Abstract
Background
Although regulated payments to encourage living kidney donation could reduce morbidity and mortality among patients waiting for a kidney transplant, doing so raises several ethical concerns.
Objective
To determine the extent to which the 3 main concerns with paying kidney donors might manifest if a regulated market were created.
Design
Cross-sectional study of participants’ willingness to donate a kidney in 12 scenarios.
Setting
Regional rail and urban trolley lines in Philadelphia County, Philadelphia, Pennsylvania.
Participants
Of 550 potential participants, 409 completed the questionnaire (response rate, 74.4%); 342 of these participants were medically eligible to donate.
Intervention
Across scenarios, researchers experimentally manipulated the amount of money that participants would receive, the participants’ risk for subsequently developing kidney failure themselves, and who would receive the donated kidney.
Measurements
The researchers determined whether payment represents an undue inducement by evaluating participants’ sensitivity to risk in relation to the payment offered or an unjust inducement by evaluating participants’ sensitivity to payment as a function of their annual income. The researchers also evaluated whether introducing payment would hinder altruistic donations by comparing participants’ willingness to donate altruistically before versus after the introduction of payments.
Results
Generalized estimating equation models revealed that participants’ willingness to donate increased significantly as their risk for kidney failure decreased, as the payment offered increased, and when the kidney recipient was a family member rather than a patient on a public waiting list (P < 0.001 for each). No statistical interactions were identified between payment and risk (odds ratio, 1.00 [95% CI, 0.96 to 1.03]) or between payment and income (odds ratio, 1.01 [CI, 0.99 to 1.03]). The proximity of these estimates to 1.0 and narrowness of the CIs suggest that payment is neither an undue nor an unjust inducement, respectively. Alerting participants to the possibility of payment did not alter their willingness to donate for altruistic reasons (P = 0.40).
Limitation
Choices revealed in hypothetical scenarios may not reflect real-world behaviors.
Conclusion
Theoretical concerns about paying persons for living kidney donation are not corroborated by empirical evidence. A real-world test of regulated payments for kidney donation is needed to definitively show whether payment provides a viable and ethical method to increase the supply of kidneys available for transplantation.
Primary Funding Source
None.
The insufficient supply of transplantable kidneys from traditional donors after neurologic determination of death (1, 2) has prompted increasing use of kidneys from the following types of donors: donors after circulatory determination of death (3), donors with risk factors for harboring transmittable infections (4), expanded-criteria donors (that is, those with risk factors, such as older age or hypertension) (5), and living donors related or unrelated to the recipient (6, 7).
Unfortunately, despite these efforts to increase the pool of kidneys, the median time to transplantation, number of patients on the waiting list, and number of patients who die while waiting for an organ continue to increase (8). Thus, for the past decade, ethicists and members of the transplant community have debated the approach of paying healthy persons to become living donors (9 –17). International black markets in organs are almost universally condemned because safeguards to protect donors are largely absent, brokers rather than donors may commandeer most of the payments, and such systems almost invariably entail wealthy travelers purchasing organs from poor natives (18, 19). By contrast, a less well-resolved ethical debate regards a regulated national market for kidneys in which donors receive payment according to a fixed and transparent schedule, organs are allocated according to standard criteria, and standards are set and monitored to ensure appropriate longitudinal care for donors (14, 20).
The potential benefits of such a regulated market are clear. Compared with lifelong dialysis, kidney transplantation from deceased donors substantially increases quality-adjusted life expectancy and is cost-saving (21, 22). Because kidney transplantation from living donors produces greater benefits (6), particularly when done before recipients initiate dialysis (7), even large payments (for example, $100 000) are estimated to be a cost-effective way to increase the supply of kidneys available for transplantation (8, 23).
However, at least 3 concerns exist with regulated payments for living kidney donation. First, payments may represent undue inducements—payments might alter a person’s perception of the risks associated with donation, thereby preventing a fully informed decision to sell a kidney. Second, payments may represent unjust inducements—payments might preferentially influence lower-income persons, thereby creating a market in which organs are acquired from poor persons and provided to those with sufficient financial and social resources to be listed for transplantation. Third, payments may dissuade altruistic donation or cause potential altruistic donors to request payment.
In this study, we did not aim to assess the conceptual strengths and weaknesses of these concerns, but rather we used empirical methods to determine the extent to which these concerns might manifest if a regulated market for kidneys were established in the United States.
Methods
Pilot Study
We developed a baseline description of living kidney donation and assessed its clarity among 51 persons awaiting jury duty in Philadelphia, Pennsylvania. Pilot study participants most commonly identified the recipient’s relationship to them (78%) and the burdens and risks associated with donation (14%) as the most important considerations governing whether they would donate. Among 7 specific donor risks presented, the possibility that participants would later develop kidney failure was most commonly (91%) cited as the most important.
Instrument Design
After clarifying the final kidney donation description to reflect the feedback from the pilot study participants, we developed scenarios in which we experimentally manipulated the factors identified as important to assess global preferences for donation (24 –27). In these scenarios (Table 1 and Appendix, available at www.annals.org), we varied risk (the percentage of living kidney donors expected to develop renal failure requiring dialysis, transplantation, or both in the future [0.1%, 1%, or 10%]), payment (the money offered for donating a kidney [$0, $10 000, or $100 000]), and recipient of the kidney (either a close family member or the next eligible patient on the waiting list). We chose 0.1% and 1% as lifetime risks for renal failure to approximate available estimates of this risk (28–30). We included the 10% risk because these studies typically excluded higher-risk donors and because donors themselves are often tolerant of greater personal risks (31). We chose the levels of payment to reflect the spectrum of values considered cost-effective (8, 14, 23).
Table 1.
Proportions of Participants Who Would Donate a Kidney, by Scenario
| Scenario Number* | Scenario Attributes | Outcome | |||
|---|---|---|---|---|---|
| Scenario Included in Packet 1, 2, or Both† | Risk for Kidney Failure, % | Payment for Donation, $ | Recipient | Participants Who Would Donate, % | |
| 1 | 1, 2 | 0.1 | 100 000 | Family | 87.4 |
| 2 | 1 | 0.1 | 10 000 | Family | 86.0 |
| 3 | 1 | 1.0 | 100 000 | Family | 84.9 |
| 4 | 1 | 0.1 | 0 | Family | 84.3 |
| 5 | 1 | 1.0 | 0 | Family | 83.2 |
| 6 | 1, 2 | 1.0 | 10 000 | Family | 79.8 |
| 7 | 1 | 10 | 100 000 | Family | 75.7 |
| 8 | 1 | 10 | 10 000 | Family | 74.6 |
| 9 | 1, 2 | 10 | 0 | Family | 74.0 |
| 10 | 2 | 1.0 | 100 000 | Waiting list | 49.0 |
| 11 | 1, 2 | 0.1 | 100 000 | Waiting list | 46.8 |
| 12 | 2 | 0.1 | 10 000 | Waiting list | 35.7 |
| 13 | 1, 2 | 1.0 | 10 000 | Waiting list | 30.1 |
| 14 | 2 | 10 | 100 000 | Waiting list | 29.9 |
| 15 | 2 | 0.1 | 0 | Waiting list | 29.9 |
| 16 | 2 | 10 | 10 000 | Waiting list | 24.2 |
| 17 | 1, 2 | 10 | 0 | Waiting list | 19.0 |
| 18 | 2 | 1.0 | 0 | Waiting list | 17.2 |
Scenarios are numbered on the basis of a ranking of the proportion of participants who would donate a kidney if the indicated risk for renal failure and the indicated financial payment were included and if the donated kidney went to the indicated recipient.
Each participant was randomly assigned to receive 1 of 2 packets of 12 scenarios each.
A full factorial design using these 3 attributes produced 18 scenarios (3 × 3 × 2). However, because responding to 18 scenarios was taxing for some pilot study participants, we randomly assigned participants in the real study to receive one of two 12-scenario packets, as shown in Table 1. In 9 of 12 scenarios in packet 1, the kidney recipient was a family member; in 9 of 12 scenarios in packet 2, the kidney recipient was the next patient on the waiting list. The 2 packets were otherwise identical, and 6 of 18 scenarios were used in both packets. This design reduced respondent burden while retaining the orthogonal relations among attributes, thereby enabling tests of all main effects and hypothesized interactions.
Participants responded to each scenario by stating their willingness to donate a kidney on a 5-point scale ranging from “definitely would not donate” to “definitely would donate.” We used a random-number generator to determine the sequence in which scenarios were presented to each participant to minimize the influence of ordering effects on overall results.
Setting and Participants
Three investigators recruited passengers on the Southeastern Pennsylvania Transit Authority regional rail system and urban trolley lines during 5 consecutive weekdays in August 2008 and June 2009. Each day, participants were recruited at a different time and on a different rail or trolley route to enhance the samples’ representativeness of the regional population and to reduce the possibility of duplicate respondents. Starting from opposite ends of the train or trolley, the investigators explained the study objectives to consecutive passengers and solicited their participation. Those who verbally consented were offered a candy bar for completing the questionnaire. The University of Pennsylvania institutional review board approved this study.
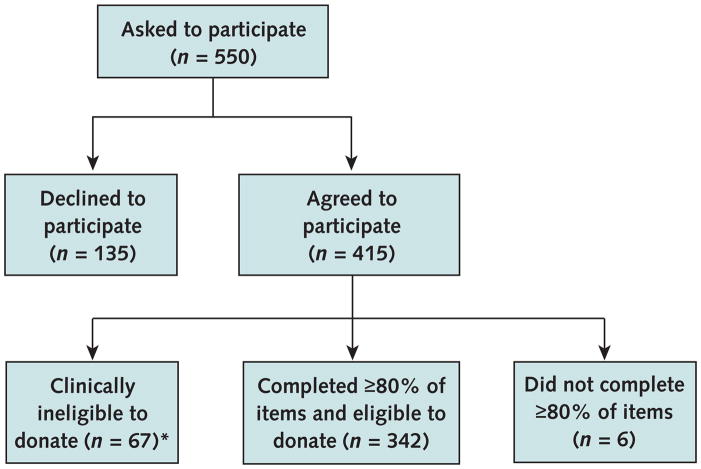
For each consenting participant, the investigator first read a standard script describing the risks, benefits, and surgical procedures involved in kidney donation and then answered participants’ questions. We excluded participants from the study if they indicated that they had any of the clinical characteristics listed in Figure 1, which would make them ineligible to donate.
Figure 1. Study flow diagram.
*Participants with any of the following criteria were considered clinically ineligible to donate: age >65 years; current kidney disease; congenital solitary kidney; family history of polycystic kidney disease; or history of heart disease, high blood pressure requiring more than 1 medication, diabetes, cancer, or hepatitis.
Statistical Analysis
We analyzed factors associated with participants’ willingness to donate by using a generalized estimating equation model with an independent working correlation structure and robust variance estimators to account for the clustering of the 12 responses made by each participant (32). We created a dichotomous outcome variable by grouping the original 5-point willingness-to-donate scale into responses of “probably” or “definitely” would donate versus “not sure,” “probably,” or “definitely” would not donate. We also created a random-effects ordinal logistic regression model in which the 5-point outcome was maintained. Because the statistical significance and relative magnitudes of the coefficients for all independent variables were similar in both models, we present only the results of the generalized estimating equation model to best reflect the discrete donation decisions that persons must make.
To determine whether money would encourage greater donation, we examined the main effect of payment on willingness to donate. We evaluated the interaction between risk and payment to examine undue inducement. If money blinds participants to their personal risk, then the effect of risk on willingness to donate should decrease as payments increase, resulting in a negative interaction. We evaluated the interaction between payment and income to examine unjust inducement. If payments influence poorer persons more than richer persons, this influence should manifest as a negative interaction between payment and income.
To determine whether introducing monetary incentives might reduce altruistic donations (that is, donations without payment), we used chi-square tests to compare the proportions of participants who were willing to donate in nonpayment scenarios among those who completed these scenarios before versus after seeing at least one other scenario that offered payment. If financial incentives reduced altruistic donation by making persons believe that charitable acts were unnecessary (33) or by causing potential altruistic donors to request payment, then lower rates of altruistic donation should be observed among persons previously alerted to the possibility of payment than among those not previously alerted.
We conducted analyses in Stata, version 10.1 (StataCorp, College Station, Texas), and SAS, version 9.2 (SAS Institute, Cary, North Carolina). We forced the following independent variables into the generalized estimating equation model: 3 manipulated scenario attributes (risk, payment, and recipient), participants’ annual household income (entered as a 6-level ordinal variable after confirming that it satisfied the linearity assumption), and 2 hypothesized interaction terms (payment-by-risk and payment-by-income) and terms for the survey packet and interval of the survey. We logarithmically transformed the risk and payment variables to improve model fit. We entered other independent variables and interactions into the model if we found an association with willingness to donate in unadjusted analyses (P < 0.15) or if inclusion of these variables modified the coefficient for the risk attribute by 15% or more. Table 2 describes participant characteristics evaluated for inclusion.
Table 2.
Participant Characteristics
| Characteristic | Participants (n = 342) | |
|---|---|---|
| Mean age (SD), y | 32.7 (12.5) | |
| Men, % | 50.6 | |
| Race, % | ||
| Black | 30.0 | |
| White | 57.9 | |
| Asian | 8.2 | |
| Other | 3.9 | |
| Annual household income, % | ||
| ≤$20 000 | 18.6 | |
| $20 001–$40 000 | 25.2 | |
| $40 001–$60 000 | 17.0 | |
| $60 001–$80 000 | 11.1 | |
| $80 001–$100 000 | 6.5 | |
| >$100 000 | 21.6 | |
| Education, % | ||
| High school or less (7–12 y) | 20.6 | |
| College (13–16 y) | 46.0 | |
| Graduate school (>16 y) | 33.4 | |
| Employment, % | ||
| Full-time | 58.5 | |
| Part-time | 20.3 | |
| None | 20.9 | |
| Have a family member with advanced kidney disease, % | 4.2 | |
| Have a friend or family member who has received an organ transplant, % | 15.1 | |
| Know a kidney donor, % | 14.6 | |
After producing the generalized estimating equation model, we estimated probabilities of donation across all possible combinations of the 4 primary independent variables: payment, risk, recipient, and income. To do so, we first used conditional standardization to adjust for differences in patterns of all other covariates among comparison groups (34).
Sample Size
The sample size for this study was guided by the hypothesized outcome that would require the most participants to detect—the payment-by-income interaction. A specific interest in comparing only participants in the highest (annual household income >$100 000) and lowest (annual household income ≤$20 000) income strata further guided our sample size. Enrolling 120 eligible participants in these 2 strata combined, with a roughly even distribution between them, would provide greater than 80% power to detect a payment-by-income interaction equivalent to a 12% change in either direction from an anticipated baseline donation rate of 40%. Enrolling a total of 300 participants (across all 6 income strata) would provide greater than 95% power to detect payment-by-income interactions of identical magnitudes when we analyzed all participants. These estimates are based on the recommendation to inflate the required sample size by 50% in order to detect an interaction term rather than a similarly sized main effect (35), and allows for a design effect (to account for the correlated nature of the 12-scenario responses per participant) (36) of 7.15. This latter choice reflects the observed design effect in our pilot study, calculated as 1 + ρ(κ − 1), in which ρ is the intraclass correlation and ρ is the number of scenarios per participant (36).
Validity
We evaluated internal consistency by assessing the proportion of responses that violated the principle of monotonicity (24, 37). This principle holds that participants should never be more willing to donate when a less favorable level of one attribute (for example, higher risk) is offered while the levels of other attributes (for example, payment and recipient) are held constant.
Role of the Funding Source
We received no specific funding for this study.
Results
Among 550 passengers who we asked to participate, 415 consented, of which 409 completed at least 80% of the scenarios (response rate, 74.4%) (Figure 1). We excluded 67 of these participants because they were clinically ineligible to donate. The remaining 342 participants indicated their willingness to donate in 4088 of 4104 scenarios they received (item response rate, 99.6%). We imputed values for the 16 missing observations by using the corresponding participant’s average willingness to donate across other scenarios. This did not change any results compared with the complete case analyses in which we excluded the 15 participants who had 1 or 2 missing ratings each.
Table 2 shows participants’ demographic characteristics. These distributions approximate those of the Philadelphia region (38), except that participants had higher education status.
Factors Associated With Willingness to Donate
In the multivariable generalized estimating equation model (Table 3), we found statistically significant associations with willingness to donate for donating to a family member rather than to the next patient on the waiting list, a lower risk for renal failure (expressed on a logarithmic scale), and a higher payment (also on a logarithmic scale) (each P < 0.001). In addition, willingness to donate was greater among women than among men (P = 0.016) and among participants with incrementally lower annual household incomes (P = 0.028). This effect of income was observed among donations to the next patient on the waiting list (P = 0.020) but not among donations to family members (P = 0.31), resulting in a significant interaction between income and recipient (odds ratio [OR], 1.26 [95% CI, 1.06 to 1.48]).
Table 3.
Factors Associated with Participants’ Willingness to Donate a Kidney
| Variable* | OR (95% CI)† | P Value‡ | |
|---|---|---|---|
| Unadjusted | Adjusted | ||
| Age (per year) | 0.99 (0.98–1.00) | 0.99 (0.97–1.00) | 0.115 |
| Female sex | 1.50 (1.08–2.07) | 1.51 (1.06–2.17) | 0.028 |
| Income level§ | 0.93 (0.85–1.01) | 0.84 (0.73–0.97) | 0.016 |
| Logarithmic increase in payment | 1.12 (1.09–1.16) | 1.16 (1.06–1.27) | 0.001 |
| Logarithmic increase in CKD risk | 0.67 (0.62–0.73) | 0.72 (0.62–0.82) | <0.001 |
| Family recipient | 9.12 (6.94–12.0) | 8.11 (4.44–14.82) | <0.001 |
| Payment-by-risk interaction | – | 1.00 (0.96–1.03) | 0.87 |
| Payment-by-income interaction | – | 1.01 (0.99–1.03) | 0.49 |
| Payment-by-recipient interaction | – | 0.87 (0.82–0.92) | <0.001 |
| Recipient-by-income interaction | – | 1.26 (1.06–1.48) | 0.007 |
| Survey packet (1 vs. 2) | – | 1.04 (0.74–1.47) | 0.81 |
| Survey period (2008 vs. 2009) | – | 1.00 (0.66–1.53) | 0.99 |
CKD = chronic kidney disease; OR = odds ratio.
Additional variables were evaluated but not selected for inclusion in the multivariable model because of their weak associations with willingness to donate: race, education, employment, having a family member with CKD, having a family member who received a transplant, and knowing an organ donor.
The magnitude of the ORs may overestimate the rate ratios because donation decisions were common. The adjusted ORs are adjusted for all variables in the table.
Reported P values are from the full multivariable model.
Income is categorized into 6 increasing strata; the reference stratum reflects an annual household income ≤$20 000.
Undue Inducement
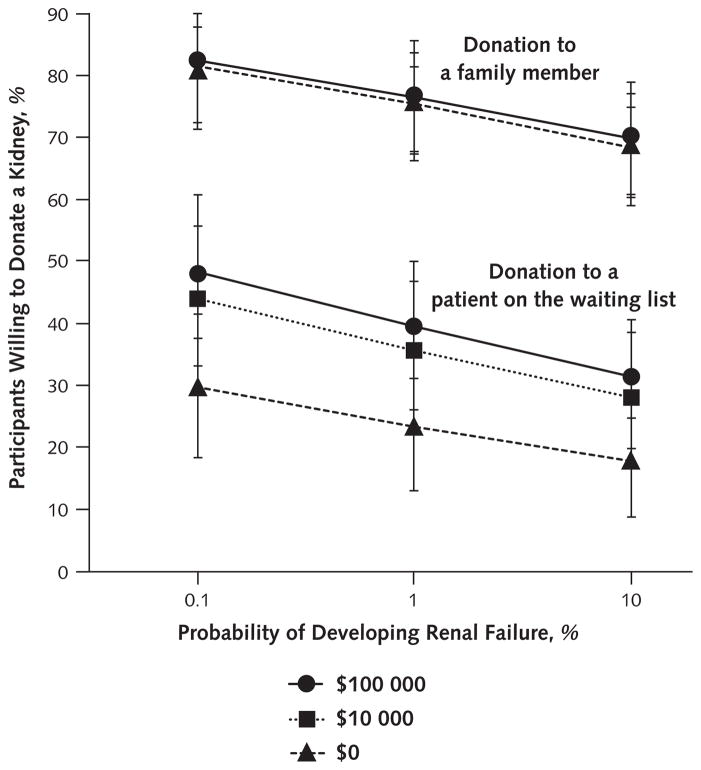
We found no evidence of an interaction between payment and risk across all scenarios (OR, 1.00 [CI, 0.96 to 1.03]) or when stratified among scenarios in which the recipient was a family member (OR, 0.99 [CI, 0.94 to 1.04]) or the next patient on the waiting list (OR, 0.97 [CI, 0.93 to 1.01]) (Figure 2). The magnitude of reductions in willingness to donate associated with increased risk for renal failure was virtually identical across payment levels (Figure 2). These results suggest that payment is not an undue inducement for living kidney donation. Similarly, we found no evidence of undue inducement when we restricted the analyses to the 70 least-educated participants (those with high school education or less) (payment-by-risk interaction: OR, 1.02 [CI, 0.96 to 1.08]).
Figure 2. Adjusted proportions of participants willing to donate a kidney to family members and to patients on the waiting list as functions of payment and risk.
Scenarios in which donors would receive payment of $100 000, payment of $10 000, or no payment are illustrated. As evident from the roughly parallel nature of the lines within each recipient group, no interaction between risk and payment occurred when the recipient was a family member (odds ratio, 0.99 [95% CI, 0.94–1.04]) or when the recipient was the next patient on the waiting list (odds ratio, 0.97 [CI, 0.93–1.01]).
By contrast, we found a significant interaction (OR, 0.87 [CI, 0.82 to 0.97]) between payment and recipient. The conditionally adjusted probabilities of donating to the next patient on the waiting list increased significantly with increasing levels of payment (29.8% [CI, 19.5% to 42.7%] for $0, 44.1% [CI, 33.1% to 55.7%] for $10 000, and 47.9% [CI, 36.4% to 59.6%] for $100 000), whereas the probabilities of donating to a family member increased only marginally and nonsignificantly with increasing levels of payment (81.2% [CI, 72.3% to 87.8%) for $0, 82.1% [CI, 74.2% to 88.0%] for $10 000, and 82.4% [CI, 74.2% to 88.4%] for $100 000).
Unjust Inducement
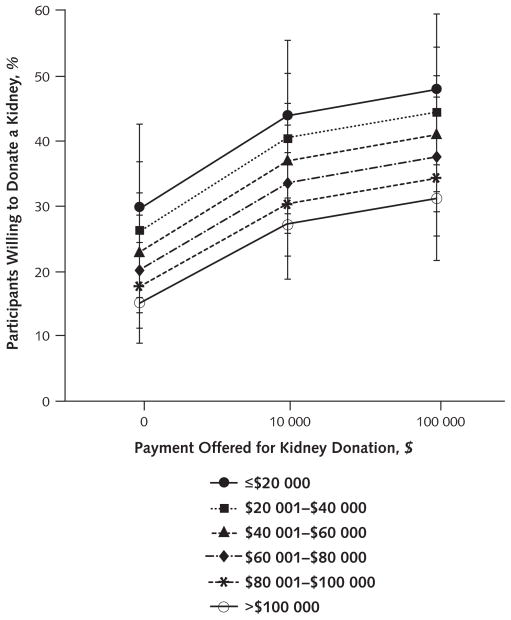
Higher payments increased the probabilities of donating but did so evenly across the 6 income strata, such that no evidence of an interaction between payment and income was found (OR, 1.01 [CI, 0.99 to 1.03]) (Figure 3). Even when we restricted analyses to the 57 participants in the lowest income stratum (annual household income ≤$20 000) and the 66 participants in the highest income stratum (annual household income >$100 000), no significant interaction emerged (OR, 0.99 [CI, 0.97 to 1.02]) (Figure 3). Among participants in the lowest income stratum, conditionally adjusted donation rates were 29.8% (CI, 19.5% to 42.7%) for $0, 44.1% (CI, 33.1% to 55.7%) for $10 000, and 47.9% (CI, 36.4% to 59.6%) for $100 000. Among participants in the highest income stratum, the rates were 15.2% (CI, 9.0% to 24.5%), 27.5% (CI, 18.8% to 38.2%), and 31.3% (CI, 21.7% to 42.9%), respectively. These results suggest that payment is not an unjust inducement for living kidney donation.
Figure 3.
Adjusted proportions of participants willing to donate a kidney to a patient on the waiting list as a function of income and payment.
Participants whose annual household incomes were ≤$20 000 through >$100 000 are illustrated. The plotted proportions have been adjusted for risk for renal failure, donor age, donor sex, version of the survey packet received, and interval of participant recruitment. Error bars represent 95% CIs around each adjusted proportion. As evident from the roughly parallel nature of the lines, no interaction between payment and income occurred (odds ratio, 1.01 [95% CI, 0.99 –1.03]).
Payment and Altruistic Donation
We found no evidence that the introduction of payment for organs would reduce altruistic donation. The proportions of scenarios in which participants were willing to donate without payment were similar when we presented these scenarios to participants before (64 of 123 participants [52.0%]) or after (523 of 933 participants [56.1%]) the introduction of monetary incentives (chi-square = 0.71; P = 0.40).
Response Validity
All 3 primary attributes were in the hypothesized direction, supporting the face validity of the results. Of the 3078 possible violations of monotonicity, 214 violations on the risk scale (7.0%) and 273 violations on the payment scale (8.9%) were observed. The median number of violations per participant was 0 (interquartile range, 0 to 1) for both scales.
Discussion
We found no evidence that any of the 3 main concerns with a regulated system of payments for living kidney donation would manifest if such a market were established. Providing payments did not dull persons’ sensitivity to the risks associated with donor nephrectomy, suggesting that payment does not represent an undue inducement— one that would make rational choice difficult. Furthermore, providing payments did not preferentially motivate poorer persons to sell a kidney, suggesting that payment does not represent an unjust inducement— one that would put substantially more pressure on poorer persons than on wealthier persons.
Similar to real-world observations from Iran’s partially regulated kidney market (39, 40), we found that poorer persons were more likely than wealthier persons to consider donation to an unrelated donor. However, contrary to both our hypotheses and concerns expressed about the Iranian market (40), we found that poorer persons were more willing to donate independent of payment (Figure 3). Even after restricting our analyses to the poorest and wealthiest participants, we found no evidence that payment influenced these 2 groups differently. This result is consistent with previous observations that payment does not preferentially motivate clinical research participation among poor persons (25). Thus, our results do not corroborate concerns about the ethics of payment per se, but rather they suggest that poorer persons may contribute disproportionately to the supply of organs with or without payment. Reasons for these behaviors, perhaps including differences in the opportunity costs of donating among richer and poorer patients, merit future study.
We also found no evidence that introducing monetary incentives would “crowd out” a person’s altruistic incentives to donate. This result is consistent with a previous public survey that found that payments would encourage kidney donation for monetary reasons far more commonly than it would discourage donation for altruistic reasons (41). Together, these studies cast substantial doubt on the concern that offering payments would undermine altruistic donation. They suggest that systems allowing payment for kidney donors would produce more transplantable organs than systems barring it.
Our study has several strengths. First, by experimentally manipulating the presentation of factors associated with donation decisions within participants, we forced persons to reveal their preferences and presumed behaviors (26, 27). This approach contrasts with questionnaires that merely query stated preferences. Second, our study had substantial power to detect even minimal statistical interactions between payment and income and between payment and risk, as reflected by the narrowness of the CIs (42) surrounding the point estimates of these interactions (Table 3). Thus, it is unlikely that we did not detect a true effect of payment as either an undue or an unjust inducement. Third, the high response rates to the survey and component items reduce the possibilities of important nonresponse biases. Fourth, the validity of the results is suggested by the low proportion of internally inconsistent responses (24, 37).
An important limitation of our study is that participants’ responses to hypothetical offers may not reflect the decisions they would make if they were truly offered payment for a kidney. For example, hypothetical offers of payment may be insufficient to blunt a person’s perception of risk, but real money might do just that. However, it seems unlikely that our study failed to detect real effects of money because participants clearly paid attention to and were influenced by money. We found that larger payments encouraged donation in general, and, as expected, payments were particularly important when participants contemplated donating to strangers. Furthermore, the experimental presentation of structured vignettes has been shown to produce valid results in other settings (43). Nonetheless, evaluating responses to hypothetical situations can take us only so far; ultimately, the effects of payment will need to be tested in natural settings.
Another possible limitation of the study regards the diversity of participants. Our sample contained a greater percentage of highly educated persons than expected from the general public. However, we found no relation between education and any outcome measure, and none of our results changed when analyses were restricted to the least-educated members of the sample. Similarly, it is possible that we did not enroll persons at the very extremes of the U.S. income distribution. However, our sample included roughly equal proportions of participants in each of the 6 predetermined income brackets, and we found unchanged results when we limited analyses to the highest versus lowest brackets.
Finally, some might believe our study is limited in that we did not address the views that payment for kidney donation is intrinsically unethical because it represents “commodification” of the body or that introducing payments for organs could have broader social ramifications, such as curtailing a person’s general selflessness. However, these arguments apply equally to payments for surrogate motherhood or clinical research participation—activities that carry similar if not greater risks than kidney donation (11) yet are legal in most nations. Thus, regardless of the merits of these arguments, regulated kidney sales are difficult to challenge on these grounds.
Our study adds evidence to what has been a largely theoretical debate about the propriety of paying persons to become living kidney donors. The results both corroborate predictions that payments could effectively increase the supply of transplantable kidneys (8, 14) and cast doubt on intuitions that payments would be undue or unjust, or would undermine a person’s otherwise altruistic behaviors (10, 16). Because participants’ responses to our questionnaires did not carry real-world consequences, our results are insufficient to support the establishment of a national system of regulated payments for kidney donation. Instead, because these and other empirical results counter theoretical concerns about regulated payments, we recommend proceeding with a highly controlled and geographically limited test of such payments that is explicitly designed to detect both intended and unintended consequences of real-world payments for living kidney donation.
Context
Persons who need kidney transplants outnumber available kidneys. Payment to donors could encourage kidney donation, but it might create unethical inducements. People might not fully consider the risk of donation, or disadvantaged persons might feel pressure to donate. Payment might deter donations for altruistic reasons.
Contribution
Researchers surveyed persons riding Philadelphia-area public transportation about whether they would donate a kidney under a range of scenarios that did and did not include various payments. Responses suggested that payment would not create undue or unjust incentives for donation or alter a person’s willingness to donate just to help another person.
Caution
Responses may not reflect what people would actually do if confronted with an opportunity for kidney donation.
—The Editors
Acknowledgments
The authors thank Thomas R. Ten Have, PhD, and Mark Cary, PhD, for their assistance in the design and analysis of this study.
Grant Support: By a Greenwall Foundation Faculty Scholar Award in Bioethics (Dr. Halpern), the National Institutes of Health (Dr. Reese; grant K23-078688-01), and internships from the University of Pennsylvania Center for Bioethics (Ms. Raz, Ms. Kohn, and Mr. Rey).
Footnotes
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M09-2133.
Reproducible Research Statement: Study protocol, statistical code, and data set: Available from Dr. Halpern (scott.halpern@uphs.upenn.edu).
Author Contributions: Conception and design: S.D. Halpern, A. Raz, D.A. Asch, P. Reese.
Analysis and interpretation of the data: S.D. Halpern, M. Rey, D.A. Asch, P. Reese.
Drafting of the article: S.D. Halpern, P. Reese.
Critical revision of the article for important intellectual content: S.D. Halpern, A. Raz, D.A. Asch.
Final approval of the article: S.D. Halpern, A. Raz, D.A. Asch, P. Reese.
Statistical expertise: S.D. Halpern.
Obtaining of funding: S.D. Halpern.
Administrative, technical, or logistic support: S.D. Halpern, A. Raz.
Collection and assembly of data: R. Kohn, M. Rey.
References
- 1.Guadagnoli E, Christiansen CL, Beasley CL. Potential organ-donor supply and efficiency of organ procurement organizations. Health Care Financ Rev. 2003;24:101–10. [PMC free article] [PubMed] [Google Scholar]
- 2.Sheehy E, Conrad SL, Brigham LE, Luskin R, Weber P, Eakin M, et al. Estimating the number of potential organ donors in the United States. N Engl J Med. 2003;349:667–74. doi: 10.1056/NEJMsa021271. [DOI] [PubMed] [Google Scholar]
- 3.Weber M, Dindo D, Demartines N, Ambühl PM, Clavien PA. Kidney transplantation from donors without a heartbeat. N Engl J Med. 2002;347:248–55. doi: 10.1056/NEJMoa020274. [DOI] [PubMed] [Google Scholar]
- 4.Halpern SD, Shaked A, Hasz RD, Caplan AL. Informing candidates for solid-organ transplantation about donor risk factors. N Engl J Med. 2008;358:2832–7. doi: 10.1056/NEJMsb0800674. [DOI] [PubMed] [Google Scholar]
- 5.Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, et al. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation. 2002;74:1281–6. doi: 10.1097/00007890-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 6.Chkhotua AB, Klein T, Shabtai E, Yussim A, Bar-Nathan N, Shaharabani E, et al. Kidney transplantation from living-unrelated donors: comparison of outcome with living-related and cadaveric transplants under current immunosuppressive protocols. Urology. 2003;62:1002–6. doi: 10.1016/s0090-4295(03)00760-x. [DOI] [PubMed] [Google Scholar]
- 7.Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med. 2001;344:726–31. doi: 10.1056/NEJM200103083441004. [DOI] [PubMed] [Google Scholar]
- 8.Becker GS, Elias JJ. Introducing incentives in the market for live and cadaveric organ donations. J Econ Perspect. 2007;21:3–24. doi: 10.1257/jep.21.3.3. [DOI] [PubMed] [Google Scholar]
- 9.Boulware LE, Troll MU, Wang NY, Powe NR. Public attitudes toward incentives for organ donation: a national study of different racial/ethnic and income groups. Am J Transplant. 2006;6:2774–85. doi: 10.1111/j.1600-6143.2006.01532.x. [DOI] [PubMed] [Google Scholar]
- 10.Delmonico FL, Arnold R, Scheper-Hughes N, Siminoff LA, Kahn J, Youngner SJ. Ethical incentives—not payment—for organ donation. N Engl J Med. 2002;346:2002–5. doi: 10.1056/NEJMsb013216. [DOI] [PubMed] [Google Scholar]
- 11.Friedman AL. Payment for living organ donation should be legalised. BMJ. 2006;333:746–8. doi: 10.1136/bmj.38961.475718.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hippen BE. In defense of a regulated market in kidneys from living vendors. J Med Philos. 2005;30:593–626. doi: 10.1080/03605310500421397. [DOI] [PubMed] [Google Scholar]
- 13.Israni AK, Halpern SD, Zink S, Sidhwani SA, Caplan A. Incentive models to increase living kidney donation: encouraging without coercing. Am J Transplant. 2005;5:15–20. doi: 10.1111/j.1600-6143.2004.00656.x. [DOI] [PubMed] [Google Scholar]
- 14.Matas AJ. The case for living kidney sales: rationale, objections and concerns. Am J Transplant. 2004;4:2007–17. doi: 10.1111/j.1600-6143.2004.00664.x. [DOI] [PubMed] [Google Scholar]
- 15.Radcliffe-Richards J, Daar AS, Guttmann RD, Hoffenberg R, Kennedy I, Lock M, et al. The case for allowing kidney sales. International Forum for Transplant Ethics. Lancet. 1998;351:1950–2. doi: 10.1016/s0140-6736(97)08211-1. [DOI] [PubMed] [Google Scholar]
- 16.Rothman SM, Rothman DJ. The hidden cost of organ sale. Am J Transplant. 2006;6:1524–8. doi: 10.1111/j.1600-6143.2006.01325.x. [DOI] [PubMed] [Google Scholar]
- 17.Veatch RM. Why liberals should accept financial incentives for organ procurement. Kennedy Inst Ethics J. 2003;13:19–36. doi: 10.1353/ken.2003.0007. [DOI] [PubMed] [Google Scholar]
- 18.Scheper-Hughes N. Keeping an eye on the global traffic in human organs. Lancet. 2003;361:1645–8. doi: 10.1016/S0140-6736(03)13305-3. [DOI] [PubMed] [Google Scholar]
- 19.Goyal M, Mehta RL, Schneiderman LJ, Sehgal AR. Economic and health consequences of selling a kidney in India. JAMA. 2002;288:1589–93. doi: 10.1001/jama.288.13.1589. [DOI] [PubMed] [Google Scholar]
- 20.Matas AJ. Design of a regulated system of compensation for living kidney donors. Clin Transplant. 2008;22:378–84. doi: 10.1111/j.1399-0012.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- 21.Schnitzler MA, Lentine KL, Burroughs TE. The cost effectiveness of deceased organ donation [Letter] Transplantation. 2005;80:1636–7. doi: 10.1097/01.tp.0000179637.37276.5a. [DOI] [PubMed] [Google Scholar]
- 22.Schnitzler MA, Whiting JF, Brennan DC, Lentine KL, Desai NM, Chapman W, et al. The life-years saved by a deceased organ donor. Am J Transplant. 2005;5:2289–96. doi: 10.1111/j.1600-6143.2005.01021.x. [DOI] [PubMed] [Google Scholar]
- 23.Matas AJ, Schnitzler M. Payment for living donor (vendor) kidneys: a cost-effectiveness analysis. Am J Transplant. 2004;4:216–21. doi: 10.1046/j.1600-6143.2003.00290.x. [DOI] [PubMed] [Google Scholar]
- 24.Halpern SD, Berns JS, Israni AK. Willingness of patients to switch from conventional to daily hemodialysis: looking before we leap. Am J Med. 2004;116:606–12. doi: 10.1016/j.amjmed.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Halpern SD, Karlawish JH, Casarett D, Berlin JA, Asch DA. Empirical assessment of whether moderate payments are undue or unjust inducements for participation in clinical trials. Arch Intern Med. 2004;164:801–3. doi: 10.1001/archinte.164.7.801. [DOI] [PubMed] [Google Scholar]
- 26.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320:1530–3. doi: 10.1136/bmj.320.7248.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan M, McIntosh E, Shackley P. Methodological issues in the application of conjoint analysis in health care. Health Econ. 1998;7:373–8. doi: 10.1002/(sici)1099-1050(199806)7:4<373::aid-hec348>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 28.Fehrman-Ekholm I, Dunér F, Brink B, Tydén G, Elinder CG. No evidence of accelerated loss of kidney function in living kidney donors: results from a cross-sectional follow-up. Transplantation. 2001;72:444–9. doi: 10.1097/00007890-200108150-00015. [DOI] [PubMed] [Google Scholar]
- 29.Soneji ND, Vyas J, Papalois VE. Long-term donor outcomes after living kidney donation. Exp Clin Transplant. 2008;6:215–23. [PubMed] [Google Scholar]
- 30.Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360:459–69. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young A, Karpinski M, Treleaven D, Waterman A, Parikh CR, Thiessen-Philbrook H, et al. Differences in tolerance for health risk to the living donor among potential donors, recipients, and transplant professionals. Kidney Int. 2008;73:1159–66. doi: 10.1038/ki.2008.65. [DOI] [PubMed] [Google Scholar]
- 32.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 33.Heyman J, Ariely D. Effort for payment. A tale of two markets. Psychol Sci. 2004;15:787–93. doi: 10.1111/j.0956-7976.2004.00757.x. [DOI] [PubMed] [Google Scholar]
- 34.Localio AR, Margolis DJ, Berlin JA. Relative risks and confidence intervals were easily computed indirectly from multivariable logistic regression. J Clin Epidemiol. 2007;60:874–82. doi: 10.1016/j.jclinepi.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Byar DP, Piantadosi S. Factorial designs for randomized clinical trials. Cancer Treat Rep. 1985;69:1055–63. [PubMed] [Google Scholar]
- 36.Kerry SM, Bland JM. The intracluster correlation coefficient in cluster randomisation. BMJ. 1998;316:1455. doi: 10.1136/bmj.316.7142.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips KA, Maddala T, Johnson FR. Measuring preferences for health care interventions using conjoint analysis: an application to HIV testing. Health Serv Res. 2002;37:1681–705. doi: 10.1111/1475-6773.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.U.S. Census Bureau. State and county quick facts. Philadelphia County, Pennsylvania: Accessed at http://quickfacts.census.gov/qfd/states/42/42101.html on 22 January 2010. [Google Scholar]
- 39.Ghods AJ, Savaj S. Iranian model of paid and regulated living-unrelated kidney donation. Clin J Am Soc Nephrol. 2006;1:1136–45. doi: 10.2215/CJN.00700206. [DOI] [PubMed] [Google Scholar]
- 40.Harmon W, Delmonico F. Payment for kidneys: a government-regulated system is not ethically achievable. Clin J Am Soc Nephrol. 2006;1:1146–7. doi: 10.2215/CJN.03050906. [DOI] [PubMed] [Google Scholar]
- 41.Jasper JD, Nickerson CA, Hershey JC, Asch DA. The public’s attitudes toward incentives for organ donation. Transplant Proc. 1999;31:2181–4. doi: 10.1016/s0041-1345(99)00301-2. [DOI] [PubMed] [Google Scholar]
- 42.Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med. 1994;121:200–6. doi: 10.7326/0003-4819-121-3-199408010-00008. [DOI] [PubMed] [Google Scholar]
- 43.Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA. 2000;283:1715–22. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]