV infections profoundly impair γδ TCR repertoires [6, 9, 19, 24], it was important to determine whether improved immune competence during antiretroviral treatment of the SIVmac/BCG-coinfected macaques restored a broad or limited repertoire of Vγ2Vδ2+ T cells in response to active BCG coinfection. To address this issue, we examined the clonality of Vγ2Vδ2+ T cells that were expanded over time after BCG coinfection and ongoing antiretroviral treatment. Because spectrotyping analyses of TCR CDR3 profiles and flow-cytometry analyses could not optimally determine the diversity of clonal expansion of these T cells, we analyzed clonotypic TCR sequences by sequencing junctional regions of Vδ2-bearing cDNA derived from expanded Vγ2Vδ2+ T cells after BCG reinfection and antiretroviral treatment. Vδ2+ T cells in the SIVmac-infected macaques were polyclonal before and after BCG reinfection during antiretroviral treatment (figure 5). Polyclonal sequences of Vδ2+ T cells were identified at the time a recall expansion of Vγ2Vδ2+ T cells was observed during BCG coinfection and antiretroviral treatment (figures 3 and 5). These results therefore suggest that improvement of immune function during effective antiretroviral treatment is able to restore a broad repertoire of Vγ2Vδ2+ T cells in response to BCG coinfection.
Figure 5.
Vδ2+ T cells were polyclonal before and after cellular expansion during bacille Calmette-Guérin (BCG) reinfection and antiretroviral treatment in simian immunodeficiency virus (SIV)mac-infected macaques. Restored recall expansions of Vγ2Vδ2+ T cells were mounted on a background of polyclonal γδ T cell receptor (TCR) repertoire. Polyclonal expansions of Vδ2 T cells were seen after antiretroviral treatment during BCG reinfection (figure 3A). Note that polyclonal sequences were demonstrated during week 7 after BCG reinfection, the time point at which a restored expansion of Vγ2Vδ2+ T cells was seen in 2 SIVmac/BCG-coinfected macaques treated with tenofovir and indinavir. Frequencies were expressed as the no. of individual clones per the total no. of analyzed clones. Similar data indicating polyclonal expansions of Vδ2+ T cells were also observed in samples from 2 other tenofovir-treated SIVmac/BCG-coinfected macaques. D indicates a diversity region; N indicates nontemplate nucleotides incorporated into TCR genes. The D+N+D+N region is displayed on the basis of the observation that TCR δ diversity is generated by 1–3 Dδ segments in tandem, by imprecise V-D and D–J joining, by exonuclease nibbling of the joining ends, and by the incorporation of random N nucleotide additions [25].
DISCUSSION
The rapid recall immune responses of Vγ2Vδ2+ T cells during BCG coinfection and antiretroviral treatment probably represent a restored ability of these cells to undergo clonal expansion rather than a redistribution of these cells from lymphoid tissues into circulation. The recall expansion of Vγ2Vδ2+ T cells after BCG reinfection usually occurs within 5–7 days and can last for months after an intravenous inoculation of normal SIV-negative macaques with large BCG inocula [3, 8]. The major expansion of BCG-specific Vγ2Vδ2+ T cells during active BCG coinfection and antiretroviral treatment may differ in nature and magnitude from the early increase in memory CD4+ T cells after receipt of HAART by HIV-1–infected humans with no coinfections [26].
The restoration of Vγ2Vδ2+ T cell expansions after active BCG coinfection and antiretroviral treatment is likely a result of the restored T helper (Th) function of BCG-stimulated CD4+ T cells. In vitro studies have shown that CD4+ Th function is required for Vγ2Vδ2+ T cells to proliferate and expand in response to phosphoantigen stimulation [6, 15–18]. In the present in vivo study, we have observed that increases in CD4+ T cell counts after active BCG coinfection and antiretroviral treatment correlate temporally with the rapid restoration of Vγ2Vδ2+ T cell expansions. In addition, potent responses of PPD-specific IFN-γ–producing CD4+ T cells go hand in hand with the restored expansion of Vγ2Vδ2+ T cells. These findings suggest that mycobacterium-specific CD4+ T cells with IFN-γ–producing effector function play an important role in the restoration of Vγ2Vδ2+ T cell expansions. In fact, studies in humans and animals have demonstrated that the BCG organism itself and PPD are potent stimulators of CD4+ T cells [23, 27]. An expansion of mycobacteria-specific CD4+ T cells may result in the production of various cytokines required for clonal expansion of Vγ2Vδ2+ T cells. Given that human interleukin (IL)–2 and IL-15 have been shown to facilitate the in vitro proliferation of Vγ2Vδ2+ T cells [28, 29], these cytokines and other T cell–growth cytokines may expand Vγ2Vδ2+ T cells during antiretroviral treatment in SIVmac/BCG-coinfected macaques. However, in SIVmac-infected macaques not treated with antiretrovirals, such a potent stimulation and activation of CD4+ T cells has a detrimental effect on Th cells themselves. BCG-mediated activation of CD4+ T cells enhances SIV replication and destruction of CD4+ T cells [23]. As a result, SIVmac/BCG-coinfection in untreated macaques induces a profound depletion and suppression of CD4+ T cells [21]. Such a BCG-induced exacerbation of CD4+ T cell deficiency in the absence of antiretroviral treatment can certainly compromise the development of adaptive Vγ2Vδ2+ T cell responses in SIVmac/BCG-coinfected macaques. Effective antiretroviral treatment in SIVmac/BCG-coinfected macaques is likely to provide an immune competent environment in which activated and expanded CD4+ T cells are protected from SIV-mediated destruction or depletion. Therefore, the immune competence of mycobacteria-specific CD4+ T cells during antiretroviral treatment may facilitate the development of adaptive Vγ2Vδ2+ T cell responses in SIVmac/BCG-coinfected macaques.
Improved immune function after antiretroviral treatment in SIVmac/BCG-coinfected macaques is able to restore a broad repertoire of Vγ2Vδ2+ T cells in response to active BCG coinfection. Earlier studies in HIV-1–infected humans and SIV-infected macaques have demonstrated that Vγ2Vδ2+ T cells, including those γδ cells with CD45−CD27− phenotypes, are particularly susceptible to deletion and functional impairment or anergy during virus infection [6, 9, 16, 19, 24]. It is not known whether immune reconstitution after receipt of HAART by HIV-1–infected humans can restore a broad or incomplete repertoire of Vγ2Vδ2+ T cells. The extent of the restored repertoire of Vγ2Vδ2+ T cells after antiretroviral treatment of HIV-infected individuals can readily be determined at the time an expansion of mycobacterium-specific Vγ2Vδ2+ T cells is observed during active mycobacterial coinfection. Our experiments with SIVmac/BCG-coinfected macaques suggest that immune reconstitution by antiretroviral treatment can facilitate the polyclonal expansion of Vγ2Vδ2+ T cells from the existing repertoire of HIV-infected individuals. Interestingly, detectable Vδ2 clonotypes identified during a restored expansion of Vγ2Vδ2+ T cells were different from those seen at weeks 0 and 2 after BCG reinfection. The Vδ2 clonotypes also differed from the dominant clones detected during their primary expansion. These observations suggest that this γδ T cell subpopulation has a large repertoire.
The restored immune responses of Vγ2Vδ2+ T cells in SIVmac/BCG-coinfected macaques may contribute to immune protection against SIVmac-related tuberculosis-like disease. We have recently demonstrated that, in BCG-vaccinated macaques, a rapid recall expansion of Vγ2Vδ2+ T cells in the lungs after M. tuberculosis aerosol challenge is associated with declines in M. tuberculosis burden and immune protection against a fatal form of tuberculosis [3]. Expanded populations of CD4+ T cells likely restore effective immunity against a tuberculosis-like disease in SIVmac/BCG-coinfected macaques. However, the rapidly expanded Vγ2Vδ2+ T cell subpopulations may act in concert with CD4+ T cells or other immune cells and produce various anti-mycobacterial cytokines, including IFN-γ and tumor necrosis factor–α [30]. Macaque Vγ2Vδ2+ T cells, like their human counterparts, produce the anti-mycobacterial molecule granulysin (data not shown). Recent in vitro studies of human cells have shown that granulysin can kill M. tuberculosis organisms [31] and that Vγ2Vδ2+ T cells can have bactericidal effects on intracellular M. tuberculosis [1, 32]. Direct or indirect antimicrobial function of Vγ2Vδ2+ T cells may help to reduce BCG loads and influence the outcome of BCG coinfection in SIVmac/BCG-coinfected macaques treated with antiretrovirals. Thus, Vγ2Vδ2+ T cell subpopulations that expand during antiretroviral treatment may contribute to protective immunity against AIDS-related mycobacterial coinfections.
Acknowledgment
We thank other members at the Chen Lab, for technical support.
Financial support: National Institutes of Health (grants RO1-RR13601 and RO1-HL64560, both to Z.W.C.).
References
- 1.Dieli F, Troye-Blomberg M, Ivanyi J, et al. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vγ9/Vδ2 T lymphocytes. J Infect Dis. 2001;184:1082–1085. doi: 10.1086/323600. [DOI] [PubMed] [Google Scholar]
- 2.Chen ZW, Letvin NL. Adaptive immune response of Vγ2Vδ2 T cells: a new paradigm. Trends Immunol. 2003;24:213–219. doi: 10.1016/s1471-4906(03)00032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen Y, Zhou D, Qiu L, et al. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, Kamath A, Das H, Li L, Bukowski JF. Antibacterial effect of human Vγ2Vδ2 T cells in vivo. J Clin Invest. 2001;108:1349–1357. doi: 10.1172/JCI13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worku S, Hoft DF. Differential effects of control and antigen-specific T cells on intracellular mycobacterial growth. Infect Immun. 2003;71:1763–1773. doi: 10.1128/IAI.71.4.1763-1773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gougeon ML, Poccia F, Boullier S. Human γδ T lymphocytes in HIV disease: effector functions and control by natural killer cell receptors. Springer Semin Immunopathol. 2000;22:251–263. doi: 10.1007/s002810000046. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Das H, Kamath A, Bukowski JF. Human Vγ2Vδ2 T cells produce IFN-γ and TNF-α with an on/off/on cycling pattern in response to live bacterial products. J Immunol. 2001;167:6195–6201. doi: 10.4049/jimmunol.167.11.6195. [DOI] [PubMed] [Google Scholar]
- 8.Lai X, Shen Y, Zhou D, et al. Immune biology of macaque lymphocyte populations during mycobacterial infection. Clin Exp Immunol. 2003;133:182–192. doi: 10.1046/j.1365-2249.2003.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enders PJ, Yin C, Martini F, et al. HIV-mediated γδ T cell depletion is specific for Vγ2+ cells expressing the Jγ1.2 segment. AIDS Res Hum Retroviruses. 2003;19:21–29. doi: 10.1089/08892220360473934. [DOI] [PubMed] [Google Scholar]
- 10.Agrati C, D’Offizi G, Narciso P, et al. γδ T cell activation by chronic HIV infection may contribute to intrahepatic vδ1 compartmentalization and hepatitis C virus disease progression independent of highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2001;17:1357–1363. doi: 10.1089/08892220152596614. [DOI] [PubMed] [Google Scholar]
- 11.Autran B, Triebel F, Katlama C, Rozenbaum W, Hercend T, Debre P. T cell receptor γ/δ+ lymphocyte subsets during HIV infection. Clin Exp Immunol. 1989;75:206–210. [PMC free article] [PubMed] [Google Scholar]
- 12.Boullier S, Cochet M, Poccia F, Gougeon ML. CDR3-independent γδ Vδ1+ T cell expansion in the peripheral blood of HIV-infected persons. J Immunol. 1995;154:1418–1431. [PubMed] [Google Scholar]
- 13.de Paoli P, Gennari D, Martelli P, et al. A subset of γδ lymphocytes is increased during HIV-1 infection. Clin Exp Immunol. 1991;83:187–191. doi: 10.1111/j.1365-2249.1991.tb05612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gioia C, Agrati C, Casetti R, et al. Lack of CD27−CD45RA−Vγ9Vδ2+ T cell effectors in immunocompromised hosts and during active pulmonary tuberculosis. J Immunol. 2002;168:1484–1489. doi: 10.4049/jimmunol.168.3.1484. [DOI] [PubMed] [Google Scholar]
- 15.Poccia F, Boullier S, Lecoeur H, et al. Peripheral Vγ9/Vδ2 T cell deletion and anergy to nonpeptidic mycobacterial antigens in asymptomatic HIV-1–infected persons. J Immunol. 1996;157:449–461. [PubMed] [Google Scholar]
- 16.Martini F, Poccia F, Goletti D, et al. Acute human immunodeficiency virus replication causes a rapid and persistent impairment of Vγ9Vδ2 T cells in chronically infected patients undergoing structured treatment interruption. J Infect Dis. 2002;186:847–850. doi: 10.1086/342410. [DOI] [PubMed] [Google Scholar]
- 17.Martini F, Urso R, Gioia C, et al. γδ T-cell anergy in human immunodeficiency virus–infected persons with opportunistic infections and recovery after highly active antiretroviral therapy. Immunology. 2000;100:481–486. doi: 10.1046/j.1365-2567.2000.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wesch D, Kabelitz D, Friese K, Pechhold K. Mycobacteria-reactive γδ T cells in HIV-infected individuals: lack of Vγ9 cell responsiveness is due to deficiency of antigen-specific CD4 T helper type 1 cells. Eur J Immunol. 1996;26:557–562. doi: 10.1002/eji.1830260309. [DOI] [PubMed] [Google Scholar]
- 19.Zhou D, Lai X, Shen Y, et al. Inhibition of adaptive Vγ2Vδ2+ T-cell responses during active mycobacterial coinfection of simian immunodeficiency virus SIVmac–infected monkeys. J Virol. 2003;77:2998–3006. doi: 10.1128/JVI.77.5.2998-3006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Institute for Laboratory Animal Research, National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 21.Shen Y, Zhou D, Chalifoux L, et al. Induction of an AIDS virus–related tuberculosis-like disease in macaques: a model of simian immunodeficiency virus–Mycobacterium coinfection. Infect Immun. 2002;70:869–877. doi: 10.1128/IAI.70.2.869-877.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen Y, Shen L, Sehgal P, et al. Antiretroviral agents restore Mycobacterium-specific T-cell immune responses and facilitate controlling a fatal tuberculosis-like disease in macaques coinfected with simian immunodeficiency virus and Mycobacterium bovis BCG. J Virol. 2001;75:8690–8696. doi: 10.1128/JVI.75.18.8690-8696.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou D, Shen Y, Chalifoux L, et al. Mycobacterium bovis bacille Calmette-Guerin enhances pathogenicity of simian immunodeficiency virus infection and accelerates progression to AIDS in macaques: a role of persistent T cell activation in AIDS pathogenesis. J Immunol. 1999;162:2204–2216. [PubMed] [Google Scholar]
- 24.Chen ZW, Shen Y, Davis IC, Shen L, Letvin NL, Fultz PN. Downregulation of macaque γδ+ T cells in lymphoid compartments after rectal infection with SIVsmmPBj14. J Med Primatol. 2000;29:143–147. doi: 10.1034/j.1600-0684.2000.290307.x. [DOI] [PubMed] [Google Scholar]
- 25.Hochstenbach F, Brenner MB. Newly identified γδ and βδ T-cell receptors. J Clin Immunol. 1990;10:1–4. doi: 10.1007/BF00917493. [DOI] [PubMed] [Google Scholar]
- 26.Yeni PG, Hammer SM, Carpenter CC, et al. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society–USA panel. JAMA. 2002;288:222–235. doi: 10.1001/jama.288.2.222. [DOI] [PubMed] [Google Scholar]
- 27.Ravn P, Boesen H, Pedersen BK, Andersen P. Human T cell responses induced by vaccination with Mycobacterium bovis bacillus Calmette-Guerin. J Immunol. 1997;158:1949–1955. [PubMed] [Google Scholar]
- 28.Boullier S, Poquet Y, Debord T, Fournie JJ, Gougeon ML. Regulation by cytokines (IL-12, IL-15, IL-4 and IL-10) of the Vγ9Vδ2 T cell response to mycobacterial phosphoantigens in responder and anergic HIV-infected persons. Eur J Immunol. 1999;29:90–99. doi: 10.1002/(SICI)1521-4141(199901)29:01<90::AID-IMMU90>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Garcia VE, Jullien D, Song M, et al. IL-15 enhances the response of human γδ T cells to nonpeptide [correction of nonpetide] microbial antigens. J Immunol. 1998;160:4322–4329. [PubMed] [Google Scholar]
- 30.Kaufmann SH. Protection against tuberculosis: cytokines, T cells, and macrophages. Ann Rheum Dis. 2002;61 Suppl 2:ii54–ii58. doi: 10.1136/ard.61.suppl_2.ii54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stenger S, Hanson DA, Teitelbaum R, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 32.Passmore JS, Glashoff RH, Lukey PT, Ress SR. Granule-dependent cytolysis of Mycobacterium tuberculosis–infected macrophages by human γδ+ T cells has no effect on intracellular mycobacterial viability. Clin Exp Immunol. 2001;126:76–83. doi: 10.1046/j.1365-2249.2001.01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]