SUMMARY
Human metapneumovirus (hMPV) is a recently discovered pathogen causing a significant portion of respiratory infections in young infants, the elderly and immunocompromised patients. Very little is known regarding the cellular signaling elicited by this virus in airway epithelial cells, the target of hMPV infection. In this study, we investigated the role of the RNA helicases RIG-I (retinoic acid inducible gene-I) and MDA-5 (melanoma differentiation-associated gene-5) as the main pattern recognition receptors (PRRs) involved in viral detection and subsequent expression of proinflammatory and antiviral genes. HMPV infection readily induced RIG-I and MDA-5 gene and protein expression in A549 cells, a type II-like alveolar epithelial cell line. Expression of dominant negative (DN) RIG-I or downregulation of RIG-I gene expression using small interfering (si)RNA significantly decreased hMPV-induced Interferon (IFN)-β, Interleukin (IL)-8, and RANTES gene transcription, by inhibiting viral-induced activation of Nuclear Factor (NF)-kB and Interferon Regulatory Factor (IRF), leading to enhanced viral replication. On the other hand, MDA-5 did not seem to play a significant role in hMPV-induced cellular responses. MAVS (mitochondrial antiviral signaling protein), an adaptor protein linking both RIG-I and MDA-5 to downstream activation of IRF-3 and NF-kB, was also necessary for hMPV-induced cellular signaling. Expression of a MAVS DN significantly reduced IFN-β and chemokine gene transcription, by inhibiting NF-kB and IRF-dependent gene transcription, in response to hMPV infection. Our results show that hMPV activates the RIG-I-MAVS signaling pathway in airway epithelial cells, leading to the expression of important proinflammatory and antiviral molecules involved in the innate immune response to viruses.
INTRODUCTION
Human metapneumovirus (hMPV) is a recently identified respiratory RNA virus belonging to the Paramyxoviridae family. HMPV is responsible for a significant portion of upper and lower respiratory tract infections in young children, the elderly and immunocompromised patients, causing bronchiolitis, croup, asthma exacerbation, and even pneumonia (Boivin et al., 2002;Esper et al., 2003;Williams et al., 2004), second only to respiratory syncytial virus (RSV) (Kahn, 2006;Principi et al., 2006;Williams et al., 2004), also a paramyxovirus. While there is an emerging literature on the clinical and epidemiological features of hMPV infection, very little is known regarding the cellular signaling activated by this pathogen in infected cells. We have recently demonstrated that hMPV is a potent stimulus for cytokine, chemokine and type I IFN production in cultured human alveolar epithelial cells, and that hMPV-induced chemokine expression is viral replication-dependent (Bao et al., 2007a). We have also shown that hMPV infection induces activation of NF-κB and IRF transcription factors (Bao et al., 2007a), which have been shown to play a fundamental role in controlling the expression of chemokines following paramyxoviral infections (Casola et al., 2001;Garofalo et al., 1996;Groskreutz et al., 2006;Spann et al., 2005). Toll-like receptors (TLRs) and RNA helicases are the pattern recognition receptors (PRRs) most commonly activated by viral infections, leading to signal cascades that regulates the expression of proinflammatory and immune mediators (Akira & Takeda, 2004;Maniatis et al., 1998). The TLRs, located in the endosomal cellular compartment, operate mainly in plasmacytoid dendritic cells, while two DExD/H box RNA helicases, RIG-I and MDA-5 have been identified to be essential for IFN induction by several viruses including Newcastle (NDV), Sendai (SeV) and hepatitis C (HCV) (Andrejeva et al., 2004;Breiman et al., 2005;tenOever et al., 2004). Both RIG-I and MDA-5 share two homologous CARD domains and a helicase domain that is required for their interaction with viral RNA (Andrejeva et al., 2004;Pichlmair et al., 2006). It has been recently shown that RIG-I is responsible for sensing any viral RNA bearing 5′-triphosphate, while MDA-5 functions as a dsRNA sensor (Hornung et al., 2006;Pichlmair et al., 2006). The CARD domain of RNA helicases mediate its interaction with the CARD domain of the adaptor molecule IPS-1/MAVS/VISA/Cardif leading to subsequent activation of important transcription factors, such as IRFs and NF-κB.
In the present study, we show that hMPV infection readily activated the expression of RIG-I and MDA-5 in human A549 cells, an alveolar type II like cell line. Overexpression of dominant negative mutant RIG-I and gene suppression by small interfering RNA (siRNA) lead to reduction in the expression of type I IFN, as well cytokines and chemokines, indicating a critical role for RIG-I in hMPV-induced cellular signaling. RIG-I gene silencing significantly inhibited hMPV-induced IRF and NF-κB activation, indicating that RIG-I play a major role in regulating the induction of these important transcription factors in response to hMPV infection. Expression of a MAVS protein lacking the N-terminal CARD domain, which acts as a dominant negative mutant, significantly decreased viral-induced IRF- and NF-κB-dependent gene transcription, including IFN-β and chemokine genes, suggesting a critical role of MAVS in hMPV-induced signaling pathways as well. A detailed understanding of the mechanism by which this viral infection triggers the innate immune response is helpful for future designing of novel therapeutic interventions and effective vaccination strategies.
MATERIALS & METHODS
Viral preparation
LLC-MK2 cells (ATCC, Manassas, VA) were maintained in MEM (Invitrogen GIBCO, Carlsbad, CA) supplemented with 10 % fetal bovine serum and Penicillin and Streptomycin (100U/ml). The hMPV strain CAN97-83 was obtained from Guy Boivin at the Research Center in Infectious Diseases, Regional Virology Laboratory, Laval University, Quebec, Canada. Virus was propagated in LLC-MK2 cells at 35°C in the absence of serum and in the presence of 1 μg of trypsin/ml (Worthington, Lakewood, NJ), and was sucrose purified, as previously described (Guerrero-Plata et al., 2005a). Viral titer was determined by immunostaining in LLC-MK2 cells, as previously described (Guerrero-Plata et al., 2006). LPS, assayed using the limulus hemocyanic agglutination assay, was not detected. Virus pools were aliquot, quick frozen on dry ice/alcohol, and stored at −70 °C until used.
Cell culture and infection with hMPV
A549 cells, human alveolar type II like epithelial cells, 293 cells, a human embryonic kidney epithelial cell line, and Vero cells, an African green monkey kidney cell line (all from American Type Culture Collection, Manassas, VA) were maintained in F-12K or MEM medium, containing 10% (vol/vol) fetal bovine serum, 10 mM glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Cell monolayers were infected with hMPV at multiplicity of infection (MOI) of 3 (unless otherwise stated), as described (Garofalo et al., 1996). An equivalent amount of 30% sucrose was added to uninfected A549 cells, as control.
RNA interference
Transfections of siRNA into A549 cells were carried out at a final concentration of 100 nM, targeting RIG-I, MDA-5, TLR3 or a scrambled negative control (Dharmacon, Lafayette, CO), using A549 transfection reagent (Altogen Biosystems, Las Vegas, NV) according to the manufacturer’s recommendations. 48 to 72 hours later, A549 cells were mock infected or infected with hMPV for 18 h at a MOI of 3.
RNA extraction and Real Time PCR
Total RNA was extracted from control and infected A549 cells by Rneasy kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Gene mRNAs were amplified by Quantitative Real Time PCR (Q-RT-PCR) using Applied Biosystems assays-on-demand 20 X mix of primers and TaqMan® MGB probes (FAM™-dye labeled) for target genes, and18S rRNA (VIC™–dye labeled probe) TaqMan® assay reagent (P/N 4319413E) for control. Separate tubes (singleplex) one-step RT-PCR was performed with 80 ng RNA for both target genes and endogenous control. The cycling parameters for one-step RT-PCR were: reverse transcription 48°C for 30 min, AmpliTaq activation 95°C for 10 min, denaturation 95°C for 15 sec and annealing/extension 60°C for 1 min (repeat 30 times) on ABI7000. Duplicate CT values were analyzed in Microsoft Excel using the comparative CT (CT) method as described by the manufacturer (Applied Biosystems). The amount of target (2−ΔΔCT) was obtained by normalizing to endogenous reference (18S) sample.
Plasmid preparation and transfections
Plasmids containing human IFN-β, RANTES, and IL-8 promoters, as well as multimers of the RANTES ISRE site or IL-8 NF-kB site, linked to the luciferase reporter gene have been previously described (Casola et al., 2000;Casola et al., 2001;Casola et al., 2002). Dominant negative (DN) RIG-I plasmids and full-length MAVS plasmid were previously described (Foy et al., 2005). Flag-tagged dominant negative MAVS in pEFTaK vector was created by deletion of the CARD domain, using full-length MAVS as a template. The primers used to generate CARD-deleted MAVS were: forward, 5′-AGCGGCCGCTCCGTTTGCTGAAGACAAGACCTATAAGTATTGTGAGCTAGTTGATCTCGCGGACGAAGTGGCC-3′, and reverse, 5′-TGTTCGAATGGGTGACCTAGTGCA-3′.
The resulting PCR product was initially cloned into TOPO cloning vector (Invitrogen, Carlsbad, CA) and then subcloned into pEFTaK vector using NotI and BstBI restriction enzymes. DNA sequencing of the constructs was performed before use. Logarithmically growing A549 cells or 293 cells were transfected in triplicate in 60 mm dishes using FuGene 6 (Roche, Indianapolis, Ind.). One ug of the reporter gene plasmid, and 0.2 ug of either empty vector plasmid, or DN expression plasmid (RIG-I or MAVS) were premixed with FuGene 6 in a 1:3 ratio (ug/ul), and added to the cells in 3 ml of regular medium. After 30 hours of transfection, cells were infected with hMPV for 15 or 24 hours. Cells were then lysed to measure independently luciferase and β-galactosidase reporter activity, as previously described (Casola et al., 2000). Luciferase was normalized to the internal control β-galactosidase activity. All experiments were performed at least two to three times.
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts from control and infected A549 cells were prepared using hypotonic/nonionic detergent lysis, as previously described (Bao et al., 2007a). Protein were normalized by protein assay (Protein Reagent, Bio-Rad, Hercules, CA) and used to bind to duplex oligonucleotides corresponding to either the RANTES ISRE, IL-8 NF-κB binding site or an Oct consensus site (Panomics), as previously described (Casola et al., 2000;Casola et al., 2001). Ten micrograms of nuclear proteins were incubated with the radiolabeled probe for 15 min at room temperature and then fractionated by 4% nondenaturing PAGE in 0.5x TBE buffer (22 mM Tris-HCl, 22 mM boric acid, 0.25 mM EDTA, pH 8) at 120 volts. After electrophoretic separation, gels were dried and exposed for autoradiography with Kodak XAR film at −70 °C using intensifying screens. Quantification of the band intensity was done by gel exposure to PhosphoImager and analysis by ImageQuant software (Molecular Dynamics).
Western blotting
Total cell lysates were prepared by adding ice-cold lysis buffer (50 mM Tric-HCl, pH 7.4, 150 mM NaCl, 1mM EGTA, 0.25% sodium deoxycholate, 1 mM Na3VO4, 1 mM NaF, 1% Triton X-100 and 1 μg/ml of aprotinin, leupeptin and pepstatin). After incubation on ice for 10 min, the lysates were collected and detergent insoluble materials were removed by centrifugation at 4o C at 14,000 g. Proteins (30 g per sample) were then boiled in 3X Laemmli buffer for 2 min and resolved on SDS-PAGE. Proteins were transferred onto Hybond-ECL nitrocellulose membrane (Amersham Pharmacia Biotech) and nonspecific binding sites were blocked by immersing the membrane in TBST blocking solution [10mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.05% Tween-20 (v/v)] containing 5% skim milk powder for 60 minutes. After a short wash in TBST, the membranes were incubated with the primary antibody overnight at 4oC (RIG-I from ABGENT and MDA-5 from IMGENEX), followed by an anti-rabbit or anti-goat peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology) diluted in TBST for 60 min at room temperature. After washing, the proteins were detected using ECL (Amersham Pharmacia Biotech) according to manufacturer’s protocol.
Statistical analysis
A two tailed student’s t-test using a 95% confidence levels were performed on all experiments. Significance is designated as follows: * p<0.05
RESULTS
Role of RNA helicases in hMPV-induced gene expression in airway epithelial cells
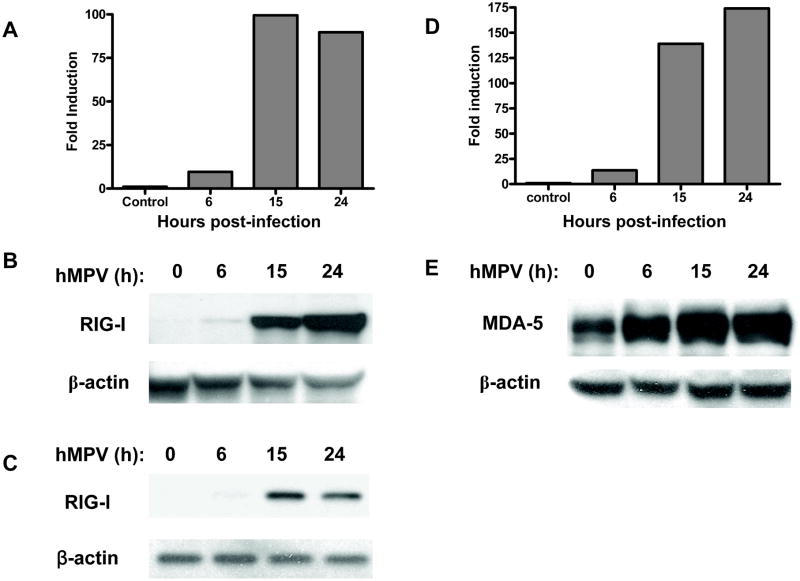
The RNA helicase, RIG-I, has been shown to recognize several RNA virus, including Newcastle disease virus (NDV), Sendai virus (SeV), vesicular stomatitis virus (VSV), hepatitis C, Japanese encephalitis virus, hepatitis C virus and respiratory syncytial virus (RSV) (Chang et al., 2006;Foy et al., 2005;Kato et al., 2005;Liu et al., 2007). On the other hand, MDA-5 has been shown to be essential in detecting picornaviruses (Kato et al., 2006) and to be the target of IFN inhibitory activity of paramyxovirus V protein (Andrejeva et al., 2004;Childs et al., 2007;Kawai et al., 2005). The role of RIG-I and MDA-5 in detecting hMPV infection and initiating cellular signaling is currently unknown. To investigate whether hMPV infection of A549 cells induced RIG-I and MDA-5 expression, total RNA was extracted from cells uninfected and infected for various length of time, and used to amplify RIG-I and MDA-5 gene by Q-RT-PCR. As shown in Fig. 1A and D, hMPV infection caused a marked increase in the expression of both genes, starting around 6 h post-infection (p.i.) and peaking between 15 and 24 h p.i. To determine whether RIG-I and MDA-5 change in mRNA levels were paralleled by changes in protein synthesis, we performed Western blot analysis of total cell lysates prepared from A549 cells uninfected or infected for various lengths of time. As shown in Fig. 1B and E, hMPV-induced RIG-I and MDA-5 protein induction followed a kinetic similar to the one seen for gene expression, with up-regulation occurring around 6 p.i. and progressive increase up to 24 h p.i.
Fig. 1. hMPV-induced RIG-I and MDA-5 gene and protein expression in A549 cells.
Total RNA was extracted from uninfected or infected A549 cells at 6, 15, and 24 h p.i. and used for Q-RT-PCR to determine changes in RIG-I (A) and MDA-5 (D) expression at different time points as indicated. Total cell lysates were prepared from uninfected or infected cells at various times p.i. Equivalent amounts of proteins were subjected to 8% SDS-PAGE, followed by Western blot analysis of RIG-I (B and C) and MDA-5 (E) expression. Membranes were stripped and reprobed with anti-β-actin antibody to control for equal loading of the samples. Data are representative of three separate experiments.
Since RIG-I is an interferon-inducible gene (Imaizumi et al., 2004), we asked the question whether its induction following hMPV infection was directly due to the virus or was dependent on the autocrine/paracrine induction of type I interferon (IFN). To do so, we investigated RIG-I expression in Vero cells, which lack type I IFN production. Western blot analysis of total cell lysates prepared from Vero cells uninfected or infected for various lengths of time showed that hMPV infection was able to induce RIG-I expression with a kinetics similar to the one observed in airway epithelial cells, starting at 6 h p.i., however the magnitude of induction was less in Vero cells and there was a decline in RIG-I expression at 24 h p.i. (Fig. 1C), indicating that the hMPV directly induces RIG-I expression and that type I IFN enhances it, likely through an autocrine/paracrine pathway.
We had previously shown that A549 cells secrete a variety of cytokines and chemokines, as well as type I IFNs, upon hMPV infection (Bao et al., 2007a). To investigate the functional significance of RIG-I in initiating cellular signaling leading to the expression of these important immune mediators, we investigated the effect of expressing a CARD-deleted mutant RIG-I, which acts as a dominant negative mutant by preventing interaction with the adaptor molecule MAVS (Liu et al., 2007), on hMPV-induced IFN-β, RANTES and IL-8 gene transcription. A549 cells were cotransfected with a construct containing either IFN-β, RANTES or IL-8 gene promoter, linked to the luciferase reporter gene, and an expression plasmid containing RIG-I DN or the corresponding empty vector. Expression of the dominant negative mutant RIG-I significantly reduced hMPV-induced luciferase activity of the IFN-β, RANTES and IL-8 gene promoter constructs by approximately 60%, 70%, and 55%, respectively (Fig. 2), indicating an important role of RIG-I in viral-induced gene transcription.
Fig. 2. Inhibition of hMPV-induced interferon-β and chemokines gene transcription by expression of dominant negative RIG-I.
A549 cells were cotransfected with a luciferase reporter plasmid containing INF-β (A), RANTES (B) or IL-8 (C) promoter together with a RIG-I dominant negative mutant expression plasmid (RIG DN), or the corresponding empty vector (EV). Cells were infected with hMPV and harvested at 15 or 24 h p.i to measure luciferase activity. Uninfected plates served as controls. For each plate luciferase was normalized to the β-galactosidase reporter activity. Data are expressed as mean ± standard deviation of normalized luciferase activity. Bars represent the mean value of triplicate samples of one experiment, n = at least two experiments. *, P< 0.05 relative to hMPV-infected EV-treated cells.
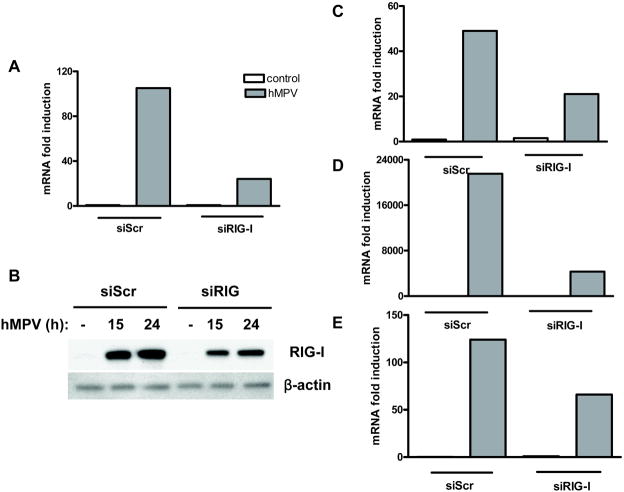
To confirm the role of RIG-I in hMPV-induced cellular signaling, we investigated cytokine, chemokine and IFN-β gene expression in A549 cells transfected with either a scramble siRNA, as control, or one targeting RIG-I and infected with hMPV. Our results show that treatment of A549 cells with siRNA targeting RIG-I effectively blocked RIG-I gene expression (~80%) and protein induction (~40 to 50%), as well as INF-β, RANTES, and IL-8 gene expression in response to hMPV infection by ~60%, 80% and 50%, respectively (Fig. 3).
Fig. 3. Effect of RIG-I gene silencing on hMPV-induced interferon-β and chemokine gene expression.
A549 cells were transfected with 100 nM siRNA targeting RIG-I (siRIG) or a scrambled negative control (siScr). A549 cells were mock infected or infected with hMPV for 18 hours at a multiplicity of infection (MOI) of 3 and harvested to prepare either total RNA or total cell lysates. Total RNA was harvested for analysis of RIG-I (A), IFN-β (C), RANTES (D), and IL-8 (E) specific gene expression by Q-RT-PCR. Total cell lysates were subjected to 8% SDS-PAGE, followed by Western blot analysis of RIG-I (B) expression. Membrane was stripped and reprobed with anti-β-actin antibody to control for equal loading of the samples. Results are representative of three separate experiments.
To determine the impact of RIG-I inhibition on other immune mediator production, supernatants of A549 cells transfected with either the scramble or RIG-I siRNA and infected with hMPV were assayed for cytokine and chemokine secretion by Bio-Plex. There was significant reduced secretion of cytokines, such as IL-6, and other chemokines, such as MIP-1α, -β- and MCP-1, in cells where RIG-I expression was silenced, compared to control cells, in response to hMPV infection, as shown in Table I.
Table I.
Effect of RIG-I silencing on hMPV-induced chemokines and cytokines secretiona
| Gene symbol | SiScr 15 h | SiScr 24 h | SiScr 48 h | SiRIG 15 h | SiRIG 24 h | SiRIG 48 h |
|---|---|---|---|---|---|---|
| IP-10 | 175 | 574 | 2885 | 114* | 358* | 927* |
| MCP-1 | 301 | 3485 | 1474 | 240 | 2273* | 1118* |
| MIP-1α | 1.38 | 14.45 | 53 | 1.51 | 12 | 39* |
| MIP-1β | 3 | 24 | 70 | 2 | 21 | 47* |
| IL-6 | 117 | 263 | 2726 | 127 | 276 | 1730* |
Supernatants from hMPV-infected cells, treated with scramble (siScr) or RIG-I (siRIG) siRNA, were harvested at the indicated time points of infection. Chemokine and cytokine secretion was measured by Bio-Plex assay. Values are expressed as picograms/ml. Results are representative of three separate experiments.
P< 0.05, relative to siScr-treated cells.
The reduction of type I IFN production, and likely IFN-dependent antiviral gene expression, resulted in enhanced viral replication, as shown by increased viral titers in cells infected with hMPV and transfected with RIG-I siRNA, compared to cells transfected with the scramble siRNA, as shown in Table II.
Table II.
Effect of RIG-I silencing on hMPV replicationa
| siRNA | 15 h | 24 h | 48 h |
|---|---|---|---|
| siScr | 1.5×105 | 3.6×105 | 8.1×105 |
| siRIG-I | 2.7×105 | 1×106 | 2.5×106 |
A549 cells were transfected with scramble (siScr) or RIG-I (siRIG) siRNA, infected with hMPV, MOI of 1, and harvested at the indicated time points to determine viral titers. Values are expressed as pfu/ml and represent the average of two independent experiments.
To investigate the role of MDA-5 in hMPV-induced cellular signaling, similar experiments as described above were performed in A549 cells transfected with specific siRNA targeting MDA-5. Our results show that effective silencing of MDA-5 gene expression (~80%) did not lead to a significant reduction of hMPV-induced cytokine or chemokine production or changes in viral replication (data not shown), indicating that MDA-5 does not play a major role in initiating cellular responses following hMPV infection.
Since inhibition of RIG-I expression did not completely abolished hMPV-induced cytokine and chemokine production and since we have recently showed that Toll-like receptor (TLR)-3 plays a role in cellular signaling in response to another paramyxovirus infection (Liu et al., 2007), we investigated whether it was involved in hMPV-induced signaling too. A549 cells were transfected with specific siRNA targeting TLR-3 or scrambled siRNA, infected with hMPV for 18 h and harvested to measure TLR-3 and chemokine gene expression. Our results show that effective silencing of TLR-3 gene expression (~80–90%) did not lead to a significant inhibition of hMPV-induced chemokine induction (data not shown), indicating that TLR-3 does not play a major role in initiating cellular responses following hMPV infection.
RIG-I is required for hMPV-induced IRF and NF-κB activation
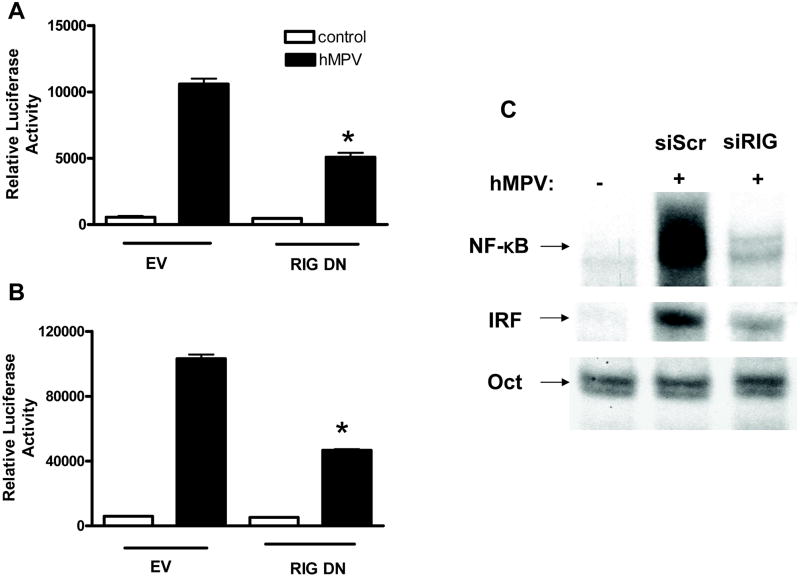
Transcription factors of the IRF family have been shown to play an essential role in viral-induced expression of type I IFN genes (reviewed in (Barnes et al., 2002)). They also regulate the induction of several other genes involved in the immune/inflammatory response to viral infections, including the chemokine RANTES [Reviewed in (Barnes et al., 2002)]. Similarly, a number of paramyxovirus-inducible inflammatory and immunoregulatory genes require NF-κB for their transcription, as we have shown in vitro for IL-8 (Garofalo et al., 1996), RANTES (Casola et al., 2001), as well as other chemokines and cytokines, secreted proteins and signaling molecules (Tian B et al., 2002). To determine whether RIG-I inhibition specifically affected hMPV-induced NF-κB- and IRF-dependent gene transcription, A549 cells were transiently cotransfected with a construct containing multiple copies of either the IL-8 NF-κB site (Casola et al., 2000) or the RANTES ISRE site (Casola et al., 2002) binding IRF proteins (Casola et al., 2001), linked to the luciferase reporter gene, and the RIG-I DN expression plasmid or the empty vector. Expression of the dominant negative mutant RIG-I significantly reduced both hMPV-induced IRF- and NF-κB- dependent gene transcription by ~55 to 60%, as shown in Fig. 4A and B, respectively, confirming the role of RIG-I as a central molecule initiating cellular signaling in response to hMPV infection.
Fig. 4. Effect of RIG-I inhibition on hMPV-induced NF-κB and IRF activation.
A549 cells or 293 cells were cotransfected with a luciferase reporter plasmid containing multimers of the RANTES ISRE site (A) or IL-8 NF-κB site (B) together with a RIG-I DN expression plasmid or an empty vector (EV). Cells were infected with hMPV and harvested at 15 h p.i. to measure luciferase activity. Uninfected plates served as controls. For each plate luciferase was normalized to the β-galactosidase reporter activity. Data are expressed as mean ± standard deviation of normalized luciferase activity. Bars represent the mean value of triplicate samples of one experiment, n = at least two experiments. *, P< 0.05 relative to hMPV-infected EV-treated cells. (C) A549 cells were transfected with scrambled siRNA (siSCR) or specific siRNA against RIG-I (siRIG) and mock infected or infected with hMPV for 15 h. Nuclear extracts were harvested and subjected to EMSA with 32P-labeled oligonucleotide probes corresponding to the IL-8 promoter NF-kB site, the RANTES promoter ISRE site or an Oct synthetic probe. Results are representative of two separate experiments.
NF-κB is a superfamily of ubiquitous transcription factors composed of NF-kB1 or p50, NF-kB2 or p52, Rel A or p65, Rel B and c-Rel proteins, which can form homo and heterodimers and produce complexes with various transcriptional activities. NF-κB inducing stimuli cause phosphorylation of inhibitory proteins called I Bs, through activation of IκB kinase (IKK) complex (Karin & Delhase, 2000), with subsequent IκB proteolytic degradation (Henkel et al., 1993), event that allows NF-κB to enter the nucleus and bind to target gene promoters. We have recently shown that hMPV infection of airway epithelial cells induces a time-dependent increase in p50 and p65 nuclear translocation with parallel changes in DNA binding activity (Bao et al., 2007a). To investigate the effect of RIG-I inhibition on NF-κB activation, A549 cells were transfected with either the scramble or RIG-I siRNA, infected with hMPV and harvested at 15 h p.i. to prepare nuclear extracts. Gel mobility shift assay was used to detect changes in abundance of DNA-binding proteins that recognized the NF-κB binding site of the IL-8 gene promoter, as previously described (Bao et al., 2007a). A consensus sequence for Oct proteins, whose DNA- binding do not change in response to viral infections, was used as an internal control. As shown in Fig. 4C, hMPV infection markedly increased binding to the IL-8 promoter NF-κB site, compared to uninfected cells, and RIG-I silencing significantly reduced the inducible binding of ~ 65% (quantified by PhosphoImager analysis). There was no change in Oct DNA-binding activity, demonstrating specificity of the RIG-I siRNA on NF-κB activation.
Among the different members of the IRF family, IRF-1, -3, -5 and -7 have been identified as direct transducers of viral-induced signaling, with IRF-3 being necessary for IFN-β and RANTES gene expression in response to paramyxovirus infections (Casola et al., 2001). We have recently shown that hMPV infection leads to a time- and replication-dependent activation of IRF-1, -3 and -7 in airway epithelial cells, resulting in increased nuclear translocation and binding to the RANTES promoter ISRE site (Bao et al., 2007a). Downregulation of RIG-I expression by siRNA resulted in ~50% reduction of hMPV-induced IRF binding to the RANTES ISRE site (quantified by PhosphoImager analysis), as shown in Fig. 4C. Altogether, these data confirm that RIG-I plays an important role in NF-kB and IRF activation, and subsequent gene expression, in response to hMPV infection.
MAVS is necessary for hMPV-induced cellular signaling
MAVS is a mitochondrial adaptor protein linking RIG-I and MDA-5 to downstream kinases responsible for NF-kB and IRF activation, leading to proinflammatory and antiviral gene transcription (Johnson & Gale, Jr., 2006;Seth et al., 2006;Sun et al., 2006;Werts et al., 2006). To determine whether MAVS was necessary for hMPV-induced signaling downstream of RIG-I, we investigated the effect of expressing a CARD-deleted mutant MAVS, which acts as a dominant negative mutant by preventing interaction with RIG-I, on hMPV-induced IFN-β, RANTES and IL-8 gene transcription. A549 cells were cotransfected with the IFN-β, RANTES or IL-8 promoter reporter construct and the MAVS DN expression plasmid or the empty vector. Expression of the dominant negative mutant MAVS increased the basal activity of the IL-8 promoter, however it significantly reduced hMPV-induced activity of the IFN-β, RANTES and IL-8 gene promoter constructs by approximately 60%, 66%, and 70%, respectively (Fig. 5A–C).
Fig. 5. Inhibition of hMPV-induced interferon-β and chemokines gene transcription by expression of dominant negative MAVS.
A549 or 293 cells were cotransfected with a luciferase reporter plasmid containing either the INF-β (A), RANTES (B), IL-8 (C) promoter or multimers of the RANTES ISRE (D) or IL-8 NF-κB site (E) together with a MAVS dominant negative mutant expression plasmid (MAVS DN), or the corresponding empty vector (EV). Cells were infected with hMPV and harvested by 15 or 24 h p.i to measure luciferase activity. Uninfected plates served as controls. For each plate luciferase was normalized to the β-galactosidase reporter activity. Data are expressed as mean ± standard deviation of normalized luciferase activity. Bars represent the mean value of triplicate samples of one experiment, n = at least two experiments. *, P< 0.05 relative to hMPV-infected EV-treated cells.
Similarly, cotransfection of the MAVS DN plasmid with either the IL-8 NF-κB or the RANTES ISRE reporter constructs resulted in enhanced basal activation of the NF-κB-driven promoter, but also in a significant reduction of both hMPV-induced NF-κB- and IRF-dependent luciferase activity by approximately 70% and 85%, respectively (Fig. 5D–E). Overall, these results confirm the important role of the RIG-I-MAVS pathway in initiating cellular signaling in response to hMPV infection.
DISCUSSION
Respiratory tract infections are a leading cause of morbidity and mortality worldwide. Human metapneumovirus (hMPV) is a recently identified RNA virus, belonging to the Paramixoviridae family, and a major cause of lower respiratory tract infections in children, elderly and immunocompromised patients and therefore it is currently considered a substantial public health problem (Kahn, 2006;Principi et al., 2006;Williams et al., 2004). The innate immune response represents a critical component of the host defense against viruses and is coordinated at the cellular level by activation of transcription factors that regulate the expression of inducible gene products with antiviral and/or inflammatory activity. Viruses contain conserved structural moieties, known as pathogen associated molecular pattern (PAMPs), that are recognized by several families of PRRs, in particular TLRs and RNA helicases. Their relative contribution in virus-triggered cellular signaling is stimulus- and cell-type-dependent [Reviewed in (Seth et al., 2006)].
In this study, we show for the first time that hMPV infection of airway epithelial cells induces the expression of the RNA helicases RIG-I and MDA-5 and that RIG-I, but not MDA-5, plays a fundamental role in hMPV-induced cellular signaling, as inhibition of RIG-I expression significantly decreases activation of IRF and NF-kB transcription factors and production of type I IFN and proinflammatory cytokines and chemokines. RIG-I-dependent signaling was necessary to induce a cellular antiviral state, as reduction of RIG-I expression resulted in enhanced hMPV replication. We have recently reported similar findings in airway epithelial cells infected with RSV, also a paramyxovirus and the most common cause of lower respiratory tract infections in children (Liu et al., 2007). A549 cells infected with RSV showed a rapid induction of RIG-I expression and RIG-I binding of viral RNA, and siRNA-mediated RIG-I silencing inhibited activation of both NF-kB and IRF-3 activation, as well as IFN-β and chemokine gene expression (Liu et al., 2007). Although we do not know the exact moiety that mediates RIG-I activation in the course of hMPV infection, we found that RIG-I does bind hMPV RNA in vitro (data not shown). Together with RIG-I, hMPV also induced MDA-5 expression, however we did not find a significant role of this helicase in hMPV-induced cellular signaling. Our findings are in agreement with recent studies indicating that RIG-I is the necessary trigger of innate immune defenses in response to a variety of RNA viruses including other paramyxoviruses, such as NDV and SeV, as well as the orthomyxovirus influenza A/B (Loo et al., 2008). Loo et al. showed that RSV, NDV and SeV infection of MDA-5 −/− mouse embryonic fibroblasts (MEFs) was able to induce IFN and IFN-stimulated gene (ISG) expression, which was almost completely abolished in RIG-I −/− MEFs (Loo et al., 2008). Interestingly, individual RIG-I or MDA-5 presence was not required for ISG expression in response to dengue virus or reovirus, although double knock-out cells showed significant impairment of ISG expression following infection with both viruses (Loo et al., 2008), indicating variable requirements of RIG-I and MDA-5 in initiating cellular signaling in response to different RNA viruses.
The incomplete abrogation of cellular responses by RIG-I gene silencing suggests the possible involvement of other pathways in hMPV-induced cellular signaling. While the role of TLRs remains speculative in non-immune cells, several studies have shown that TLR-3 is inducible in human epithelial cells and can play a role in regulating proinflammatory response against several viruses, including RSV (Groskreutz et al., 2006;Le et al., 2007;Liu et al., 2007;Rudd et al., 2006). We have also recently shown that hMPV infection of airway epithelial cells does induce TLR-3 expression, as well the expression of other signaling molecules related to TLR signaling, including TRIF (Bao et al., 2007b). However, inhibition of TLR-3 expression did not affect hMPV-induced chemokine gene expression, indicating that it does not play a significant role in hMPV-induced signaling in airway epithelial cells. Whether other TLRs plays a role in hMPV-dependent cellular activation needs further investigation.
MAVS is a recently identified adaptor protein which recruits RIG-I and MDA-5 to the outer membrane of mitochondria as part of a signaling complex that activates NF-κB and IRF, leading to IFN and ISG expression (Kawai et al., 2005;Seth et al., 2005). MAVS signaling is initiated by ligand-induced interaction of its N-terminal CARD domain with the CARD domain of either RIG-I or MDA-5. In our study, over-expression of CARD-deleted MAVS greatly diminished hMPV-induced IFN and chemokine promoter activation and more specifically IRF and NF-κB- dependent gene transcriptions, indicating a critical role of MAVS in hMPV-induced signaling in airway epithelial cells. Although RIG-I and MDA-5 role in viral recognition and innate immune signaling appears to be different, MAVS seems to be an essential feature of host immunity to RNA virus infection (Sun et al., 2006). In MEFs lacking MAVS expression, infection with SeV, influenza, reovirus and dengue did not induce IRF-3 nuclear translocation, IFN promoter activation and ISG expression (Loo et al., 2008), indicating that MAVS is necessary for innate immune responses to RNA viruses. Recent studies have emphasized the importance of mitochondrial location for MAVS function, implying that other mitochondrial proteins play a fundamental role in regulation of innate immune responses to RNA viruses (Chen et al., 2007;Li et al., 2005;Seth et al., 2005). Interestingly, both hMPV and RSV share RIG-I as the PRR recognizing their infection in airway epithelial cells, however, we and others have reported distinct ability to induce cytokine and chemokine, as well as IFN production between RSV and hMPV both in vitro and in vivo (Bao et al., 2007b;Guerrero-Plata et al., 2005a;Guerrero-Plata et al., 2005b;Guerrero-Plata et al., 2006). Whether this difference could be attributed to a differential recruitment of signaling molecules, such as TRAF-3 (Saha & Cheng, 2006), to the RIG-I/MAVS signaling complex, or is due to the activation of additional distinct anti-viral signaling pathways remains to be investigated.
Acknowledgments
This work was supported by grants NIEHS 06676 and NIAID P01 062885. X. B. was supported by the NIAID training grant T32 AI07536.
References
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, Randall RE. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci U S A. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Liu T, Spetch L, Kolli D, Garofalo RP, Casola A. Airway epithelial cell response to human metapneumovirus infection. Virology. 2007a;368:91–101. doi: 10.1016/j.virol.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Liu T, Sinha M, Hong C, Luxon BA, Garofalo RP, Casola A. Identification of human metapneumovirus-induced gene networks in airway epithelial cells by microarrays Analysis. Virology. 2007b doi: 10.1016/j.virol.2007.12.024. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes B, Lubyova B, Pitha PM. On the role of IRF in host defense. J Interferon Cytokine Res. 2002;22:59–71. doi: 10.1089/107999002753452665. [DOI] [PubMed] [Google Scholar]
- Boivin G, Abed Y, Pelletier G, Ruel L, Moisan D, Cote S, Peret TC, Erdman DD, Anderson LJ. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:1330–1334. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- Breiman A, Grandvaux N, Lin R, Ottone C, Akira S, Yoneyama M, Fujita T, Hiscott J, Meurs EF. Inhibition of RIG-I-dependent signaling to the interferon pathway during hepatitis C virus expression and restoration of signaling by IKKepsilon. J Virol. 2005;79:3969–3978. doi: 10.1128/JVI.79.7.3969-3978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casola A, Garofalo RP, Haeberle H, Elliott TF, Lin A, Jamaluddin M, Brasier AR. Multiple cis regulatory elements control RANTES promoter activity in alveolar epithelial cells infected with respiratory syncytial virus. J Virol. 2001;75:6428–6439. doi: 10.1128/JVI.75.14.6428-6439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casola A, Garofalo RP, Jamaluddin M, Vlahopoulos S, Brasier AR. Requirement of a novel upstream response element in RSV induction of interleukin-8 gene expression: stimulus-specific differences with cytokine activation. J Immunol. 2000;164:5944–5951. doi: 10.4049/jimmunol.164.11.5944. [DOI] [PubMed] [Google Scholar]
- Casola A, Henderson A, Liu T, Garofalo RP, Brasier AR. Regulation of RANTES promoter activation in alveolar epithelial cells after cytokine stimulation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1280–L1290. doi: 10.1152/ajplung.00162.2002. [DOI] [PubMed] [Google Scholar]
- Chang TH, Liao CL, Lin YL. Flavivirus induces interferon-beta gene expression through a pathway involving RIG-I-dependent IRF-3 and PI3K-dependent NF-kappaB activation. Microbes Infect. 2006;8:157–171. doi: 10.1016/j.micinf.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Chen Z, Benureau Y, Rijnbrand R, Yi J, Wang T, Warter L, Lanford RE, Weinman SA, Lemon SM, Martin A, Li K. GB virus B disrupts RIG-I signaling by NS3/4A-mediated cleavage of the adaptor protein MAVS. J Virol. 2007;81:964–976. doi: 10.1128/JVI.02076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs K, Stock N, Ross C, Andrejeva J, Hilton L, Skinner M, Randall R, Goodbourn S. MDA-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology. 2007;359:190–200. doi: 10.1016/j.virol.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Esper F, Boucher D, Weibel C, Martinello RA, Kahn JS. Human metapneumovirus infection in the United States: clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics. 2003;111:1407–1410. doi: 10.1542/peds.111.6.1407. [DOI] [PubMed] [Google Scholar]
- Foy E, Li K, Sumpter R, Jr, Loo YM, Johnson CL, Wang C, Fish PM, Yoneyama M, Fujita T, Lemon SM, Gale M., Jr Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc Natl Acad Sci U S A. 2005;102:2986–2991. doi: 10.1073/pnas.0408707102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo RP, Sabry M, Jamaluddin M, Yu RK, Casola A, Ogra PL, Brasier AR. Transcriptional activation of the interleukin-8 gene by respiratory syncytial virus infection in alveolar epithelial cells: Nuclear translocation of the RelA transcription factor as a mechanism producing airway mucosal inflammation. J Virol. 1996;70:8773–8781. doi: 10.1128/jvi.70.12.8773-8781.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groskreutz DJ, Monick MM, Powers LS, Yarovinsky TO, Look DC, unninghake GW. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol. 2006;176:1733–1740. doi: 10.4049/jimmunol.176.3.1733. [DOI] [PubMed] [Google Scholar]
- Guerrero-Plata A, Baron S, Poast JS, Adegboyega PA, Casola A, Garofalo RP. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J Virol. 2005a;79:10190–10199. doi: 10.1128/JVI.79.16.10190-10199.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A, Casola A, Garofalo RP. Human metapneumovirus induces a profile of lung cytokines distinct from that of respiratory syncytial virus. J Virol. 2005b;79:14992–14997. doi: 10.1128/JVI.79.23.14992-14997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A, Casola A, Suarez G, Yu X, Spetch L, Peeples ME, Garofalo RP. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am J Respir Cell Mol Biol. 2006;34:320–329. doi: 10.1165/rcmb.2005-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel T, Machleidt T, Alkalay I, Kronke M, Ben-Neriah Y, Baeuerle PA. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Hatakeyama M, Yamashita K, Yoshida H, Ishikawa A, Taima K, Satoh K, Mori F, Wakabayashi K. Interferon-gamma induces retinoic acid-inducible gene-I in endothelial cells. Endothelium. 2004;11:169–173. doi: 10.1080/10623320490512156. [DOI] [PubMed] [Google Scholar]
- Johnson CL, Gale M., Jr CARD games between virus and host get a new player. Trends Immunol. 2006;27:1–4. doi: 10.1016/j.it.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Kahn JS. Epidemiology of human metapneumovirus. Clin Microbiol Rev. 2006;19:546–557. doi: 10.1128/CMR.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, Akira S. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- Le GR, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, Si-Tahar M. Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol. 2007;178:3368–3372. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- Li XD, Sun L, Seth RB, Pineda G, Chen ZJ. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc Natl Acad Sci U S A. 2005;102:17717–17722. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic Acid-inducible gene I mediates early antiviral response and toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, Akira S, Gill MA, Garcia-Sastre A, Katze MG, Gale M., Jr Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, Falvo JV, Kim TH, Kim TK, Lin CH, Parekh BS, Wathelet MG. Structure and function of the interferon-beta enhanceosome. Cold Spring Harb Symp Quant Biol. 1998;63:609–620. doi: 10.1101/sqb.1998.63.609. [DOI] [PubMed] [Google Scholar]
- Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- Principi N, Bosis S, Esposito S. Human metapneumovirus in paediatric patients. Clin Microbiol Infect. 2006;12:301–308. doi: 10.1111/j.1469-0691.2005.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd BD, Smit JJ, Flavell RA, Alexopoulou L, Schaller MA, Gruber A, Berlin AA, Lukacs NW. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J Immunol. 2006;176:1937–1942. doi: 10.4049/jimmunol.176.3.1937. [DOI] [PubMed] [Google Scholar]
- Saha SK, Cheng G. TRAF3: A New Regulator of Type I Interferons. Cell Cycle. 2006:5. doi: 10.4161/cc.5.8.2637. [DOI] [PubMed] [Google Scholar]
- Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16:141–147. doi: 10.1038/sj.cr.7310019. [DOI] [PubMed] [Google Scholar]
- Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Spann KM, Tran KC, Collins PL. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J Virol. 2005;79:5353–5362. doi: 10.1128/JVI.79.9.5353-5362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, Chen ZJ. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- tenOever BR, Sharma S, Zou W, Sun Q, Grandvaux N, Julkunen I, Hemmi H, Yamamoto M, Akira S, Yeh WC, Lin R, Hiscott J. Activation of TBK1 and IKKvarepsilon kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity. J Virol. 2004;78:10636–10649. doi: 10.1128/JVI.78.19.10636-10649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Zhang Y, Luxon B, Garofalo RP, Casola A, Sinha M, Brasier AR. Identification of NF-kB dependent gene networks in respiratory syncytial virus-infected cells. J Virol. 2002;76:6800–6814. doi: 10.1128/JVI.76.13.6800-6814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werts C, Girardin SE, Philpott DJ. TIR, CARD and PYRIN: three domains for an antimicrobial triad. Cell Death Differ. 2006;13:798–815. doi: 10.1038/sj.cdd.4401890. [DOI] [PubMed] [Google Scholar]
- Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, Wright PF, Crowe JE., Jr Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]