Abstract
A fundamental step in embryonic development is cell differentiation whereby highly specialised cell types are developed from a single undifferentiated, fertilised egg. One of the earliest lineages to form in the mammalian conceptus is the trophoblast, which contributes exclusively to the extraembryonic structures that form the placenta. Trophoblast giant cells (TGCs) in the rodent placenta form the outermost layer of the extraembryonic compartment, establish direct contact with maternal cells, and produce a number of pregnancy-specific cytokine hormones. Giant cells differentiate from proliferative trophoblasts as they exit the cell cycle and enter a genome-amplifying endocycle. Normal differentiation of secondary TGCs is a critical step toward the formation of the placenta and normal embryonic development. Trophoblast development is also of particular interest to the developmental biologist and immunobiologist, as these cells constitute the immediate cellular boundary between the embryonic and maternal tissues. Abnormalities in the development of secondary TGCs results in severe malfunction of the placenta. Herein we review new information that has been accumulated recently regarding the molecular and cellular regulation of trophoblast and placenta development. In particular, we discuss the molecular aspects of murine TGC differentiation. We also focus on the role of growth and transcription factors in TGC development.
Keywords: Trophoblast cells, Placenta, Growth factors, Transcription factors
1. Development of murine trophoblast cell subtypes
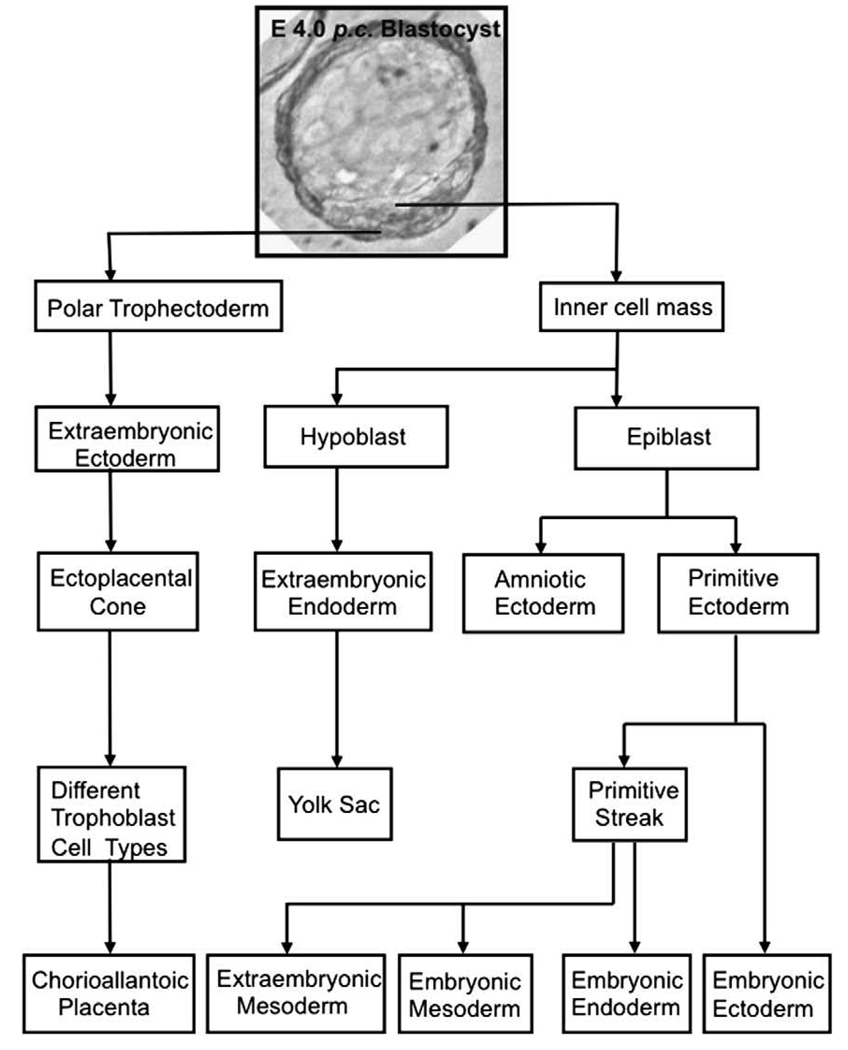
By the end of the pre-implantation period of development the murine blastocyst cavity is surrounded by the trophectoderm (TE), which develops into different types of trophoblast cells after implantation (Figs. 1 and 2). Polar TE overlies the inner cell mass (ICM) and gives rise to trophoblast stem cells (TSCs). Shortly after implantation, the mural TE, on the side away from the ICM, differentiates into primary TGCs. Polar TE differentiate later into secondary TGCs (Figs. 1 and 2). Primary TGCs form an anastomosing network of blood sinuses at the periphery of the embryo and facilitate the diffusion of oxygen and nutrients from the maternal circulation into the embryo (Bevilacqua and Abrahamsohn, 1988). Secondary TGCs arise from the polar TE-derived ectoplacental cone (EPC) from around day 7–7.5 post-coitum (p.c.) onwards (Figs. 2 and 3).
Fig. 1.
Overview of lineage relationships in early mouse embryo. Note that the ectoplacental cone is one of the derivatives of the polar trophectoderm-derived extraembryonic ectoderm. The ectoplacental cone differentiates into different trophoblast cell types, which form the chorioallantoic placenta.
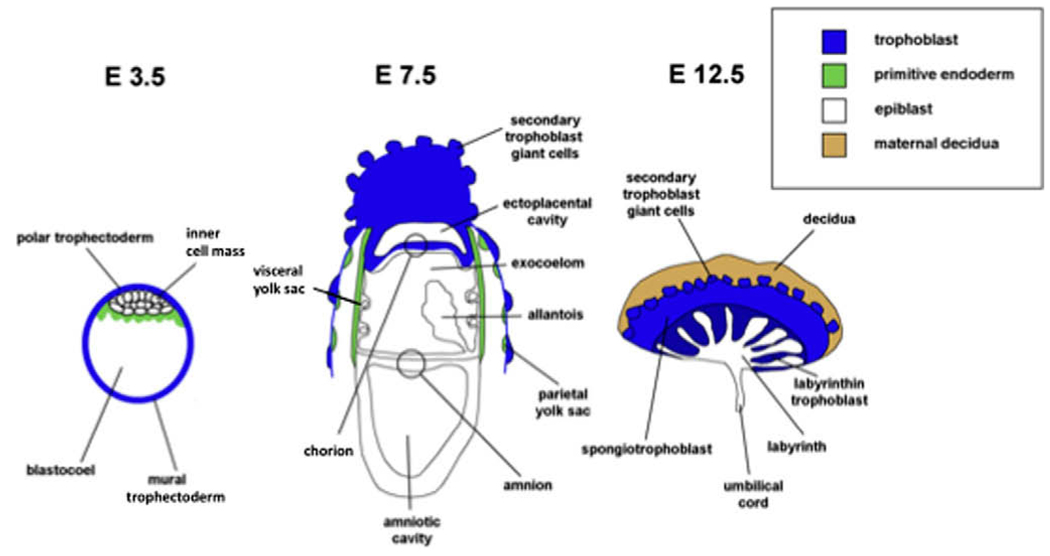
Fig. 2.
Diagram of cell lineage relationships in early mouse development, the major derivatives of the primitive endoderm (green) and trophectoderm (blue) and the major layers forming the later placenta in postimplantation development.
Fig. 3.
Paraffin sections of mouse embryo stained with haematoxylin and eosin. The mesometrial pole is positioned towards the top, and the antimesometrial pole to the bottom in B and C. Scale Bars 10 µm. (A) a 6.5 day early egg cylinder embryo. (B) day 7.5 embryo showing clear distinction between embryonic and extraembryonic levels, enlarged exocoelom, amniotic and chorionic folds. Note that exocoelom interrupting the proamniotic cavity and Reichert’s membrane is becoming clearer. (C) day 8.5 embryo showing increased number of secondary TGCs surrounding the conceptus, and the contact between the allantois and the chorion. Abbreviation: Allantois (Al), amnion (Am), amniotic cavity (AC), Chorion (Ch), Decidua (Dc), distal endoderm (DE), Ectoplacental cone (EPC), ectoplacental cavity (EC), exocoelom (Ex), extraembryonic ectoderm (EXE), Neural ectoderm (NE), Primitive streak (PS), proamniotic cavity (PC), proximal endoderm (PE), Reichert’s membrane (RM), Yolk cavity (YC), yolk sac (YS). Secondary and primary trophoblast giant cells (2° TGC, 1° TGC). Scale Bars: 10 µm.
TGCs have characteristic large polyploid nuclei due to continuous rounds of DNA replication, without intervening mitosis (Gardner and Davies, 1993; MacAuley et al., 1998; Nakayama et al., 1998.) There are no direct cell lineage tracing experiments to date to support the differentiation of the EPC into secondary TGCs. However, spontaneous differentiation of cultured EPCs to secondary TGCs provides supportive evidence for this model (Romagnano and Babiarz, 1990; Sutherland et al., 1991, 1993; El-Hashash and Kimber, 2004, 2006).
TGCs mediate embryo implantation and conceptus invasion into the uterus, which are facilitated by proteinases, such as urokinase-type plasminogen activator (UPA) and gelatinase A,B/matrix metalloproteinase-9 (MMP-9) (Teesalu et al., 1996; Alexander et al., 1996), as well as by chorionic gonadotrophin hormone (Prast et al., 2008). In contrast, decidual metalloproteinase inhibitors, such as TIMP-1 and TIMP3 counteract TGC-derived proteinases and limit TGC invasion into the uterine stroma to several hundred microns in rodents (Alexander et al., 1996; Cross et al., 2003). This is a critical process, since unlimited TGC invasion could extend beyond the endometrium and thus give rise to tumour-like outgrowths, whereas the embryo will not receive enough nutrients if invasion is insufficient.
Most recently, Kuang et al. (2009) identified CXCL14, a member of the chemokine family, as an important paracrine/autocrine modulator regulating trophoblast outgrowth at the maternal-fetal interface during the process of pregnancy establishment. Uterine CXCL14 expression was specifically induced at the embryo implantation site and expanded with the subsequent decidualization process in a spatiotemporal manner. The implanting embryo also showed a highlighted expression of CXCL14 in the blastocyst TE and its derived EPCs during postimplantation development. In vitro functional study revealed that CXCL14 could significantly inhibit both primary and secondary TGC attachment and outgrowth, correlated with a stage-dependant downregulation of MMP-2 and/or MMP-9 activity. Moreover, it was found that biotinylated CXCL14 could specifically bind to trophoblast cells in vitro and in vivo, suggesting trophoblast, perhaps expressing the unidentified CXCL14 receptor, is a bioactive target of CXCL14 (Kuang et al., 2009).
Furthermore, recent studies found that brain-derived neurotrophic factor (BDNF) promotes implantation and subsequent placental development by stimulating trophoblast cell growth and survival. BDNF and neurotrophin-4/5, together with their receptor, tyrosine kinase B (TrkB), are expressed in TE cells of blastocysts during implantation. Treatment with BDNF promotes blastocyst outgrowth, but not adhesion, in vitro and increased levels of the cell invasion marker MMP-9 in cultured blastocysts through the phosphatidylinositol 3-kinase pathway (Kawamura et al., 2009).
In addition, primary and secondary TGCs produce several cytokines and prolactins, which upregulate maternal blood flow to the implantation site, and promote the production of ovarian progesterone and lactogens. The latter are necessary for physiological adaptations in the mother during pregnancy (Linzer and Fisher, 1999; Cross et al., 2002). Recently, the previously assumed to be homogenous population of TGCs has been shown to consist of several distinct subsets based on expression of placental lactogens, placental specific cathepsin and location, while each of these population now appears to have a separate origin (Simmons et al., 2007).
2. Formation of murine chorioallantoic placenta
The chorioallantoic placenta is composed of an outer trophoblast-derived epithelium, and an underlying vascular network and stroma, which are mesodermal in origin (Downs, 1998; Cross et al., 2003). By day 9.5–10 p.c., the allantois with its associated vessels fuses with the chorionic plate to form a highly folded labyrinthine layer, which provides a large surface area for gas and nutrient exchange. The labyrinthine layer divides into two syncytiotrophoblast layers that separate the fetal blood vessels from the maternal blood spaces (Figs. 2 and 3).
Three distinctive subtypes of trophoblast cells form the placenta: the innermost labyrinthine layer, intermediate spongiotrophoblast and the outermost layer of secondary TGCs, each with specialised endocrine, vascular, immunological or transport functions during gestation (Figs. 2 and 3; Rinkenberger et al., 1997; Cross, 2000; Cross et al., 2003). Secondary TGCs appear on day 7.5 p.c. to first mediate implantation. The spongiotrophoblast layer forms around day 9.5 p.c. as the result of the expansion and flattening of the EPC. The EPC origin of spongiotrophoblasts is supported by their expression of several EPC-restricted genes, e.g. Flt1 and Tpbpa (Cross et al., 2002).
Glycogen trophoblasts, a suggested specialised subtype of spongiotrophoblast cells, appear within the spongiotrophoblast after day 12.5 p.c., and express typical spongiotrophoblast markers (Adamson et al., 2002). Another trophoblast subtype, syncytiotrophoblast cells, constitutes the nutrient transport surface within the labyrinthine layer (Cross, 2000). By day 10 p.c., the murine placenta consists of: (from inside to outside) the chorionic plate, the branched labyrinthine layer containing maternal blood, the junctional zone of spongiotrophoblast layer, and the TGC layer (Fig. 2).
3. Models to study murine trophoblast differentiation
Several in vitro and gene knockout models have been developed to study the development and differentiation of murine trophoblast cells. Mouse blastocyst can grow in vitro in a serum-containing medium on a defined culture substrate such as vitronectin, fibronectin, laminin or collagen. This allows study of the role particular substrates, e.g. fibronectin, laminin and collagen; play in primary trophoblast development (Armant et al., 1986; Sutherland et al., 1988). It is possible to investigate the interaction between trophoblast cells and molecules usually present in their adhesion and migration pathway, such as within the epithelial basement membrane or stromal extracellular matrix (ECM). Such studies led to the discovery of the importance of the proteoglycans, integrins and other ECM molecules in mouse implantation (Sutherland et al., 1993; Rout et al., 2004). They also facilitated investigation of the effect of growth factors on primary trophoblast outgrowth, including Tumour Necrosis Factor-α (TNF-α; Whiteside et al., 2003), Transforming Growth Factor-α and -β (TGF-α and TGF-β), Parathyroid Hormone-related protein (PTHrP; Nowak et al., 1999), Epidermal Growth Factor (EGF), and Fibroblast Growth Factor-2 (FGF-2; Haimovici and Anderson, 1993).
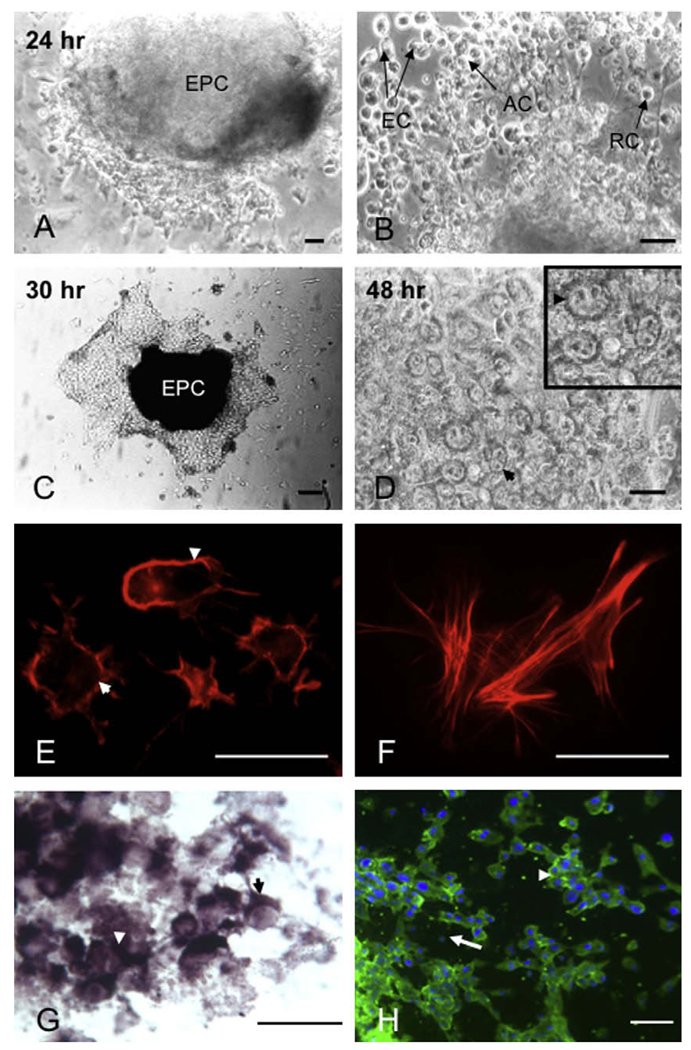
In contrast to primary TGCs, studies on secondary TGC differentiation remain limited, probably due to the difficulties of isolating EPC explants free of any attached maternal or embryonic tissues. In these studies, EPC explants were isolated from murine embryos, dissected from the extraembryonic ectoderm (EXE) and attached trophoblast cells and grown in a culture medium supplemented with serum on an ECM substrate (Sutherland et al., 1993; Parast et al., 2001; Goncalves et al., 2003). We (El-Hashash and Kimber, 2004) and others (Romagnano and Babiarz, 1990) have successfully grown EPC explants in a serum-free medium to characterize and study secondary TGC differentiation. The explants attach to the ECM substrate and form outgrowths that spread as a monolayer of flattened TGCs (Fig. 4A–D). Primary/secondary TGCs are identified by their characteristic large nuclei due to DNA endoreduplication and expression of several TGC-specific genetic markers (Fig. 5).
Fig. 4.
(A–D) Phase contrast micrographs show cellular morphology of EPC secondary trophoblast outgrowths in a serum-containing (A and B), or serum-free (C and D) medium at different time points after attachment. Note giant nuclei that occupied most of the cell in (B). (C and D) Outgrowths on 100 µg/ml FN substrate with 2% Nutridoma-HU in a serum-free medium. Inserted box in (D) showed multinucleated cells with irregular-shape nuclei, and arrowheads refer to multinucleated cell and giant nucleus. (E and F) Actin cytoskeleton organisation in secondary trophoblast cells stained with Texas Red Phalloidin. Note peripheral actin (E; arrowheads) and intense central and peripheral stress fibres (F) in cell growing in culture for 2 and 3 days, respectively. (G and H) Expression of PL-II mRNA (G) and protein (H) by secondary trophoblast cells in vitro. Blue nuclear labelling with DAPI (H). Note strong staining over the cytoplasm corresponding to the site of PL-II transcript localization (arrowheads; G), and cell spreading in clusters with giant (arrowhead) rather than small (arrow) nuclei in (H). Ectoplacental cone explant (EPC), aggregated cells (AC), rounded cells (RC), elongated cells (EC). Scale bars represent; 50µm (A), 30 µm (B and D), 250 µm (C), 10 µm (E–H).
Fig. 5.
Staining of longitudinal sections of E 8.5 mouse embryos in utero (for insets 1 and 2 in Fig. 3C) for different markers of secondary TGCs: with BOO6 antibody (A and B; anti-B-blood group antigen), HOO1 antibody (C and D; anti-Le-b/Le-y blood group antigens), anti-α7 antibody (E and F), anti-PL-II antibody (G and H), and AP-2γ (I and J). The mesometrial pole is positioned towards the top, and the antimesometrial pole to the bottom. Amnion (Am), chorionic plate (CP), decidua (Dc), distal endoderm (DE), ectoplacental cone (EPC), embryo (Em), proximal endoderm (PE), secondary TGC (2°TGC). Scale Bars: 10 µm.
Furthermore, advancements in understanding trophoblast differentiation have been achieved through development of model systems, e.g. the Rcho-1 and TSC lines, and improvements in knockout and knockdown technology. Homologous recombination-based gene targeting has enhanced analysis of gene function by generation of mice with defined germ-line mutations, e.g. null mutation of individual genes. This has lead to identification of the function of several trophoblast-regulating genes; e.g. Hand1, Mash-2 and AP-2γ. Moreover, site-specific recombinases and their target sequences such as the Cre/loxP system provides a tool to study gene function by inactivating genes in a tissue specific and/or inducible manner (Kuhn and Torres, 2002), where tissue specific promoter sequences are established.
4. Characteristics of TGC differentiation
The successful establishment of placentation depends critically on the orderly differentiation of TGCs, which is thought to coincide with a type of the epithelial–mesenchymal transition (EMT; reviewed in Sutherland 2003). EMT is characterized by destabilised cell–cell interactions and an increase in cell protrusive activity, followed by trophoblast reepithelisation, little or no cell motility, and stabilised cell–cell and cell–matrix interactions (Parast et al., 2001). Other characteristics of TGC differentiation include rearrangement of the cytoskeletal architecture, exit from the cell cycle, DNA endoreduplication and production of trophoblast-specific proteins such as placental prolactins.
4.1. Changes in the actin cytoskeleton
Like other Eukaryotic cells, trophoblasts are able to adopt a variety of shapes, and carry out coordinated movements depending on their highly dynamic cytoskeleton: actin filaments, microtubules and intermediate filaments (Fig. 4E and F). Undifferentiated trophoblast cells contain little organised actin and few peripheral focal complexes, and exhibit high membrane protrusive activity in vitro (Parast et al., 2001). In differentiated TGCs, cell junctions and the actin cytoskeleton become highly organised (Bevilacqua and Abrahamsohn, 1988, 1989; Parast et al., 2001). Both stability and organisation of cell–cell interactions increase, in correlation with changes in RhoGTPase activity (Parast et al., 2001). A few factors have been identified to regulate trophoblast cellular differentiation. Insulin growth factor-1 (IGF-1) increases the formation and organisation of actin stress fibres and lamellipodia (Kabir-Salmani et al., 2002). Leukaemia inhibitory factor (LIF) regulates TGC differentiation via Janus kinase 1-signal transducer and activator of transcription-suppressor of cytokine signalling 3 pathways (Takahashi et al., 2008). Moreover, PTHrP increases actin expression and stress fibre formation and organisation in trophoblast cells in vitro (El-Hashash and Kimber, 2006).
4.2. Cell cycle exit and DNA endoreduplication
The two distinct cell populations within the EPC, giant and diploid non-giant cells, exhibit different cellular programmes. Mitotic cycles of non-giant spongiotrophoblasts provide a continuous source of cells. In contrast, the increase of cell size, with accompanying polyploidization due to DNA endoreduplication, is a common mode of growth for TGCs in euthe-rian mammals (Ilgren, 1983; Zybina and Zybina, 1996). DNA endoreduplication is linked to TGC differentiation and has been proposed to facilitate the acceleration of cell differentiation and to increase cell growth by shortening the cell cycle and simultaneously increasing the number of chromosomes (Gardner and Davies, 1993; Goncalves et al., 2003).
Normal cell cycle regulators, e.g. Cyclins, are expressed by murine and human trophoblasts and uncoupled during DNA endoreduplication leading to a shift from the mitotic to the endocycle (Lilly and Spradling, 1996; Korgun et al., 2006). Inhibition of Cyclin B1 protein translation, together with Cyclin D1 expression, has been suggested as a mechanism for the trophoblast endocycle (Palazon et al., 1998). Trophoblast cells fail to assemble Cyclin B/p34CDK1 complexes at the time of mitotic cycle arrest during the first endocycle, and suppress Cyclin B protein expression in the subsequent endocycles (MacAuley et al., 1998). Cyclin D3 expression decreases while Cyclin D1 increases during the differentiation into TGCs in vitro (MacAuley et al., 1998). Moreover, the initiation of S phase is associated with increasing Cyclin A/E synthesis during the endocycle, while termination of S phase involves a loss of Cyclin A/E in Rcho-1 trophoblast cells (Wilhide et al., 1995; MacAuley et al., 1998). Similarly, the progression into endocycle is due to the absence of transcription for mitotic Cyclins A/B (Lehner and O’Farrell, 1990), and periodic expression of the S phase-gene Cyclin E, which is also required continuously through S phase (Lilly and Spradling, 1996) in Drosophila.
Furthermore, more critical steps in programmed endoreduplication in trophoblast cells have been discovered recently; with the finding that Cyclin-dependent kinase (CDK) activity regulates the transition from mitotic cell cycles to endocycles when TSCs differentiate into TGCs (Ullah et al., 2008). Selective inhibition of CDK1 activity, either by a chemical inhibitor called RO3306, or by developmentally programmed induction of the CDK-specific inhibitor p57 causes TSCs to endoreduplicate and differentiate into TGCs. Multiple rounds of genome duplication are then sustained by CDK2 and oscillating levels of p57. Induction of the CDK-specific inhibitor p21 serves to prevent apoptosis in cells undergoing multiple rounds of endoreduplication. Thus, endoreduplication in TSCs is triggered by p57 inhibition of CDK1 with concomitant suppression of the DNA damage response by p21 (Ullah et al., 2008).
4.3. Endocrine differentiation
In addition to accommodating the nutritional and growth-regulatory needs of the developing foetus, trophoblast cells are unique endocrine organ, which produce prolactin (PRL)-related and growth hormones, including prolactin like proteins (PLP-A,-C,-E,-F,-N.), proliferin (PLF), and PLF-related protein. These proteins are expressed during mid- and late gestation and have been intensively reviewed (Soares et al., 2007; Simmons et al., 2008). Among PRL hormones, placental lactogen-1 and -2 (PL-I and PL-II) are of particular interest for TGC differentiation (Figs. 4G and H and Fig. 6G and H).
Fig. 6.
Model for key steps of secondary TGC differentiation, and changes in the expression pattern of key trophoblast subtype-specific transcription factors involved in each step. Arrows ↑ and ↓ refer to upregulation and downregulation expression.
The PL-II is a non-glycosylated single-chain polypeptide, and shares significant amino acid sequence homology with PL-I (Duckworth et al., 1986; Robertson et al., 1982). While PL-I synthesis occurs during early-mid pregnancy, PL-II is specifically produced by secondary TGCs and appears in the murine maternal circulation on day 9 p.c., and increases until about day 14 p.c. It then either remains relatively constant in some mouse strains or continues increasing until the end of pregnancy in others (Faria et al., 1991; Soares et al., 1982; Soares and Talamantes, 1983). PL-II enters the fetal compartment and binds to the widely expressed PRL receptor in the developing foetus (Soares and Talamantes, 1983; Ogren and Talamantes, 1988), and co-localizes with IGF-2 in murine placenta (Ishida et al., 2007).
PL-I and PL-II display a wide variety of PRL-like activities on different maternal target tissues. They promote mammary gland development, lactogenesis and maintenance of the corpus luteum and its production of progesterone (Colosi et al., 1988; Galosy and Talamantes, 1995). Several proteins regulate PL-II production, including EGF (Yamaguchi et al., 1992), interleukins-1 and -6 (IL-1, IL-6; Yamaguchi et al., 1996), and decidual-derived calcyclin (Richard et al., 1998).
5. Molecular pathways regulating TGC differentiation
In the last two decades, the purification, cloning and characterisation of several transcription factors, together with improving transgenic mouse technologies, have increased the knowledge of the genetic basis of trophoblast differentiation. Many recently discovered cell type specific transcription factors are now known to regulate trophoblast cell development. This has been extensively reviewed (Cross et al., 2002, 2003; Simmons and Cross, 2005), and a brief summary of the most investigated factors is described in Table 1.
Table 1.
Summary of the main known transcription and related factor that regulate trophoblast development (not a comprehensive review).
| Transcription factors (family) | Source | Placental phenotype (?*) | Biological action related to trophoblast cells | References |
|---|---|---|---|---|
| Hand 1 (bHLH) | EPC, TGCs, spongiotrophoblasts | Block of TGC differentiation | Stimulates TGC formation | Riley et al. (1998) Cross et al. (1995) |
| Mash 2 (bHLH) | TS cells in the chorion, spongiotrophoblasts, EPC | Loss of spongio- trophoblasts, increased TGC formation | Blocks TGC differentiation or sustains spongio- trophoblast proliferation | Cross et al. (1995) Guillemot et al. (1994) |
| Stra 13 (bHLH) | TGCs | ? | Induces TGC differentiation in vitro | Hughes et al (2004) |
| E-factor (Alf1and Itf2; bHLH) | TS cells in the chorion, spongiotrophoblasts, EPC | ? | Acts as obligate dimer with Mash2 and affects on TGC differentiation | Zhuang et al (1996) |
| Tfeb (bHLH-zipper protein) | Labyrinthine trophoblastcells | Small labyrinth | Affects on placentalvascularization | Steingrimsson et al. (1998). |
| dx2 (Homeodomain) | TE, exteraembryonic tissues | Defected TE Development and failure of implantation | Regulates TE differentiation | Chawengsaksophak et al (1997) |
| PEM (Homeodomain) | Secondary TGCs, chorion | ? | Regulates differentiation of embryonic stem cells | Fan et al. (1999) |
| Dlx3(Homeodomain) | EPC, labyrinthine trophoblast cells, chorionic plates | Small labyrinth | Promotes labyrinthine trophoblast development and vascularization of the placenta | Morasso et al. (1999) |
| Esx1(Homeodomain) | Labyrinthine trophoblast cells, chorion | Placental hyperplasi, vascularization defects | Promotes labyrinthine trophoblast development and vascularization of the placenta | Li and Behringer (1998) |
| Eomes (T-box protein) | TE, EXE | TE does not differentiate into trophoblast, and embryos arrested at blastocyst stage | Maintains TS cell fate | Russ et al. (2000) |
| Ets2 (Ets protein) | TE, EXE | Increased TS cell differentiation and decreased TS self-renewal. | Stimulates EPC proliferation and MMP-9 production by TGC, and is essential for TS cell self-renewal | Yamamoto et al (1998), Wen et al. (2007) |
| I-mfa (I-mfa/HIC protein) | EPC, chorion, secondary TGCs | Reduced TGC differentiation | Promotes TGC differentiation and survival in vitro | Kraut et al. (1998) |
| Jun B (b-Zip protein) | TGCs, EPC, allantois | Small labyrinth (owing to trophoblast defects) | Stimulates the formation of labyrinthine trophoblast cells | Schorpp-Kistner et al. (1999) |
| mSNA(Zinc finger protein) | Spongiotrophoblast cells,EPC | ? | Blocks TGC differentiation | Nakayama et al(1998) |
| AP-2γ(AP-2 protein) | TE, TGCs, EPC, EXE, spongiotrophoblast cells, labyrinthine trophoblast cells | Reduced proliferation of the EPC and TGCs, failure of labyrinth formation | Essential for extraembryonic membrane development | Auman et al. (2002) |
| TEAD4(TEAD family) | TSCs, ESCs, EPC, TGCs, chorion | Tead4(−/−) morulae do not produce TSCs or trophectoderm, and do not implant into the uterine endometrium | Required for specification of the trophectoderm lineage | Yagi et al. (2007) |
| HOP/NECC1 (Homeodomain-only protein | TGCs, spongiotrophoblasts | Placenta exhibites marked propagation of TGCs and reduction of spongiotrophoblast cells | Inhibits TGC differentiation | Asanoma et al. (2007) |
?*, not known.
The general consensus is that the molecular differentiation of secondary TGCs is a multistep process. This has encouraged numerous studies on the expression and function of transcription factors that have been implicated in each step (Fig. 6; Cross, 2000; Simmons and Cross, 2005). Major transcription factors, including Mash2, Eomes, cdx2, and Err2, as well as Id1, Id2 and E-factors regulate the development of TSCs of day 6.5 p.c. EXE. Differentiation of the EXE into the EPC is stimulated by the suppression of the above transcription factors except Mash2, and upregulation of Hand1 and mSNA. Downregulation of Mash2 and mSNA, together with upregulation of Stra13, may trigger the differentiation of the EPC and spongiotrophoblasts into secondary TGCs (Fig 6, Table 1).
Increasing attention has also been paid to the role of growth factors and cytokines in trophoblast development. This review will not provide a comprehensive list of growth factors/cytokines, which are required for trophoblast development because the topic is well covered elsewhere (Morrish et al., 2007; Guzeloglu-Kayisli et al., 2009). A brief summary of the effect of some of the intensively studied growth factors on trophoblast cells is shown in Table 2. The role of growth factors/cytokines in controlling trophoblast development, together with trophoblast-regulatory genes, will be discussed for TSCs and each trophoblast subtypes below.
Table 2.
Summary of the main known growth factors that affect on trophoblast development (not a comprehensive review).
| Growth factors/cytokines | Source | Target | Biological action on trophoblast cells | Placental phenotype?* | References |
|---|---|---|---|---|---|
| EGF (Epidermal Growth factor) | Uterine tissues, macrophages | Trophoblast and uterine pithelial endothelial and fibroblast cells | Enhances trophoblast outgrowth/proliferation, and regulates the expression of uPA and the MMP-9 by trophoblast cells | EGF receptor (Egfr) null mice have small labyrinth/ spongio- trophoblast | (Haimovici and Anderson (1993), Sappino et al. (1989), Dackor et al. (2007), Threadgill et al. (1995) |
| TGF-α (Transformation Growth factor-α) | Uterine tissues: macrophages, epithelial and platelet cells, | Trophoblast and uterine epithelial, endothelial and fibroblast cells | Enhances trophoblast outgrowth in vitro | ? | Haimovici and Anderson (1993) |
| TGF-β (Transformation Growth Factor-β) | Uterine deciduas and luminal epithelial cells | Epithelial cells, endothelial cells, fibroblasts | Stimulates trophoblast outgrowth, and inhibits trophoblast cell invasion in vitro | ? | Nowak et al. (1999), Zhao et al. (2006) |
| Nodal | Spongio- trophoblasts | Spongio-trophoblasts, TGCs | Inhibits TGC differentiation | Increased TGC differentiation | Ma et al. (2001) |
| FGF-2 (Fibroblast Growth Factor-2) | Uterine tissues: macrophages, endothelial, fibroblast and platelets, cells | Trophoblast and epithelial, endothelial, macrophage and fibroblast cells of the uterus | Increases blastocyst attachment and enhances trophoblast outgrowth and invasion in vitro | ? | Haimovici and Anderson (1993), Taniguchi et al. (1998) |
| FGF-4(Fibroblast Growth Factor-4) | ICM cells | TE cells | Stimulates the proliferation of polar TE cells | Failure of trophoblast proliferation | Chai et al.(1998), Goldin and Papaioannou (2003) |
| FGF-7(Fibroblast Growth Factor-7) | Stromal cells of several tissues | Trophoblast cells | Enhances the spreading of trophoblast cells in vitro | ? | Taniguchi et al. (1998) |
| FGF-10(Fibroblast Growth Factor-10) | Decidual and trophoblast cells | Trophoblast cells | Promotes invasion and outgrowth of trophoblast cells | ? | Natanson-Yaron et al. (2007) |
| HGF (Hepatocyte Growth Factor) | Uterine epithelial cells | Trophoblast cells | Regulates trophoblast invasion in vitro | Small labyrinth | Cartwright et al. (1999), Uehara et al. (1995) |
| LIF (Leukaemia Inhibitory Factor) | Uterine glandular epithelium | Trophoblast cells | Stimulates trophoblast differentiation and regulates the expression of uPA and the MMP-9 by trophoblasts cells | LIF receptor null mice have small labyrinth and vascular lesions | Sappino et al. (1989), Harvey et al. (1995), Ware et al. (1995), Takahashi et al. (2008) |
| HB-EGF (Heparin-binding EGF-like Growth Factor) | Uterine luminal epithelium | TE, trophoblast cells | Promotes trophoblast outgrowth, and stimulates zona hatching | ? | Das et al. (1994) |
| PDGF (Platelet-derived Growth Factor) | Uterine tissues: macrophages, endothelial, fibroblast and platelets cells | Trophoblast cells, epithelial, fibroblast and macrophage cells of the uterus | Enhances trophoblast outgrowth in vitro | Small labyrinth | Haimovici and Anderson (1993), Ohlsson et al. (1999) |
| VEGF (Vascular endothelial growth Factor) | Uterine tissues, trophoblast cells | Trophoblast cells | Promotes the proliferation and migration of human trophoblast cells | ? | Ahmed and Perkins (2000), Allen et al. (2007) |
| IGF (Insulin-like Growth Factor) | TGCs, glycogen cells, labyrinthine and spongio- trophoblasts | Trophoblast cells | Plays a role in trophoblast cell invasion | Disruptiation of the development of glycogen trophoblast cells | Lopez et al. (1996), Carter et al. (2006) |
| PTHrP(Parathyroid Hormone-related) Protein | Uterine tissues: stromal cells, luminal and glandular epithelium, and TGCs | TGCs | Reduces proliferation, decreases apoptosis, and enhances differentiation of secondary TGCs | ? | El-Hashash et al. (2005), El-Hashash and Kimber (2006) |
| Insulin | ? | ? | Stimulates trophoblast mitogenesis and downregulates metabolic responses. | ? | Navarrete Santos et al. (2008) |
| PlGF(Placenta Growth Factor) | Trophoblast cells, maternal Natural Killer (uNK) cells | Trophoblast and uNK cells | Controls intravascular invasion, cell proliferation and differentiation of trophoblast cells | Neither implantation sites nor uNK cells are developed | Tayade et al. (2007) |
| CSF2(colony-stimulating factor) | Uterine stromal cells, uNK cells and decidua, and trophoblast cells | Trophoblast cells | Controls placental gene expression and trophoblast proliferation | Dysmorphic placenta with altered trophoblast cell composition | Sferruzzi-Perri et al. (2009) |
?*, not known.
5.1. Trophoblast stem cells (TSCs)
TSCs can be isolated from murine blastocysts or at an early postimplantation stage of development in the chorion, and can give rise to all differentiated trophoblast cell subtypes (Tanaka et al., 1998; Oda et al., 2006), a process regulated by the MAPK (e.g. MAPK14; Winger et al., 2007) signalling pathway. FGF-4, which is a paracrine factor produced by the ICM/epiblast and signals through MAPK, controls trophoblast proliferation. Its cognate receptor, FGFR2, is expressed in trophoblastic tissues, including the EXE and chorion (Rossant and Cross, 2001). Survival of mouse blastocyst-derived TSCs requires FGF-4 and embryonic fibroblast-conditioned medium (supplying TGF-β and Activin-A; Erlebacher et al., 2004). In the presence of these factors, mouse TSCs exhibit sustained undifferentiated proliferation, without significant expression of the phenotypic markers of placental trophoblasts, such as PL-1/PL-II, or placental prolactin-related proteins. Removal of FGF-4 results in the arrest of cell proliferation, rapid TGC formation and onset of hormone gene transcription (Tanaka et al., 1998). Consistent with these findings, FGF-4/FGR2 mutant mice show failure in trophoblast proliferation (Feldman et al., 1995; Goldin and Papaioannou, 2003). Fgf-4 expression is regulated by the transcription factor Sox-2. If Sox-2 is knocked down, fgf-4 and fgf-r2 expression is greatly diminished, and trophectoderm does not form (Keramari M and Kimber SJ, unpublished data).
Most Recently, Abell et al. (2009) have shown that TSC maintenance by FGF-4 requires MEKK4 activation of Jun N-terminal kinase. Mice harboring a point mutation that renders inactive the mitogen-activated protein kinase kinase kinase-4 (MEKK4) exhibit dysregulated placental development with increased trophoblast invasion. Isolated MEKK4 kinase-inactive TSCs cultured under undifferentiating, self-renewing conditions in the presence of FGF-4 display increased expression of Slug, Twist, and MMP-2, loss of E-cadherin, and hyper-invasion of ECM, each a hallmark of EMT (Abell et al., 2009).
Furthermore, it has been shown that SUMO-specific protease 2 (SENP2) is essential for modulating p53-Mdm2 in development of TSC niches and lineages (Chiu et al., 2008). SENP2 modifies proteins by removing SUMO from its substrates, and is highly expressed in trophoblast cells. SENP2 null mice have a deficiency in cell cycle progression, particularly in the G–S transition, which is required for mitotic and endoreduplication cell cycles in trophoblast proliferation and differentiation, respectively. Also, SENP2 ablation disturbs the p53-Mdm2 pathway, affecting the expansion of trophoblast progenitors and their maturation (Chiu et al., 2008).
The T-box protein eomesodermin (Eomes) and the caudal-type homeodomain protein Cdx2 transcription factors are expressed in the TE and maintain TSC fate, and both Eomes and Cdx2 homozygous mutant embryos die around the time of implantation (Russ et al., 2000; Chawengsaksophak et al., 1997; Tanaka et al., 1998) A block in early TE differentiation occurs in Eomes mutant blastocysts; while in the complete absence of Cdx2, TE cell identity cannot be maintained in the blastocyst (Strumpf et al., 2005). The latter study demonstrated that Cdx2 is required for correct cell fate specification and differentiation of the TE and is essential for segregation of the ICM and TE lineages at the blastocyst stage by ensuring repression of Oct4 and Nanog in the TE (Strumpf et al., 2005). A recent study also showed that Cdx2 plays a critical role in cell polarity and in cell allocation and specification of TE and ICM in the mouse embryo by upregulating atypical PKC (aPKC) and thus controlling asymmetric cell division (Jedrusik et al., 2008). TSC differentiation into TGCs or spongiotrophoblasts is associated with suppression of Eomes and cdx2 expression (Tanaka et al., 1998).
Oct4, is a member of the POU family of transcription factors, produced by the preimplantation embryo and high in ICM and inhibits trophoblast cell fate (Palmieri et al., 1994). All blastomeres adopt a trophoblast cell fate, regardless of their position in the conceptus of Oct4 null mutants, suggesting the importance of Oct4 for maintaining of ICM fate (Nichols et al., 1998). Ets2, a regulator of cdx2, is essential for TSC self-renewal (Wen et al., 2007), while Tead4 is an early transcription factor required for specification and development of the TE lineage, which includes expression of Cdx2 (Yagi et al., 2007; Nishioka et al., 2008). In addition, Err2 transcription factor is controlled by FGF-10 signalling, and is an important regulator of TSC fate (Luo et al., 1997; Tremblay et al., 2001).
AP-2γ, an essential regulator of TSC fate controlled by FGF signalling is of particular interest for TGC differentiation, since it activates the human PL promoter (Richardson et al., 2000). It is present in all embryonic cells during pre-implantation development and, together with Eomes, is expressed in all trophoblast lineages throughout placental development (Auman et al., 2002). In postimplantation stages, AP-2γ expression is restricted to the extraembryonic lineages: primary TGCs and diploid cells of the polar TE, EXE and EPC, and increases in all the derivatives of the trophoblast lineage during chorioallantoic fusion (Sapin et al., 2000). AP-2γ knockout conceptuses show a reduced cell proliferation of the EXE and the EPC, low number of TGCs, failure in the formation of the labyrinth layer, as well as repression of the FGF-4 responsive genes: Eomes and cdx2 (Auman et al., 2002). This also leads to a significant retardation of the growth of mutant embryos by day 7.5 p.c. and their re-absorption by day 9.5 p.c. (Werling and Schorle, 2002).
Nodal, a member of TGF-β family, is expressed by the epiblast as well as later on in TGCs (Ma et al., 2001), and maintains FGF-4 expression in the epiblast. It also induces proteases (Furin and Pace-4) in the EXE, which cleave Nodal to its active form. Nodal and FGF targets in the EXE include TSC genes indicating that they co-operate to maintain TSC fate (Guzman-Ayala et al., 2004). Differentiated TGCs increase in Nodal-deficient mouse embryo, indicating its importance for TGC differentiation (Ma et al., 2001). Furthermore, FGF/Nodal signalling actively inhibits TSC differentiation into giant cells (Hughes et al., 2004). In addition, recent studies showed that Hand1 release from the nucleolus controls differentiation of TSCs into TGCs, and this switch is controlled by the antagonistic activities of the I-mfa domain-containing protein HICp40 and Polo-like kinase-4 (Plk4; Martindill et al., 2007; Martindill and Riley, 2008).
Recently, there have been efforts to directly derive TSCs from human embryos (Rossant, 2001). However, the methods developed to successfully derive TSCs from mouse embryos have not been reported to yielded comparable cells from human blastocysts. This may be due to the diverse growth factor requirements and regulatory pathways for embryonic stem cell (ESC) derivation and differentiation between mouse and human. For example, studies on nonhuman primate (Thomson et al., 1995) and human (Thomson et al., 1998) showed that whereas LIF is critical for sustaining mouse ESCs, it is ineffectual in the human system. Subsequent studies showed that the pathways that sustain undifferentiated growth in human ESCs have rely primarily on FGF-2 and TGF-β/Activin signalling, directing suppression of BMP signalling, for undifferentiated growth of ESCs (Xu et al., 2005). In the continuous presence of FGF-2 and mouse embryonic fibroblast-conditioned medium (which typically sustain undifferentiated human ESC growth), addition of BMP-4 (or related ligands for BMP receptor IB, BMP-2, -7, or GDF-5) resultes in the differentiation of human ESCs to cells of a uniform epithelial appearance (Xu et al., 2002). Thus, It is reasonable to predict that TSCs could eventually be derived from human embryos given sufficient material with which to evaluate a broad range of culture conditions; however, the development of alternative approaches becomes necessary for progress beyond the mouse model (Douglas et al., 2009).
5.2. Spongiotrophoblast cells
The attributed origin of spongiotrophoblast from the EPC is largely based on the similarity in gene expression patterns, e.g. Mash-2, Tpbp/4311 and Flt1. Mash2, a basic helix-loop-helix (bHLH) transcription factor, is predominantly expressed throughout murine pre- and postimplantation stages in the EPC, spongiotrophoblasts and the chorion, but is undetectable in primary and secondary TGCs and the yolk sac (Guillemot et al., 1994; Nakayama et al., 1997). Mash-2 transiently maintains the proliferation of trophoblast cells in an FGF-4-independent manner (Hughes et al., 2004), and sustains spongiotrophoblast proliferation and survival (Guillemot et al., 1994; Cross et al., 1995; Tanaka et al., 1997). Mash-2-deficient conceptuses show premature loss of the EPC, absence of spongiotrophoblasts by E10.5 p.c., enlargement of TGC layer and a reduction in the formation of labyrinth layer (Guillemot et al., 1994; Tanaka et al., 1997). Mash-2 mutant mice can be rescued through aggregation of diploid Mash-2−/−, and tetraploid wild-type embryos and are viable and fertile, suggesting no function for Mash-2 in the developing embryo itself (Guillemot et al., 1994). Neither zygotic nor maternal Mash-2 transcripts are required for early trophoblast development, as indicated by the normal development of mutant embryos derived from Mash-2−/− females (Rossant et al., 1998).
Two bHLH factors, Alf1/HEB and Itf2, are obligate DNA binding partners for Mash-2. They are only expressed in the inner region of the EPC and chorion (Scott et al., 2000), implying that Mash-2 function is limited to the proliferative inner EPC cells that probably maintain spongiotrophoblast layers (Cross et al., 2003). The genes Tpbp/4311 (Lescisin et al., 1988) and Flt1 (He et al., 1999) are also expressed by both the EPC and spongiotrophoblast cells, but are absent in the EXE, TGCs and labyrinth. The expression levels of Tpbp/4311 and Flt1 increase with embryonic development, and their functions are still unclear. In addition, recent studies identified the putative transcriptional regulator BPTF/FAC1, which is expressed in embryonic and extra-embryonic tissues of the early mouse conceptus, as an essential regulator for the differentiation of EPC-derived trophoblast cells (Goller et al., 2008). BPTF/FAC1-deficient mouse embryos form apparently normal blastocysts that implant and develop epiblast, visceral endoderm, and the EXE including TSCs. However, the EPC is drastically reduced or absent in mutants, which may cause the embryonic lethality (Goller et al., 2008).
Furthermore, Epidermal Growth Factor Receptor (EGFR) has been recently shown to promote growth of the placental spongiotrophoblast layer in mice. Also, EGFR expressed in the uterine stroma may play an underappreciated role in preparation of the uterus for embryo implantation (Dackor et al., 2009a). Egfr homozygous placentas have a reduced spongiotrophoblast layer in some strains, while spongiotrophoblasts and glycogen cells are almost completely absent in others (Dackor et al., 2009b).
5.3. Trophoblast giant cells (TGCs)
In the absence of FGF, cultured primary trophoblast cells spontaneously differentiate into giant cells, suggesting that TGC differentiation is a “default pathway” of trophoblast differentiation. Overexpression of Hand1, a bHLH transcription factor, induces giant cell differentiation in Rcho-1 cells in vitro, which is inhibited upon ectopic expression of Mash-2 (Cross et al., 1995; Scott et al., 2000). Hand1 null embryos arrest in development around day 7.5–8 p.c., and show a diminished number of primary and secondary TGCs, a reduced EPC, and the absence of PL expression (Riley et al., 1998). Hand1−/− TSCs show reduced levels of TGC differentiation (Hemberger et al., 2004).
The expression pattern and activity of Hand1 and Mash-2 have been the subject of controversy. Hand1 expression overlaps the expression of Mash-2 in the EPC and spongiotrophoblasts, and layers of the placenta containing giant cells precursors in murine postimplantation stages (Scott et al., 2000). Contrary to Mash-2, Hand1 is highly expressed in primary and secondary TGCs, but is absent from the chorion (Scott et al., 2000). Hand1 and Mash-2 show mutually antagonistic activities on trophoblast cell differentiation in vitro, where Hand1 competes with Mash-2 for binding to E-factors such as ITF-2, ALF1 or E47, and thus competes away Mash-2 function (Scott et al., 2000). Hand1 is likely to be required for turning off Mash-2 expression, since Hand1 knockout mice show persistent Mash-2 expression (Riley et al., 1998). However, Scott et al. (2000) suggested that the function of Hand1 is merely to inhibit Mash-2 activity, as the defects of Hand1 mutants are not rescued in Hand1; Mash-2 double mutant mice. Ectopic expression of Hand1 induces TGC differentiation even in the presence of FGF-4 (Hughes et al., 2004).
Groucho-related gene 3 (Grg3) is a mouse homologue of the Drosophila Groucho corepressor protein, which, together with the other Notch signalling pathway member Hairy/Enhancer of Split (homologous to mammalian Hes), is involved in developmental processes, usually by downregulation of the Hairy/Enhancer of Split downstream gene Achaete-scute (mammalian Mash). Several Notch signalling pathway members were shown to be expressed in mouse placenta, including Notch2, Hes2, Hes3, Grg2, Grg3 and Mash2 (Nakayama et al., 1997). Grg3 is expressed in secondary TGCs and in the labyrinth, but not in the spongiotrophoblast layer. Homozygous knockout mice for Grg3 dying in utero between day 13.5 p.c. and day 15.5 p.c. show marked growth retardation, which is most probably related to the observed underdevelopment of the Grg3 deficient trophoblast. In Grg3 deficient placenta, the number of secondary TGCs is greatly reduced, suggesting that Grg3 is necessary for proper differentiation into secondary TGCs (Morrish et al., 2007). The hypothetical mechanism of Grg3 action could be interaction with Hes2 and downregulation of Mash2 expression, which would inhibit differentiation into spongiotrophoblast and shift it towards secondary TGCs (Morrish et al., 2007).
Retinoic acid and its inducible bHLH factor, Stra13, stimulate TGC differentiation. RA-treated TSCs show arrested cell proliferation and enhanced giant cell differentiation (Yan et al., 2001). Stra13 is expressed by TGCs in vivo (Boudjelal et al., 1997) and increases during TGC differentiation (Hughes et al., 2004). Overexpression of Stra13 overrides FGF signalling in order to promote TSC differentiation into TGCs (Hughes et al., 2004). Similarly, GATA2 and GATA3 have been implicated in the regulation of trophoblast-specific genes. Recently, Ray et al. (2009) found that GATA3 directly represses Gata2 in undifferentiated trophoblast cells, and a switch in chromatin occupancy between GATA3 and GATA2 (GATA3/GATA2 switch) induces transcription during trophoblast differentiation. Thus, GATA3/GATA2 switch provides an important mechanism for the transcriptional regulation of other trophoblast-specific genes.
In addition, BDNF and neurotrophin-4/5 proteins as well as their receptor TrkB, which are expressed after implantation in trophoblast cells and placentas, are also essential for trophoblast cell development (Kawamura et al., 2009). Treatment of cultured trophoblast cells with the TrkB ectodomain, or a Trk receptor inhibitor K252a, suppresses cell growth as reflected by decreased proliferation and increased apoptosis. Studies using the specific inhibitors indicated the importance of the phosphatidylinositol 3-kinase/Akt pathway in mediating the action of TrkB ligands. Also, in vivo studies in pregnant mice further demonstrated that treatment with K252a suppresses placental development accompanied by increases in trophoblast cell apoptosis and decreases in placental labyrinth zone at mid-gestation (Kawamura et al., 2009).
Furthermore a recent study by Schulz et al. (2009) suggested that leptin influences TGC differentiation. Leptin accelerates disappearance of non-giant cells while inhibiting terminal differentiation of committed giant cells, possibly by maintaining cells in an intermediate stage of differentiation. In trophoblast culture, leptin stimulates the phosphorylation of MEK (MAP2K1), increases the concentration of the suppressor of cytokine signalling 3 (SOCS3) protein, and upregulates metalloproteinase activity. In addition, leptin stimulates the activity of some genes with roles in cell motility, including Stmn, a gene linked to invasiveness in other cell types, as well as several genes associated with MAPK and RhoGTPase signalling (Schulz et al., 2009).
Evidence from our laboratory that PTHrP growth factor regulates TGC differentiation has shed new light on the mechanisms by which PTHrP can regulate cell differentiation. PTHrP, a 139- to 173-amino acid multifunctional protein, is widely expressed in different cell types, in which it acts as a paracrine, autocrine or intracrine factor, signalling through its cognate PTHR1 receptor (Wysolmerski and Stewart, 1998). PTHrP acts in a stage-specific manner on the EPC precursors to induce their differentiation into secondary TGCs through several parallel processes. Firstly, PTHrP shifts the trophoblast cell cycle into endocycle (El-Hashash et al., 2005). DNA endoreduplication of TGCs, a process closely linked to the onset of their differentiation, is accompanied with upregulation of Cyclin D1 and repression of Cyclin B expression (MacAuley et al., 1998; Palazon et al., 1998). PTHrP dramatically downregulates Cyclin B1, increases Cyclin D1 expression, and inhibits the expression of mSNA, a transcription factor that represses switching from mitosis to endoreduplication and TGC differentiation (Nakayama et al., 1998; El-Hashash et al., 2005). This possible stimulation of the mitotic-endocycle transition is supported by increasing DNA synthesis, number of TGCs, and upregulation of RhoA, a stimulator of Cyclin D1 expression in mid-G1 phase and of G1 phase progression (Welsh et al., 2001) in PTHrP–treated cultures (El-Hashash et al., 2005; El-Hashash and Kimber, 2006).
Secondly, PTHrP affects critical regulators of TGC differentiation. We identified several target genes for PTHrP that have not been reported in other cell types before, e.g. Eomes, Mash-2, mSNA, AP-2γ and Stra13. PTHrP downregulates TSC-maintaining Eomes and spongiotrophoblast-specific genes Mash-2 and mSNA, and upregulates TGC differentiation-stimulating genes AP-2γ, Stra13 and PL-II (El-Hashash et al., 2005). Thus, PTHrP may regulate TGC differentiation directly from trophoblast precursors in the EPC and by terminal differentiation of intermediate diploid trophoblast populations of the spongiotrophoblast. Finally, PTHrP induces the organisation of actin fibres, probably through controlling EphB/EphrinB-RhoA signalling, resulting in the characteristic flattened, fibroblastic morphology of differentiated TGCs. This is accompanied by downregulation of E-cadherin expression, and an increase in cell spreading in culture (El-Hashash and Kimber, 2006). Several other factors regulate TGC development, including LIF, TGF-α, EGF, FGF-2 HB-EGF, FGF-7, HGF and PDGF, and are summarized in Table 2.
5.4. Glycogen trophoblast cells and syncytiotrophoblasts
It is widely thought that glycogen trophoblast cells (GTCs) are a specialised subtype of spongiotrophoblast, since they express the Tpbp/4311, a gene exclusively expressed by spongiotrophoblast cells (Adamson et al., 2002). GTCs first appear within the spongiotrophoblast layer, from which they migrate into the maternal decidual tissue (Adamson et al., 2002). They specifically express Connexin31 and increase in number from day 12.5 p.c. to day 16.5 p.c. in mouse (Coan et al., 2006). Little is known about the regulators of GTC development; however, GTC number is remarkably changed in Igf2 (Lopez et al., 1996), and p57kip2 (Takahashi et al., 2000) mutant mice, suggesting direct or indirect regulation by these factors. Moreover, a recent study showed that genetic deficiency in granulocyte–macrophage colony-stimulating factor (CSF2) alters placental gene expression and GTC and giant cell abundance in mice (Sferruzzi-Perri et al., 2009). The CSF2 ligand and the CSF2 receptor alpha subunit are predominantly synthesized in the placental junctional zone. Altered placental structure in Csf2 null mice at E15 is characterized by an expanded junctional zone and by increased Cx31(+) glycogen cells and cyclin-dependent kinase inhibitor 1C (CDKN1C(+), P57(Kip2+)) giant cells, accompanied by elevated junctional zone transcription of genes controlling spongiotrophoblast and giant cell differentiation and secretory function (Ascl2, Hand1, Prl3d1, and Prl2c2; Sferruzzi-Perri et al., 2009).
In most mammalian species, a key process of placenta development is the fusion of trophoblast cells into a highly specialised, multinucleated syncytiotrophoblast layer, through which most of the materno-fetal exchanges take place. Little is known about this process; however, a recent study has suggested an essential role for syncytin-A in trophoblast cell differentiation and syncytiotrophoblast morphogenesis during placenta development (Dupressoir et al., 2009). Mouse syncytin-A and syncytin-B are two fusogenic placenta-specific murine envelope genes of retroviral origin, and have in vitro cell–cell fusion activity (Dupressoir et al., 2005). Using gene knockout approaches, Dupressoir et al. (2009) found that homozygous syncytin-A null mouse embryos die in utero between 11.5 and 13.5 days of gestation, with specific disruption of the architecture of the syncytiotrophoblast-containing labyrinth, and the trophoblast cells failing to fuse into an interhemal syncytial layer. Lack of syncytin-A-mediated trophoblast cell fusion is associated with cell overexpansion at the expense of fetal blood vessel spaces and with apoptosis, adding to the observed materno-fetal interface structural defects to provoke decreased vascularization, inhibition of placental transport, and fetal growth retardation (Dupressoir et al., 2009). Similarly, syncytin glycoproteins are expressed in human trophoblastic cells and are involved in trophoblastic cell fusion (Malassine et al., 2005, 2007; Frendo et al., 2003).
Differentiated syncytiotrophoblasts (SynTs) comprise the bulk of the trophoblast component in the labyrinthine layer after fusion of allantoic mesoderm with the basal surface of the chorion (Cross, 2000). Gcm1 is a key transcription factor, which regulates both morphogenesis and differentiation of SynTs (Anson-Cartwright et al., 2000). Gcm1-deficient mouse embryos show failure of SynT formation and villous morphogenesis is not initiated (Anson-Cartwright et al., 2000). Gcm1 expression is mainly restricted to the murine placenta starting from day 7.5 p.c. (Basyuk et al., 1999; Anson-Cartwright et al., 2000) and is maintained by unknown signals from the allantois (Hunter et al., 1999). Gcm1 and Syncytin-A, a gene expressed in SynTs, stimulates TSC differentiation into SynTs, but not toward TGCs (Anson-Cartwright et al., 2000; Gong et al., 2007). Recently, the RNA-binding protein HuR has been found to control the differentiation of SynTs. HuR’s absence impairs the invagination of allantoic capillaries into the chorionic trophoblast layer and the differentiation of SynTs that controls the morphogenesis and vascularization of the placental labyrinth and fetal support. (Katsanou et al., 2009). Other genes, including Esx1 (Li and Behringer, 1998), Dlx3 (Morasso et al., 1999) and Pparγ (Barak et al., 1999) are also expressed exclusively in the labyrinthine layer of mature placenta and are critical for its development. The regulation and function of these genes are still unknown.
Like other trophoblast subtypes, SynT differentiation and branching of chorionic villi are regulated by intercellular signalling, including FGF/Fgfr, HGF/Met, EGF/Egfr and Wnt/Fzd (Cross et al., 2003; Cross, 2005). A hypomorphic mutation in Fgfr2 results in defects of chorioallantoic branching (Xu et al., 1998). Likewise, mutant mice for Hgf (Uehara et al., 1995) or its c-Met receptor (Bladt et al., 1995), Fra1, JunB or genes encoding signalling adaptor proteins (Grb2, Mek1, p38, Sos1; downstream targets of receptor tyrosine kinases; Rossant and Cross, 2001) show a similar phenotype. Recently, Nadeau et al. (2009) found that Map2k1 and Map2k2, which encode dual-specificity kinases responsible for ERK/MAP kinase activation that is essential for growth factor signalling, are also required for normal development of syncytiotrophoblasts during placentation. Map2k2 haploinsufficiency affects the normal development of placenta in the absence of one Map2k1 allele. Most Map2k1+/− Map2k2+/− double embryos die during gestation because of placenta defects restricted to extra-embryonic tissues. Moreover, the deletion of one or both Map2k2 alleles in the context of one null Map2k1 allele leads to the formation of multinucleated trophoblast giant (MTG) cells. MTG cells are derived from Gcm1-expressing SynTs, which are affected in their ability to form the uniform SynT layer II lining the maternal sinuses (Nadeau et al., 2009). In addition, Wnt signalling is required for chorioallantoic attachment and labyrinth development. A failure in chorioallantoic attachment is reported in Wnt7b−/− mouse embryos (Parr et al., 2001). A gene encoding a Wnt receptor, Fzd5, is expressed in labyrinth trophoblasts and is essential for placental angiogenesis and yolk sac formation (Ishikawa et al., 2001), while allantois-derived Wnt2 is required later in labyrinth development (Monkley et al., 1996).
Furthermore, studies by Dackor et al. (2007, 2009a,2009b) highlighted the importance of EGFR, which is a member of the ERBB family of receptor tyrosine kinases, in placental development. In mice that are Egfr(tm1Mag) nullizygous, placentas have fewer proliferative trophoblasts than wild-type and exhibit strain-specific defects in the spongiotrophoblast and labyrinth layers (Dackor et al., 2007, 2009a). Moreover, increasing the levels of EGFR signalling by using hypermorphic Egfr(Dsk5) allele results in larger placental size with a more prominent spongiotrophoblast layer and increased expression of glycogen cell-specific genes (Dackor et al., 2009b). This study also demonstrated that mice with increased levels of EGFR signalling exhibit an extensive level of genetic background-dependent phenotypic variability, and EGFR expressed in the uterine stroma may play an underappreciated role in preparation of the uterus for embryo implantation (Dackor et al., 2009b).
Recent studies showed that several members of the Notch signalling pathway, as well as the potential Notch target Mash2, are necessary for the proper development of a functional placenta. Notch2 regulates maternal blood sinuses formation; and Notch1, Notch1/4, Hey1/2, Dll4 and RBPJκ play important roles in vascular morphogenesis of the placenta. Additionally there are several reports describing an expression of other Notch pathway members: Dll1, Hes1, Tle1–3, in the placenta. However, the molecular processes by which members of the Notch pathway exert their roles are still not well understood (Gasperowicz and Otto, 2008).
6. Conclusions and future directions
Rapid progress in understanding placental development and its regulatory molecules has been achieved in the last decade. However, much more work to complete the analysis of both the molecular and cellular differentiation of TGCs remains to be done. Our current understanding of the process of trophoblast differentiation confirms that it has several aspects that are highly relevant to other morphogenetic and differentiation events. This is clearly evidenced by recent findings that trophoblast differentiation involves several molecular and cellular mechanisms that have been discovered during the differentiation of other embryonic tissues. This includes, among others, Hand1 expression, which is important for TGC and cardiac differentiation, and Eomes expression that is common between trophoblast and mesodermal cells. In addition, an analysis with a focus on placenta development of already existing Notch pathway mutants as well as the generation of mouse strains carrying targeted mutations in further members of the pathway is awaited for a better understanding of the Notch pathway function in placentation.
Several other general conclusions have been revealed from recent accumulated evidence of placenta and trophoblast development. Placental development, as does development of other embryonic organs, progresses through several steps, and several key regulators of these steps have now been identified. However, further work will be needed to discover up-stream and downstream genes involved in each step. Another exciting aspect of murine placenta regulatory genes is the discovery of human homologues of several of them. This will allow scientists to explore whether human placental pathologies are associated with changes in the expression of these genes. Further studies are required to understand the crosstalk between the epiblast and the developing trophoblast population during early trophoblast differentiation and the signalling exchange between the maternal system and the different cell types of the developing placenta. In addition, linking the molecular and cellular mechanisms of trophoblast differentiation will not only help in fully understanding trophoblast differentiation but also will facilitate discovery of critical changes that lead to developmental defects in the placenta.
Acknowledgments
This work was supported by the University of Manchester Research Scholarship (URS) and Overseas Research Scholarship (ORS) awards to AHKE.
REFERENCES
- Abell AN, Granger DA, Johnson N, Vincent-Jordan N, Dibble C, Johnson GL. Trophoblast stem cell maintenance by fibroblast growth factor 4 requires MEKK4 activation of Jun N-terminal kinase. Mol. Cell Biol. 2009;29(10):2748–2761. doi: 10.1128/MCB.01391-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev. Biol. 2002;250(2):358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- Ahmed A, Perkins J. Angiogenesis and intrauterine growth restriction. Baillieres Best. Pract. Res. Clin. Obstet. Gynacol. 2000;14:981–998. doi: 10.1053/beog.2000.0139. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Howard EW, Bissell MJ, Werb Z. Rescue of mammary epithelial cell apoptosis and entactin degradation by a TIMP-1 transgene. J. Cell Biol. 1996;135:1669–1677. doi: 10.1083/jcb.135.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen WR, Gower S, Wilsher S. Immunohistochemical localization of vascular endothelial growth factor (VEGF) and its two receptors (Flt-I and KDR) in the endometrium and placenta of the mare during the oestrous cycle and pregnancy. Reprod. Domest. Anim. 2007;42(5):516–526. doi: 10.1111/j.1439-0531.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- Anson-Cartwright L, Dawson K, Holmyard D, Fisher S, Lazzarini R, Cross JC. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat. Genet. 2000;25(3):311–314. doi: 10.1038/77076. [DOI] [PubMed] [Google Scholar]
- Armant DR, Kaplan H, Lennarz WJ. Fibronectin and laminin promote in vitro attachment and outgrowth of mouse blastocysts. Dev. Biol. 1986;116:519–523. doi: 10.1016/0012-1606(86)90152-1. [DOI] [PubMed] [Google Scholar]
- Asanoma K, Kato H, Yamaguchi S, Shin C, Liu Z, Kato K, Inoue T, Miyanari Y, Yoshikawa K, Sonoda K, Fukushima K, Wake N. HOP/NECC1, a novel regulator of mouse trophoblast differentiation. J. Biol. Chem. 2007;282(33):24065–24074. doi: 10.1074/jbc.M701380200. [DOI] [PubMed] [Google Scholar]
- Auman HJ, Nottoli T, Lakiza O, Winger Q, Donaldson S, Williams T. Transcription factor AP-2γ is essential in the extra-embryonic lineages for early postimplantation development. Development. 2002;129:2733–2747. doi: 10.1242/dev.129.11.2733. [DOI] [PubMed] [Google Scholar]
- Barak Y, Nelson M, Ong E, Jones Y, Ruiz-Lozano P, Chien K, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell. 1999;4(4):585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- Basyuk E, Cross J, Corbin J, Nakayama H, Hunter P, Nait-Oumesmar B, Lazzarini RA. Murine Gcm1 gene is expressed in a subset of placental trophoblast cells. Dev. Dyn. 1999;214(4):303–311. doi: 10.1002/(SICI)1097-0177(199904)214:4<303::AID-AJA3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Bevilacqua EM, Abrahamsohn PA. Ultrastructure of mouse trophoblast giant cell transformation during the invasive stage of implantation of the mouse embryo. J. Morphol. 1988;198:341–351. doi: 10.1002/jmor.1051980308. [DOI] [PubMed] [Google Scholar]
- Bevilacqua EM, Abrahamsohn PA. Trophoblast invasion during implantation of the mouse embryo. Arch. Biol. Med. Exp. 1989;22:107–118. [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376(6543):768–4671. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Boudjelal M, Taneja R, Matsubara S, Bouillet P, Dolle P, Chambon P. Overexpression of Stra13, a novel retinoic acid-inducible gene of the basic helix-loop-helix family inhibits mesodermal and promotes neuronal differentiation of P19 cells. Genes Dev. 1997;11:2052–2065. doi: 10.1101/gad.11.16.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A, Nygard K, Mazzuca D, Han V. The expression of insulin-like growth factor and insulin-like growth factor binding protein mRNAs in mouse placenta. Placenta. 2006;27:278–290. doi: 10.1016/j.placenta.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Cartwright JE, Holden DP, Whitley G. Hepatocyte growth factor regulates trophoblast cell motility and invasion: a role for nitric oxide. Br. J. Pharmacol. 1999;128:181–189. doi: 10.1038/sj.bjp.0702757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai N, Patel Y, Jacobson K, McMahon J, McMahon A, Rappolee DA. FGF is an essential regulator of the fifth cell division in preimplantation mouse embryos. Dev. Biol. 1998;198:105–115. doi: 10.1006/dbio.1997.8858. [DOI] [PubMed] [Google Scholar]
- Chawengsaksophak K, James R, Hammond VE, Kontgen F, Beck F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997;386:84–87. doi: 10.1038/386084a0. [DOI] [PubMed] [Google Scholar]
- Chiu SY, Asai N, Costantini F, Hsu W. SUMO-specific protease 2 is essential for modulating p53-Mdm2 in development of trophoblast stem cell niches and lineages. PLoS Biol. 2008;6(12):e310. doi: 10.1371/journal.pbio.0060310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan PM, Conroy N, Burton GJ, Ferguson-Smith AC. Origin and characteristics of glycogen cells in the developing murine placenta. Dev. Dyn. 2006;235(12):3280–3294. doi: 10.1002/dvdy.20981. [DOI] [PubMed] [Google Scholar]
- Colosi P, Ogren L, Southard JN, Thordarson G, Linzer DIH, Talamantes F. Biological, immunological, and binding properties of recombinant mouse placental lactogen-1. Endocrinology. 1988;123:2662–2667. doi: 10.1210/endo-123-6-2662. [DOI] [PubMed] [Google Scholar]
- Cross JC. Genetic insight into trophoblast differentiation and placental morphogenesis. Sem. Cell Dev. Biol. 2000;11:105–113. doi: 10.1006/scdb.2000.0156. [DOI] [PubMed] [Google Scholar]
- Cross JC. How to make a placenta: mechanisms of trophoblast cell differentiation in mice – a review. Placenta. 2005;26 Suppl. A:S3–S9. doi: 10.1016/j.placenta.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Cross JC, Flannery ML, Steingrimsson E, Jenkins NA, Copeland NG, Rutter WJ, Werb Z. Hxt encodes a basic helix-loope-helix transcription factor that regulates trophoblast cell development. Development. 1995;121:2513–2523. doi: 10.1242/dev.121.8.2513. [DOI] [PubMed] [Google Scholar]
- Cross JC, Anson-Cartwright L, Scott IC. Transcription factors underlying the development and endocrine functions of the placenta. Recent Prog. Horm. Res. 2002;57:221–234. doi: 10.1210/rp.57.1.221. [DOI] [PubMed] [Google Scholar]
- Cross JC, Baczyk D, Dobric N, Hemberger M, Hughes M, Simmons DG, Yamamoto H, Kingdom JCP. Genes, development and evolution of the placenta. Placenta. 2003;24:123–130. doi: 10.1053/plac.2002.0887. [DOI] [PubMed] [Google Scholar]
- Dackor J, Strunk K, Wehmeyer M, Threadgill DW. Altered trophoblast proliferation is insufficient to account for placental dysfunction in Egfr null embryos. Placenta. 2007;28(11–12):1211–1218. doi: 10.1016/j.placenta.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackor J, Caron KM, Threadgill DW. Placental and embryonic growth restriction in mice with reduced function epidermal growth factor receptor alleles. Genetics. 2009a doi: 10.1534/genetics.109.104372. Jun 29 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackor J, Li M, Threadgill DW. Placental overgrowth and fertility defects in mice with a hypermorphic allele of epidermal growth factor receptor. Mamm. Genome. 2009b;20(6):339–349. doi: 10.1007/s00335-009-9189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Wang X, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- Downs KM. The murine allantois. Curr. Top Dev. Biol. 1998;39:1–33. doi: 10.1016/s0070-2153(08)60451-2. [DOI] [PubMed] [Google Scholar]
- Douglas GC, VandeVoort CA, Kumar P, Chang T, Golos TG. Trophoblast stem cells: models for investigating trophectoderm differentiation and placental development. Endocr. Rev. 2009;30(3):228–240. doi: 10.1210/er.2009-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth ML, Kirk KL, Friesen HG. Isolation and identification of cDNA clone of rat placental lactogen II. J. Biol. Chem. 1986;261:10871–10878. [PubMed] [Google Scholar]
- Dupressoir A, Marceau G, Vernochet C, Bénit L, Kanellopoulos C, Sapin V, Heidmann T. Syncytin-A and syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Proc. Natl. Acad. Sci. USA. 2005;102:725–730. doi: 10.1073/pnas.0406509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupressoir A, Vernochet C, Bawa O, Harper F, Pierron G, Opolon P, Heidmann T. Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc. Natl. Acad. Sci. USA. 2009;106(29):12127–12132. doi: 10.1073/pnas.0902925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hashash AH, Kimber SJ. Trophoblast differentiation in vitro: establishment and characterisation of a serum-free culture model for murine secondary trophoblast giant cells. Reproduction. 2004;128(1):53–71. doi: 10.1530/rep.1.00149. [DOI] [PubMed] [Google Scholar]
- El-Hashash AH, Kimber SJ. PTHrP induces changes in cell cytoskeleton and E-cadherin and regulates Eph/Ephrin kinases and RhoGTPases in murine secondary trophoblast cells. Dev. Biol. 2006;290(1):13–31. doi: 10.1016/j.ydbio.2005.10.010. [DOI] [PubMed] [Google Scholar]
- El-Hashash AH, Esbrit P, Kimber SJ. PTHrP promotes murine secondary trophoblast giant cell differentiation through induction of endocycle, upregulation of giant-cell-promoting transcription factors and suppression of other trophoblast cell types. Differentiation. 2005;73(4):154–174. doi: 10.1111/j.1432-0436.2005.00013.x. [DOI] [PubMed] [Google Scholar]
- Erlebacher A, Price KA, Glimcher LH. Maintenance of mouse trophoblast stem cell proliferation by TGF-[β]/activin. Dev. Biol. 2004;275:158–169. doi: 10.1016/j.ydbio.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Fan Y, Melhem MF, Chaillet JR. Forced expression of the homeobox-containing gene Pem blocks differentiation of embryonic stem cells. Dev. Biol. 1999;210(2):481–496. doi: 10.1006/dbio.1999.9279. [DOI] [PubMed] [Google Scholar]
- Faria TN, Ogren L, Talamantes F, Linzer DI, Soares MJ. Localization of placental lactogen-I in trophoblast giant cells of the mouse placenta. Biol. Reprod. 1991;44:327–331. doi: 10.1095/biolreprod44.2.327. [DOI] [PubMed] [Google Scholar]
- Feldman B, Poueymirou W, Papaioannou VE, DeChiara TM, Goldfarb M. Requirement of FGF-4 for postimplantation mouse development. Science. 1995;267(5195):246–249. doi: 10.1126/science.7809630. [DOI] [PubMed] [Google Scholar]
- Frendo JL, Olivierm D, Cheynet V, Blond J, Bouton O, Vidaud M, Rabreau M, Evain-Brion D, Mallet F. Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol. Cell Biol. 2003;23(10):3566–3574. doi: 10.1128/MCB.23.10.3566-3574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galosy SS, Talamantes F. Luteotropic actions of placental lactogens at midpregnancy in the mouse. Endocrinology. 1995;136:3993–4003. doi: 10.1210/endo.136.9.7649108. [DOI] [PubMed] [Google Scholar]
- Gardner RL, Davies TJ. Lack of coupling between onset of giant transformation and genome endoreduplication in the mural trophectoderm of the mouse blastocyst. J. Exp. Zool. 1993;265:54–60. doi: 10.1002/jez.1402650108. [DOI] [PubMed] [Google Scholar]
- Gasperowicz M, Otto F. The notch signalling pathway in the development of the mouse placenta. Placenta. 2008;29(8):651–659. doi: 10.1016/j.placenta.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Goldin SN, Papaioannou VE. Paracrine action of FGF4 during periimplantation development maintains trophectoderm and primitive endoderm. Genesis. 2003;36(1):40–47. doi: 10.1002/gene.10192. [DOI] [PubMed] [Google Scholar]
- Goller T, Vauti F, Ramasamy S, Arnold HH. Transcriptional regulator BPTF/FAC1 is essential for trophoblast differentiation during early mouse development. Mol. Cell Biol. 2008;28(22):6819–6827. doi: 10.1128/MCB.01058-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves CR, Antonini S, Vianna-Morgante AM, Machado-Santelli GM, Bevilacqua E. Developmental changes in the ploidy of mouse implanting trophoblast cells in vitro. Histochem. Cell Biol. 2003;119:189–198. doi: 10.1007/s00418-003-0500-0. [DOI] [PubMed] [Google Scholar]
- Gong R, Huang L, Shi J, Luo K, Qiu G, Feng H, Tien P, Xiao G. Syncytin-A mediates the formation of syncytiotrophoblast involved in mouse placental development. Cell Physiol. Biochem. 2007;20(5):517–526. doi: 10.1159/000107535. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Nagy A, Auerbach A, Rossant J, Joyner AL. Essential role of Mash-2 in extraembryonic development. Nature. 1994;371:333–336. doi: 10.1038/371333a0. [DOI] [PubMed] [Google Scholar]
- Guzeloglu-Kayisli O, Kayisli UA, Taylor HS. The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin. Reprod. Med. 2009;27(1):62–79. doi: 10.1055/s-0028-1108011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Ayala M, Ben-Haim N, Beck S, Constam DB. Nodal protein processing and fibroblast growth factor 4 synergize to maintain a trophoblast stem cell microenvironment. Proc. Natl. Acad. Sci. USA. 2004;101(44):15656–15660. doi: 10.1073/pnas.0405429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovici F, Anderson DJ. Effects of growth factors and growth factor-extracellular matrix interactions on mouse trophoblast outgrowth in vitro. Biol. Reprod. 1993;49:124–130. doi: 10.1095/biolreprod49.1.124. [DOI] [PubMed] [Google Scholar]
- Harvey MB, Leco K, Arcellana-Panlilio M, Zhang X, Edwards D, Schultz G. Proteinase expression in early mouse embryos is regulated by leukaemia inhibitory factor and epidermal growth factor. Development. 1995;121:1005–1014. doi: 10.1242/dev.121.4.1005. [DOI] [PubMed] [Google Scholar]
- He Y, Smith S, Day K, Clark D, Licence D, Charnock-Jones DS. Alternative splicing of vascular endothelial growth factor (VEGF)-R1 (FLT-1) pre-mRNA is important for the regulation of VEGF activity. Mol. Endocrinol. 1999;13:537–545. doi: 10.1210/mend.13.4.0265. [DOI] [PubMed] [Google Scholar]
- Hemberger M, Hughes M, Cross JC. Trophoblast stem cells differentiate in vitro into invasive trophoblast giant cells. Dev. Biol. 2004;271:362–371. doi: 10.1016/j.ydbio.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Hughes M, Dobric N, Scott IC, Su L, Starovic M, St-Pierre B, Egan SE, Kingdom JC, Cross JC. The Hand1, Stra13 and Gcm1 transcription factors override FGF signalling to promote terminal differentiation of trophoblast stem cells. Dev. Biol. 2004;271:26–37. doi: 10.1016/j.ydbio.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Hunter PJ, Swanson B, Haendel M, Lyons G, Cross JC. Mrj encodes a DnaJ-related co-chaperone that is essential for murine placental development. Development. 1999;126(6):1247–1258. doi: 10.1242/dev.126.6.1247. [DOI] [PubMed] [Google Scholar]
- Ilgren EB. Control of trophoblast growth. Placenta. 1983;4:307–328. doi: 10.1016/s0143-4004(83)80010-1. [DOI] [PubMed] [Google Scholar]
- Ishida M, Ohashi S, Kizaki Y, Naito J, Horiguchi K, Harigaya T. Expression profiling of mouse placental lactogen II and its correlative genes using a cDNA microarray analysis in the developmental mouse placenta. J. Reprod. Dev. 2007;53(1):69–76. doi: 10.1262/jrd.18002. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Tamai Y, Zorn A, Yoshida H, Seldin M, Nishikawa S, Taketo M. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001;128(1):25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- Jedrusik A, Parfitt DE, Guo G, Skamagki M, Grabarek JB, Johnson MH, Robson P, Zernicka-Goetz M. Role of Cdx2 and cell polarity in cell allocation and specification of trophectoderm and inner cell mass in the mouse embryo. Genes Dev. 2008;22(19):2692–2706. doi: 10.1101/gad.486108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir-Salmani M, Shiokawa S, Akimoto Y, Hasan-Nejad H, Sakai K, Nagamatsu S, Sakai K, Nakamura Y, Hosseini A, Iwashita M. Characterization of morphological and cytoskeletal changes in trophoblast cells induced by insulinlike growth factor-I.J. Clin. Endocrinol. Metab. 2002;87:5751–5759. doi: 10.1210/jc.2002-020550. [DOI] [PubMed] [Google Scholar]
- Katsanou V, Milatos S, Yiakouvaki A, Sgantzis N, Kotsoni A, Alexiou M, Harokopos V, Aidinis V, Hemberger M, Kontoyiannis DL. The RNA-binding protein Elavl1/HuR is essential for placental branching morphogenesis and embryonic development. Mol. Cell Biol. 2009;29(10):2762–2776. doi: 10.1128/MCB.01393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Kawamura N, Sato W, Fukuda J, Kumagai J, Tanaka T. Brain-derived neurotrophic factor promotes implantation and subsequent placental development by stimulating trophoblast cell growth and survival. Endocrinology. 2009;150(8):3774–3782. doi: 10.1210/en.2009-0213. [DOI] [PubMed] [Google Scholar]
- Korgun ET, Celik-Ozenci C, Acar N, Cayli S, Desoye G, Demir R. Location of cell cycle regulators cyclin B1, cyclin A, PCNA, Ki67 and cell cycle inhibitors p21, p27 and p57 in human first trimester placenta and deciduas. Histochem. Cell Biol. 2006;125(6):615–624. doi: 10.1007/s00418-006-0160-y. [DOI] [PubMed] [Google Scholar]
- Kraut N, Sinder L, Chen C, Tapscott SJ, Groundine M. Requirement of the mouse I-mfa gene for placental development and skeletal patterning. EMBO J. 1998;17:6276–6288. doi: 10.1093/emboj/17.21.6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang H, Chen Q, Fan X, Zhang Y, Zhang L, Peng H, Cao Y, Duan E. CXCL14 inhibits trophoblast outgrowth via a paracrine/autocrine manner during early pregnancy in mice. J. Cell Physiol. 2009 doi: 10.1002/jcp.21877. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Kuhn R, Torres RM. Cre/loxP recombination system and gene targeting. Methods Mol. Biol. 2002;180:175–204. doi: 10.1385/1-59259-178-7:175. [DOI] [PubMed] [Google Scholar]
- Lehner CF, O’Farrell PH. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990;61:535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescisin KR, Varmuza S, Rossant J. Isolation and characterization of a novel trophoblast-specific cDNA in the mouse. Genes Dev. 1988;2(12A):1639–1646. doi: 10.1101/gad.2.12a.1639. [DOI] [PubMed] [Google Scholar]
- Li Y, Behringer RR. Esx1 is an X-chromosome-imprinted regulator of placental development and fetal growth. Nat. Genet. 1998;20(3):309–311. doi: 10.1038/3129. [DOI] [PubMed] [Google Scholar]
- Lilly MA, Spradling AC. The Drosophila endocycle is controlled by Cyclin E and lacks a checkpoint ensuring S-phase completion. Genes Dev. 1996;10:2514–2526. doi: 10.1101/gad.10.19.2514. [DOI] [PubMed] [Google Scholar]
- Linzer DIH, Fisher SJ. The placenta and the prolactin family of hormones: regulation of the physiology of pregnancy. Mol. Endocrinol. 1999;13:837–840. doi: 10.1210/mend.13.6.0286. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Dikkes P, Zurakowski D, Villa-Komaroff L. Insulin-like growth factor II affects the appearance and glycogen content of glycogen cells in the murine placenta. Endocrinology. 1996;137(5):2100–2108. doi: 10.1210/endo.137.5.8612553. [DOI] [PubMed] [Google Scholar]
- Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguère V. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature. 1997;388(6644):778–782. doi: 10.1038/42022. [DOI] [PubMed] [Google Scholar]
- Ma GT, Soloveva V, Tzeng S-J, Lowe L, Pfendler K, Iannaccone P, Kuehn M, Linzer D. Nodal regulates trophoblast differentiation and placental development. Dev. Biol. 2001;236:124–135. doi: 10.1006/dbio.2001.0334. [DOI] [PubMed] [Google Scholar]
- MacAuley A, Cross JC, Werb Z. Reprogramming the cell cycle for endoreduplication in rodent trophoblast cells. Mol. Biol. Cell. 1998;9(4):795–807. doi: 10.1091/mbc.9.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malassine A, Handschuh K, Tsatsaris V, Gerbaud P, Cheynet V, Oriol G, Mallet F, Evain-Brion D. Expression of HERV-W Env glycoprotein (syncytin) in the extravillous trophoblast of first trimester human placenta. Placenta. 2005;26(7):556–562. doi: 10.1016/j.placenta.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Malassine A, Blaise S, Handschuh K, Lalucque H, Dupressoir A, Evain-Brion D, Heidmann T. Expression of the fusogenic HERV-FRD Env glycoprotein (syncytin 2) in human placenta is restricted to villous cytotrophoblastic cells. Placenta. 2007;28(2–3):185–191. doi: 10.1016/j.placenta.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Martindill DM, Riley PR. Cell cycle switch to endocycle: the nucleolus lends a hand. Cell Cycle. 2008;7(1):17–23. doi: 10.4161/cc.7.1.5228. [DOI] [PubMed] [Google Scholar]
- Martindill DM, Risebro C, Smart N, Franco-Viseras Mdel M, Rosario C, Swallow C, Dennis J, Riley PR. Nucleolar release of Hand1 acts as a molecular switch to determine cell fate. Nat. Cell Biol. 2007;9(10):1131–1141. doi: 10.1038/ncb1633. [DOI] [PubMed] [Google Scholar]
- Monkley SJ, Delaney S, Pennisi D, Christiansen J, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122(11):3343–3353. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- Morasso MI, Grinberg A, Robinson G, Sargent T, Mahon KA. Placental failure in mice lacking the homeobox gene Dlx3. Proc. Natl. Acad. Sci. USA. 1999;96:162–167. doi: 10.1073/pnas.96.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrish DW, Kudo Y, Caniggia I, Cross J, Evain-Brion D, Gasperowicz M, Kokozidou M, Leisser C, Takahashi K, Yoshimatsu J. Growth factors and trophoblast differentiation. Placenta. 2007;28:S121–S124. doi: 10.1016/j.placenta.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Nadeau V, Guillemette S, Bélanger LF, Jacob O, Roy S, Charron J. Map2k1 and Map2k2 genes contribute to the normal development of syncytiotrophoblasts during placentation. Development. 2009;136(8):1363–1374. doi: 10.1242/dev.031872. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Liu Y, Stifani S, Cross JC. Developmental restriction of Mash-2 expression in trophoblast correlates with potential activation of the notch-2 pathway. Dev. Genet. 1997;21(1):21–30. doi: 10.1002/(SICI)1520-6408(1997)21:1<21::AID-DVG3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Scott IC, Cross JC. The transition to endoreduplication in trophoblast giant cells is regulated by the mSNA zinc finger transcription factor. Dev. Biol. 1998;199:150–163. doi: 10.1006/dbio.1998.8914. [DOI] [PubMed] [Google Scholar]
- Natanson-Yaron S, Anteby E, Greenfield C, Goldman-Wohl D, Hamani Y, Hochner-Celnikier D, Yagel S. FGF 10 and Sprouty 2 modulate trophoblast invasion and branching morphogenesis. Mol. Hum. Reprod. 2007;13(7):511–519. doi: 10.1093/molehr/gam034. [DOI] [PubMed] [Google Scholar]
- Navarrete Santos A, Ramin N, Tonack S, Fischer B. Cell lineage-specific signalling of insulin and insulin like growth factor (IGF) 1 in rabbit blastocysts. Endocrinology. 2008;149(2):515–524. doi: 10.1210/en.2007-0821. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:375–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Nishioka N, Yamamoto S, Kiyonari H, Sato H, Sawada A, Ota M, Nakao K, Sasaki H. Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mech. Dev. 2008;125(3–4):270–283. doi: 10.1016/j.mod.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Nowak RA, Haimovici F, Biggers JD, Erbach GT. Transforming growth factor-β stimulates mouse blastocyst outgrowth through a mechanism involving parathyroid hormone-related protein. Biol. Reprod. 1999;60:85–93. doi: 10.1095/biolreprod60.1.85. [DOI] [PubMed] [Google Scholar]
- Oda M, Shiota K, Tanaka S. Trophoblast stem cells. Methods Enzymol. 2006;419:387–400. doi: 10.1016/S0076-6879(06)19015-1. [DOI] [PubMed] [Google Scholar]
- Ogren L, Talamantes F. Prolactins of pregnancy and their cellular source. Int. Rev. Cytol. 1988;112:1–65. doi: 10.1016/s0074-7696(08)62005-7. [DOI] [PubMed] [Google Scholar]
- Ohlsson R, Falck P, Hellström M, Lindahl P, Boström H, Franklin G, Ahrlund-Richter L, Pollard J, Soriano P, Betsholtz C. PDGFB regulates the development of the labyrinthine layer of the mouse fetal placenta. Dev. Biol. 1999;212(1):124–136. doi: 10.1006/dbio.1999.9306. [DOI] [PubMed] [Google Scholar]
- Palazon LS, Davies TJ, Gardner RL. Translational inhibition of cyclin B1 and appearance of cyclin D1 very early in the differentiation of mouse trophoblast giant cells. Mol. Human Reprod. 1998;4:1013–1020. doi: 10.1093/molehr/4.11.1013. [DOI] [PubMed] [Google Scholar]
- Palmieri SL, Peter W, Hess H, Schöler HR. Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev. Biol. 1994;166(1):259–267. doi: 10.1006/dbio.1994.1312. [DOI] [PubMed] [Google Scholar]
- Parast MM, Aeder S, Sutherland AE. Trophoblast giant cell differentiation involves changes in cytoskeleton and cell motility. Dev. Biol. 2001;230:43–60. doi: 10.1006/dbio.2000.0102. [DOI] [PubMed] [Google Scholar]
- Parr BA, Cornish V, Cybulsky M, McMahon AP. Wnt7b regulates placental development in mice. Dev. Biol. 2001;237(2):324–332. doi: 10.1006/dbio.2001.0373. [DOI] [PubMed] [Google Scholar]
- Prast J, Saleh L, Husslein H, Sonderegger S, Helmer H, Knöfler M. Human chorionic gonadotrophin stimulates trophoblast invasion through ERK and AKT signalling. Endocrinology. 2008;149(3):979–987. doi: 10.1210/en.2007-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Dutta D, Rumi MA, Kent LN, Soares MJ, Paul S. Context-dependent function of regulatory elements and a switch in chromatin occupancy between GATA3 and GATA2 regulate Gata2 transcription during trophoblast differentiation. J. Biol. Chem. 2009;284(8):4978–4988. doi: 10.1074/jbc.M807329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard L, Farnsworth E, Talamantes F. Calcyclin in mouse decidua: expression and effects on placental lactogen secretion. Biol.Reprod. 1998;59:546–552. doi: 10.1095/biolreprod59.3.546. [DOI] [PubMed] [Google Scholar]
- Richardson BD, Langland R, Bachurski C, Richards R, Kessler C, Cheng Y, Handwerger S. Activator protein-2 regulates human placental lactogen gene expression. Mol. Cell Endocrinol. 2000;160:183–192. doi: 10.1016/s0303-7207(99)00209-9. [DOI] [PubMed] [Google Scholar]
- Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat. Genet. 1998;18:271–275. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- Rinkenberger JL, Cross JC, Werb Z. Molecular genetics of implantation in the mouse. Dev. Genet. 1997;21:6–20. doi: 10.1002/(SICI)1520-6408(1997)21:1<6::AID-DVG2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Robertson MC, Gillespie B, Friesen HG. Characterization of two forms of rat placental lactogen (rPL): rPL-I and rPL-II. Endocrinology. 1982;111:1862–1866. doi: 10.1210/endo-111-6-1862. [DOI] [PubMed] [Google Scholar]
- Romagnano L, Babiarz B. The role of murine cell surface galactosyltransferase in trophoblast: laminin interaction in vitro. Dev. Biol. 1990;141:254–261. doi: 10.1016/0012-1606(90)90381-r. [DOI] [PubMed] [Google Scholar]
- Rossant J. Stem cells from the mammalian blastocyst. Stem cells. 2001;19:477–482. doi: 10.1634/stemcells.19-6-477. [DOI] [PubMed] [Google Scholar]
- Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat. Rev. Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- Rossant J, Guillemot F, Tanaka M, Latham K, Gertenstein M, Nagy A. Mash2 is expressed in oogenesis and preimplantation development but is not required for blastocyst formation. Mech. Dev. 1998;73:183–191. doi: 10.1016/s0925-4773(98)00051-3. [DOI] [PubMed] [Google Scholar]
- Rout UK, Wang J, Paria BC, Armant DR. Alpha5beta1, alphaVbeta3 and the platelet-associated integrin alphaIIbbeta3 coordinately regulate adhesion and migration of differentiating mouse trophoblast cells. Dev. Biol. 2004;268:135–151. doi: 10.1016/j.ydbio.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Russ AP, Wattler S, Colledge W, Aparicio S, Carlton M, Pearce J, Barton S, Surani M, Ryan K, Nehls M, Wilson V, Evans MJ. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature. 2000;404:95–99. doi: 10.1038/35003601. [DOI] [PubMed] [Google Scholar]
- Sapin V, Bouillet P, Oulad-Abdelghani M, Dastugue B, Chambon P, Dollè P. Differential expression of retinoic-acid inducible (Stra) genes during mouse placentation. Mech. Dev. 2000;92:295–299. doi: 10.1016/s0925-4773(00)00241-0. [DOI] [PubMed] [Google Scholar]
- Sappino AP, Hurate J, Belin D, Vassali JD. Plasminogen activators in tissue remodelling and invasion: mRNA localisation in mouse ovaries and implanting embryos. J. Cell Biol. 1989;109:312–323. doi: 10.1083/jcb.109.5.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorpp-Kistner M, Wang Z-Q, Angel P, Wagner EF. JunB is essential for mammalian placentation. EMBO J. 1999;18:934–948. doi: 10.1093/emboj/18.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz LC, Widmaier EP, Qiu J, Roberts RM. Effect of leptin on mouse trophoblast giant cells. Biol. Reprod. 2009;80(3):415–424. doi: 10.1095/biolreprod.108.073130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott IC, Anson-Cartwright L, Riley P, Reda D, Cross JC. The Hand1 basic helix-loop-helix transcription factor regulates trophoblast giant cell differentiation via multiple mechanisms. Mol. Cell Biol. 2000;20:530–541. doi: 10.1128/mcb.20.2.530-541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sferruzzi-Perri AN, Macpherson AM, Roberts CT, Robertson SA. Csf2 null mutation alters placental gene expression and trophoblast glycogen cell and giant cell abundance in mice. Biol. Reprod. 2009;81(1):207–221. doi: 10.1095/biolreprod.108.073312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev. Biol. 2005;284(1):12–24. doi: 10.1016/j.ydbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Simmons DG, Fortier AL, Cross JC. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev. Biol. 2007;304(2):567–578. doi: 10.1016/j.ydbio.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Simmons DG, Rawn S, Davies A, Hughes M, Cross JC. Spatial and temporal expression of the 23 murine prolactin/ placental lactogen-related genes is not associated with their position in the locus. BMC Genomics. 2008;9:352. doi: 10.1186/1471-2164-9-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MJ, Talamantes F. Genetic and litter size effects on serum placental lactogen in the mouse. Biol. Reprod. 1983;29:165–171. doi: 10.1095/biolreprod29.1.165. [DOI] [PubMed] [Google Scholar]
- Soares MJ, Colosi P, Talamantes F. The development and characterization of homologous radioimmunoassay for mouse placental lactogen. Endocrinology. 1982;110:668–670. doi: 10.1210/endo-110-2-668. [DOI] [PubMed] [Google Scholar]
- Soares MJ, Konno T, Alam SM. The prolactin family: effectors of pregnancy-dependent adaptations. Trends Endocrinol. Metab. 2007;18(3):114–121. doi: 10.1016/j.tem.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Steingrimsson E, Tessarollo L, Reid S, Jenkins N, Copeland NG. The bHLH-Zip transcription factor Tfeb is essential for placental vascularization. Development. 1998;125:4607–4616. doi: 10.1242/dev.125.23.4607. [DOI] [PubMed] [Google Scholar]
- Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- Sutherland AE. Mechanisms of implantation in the mouse: differentiation and functional importance of trophoblast giant cell behavior. Dev. Biol. 2003;258:241–251. doi: 10.1016/s0012-1606(03)00130-1. [DOI] [PubMed] [Google Scholar]
- Sutherland AE, Calarco P, Damasky CH. Expression and function of cell surface extracellular matrix receptors in mouse blastocyst attachment and outgrowth. J. Cell Biol. 1988;106:1331–1348. doi: 10.1083/jcb.106.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland AE, Sanerson R, Mayes M, Seibert M, Calarco P, Bernfield M, Damsky CH. Expression of syndecan, a putative low affinity fibroblast growth factor receptor, in the early mouse embryo. Development. 1991;113:339–351. doi: 10.1242/dev.113.1.339. [DOI] [PubMed] [Google Scholar]
- Sutherland AE, Calarco P, Damsky CH. Developmental regulation of integrin expression at the time of implantation in the mouse embryo. Development. 1993;119:1175–1186. doi: 10.1242/dev.119.4.1175. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kobayashi T, Kanayama N. P57(Kip2) regulates the proper development of labyrinthine and spongiotrophoblasts. Mol. Hum. Reprod. 2000;6(11):1019–1025. doi: 10.1093/molehr/6.11.1019. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Takahashi M, Carpino N, Jou S, Chao J, Tanaka S, Shigeyoshi Y, Parganas E, Ihle JN. Leukemia inhibitory factor regulates trophoblast giant cell differentiation via Janus kinase 1-signal transducer and activator of transcription 3-suppressor of cytokine signaling 3 pathway. Mol. Endocrinol. 2008;22(7):1673–1681. doi: 10.1210/me.2008-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Gertsenstein M, Rossant J, Nagy A. Mash2 acts cell autonomously in mouse spongiotrophoblast development. Dev. Biol. 1997;190:55–65. doi: 10.1006/dbio.1997.8685. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis A-K, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- Taniguchi F, Harada T, Yoshida S, Iwabe T, Onohara Y, Tanikawa M, Terakawa N. Paracrine effects of bFGF and KGF on the process of mouse blastocyst implantation. Mol. Reprod.Dev. 1998;50:54–62. doi: 10.1002/(SICI)1098-2795(199805)50:1<54::AID-MRD7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Tayade C, Hilchie D, He H, Fang Y, Moons L, Carmeliet P, Foster R, Croy BA. Genetic deletion of placenta growth factor in mice alters uterine NK cells. J. Immunol. 2007;178(7):4267–4275. doi: 10.4049/jimmunol.178.7.4267. [DOI] [PubMed] [Google Scholar]
- Teesalu T, Blasi F, Talarico D. Embryo implantation in mouse: fetomaternal coordination in the pattern of expression of uPA, uPAR, PAI-1 and alpha 2MR/LRP genes. Mech. Dev. 1996;56:103–116. doi: 10.1016/0925-4773(96)00515-1. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris C, Becker R, Hearn JP. Isolation of a primate embryonic stem cell line. Proc. Natl. Acad. Sci.USA. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro S, Waknitz M, Swiergiel J, Marshall V, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269(5221):230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Kunath T, Bergeron D, Lapointe L, Champigny C, Bader J, Rossant J, Giguère V. Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERR beta. Genes Dev. 2001;15(7):833–838. doi: 10.1101/gad.873401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373(6516):702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- Ullah Z, Kohn MJ, Yagi R, Vassilev LT, DePamphilis ML. Differentiation of trophoblast stem cells into giant cells is triggered by p57/Kip2 inhibition of CDK1 activity. Genes Dev. 2008;22(21):3024–3036. doi: 10.1101/gad.1718108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware CB, Horowitz M, Renshaw B, Hunt J, Liggitt D, Koblar S, Gliniak B, McKenna H, Papayannopoulou T, Thoma B, et al. Targeted disruption of the low-affinity leukemia inhibitory factor receptor gene causes placental, skeletal,neural and metabolic defects and results in perinatal death. Development. 1995;121(5):1283–1299. doi: 10.1242/dev.121.5.1283. [DOI] [PubMed] [Google Scholar]
- Welsh CF, Roovers K, Villanueva J, Li Y, Schwartz MA, Assoian RK. Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nat.Cell Biol. 2001;3:950–957. doi: 10.1038/ncb1101-950. [DOI] [PubMed] [Google Scholar]
- Wen F, Tynan J, Cecena G, Williams R, Múnera J, Mavrothalassitis G, Oshima RG. Ets2 is required for trophoblast stem cell self-renewal. Dev. Biol. 2007;312(1):284–299. doi: 10.1016/j.ydbio.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling U, Schorle H. Transcription factor gene AP-2γ essential for early murine development. Mol. Cell Biol. 2002;22(9):3149–3156. doi: 10.1128/MCB.22.9.3149-3156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside EJ, Boucaut K, The A, Garcia-Aragon J, Harvey M, Herington AC. Elevated concentration of TNF-alpha induces trophoblast differentiation in mouse blastocyst outgrowths. Cell Tissue Res. 2003;314(2):275–280. doi: 10.1007/s00441-003-0791-4. [DOI] [PubMed] [Google Scholar]
- Wilhide CC, Van Dan C, Dispersio J, Kenedy AA, Bray PF. Overexpression of cyclin D1 in the Dami megakaryocytic cell line causes growth arrest. Blood. 1995;86:294–304. [PubMed] [Google Scholar]
- Winger QA, Guttormsen J, Gavin H, Bhushan F. Heat shock protein 1 and the mitogen-activated protein kinase 14 pathway are important for mouse trophoblast stem cell differentiation. Biol. Reprod. 2007;76(5):884–891. doi: 10.1095/biolreprod.106.056820. [DOI] [PubMed] [Google Scholar]
- Wysolmerski JJ, Stewart AF. The physiology of parathyroid hormone-related protein: an emerging role as a developmental factor. Annu. Rev.Physiol. 1998;60:431–460. doi: 10.1146/annurev.physiol.60.1.431. [DOI] [PubMed] [Google Scholar]
- Xu RH, Chen X, Li D, Li R, Addicks G, Glennon C, Zwaka T, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat.Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- Xu RH, Peck RM, Li D, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat.Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- Xu X, Weinstein M, Li C, Naski M, Cohen R, Ornitz D, Leder P, Deng C. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development. 1998;125(4):753–765. doi: 10.1242/dev.125.4.753. [DOI] [PubMed] [Google Scholar]
- Yagi R, Kohn M, Karavanova I, Kaneko K, Vullhorst D, Depamphilis M, Buonanno A. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development. 2007;134(21):3827–3836. doi: 10.1242/dev.010223. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Ogren L, Endo H, Thordarson G, Bigsby RM, Talamantes F. Production of mouse placental lactogen-I and placental lactogen-II by the same giant cell. Endocrinology. 1992;131(4):1595–1602. doi: 10.1210/endo.131.4.1396305. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Sawada K, Miyake A. Lipopolysaccharides selectively inhibit mouse placental lactogen-II secretion through stimulation of interleukin-1 alpha (IL-1 alpha) and IL-6 production. J. Endocrinol. Invest. 1996;19(7):415–421. doi: 10.1007/BF03349885. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Flannery M, Kupriyanov S, Pearce J, McKercher S, Henkel G, Maki R, Werb Z, Oshima RG. Defective trophoblast functions in mice with a targeted mutation of Ets2. Genes Dev. 1998;12:1315–1326. doi: 10.1101/gad.12.9.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Tanaka S, Oda M, Makino T, Ohgane J, Shiota K. Retinoic acid promotes differentiation of trophoblast stem cells to a giant cell fate. Dev.Biol. 2001;235:422–432. doi: 10.1006/dbio.2001.0300. [DOI] [PubMed] [Google Scholar]
- Zhao M, Qiu W, Li Y, Zhang Z, Li D, Wang Y. Dual effect of transforming growth factor beta1 on cell adhesion and invasion in human placenta trophoblast cells. Reproduction. 2006;132(2):333–341. doi: 10.1530/rep.1.01112. [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Cheng P, Weintraub H. B-lymphocyte development is regulated by the combined dosage of three basic helix-loop-helix genes E2A, E2-2 and HEB. Mol. Cell Biol. 1996;16:2898–2905. doi: 10.1128/mcb.16.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zybina EV, Zybina TG. Polytene chromosomes in mammalian cells. Int. Rev.Cytol. 1996;165:53–119. doi: 10.1016/s0074-7696(08)62220-2. [DOI] [PubMed] [Google Scholar]