Abstract
Bioorthogonal chemical reactions are paving the way for new innovations in biology. These reactions possess extreme selectivity and biocompatibility, such that their participating reagents can form covalent bonds within richly functionalized biological systems—in some cases, living organisms. This tutorial review will summarize the history of this emerging field, as well as recent progress in the development and application of bioorthogonal copper-free click cycloaddition reactions.
Introduction
The middle of the last century was a golden era for physical organic chemistry. The goal of understanding the fundamental relationship of structure and reactivity was sufficient justification to synthesize a wide assortment of compounds that had no other perceived utility. As trends in chemistry shifted, focusing more on application-based goals, many of the findings of chemists like Huisgen and Krebs were largely academic teaching tools until they experienced a renaissance in the form of the copper-catalyzed azide–alkyne cyclooaddition, often referred to as “click” chemistry.1–3 This energized the chemical community, with researchers from materials science to biochemistry all reaching into the treasure chest of reactions scattered throughout the older literature.
Click reactions are defined more broadly as those that meet the necessary criteria of being selective, high yielding, and having good reaction kinetics. A subclass of click reactions whose components are inert to the surrounding biological milieu is termed bioorthogonal.4 This goes one step beyond the typical definition of a click reaction because of the added complications of biocompatibility. The focus of this review will be on a group of these bioorthogonal reactions that are cycloadditions lacking exogenous metal catalysts, the so-called Cu-free click reactions. Exogenous metals can have mild to severe cytotoxic effects and can thus disturb the delicate metabolic balance of the systems being studied.5
This review will first address two questions at the heart of the emerging field of bioorthogonal chemistry. The first is, why do people want to do chemical reactions in cells and animals? The second question, which is more alluring to synthetic chemists, is why are selective chemical reactions in cells and living organisms difficult? From these questions we will then explore how bioorthogonal reaction partners were designed to overcome the stated problems. This section will be followed by a discussion of the synthetic strategies of successful systems, and finally, their applications in chemical biology.
Why do people want to do chemical reactions in cells and living organisms?
While more advanced imaging systems have allowed us to see substructures of cells, these types of aerial pictures often lead to more questions than answers.6 A satellite image of a walled community can give you an idea of the size and location of a particular house, but little detail when it comes to the occupants of the house and their comings and goings. By covalently attaching tracking devices (e.g., optical probes) to occupants of various subcellular houses we are able to learn more about the interactions within cells. In principle, biomolecules within cells can be covalently labeled with probes using bioorthogonal reactions. This requires the introduction of one reaction partner into the target biomolecule, and that the reagents minimally perturb the system in which they are meant to react.4
Why is it difficult to perform a selective reaction in cells and living organisms?
After millions of years of optimization, nature has developed highly selective enzymes that allow for substrate recognition and subsequent reaction of functional groups. While this works astonishingly well for nature, it is impractical for scientists to make a new enzyme to allow for selective covalent tagging of each biomolecule being studied. So with this limitation in mind, why not perform a direct chemical reaction linking a probe to the target biomolecule, as one would in a conventional synthetic chemistry lab? To answer this question, we must first look at an organic chemistry textbook and remove any reaction that is sensitive to water (Fig. 1). Second, with an abundant supply of thiols and amines in the cell, we must also remove reagents that are prone to nucleophilic attack. Third, because of the reducing environment in the cytosol, we have to remove reactions that are sensitive to redox chemistry. If a reaction requires heat (above 37 °C), pressure, or high concentrations to work then it is also unacceptable. Lastly, some functionalities can be digested by cellular enzymes that have an “ase” in their name (e.g., esterase, phosphatase, sulfatase, etc.). Add reagent toxicity to this list and you will find a select few reactions that remain viable for performance in living systems. The remainder of this review will discuss handful of the reactions that have been engineered to fit these criteria. We will start by examining the design of the corresponding reaction components.
Fig. 1.
A general bioorthogonal reaction. The bioorthogonal functionality, oval with horizontal lines, reacts with its counterpart, oval with vertical lines, to label a biomolecule in live cells or organisms.
Design of bioorthogonal reagents
One of the most popular bioorthogonal functional groups is the azide. The small size, coupled with its inert nature towards endogenous biological functionalities, sets the azide apart. Additionally the azide has unique chemistries with other bioorthogonal functionalities, most notably phosphines and alkynes. Indeed the reaction of azides with triarylphosphines equipped with an ester—a process termed the Staudinger ligation—was the first bioorthogonal reaction ever performed in living systems.7 Like the cycloadditions that form the focus of this review, its roots can be traced to the early 1900s.8 However, the relatively slow kinetics of the Staudinger ligation limited its use in probing fast biological processes. Thus, our group, among others, looked to find a reaction whose kinetics were better suited to monitoring processes as they occurred.
Cycloadditions were viewed as ideal reactions because of tunable electronics and their intrinsic selectivity.9 Huisgen developed the [3+2] cycloaddition between an azide and an acyclic alkyne,10 but this reaction required a good deal of heat to overcome the activation barrier of deforming the alkyne’s bond angle to form the triazole (Fig. 2A). Sharpless and co-workers and Meldal and co-workers developed a Cu-catalyzed rendition of this reaction using terminal alkynes as substrates, but the metal’s cytotoxicity limits its use in living systems.1,2 To increase the rate of the cycloaddition without the need of a catalyst we took a page out of old physical organic literature and looked at ring-strain as a way to overcome the sluggish reactivity of alkynes with azides. Upon reading that cyclooctyne reacts with phenylazide “explosionsartig” (like an explosion), we decided to investigate this class of molecules.11 The bond angles of the sp-hybridized carbons in cyclooctynes is ~160°, which is distorted toward the transition state of the cycloaddition reaction, resulting in a dramatic rate acceleration (Fig. 2B).
Fig. 2.
The Cu-free reaction of azides and alkynes. (A) A comparison of bond angles between linear alkynes and triazoles. (B) A comparison of bond angles between a strained cyclooctyne and its corresponding triazole product. (C) Comparison of second-order rate constants of cyclooctynes in a model reaction with benzyl azide.
The first cyclooctyne that we evaluated, compound 1 (“OCT,” Fig. 2C), was shown to undergo cycloaddition with azides to give triazole 2, and although its reaction kinetics were vastly accelerated compared to the linear alkynes, there was room for improvement.12 Postulating that the reaction involved the lowest unoccupied molecular orbital (LUMO) of the alkyne and the highest occupied molecular orbital (HOMO) of the azide we sought to lower the LUMO of the alkyne by withdrawing electron density from the bond.13 Adding one fluorine atom to the ring system (3) increased the reaction rate constant by 3-fold.14 With the addition of a second fluorine atom to the ring, to create a compound we call DIFO (4), we observed a marked increase in rate constant, making the reaction 60 times faster than the parent compound, 1.15 The renewed interest in these cycloadditions has caught the attention of theorists, some of whom ascribe the enhanced reactivity to a decrease in the requisite distortion in the transition state coupled with better phase-transfer interactions from the fluorine atoms.16 Others claim that additional steric interactions impart extra strain compared with 1.17 While the rate of reaction for DIFO was exceptional, the compound’s solubility in water was less than ideal. This was addressed with the synthesis of a cyclooctyne with heteroatoms incorporated in and on the ring, as in dimethoxyazacyclooctyne 5.18
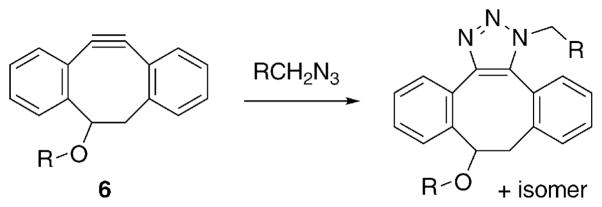
While we added fluorine atoms to our cyclooctynes to increase their reaction rates, the Boons group increased the strain-energy by making a functionalized derivative of dibenzocyclooctyne (6, Scheme 1).19,20 These systems are relatively easy to synthesize and can be derivatized at various aryl positions to enhance kinetics or solubility. As with many of these types of reagents, solubility is a significant hurdle in utility. Solubility concerns aside, dibenzocyclooctynes react with azides almost as fast as DIFO.
Scheme 1.
Reaction between a dibenzocyclooctyne and an azide.
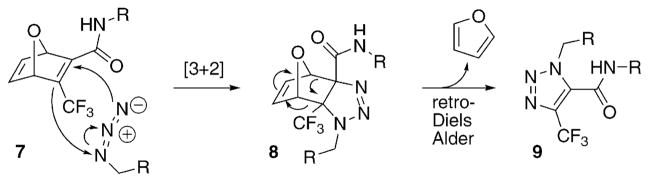
While others were working with alkynes to form triazoles upon cycloaddition with azides, Rutjes and co-workers developed an alkyne surrogate that forms a similar product (Scheme 2).21 An activated oxanorbornadiene (7) first reacts with an azide in a [3+2]-cycloaddition to give 8. This is followed immediately by a retro-Diels–Alder reaction to give a stable 1,2,3-triazole, 9. They found that further activation of the strained double bond in 7 with trifluoromethyl groups gives acceptable rate constants for the click reaction.
Scheme 2.
The two-step reaction of oxanorbornadienes with azides forms triazoles.
The Fox group explored the use of the inverse-electron-demand Diels–Alder reaction to attach a trans-cyclooctene (10, Scheme 3A) to a bipyridyltetrazine-tethered probe (11).22 While cis-cyclooctenes are relatively inert, the trans isomer is highly strained and thus remarkably reactive. The reaction produces intermediate 12, which rapidly decomposes to extrude nitrogen and to form 13 upon tautomerization. The reaction rates for these systems are orders of magnitude higher than for any other Cu-free click reaction. These alkenes are useful, but photoisomerization is always a concern when dealing with strained alkenes.
Scheme 3.
Inverse-electron-demand Diels–Alder reactions of strained alkenes. (A) The reaction between trans-cyclooctene 10, with biaryltetrazine 11. (B) The reaction between norbornene 15 with aryltetrazine 14. (C) The reaction between cyclobutene 17 with biaryltetrazine 18.
Shortly after Fox’s report, Hilderbrand and co-workers presented their work with mono-aryltetrazines (14, Scheme 3B).23 They used norbornene (15) in place of the trans-cyclooctene to give 16. Norbornene is more stable than trans-cyclooctene, but the reaction rates are not as high. Similarly, the monoaryltetrazine is not as reactive as the biaryltetrazine, but it is more stable in a biological setting. In yet another independent effort, Pipkorn and co-workers used tetrazine chemistry to assemble late-stage synthetic intermediates for cancer treatment.24 Their system took advantage of a highly strained cyclobutene-containing molecule (17, Scheme 3C) with a biaryltetrazine (18) to give 19. A head-to-head comparison of the reactions of these various alkenes and tetrazines has not been reported, but combining the best feature of each approach could add valuable tools to the field.
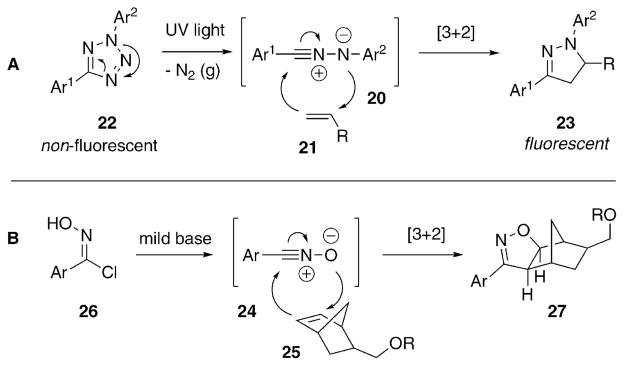
The penultimate copper-free click reaction that we will examine is another gem from Huisgen that had long been overlooked, the photoinducible dipolar cycloaddition between a nitrile imine (20) and an alkene (21, Scheme 4A).25 Lin and co-workers recently utilized this transformation as a bioorthogonal reaction, dubbing it the “photo-click” reaction.26 Terminal alkenes are quite small and, when not in conjugation with a carbonyl group, unreactive. Tetrazoles (22) are activated using relatively low energy UV-light, releasing nitrogen to produce nitrile imines, which serve as active 1,3-dipoles in cycloadditions. Nitrile imines react with alkenes to make pyrazoline cycloadducts (23). The rate of nitrogen extrusion is high so that cells need not be exposed to the potentially damaging UV-light for prolonged times. The reaction between the nitrile imine and alkene is slower, but it outcompetes degradation of the unstable nitrile imine in the presence of water. Additionally, while the starting materials are not fluorescent, the pyrazoline product is fluorescent, making the system fluorogenic, an attractive characteristic rarely seen in bioorthogonal reactions.27,28
Scheme 4.
In situ generated dipoles as bioorthogonal reagents. (A) The photo-click reaction. (B) The generation and reaction of the nitrile-oxide.
There is more than one way to generate an active dipole in situ. Carell and co-workers29 took advantage of yet another Huisgen discovery30 in their recent work with the reaction between nitrile oxides (24) and norbornenes (25, Scheme 4B). Nitrile oxides are generated by the deprotonation of the relatively stable arylchlorooximes (26) by a weak base. Upon generation, the dipoles readily react with alkenes, and the added strain of the norbornene allows the reaction to proceed rapidly to give the isoxazoline product, 27.
Synthesis of bioorthogonal reagents
After deciding on a bioorthogonal reaction to pursue, it is up to chemists to craft syntheses of the two reagents. While theoretical studies can point to new bioorthogonal reagents with highly desirable properties, a tractable synthesis is a must for any reagent to be of widespread utility. While cyclic alkynes with a ring size of less than eight atoms are unstable, often dimerizing or trimerizing, the cyclooctyne has a nice balance of stability and reactivity.31 The central challenge in synthesizing cyclooctynes is building ring-strain—about 18 kcal mol−1—into the system at a late enough stage in the synthesis so that the sensitive functionality need not withstand too many reactions. The formation of the strained cyclic alkyne is often accomplished by extrusion of a pair of leaving groups. These reactions are generally carried out in the presence of a strong base or heat. Since cyclooctynes are not new to synthetic chemistry, we will only discuss syntheses that have been used to construct the compounds used in bioorthogonal reactions.
The original bioorthogonal cyclooctyne, 1, was made from a silver-assisted opening of dibromocyclopropane 28, made in one step from cycloheptene, with alcohol 29 (Scheme 5).12,32 The trans-bromocyclooctene, 30, was subjected to elimination of the vinyl bromide under basic conditions to install the cyclooctyne. The conversion of 30 to the cyclooctyne is facilitated by the similarity in strain-energy between the two molecules. The aryl ester was saponified to acid 1, which could then be coupled to various probes for biological studies. The synthesis of 1 was completed in just three steps from 28 in 73% overall yield.
Scheme 5.
Synthesis of 1.
The synthesis to incorporate one fluorine atom onto the cyclooctyne ring was accomplished with a strategy that differed considerably from that used to construct 1. Mono-fluorination of commercially available cyclooctanone (31) to give 32 was accomplished via treatment of a silylenolether with Selectfluor (Scheme 6).14 Alkylation of monofluoroketone 32 gave a ketone intermediate that was converted to enoltriflate 33. This compound was converted to the monofluorinated cyclooctyne, 34, by elimination of the triflate with a strong base. The six-step synthesis of 34 from 31 was completed with an overall yield of 11%.
Scheme 6.
Synthesis of a monofluorinated cyclooctyne (34).
DIFO (35, Scheme 7) presented a significantly more difficult synthetic task, and the first route had ten steps.15 To allow for double fluorination alpha to the ketone, the linker moiety could no longer reside adjacent to the alkyne as in the previous compounds. Commercially available diol 36 was mono-alkylated with a functional handle, an allyl group, to be exploited later. This allowed selective oxidation of the other alcohol to form a ketone. Fluorination as before was accomplished via silylenolether 37. A second fluorination sequence was required to install the geminal fluorine atom in ketone 38. Oxidation of the allyl group to an acid afforded a site for attaching a probe, and the alkyne was again installed via enoltriflate formation and elimination to give 35. The overall yield for the ten-step synthesis was 1%.
Scheme 7.
First synthesis of DIFO (35).
A second-generation synthesis of the difluorinated cyclo-octyne was developed to make these highly reactive reagents more readily available to biologists.33 Starting with the difluorination of 1,3-cyclooctadione (39), made in a three-step sequence from a commercially available diester, the linker was then installed via a Wittig reaction to give 40 (Scheme 8). Reduction of the alkene followed by enoltriflate formation gave 41. The alkyne was installed by elimination of the triflate, and saponification of the ester to acid 42 allowed for coupling to probes. The synthesis from 39 to 42 was carried out in 6 steps with an overall yield of 36%.
Scheme 8.
More practical synthesis of DIFO analog (42).
To make the cyclooctynes more water-soluble we later developed a heteroatom rich cyclooctyne.18 The synthesis of this began with a sugar derivative. Glucose analog 43, made in a high-yielding three-step sequence from commercially available material, was converted to diene 44 via a reduction/reductive amination sequence followed by acylation of the resulting secondary amine (Scheme 9). Grubbs’ metathesis, oxidation of the allylic alcohol and hydrogenation of the alkene gave ketone 45. The ketone was converted to selenadiazole 46, which, upon vigorous heating, eliminated nitrogen and selenium to install the strained alkyne. Saponification of the ester gave alkyne 47. The route from 43 to 47 required 9 steps and was accomplished in 6% overall yield.
Scheme 9.
Synthesis of water-soluble cyclooctyne 47.
The synthesis of the dibenzocyclooctyne as reported by Boons began with known alkene 48 (made in 2 steps from phenylacetaldehyde, Scheme 10).34 Protection of the alcohol followed by bromination gave dibromide 49.19 The strained alkyne was installed by dehydrobromination, and linkage of a probe to the free alcohol was achieved by way of a carbamate to give 50. The synthesis from 48 to 50 was completed with a yield of 15% over 5 steps.
Scheme 10.
Synthesis of dibenzocyclooctyne 50.
It should be noted that, while the syntheses of strained cyclooctynes may appear straightforward on paper, each route has problematic steps that hinder widespread utility. Thus, a remaining challenge is to devise routes that are practical, even in the hands of non-experts.
Moving away from the cyclooctynes, many of the other systems are synthetically less challenging. A good example of this assertion is oxanorbornadiene 7 (Scheme 11).21 The synthesis consists of a Diels–Alder reaction between activated alkyne 51 and furan. Saponification to the acid allows for attachment of a probe moiety via standard coupling chemistry.
Scheme 11.
Synthesis of oxanorbornadiene 7.
trans-Cyclooctenes have a tremendous amount of strain (~16 kcal mol−1).35 In spite of this they are relatively easy to synthesize. The photoisomerization of cis-cyclooctene 52 to trans-cyclooctene 53 proceeds well (Scheme 12A), but reversion to the more stable cis form occurs over time.22 To make cyclobutene 17, cyclooctatetrene was reacted with maleic anhydride (Scheme 12B).24 Condensation with an amine allowed the installation of a probe. Unlike the systems described above which use the azide as a counterpart, strained alkenes rely on inverse-electron-demand Diels–Alder dienes. The dienes of choice are tetrazines (Scheme 13). Bipyridyl-tetrazine 11 (Scheme 13A)22 and biaryltetrazine 18 (Scheme 13C)24 can be made by condensing a cyanopyridine with either a second cyanopyridine or arylnitrile and hydrazine followed by oxidation. The less reactive, but more stable, mono-aryl tetrazines (14, Scheme 13B)23 were made by condensing 4-(aminomethyl)benzonitrile with formamidine acetate and hydrazine in the presence of sulfur, followed by oxidation of the resulting dihydrotetrazine.
Scheme 12.
Synthesis of reactive alkenes.
Scheme 13.
Synthesis of various tetrazines.
For the photo-click reaction, the fluorogenic diaryl tetrazoles were made by first condensing an aldehyde, 54 (Scheme 14), with phenylsulfonylhydrazine to make hydrazone 55.36 A base-mediated reaction of arylsulfonylhydrazone 55 with arenediazonium salt 56 gave 2,5-disubstituted tetrazoles (22). This sequence was used to make a variety of aryl substituted analogs. In situ photo-irradiation of these compounds expels nitrogen gas to unmask the reactive nitrile imine functionality.
Scheme 14.
Synthesis of tetrazoles.
As mentioned above, a second in situ generated dipole is the nitrile oxide. Nitrile oxides (24) can come from the reaction of chlorooximes (26) with a mild base (Scheme 15).29 Chlorooximes are made in two steps from aldehydes. The first step is the formation of an oxime with hydroxylamine and an aldehyde, 57. The chlorination of this oxime to form 26 can be accomplished with chlorine gas, or more conveniently with N-chlorosuccinimide (NCS).
Scheme 15.
Synthesis of nitrile oxides.
Use of bioorthogonal reactions in biological systems
With the bioorthogonal reactions designed and the reagents synthesized, it is time to turn our attention to biological applications. As mentioned above, the use of bioorthogonal reactions to tag biomolecules with probes requires that one reagent is somehow installed in the target molecule. Also, the reagent must be sufficiently stable so as to persist during the installation process.
There are numerous applications of cyclooctynes in the context of biological systems. Our group first showed that the cyclooctynes were able to selectively label cells that had metabolically incorporated azidosugars onto their surface as glycoproteins (Fig. 3).14 A two-step labeling protocol was employed, the first being treatment of the metabolically labeled cells with OCT-biotin, and those cells then being treated with avidin conjugated fluorescein. No change in cell viability was observed under the reaction conditions. Boons and co-workers showed that their dibenzocyclooctyne could also efficiently react with azides that had been incorporated into cell-surface glycans via non-natural sugars.20
Fig. 3.
Imaging cell-surface azidosugars with cyclooctyne probes. Azidosugars are metabolized by cells and incorporated into cell-surface glycans. The azide-labeled glycans are then reacted with a cyclooctyne-conjugated imaging probe.
Ting and co-workers introduced azides into mammalian cell-surface proteins using an enzyme named lipoic acid ligase (LplA, Fig. 4A).37 The protein could then be labeled with a fluorinated cyclooctyne-conjugated fluorescent dye.
Fig. 4.
Applications of cyclooctynes in cells. (A) LplA-mediated addition of an azido-fatty acid to a target protein and imaging with a fluorinated cyclooctyne probe. (B) Use of OCT to select for E. coli strains that incorporate high levels of an azido amino acid into cell-surface proteins. (C) Imaging an OCT–phospholipid conjugate on live cells using an azide-functionalized probe.
Copper is toxic to living cells, and as such the copper-free click reaction has been useful in applications where cell viability is a must. Tirrell and co-workers used a biotinylated-OCT tool for screening for enzymes that install azido amino acids in proteins within live Escherichia coli (Fig. 4B).38 After feeding E. coli the azido amino acid, the cells were labeled with biotinylated-OCT and fluorescent avidin. E. coli showing the brightest signal were collected, regrown, and subjected to a second round of selection. A novel enzyme that works well on azido amino acids was found using this procedure. Similarly, Zou and Yin capitalized on the non-toxic Cu-free click chemistry to label bacteriophage with probes without harming their bacterial host cells.39
Burkart and co-workers studied protein–protein interactions in non-ribosomal peptide biosynthesis by crosslinking domains using the second-generation DIFO, DIFO-2, on one domain and an azide on the other.40 de Koster and co-workers have recently used the reaction between a resin-immobilized cyclooctyne (non-fluorinated) with an azido-peptide to purify complex mixtures for proteomic analysis.41 A disulfide bond between the resin and cyclooctyne allowed for reductive removal of the purified peptides from the solid support.
The above examples share in common the placement of the azide within the target biomolecule, while the detection probe bears the cyclooctyne functionality. Neef and Schultz were the first, to our knowledge, to reverse the polarity of the reaction (Fig. 4C).42 They conjugated a phospholipid to OCT and inserted the modified lipid into live cell membranes. These cells were then imaged with azido-coumarin, a fluorogenic dye.
The final examples of the utility of cyclooctynes in biological settings give a glimpse of where these reactions are headed. Moving up from cell-culture, we have turned to model organisms to further explore the scope of these robust bioorthogonal reactions. Zebrafish were chosen as ideal organisms because they are vertebrates, have well-characterized developmental programs, and they are translucent during early stages of life.43 By bathing zebrafish embryos in media containing azidosugars, we were able to label their glycans at various stages of embryogenesis. At different time-points the zebrafish embryos were bathed in DIFO conjugated to a fluorescent dye.44 We were able to witness spatiotemporal changes in the distribution of glycans during the development process.
As the fastest of the three tetrazine ligation reactions, the process using trans-cycloctene may have interesting applications in living systems.22 To realize this goal, researchers may have to address several issues. First, the diaryltetrazines show significant cross reactivity with thiols. Additionally, both trans-cyclooctene and the tetrazine partner are rather large functionalities, which may complicate their metabolic or enzymatic incorporation into biomolecules. Those points aside, the reaction between trans-cyclooctene and diaryltetrazines was shown to be successful in a range of solvents and media, including cell lysate. As a proof of concept Fox and co-workers appended trans-cyclooctene to a thioredoxin and showed by shifts in mass spectral data that ligation with a tetrazine reagent had occurred in a buffered solution.
Hilderbrand and co-workers attached their norbornene to antibodies that were targeted to a human breast cancer cell line.23 After treating the cells with the modified antibodies in vitro, the ligation with a fluorescent dye–tetrazine conjugate produced a visible readout of cells that had been pretargeted with the antibody. More recently Hilderbrand, Weissleder and co-workers have used the trans-cyclooctene as an effective tool to accomplish the same goal.45
To evaluate the utility of the oxanorbornadiene, Rutjes and co-workers attached a derivative to a model protein, hen egg white lysozyme, via a polyethyleneglycol (PEG) linker. The modified protein was reacted with azido-coumarin resulting in fluorescent labeling.21 This technology was also used to conjugate a chelated 111 In-radioisotope to an azide-labeled cyclic peptide.46
Carell and co-workers used the [3+2]-cycloaddition reaction between a nitrile oxide and unprotected DNA adorned with norbornene to selectively label the oligonucleotides. 29 This reaction is orthogonal to the above mentioned azide reactions, so in principle, one might employ both chemistries to effect two unique modifications of DNA or other biomolecules. The nitrile-oxide reaction with norbornene was also used to modify DNA during solid-phase synthesis.
Photo-click chemistry has a good deal of promise for biological applications. The fact that the photo-click reaction is fluorogenic makes it an ideal tool for imaging of living systems.27 After incorporating an O-allyl-tyrosine into a Z-domain protein, Lin and co-workers were able to selectively modify the non-natural allyl group with an appropriate tetrazole. A range of tetrazoles were synthesized to optimize fluorescence properties including sensitivity. Using this knowledge, wild-type and mutant Z-domain proteins were imaged in living E. coli. The photo-irradiation is fast enough to avoid significant damage to the bacteria as determined by their ability to replicate.
Future directions and concluding remarks
As we get a better idea of the powers and limitations of each bioorthogonal reaction pair, we can start using them in concert to study increasingly complex interactions in biological settings. While the field of bioorthogonal chemistry has expanded rapidly, there remains a pressing need for new reagents with fewer side reactions and increased efficiencies. Where will these new reactions come from? Using history as a guide, they are found in the least likely of places, buried in a piece of purely academic scholarship that is being written up at this very moment.
Biographies

Dr John Jewett is an American Cancer Society postdoctoral fellow working under the direction of Prof. Carolyn Bertozzi on the development of new bioorthogonal reagents. He earned his AB in biophysical chemistry from Dartmouth College in 2003 and obtained his PhD at the University of Chicago in 2008 with Prof. Viresh Rawal. His doctoral research was focused on the syntheses of several members of the pederin family of natural products.

Prof. Carolyn Bertozzi is the T.Z. and Irmgard Chu Distinguished Professor of Chemistry and Professor of Molecular and Cell Biology at UC Berkeley, and Professor of Molecular and Cellular Biology at UCSF. She is also the Director of the Molecular Foundry at the Lawrence Berkeley National Laboratory and an Investigator of the Howard Hughes Medical Institute. She earned her AB in Chemistry from Harvard University in 1988 and obtained her PhD at UC Berkeley in 1993 with Prof. Mark Bednarski. She carried out postdoctoral research at UCSF with Prof. Steven Rosen and joined the faculty at UC Berkeley in 1996.
Footnotes
Part of a themed issue reviewing the latest applications of click chemistry.
References
- 1.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem, Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Tornøe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 3.For a recent review see: Tornøe CW, Meldal M. Chem Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479.
- 4.For a recent review see: Sletten EM, Bertozzi CR. Angew Chem, Int Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942.
- 5.Gaetke LM, Chow CK. Toxicology. 2003;189:147–163. doi: 10.1016/s0300-483x(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 6.Thompson RB, Ligler F. J Biomed Opt. 2002;7:531–660. [Google Scholar]
- 7.Prescher JA, Dube DH, Bertozzi CR. Nature. 2004;430:873–877. doi: 10.1038/nature02791. [DOI] [PubMed] [Google Scholar]
- 8.Staudinger H, Meyer J. Helv Chim Acta. 1919;2:635. [Google Scholar]
- 9.Padwa A. 1,3-Dipolar Cycloaddition Chemistry. Vol. 1 Wiley-Interscience Publication; New York: 1984. [Google Scholar]
- 10.Huisgen R. Angew Chem, Int Ed Engl. 1963;2:565–598. [Google Scholar]
- 11.Wittig G, Krebs A. Chem Ber. 1961;94:3260–3275. [Google Scholar]
- 12.Agard NJ, Prescher JA, Bertozzi CR. J Am Chem Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 13.Chambers RD. Fluorine in Organic Chemistry. Blackwell; Oxford: 2004. pp. 14–15.pp. 224–225. [Google Scholar]
- 14.Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR. ACS Chem Biol. 2006;1:644–648. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- 15.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc Natl Acad Sci U S A. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenebeck F, Ess DH, Jones GO, Houk KN. J Am Chem Soc. 2009;131:8121–8133. doi: 10.1021/ja9003624. [DOI] [PubMed] [Google Scholar]
- 17.Bach RD. J Am Chem Soc. 2009;131:5233–5243. doi: 10.1021/ja8094137. [DOI] [PubMed] [Google Scholar]
- 18.Sletten EM, Bertozzi CR. Org Lett. 2008;10:3097–3099. doi: 10.1021/ol801141k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bühl H, Gugel H, Kolshorn H, Meier H. Synthesis. 1978:536. [Google Scholar]
- 20.Ning X, Guo J, Wolfert M, Boons GJ. Angew Chem, Int Ed. 2008;47:2253–2255. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Berkel SS, Dirks AJ, Debets MF, van Delft FL, Cornelissen JJLM, Nolte RJM, Rutjes FPJT. Chem BioChem. 2007;8:1504–1508. doi: 10.1002/cbic.200700278. [DOI] [PubMed] [Google Scholar]
- 22.Blackman ML, Royzen M, Fox JM. J Am Chem Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devaraj NK, Weissleder R, Hilderbrand SA. Bioconjugate Chem. 2008;19:2297–2299. doi: 10.1021/bc8004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pipkorn R, Waldeck W, Didinger B, Koch M, Mueller G, Wiessler M, Braun K. J Pept Sci. 2009;15:235–241. doi: 10.1002/psc.1108. [DOI] [PubMed] [Google Scholar]
- 25.Huisgen R. Angew Chem, Int Ed Engl. 1968;7:321–328. [Google Scholar]
- 26.Song W, Yang Y, Qu J, Madden MM, Lin Q. Angew Chem, Int Ed. 2008;47:2832–2835. doi: 10.1002/anie.200705805. [DOI] [PubMed] [Google Scholar]
- 27.Song W, Wang Y, Qu J, Lin Q. J Am Chem Soc. 2008;130:9654–9655. doi: 10.1021/ja803598e. [DOI] [PubMed] [Google Scholar]
- 28.For another fluorogenic system see: Hangauer MJ, Bertozzi CR. Angew Chem, Int Ed. 2008;47:2394–2397. doi: 10.1002/anie.200704847. and references therein.
- 29.Gutsmiedl K, Wirges CT, Ehmke V, Carell T. Org Lett. 2009;11:2405–2408. doi: 10.1021/ol9005322. [DOI] [PubMed] [Google Scholar]
- 30.Huisgen R, Seidel M, Wallbillich G, Knupfer H. Tetrahedron. 1962;17:3–29. [Google Scholar]
- 31.Krebs A, Wilke J. Topics in Current Chemistry. Springer; Germany: 1983. pp. 189–233. [Google Scholar]
- 32.Reese CB, Shaw A. Chem Commun. 1970:1172–1173. [Google Scholar]
- 33.Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. J Am Chem Soc. 2008;130:11486–11493. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung ME, Mossman AB, Lyster MA. J Org Chem. 1978;43:3698–3701. [Google Scholar]
- 35.Anslyn EV, Dougherty DA. Modern physical organic chemistry. University Science; Sausalito, CA: 2006. p. 111. [Google Scholar]
- 36.Ito S, Tanaka Y, Kakehi A, Kondo KI. Bull Chem Soc Jpn. 1976;49:1920–1923. [Google Scholar]
- 37.Fernandez-Suarez M, Baruah H, Martinez-Hernandez L, Xie KT, Baskin JM, Bertozzi CR, Ting AY. Nat Biotechnol. 2007;25:1483–1487. doi: 10.1038/nbt1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Link AJ, Vink MK, Agard NJ, Prescher JA, Bertozzi CR, Tirrell DA. Proc Natl Acad Sci U S A. 2006;103:10180–10185. doi: 10.1073/pnas.0601167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou Y, Yin J. Bioorg Med Chem Lett. 2008;18:5664–5667. doi: 10.1016/j.bmcl.2008.08.085. [DOI] [PubMed] [Google Scholar]
- 40.Hur GH, Meier JL, Baskin J, Codelli JA, Bertozzi CR, Marahiel MA, Burkart MD. Chem Biol. 2009;16:372–381. doi: 10.1016/j.chembiol.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nessen MA, Kramer G, Back J, Baskin JM, Smeenk LEJ, de Koning LJ, van Maarseveen JH, de Jong L, Bertozzi CR, Hiemstra H, de Koster CG. J Proteome Res. 2009;8:3702–3711. doi: 10.1021/pr900257z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neef AB, Schultz C. Angew Chem, Int Ed. 2009;48:1498–1500. doi: 10.1002/anie.200805507. [DOI] [PubMed] [Google Scholar]
- 43.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 44.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. Science. 2008;320:664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA, Weissleder R. Angew Chem, Int Ed. 2009;48:7013–7016. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Berkel SS, Dirks AJ, Meeuwissen SA, Pingen DLL, Boerman OC, Laverman P, van Delft FL, Cornelissen JJLM, Rutjes FPJT. ChemBioChem. 2008;9:1805–1815. doi: 10.1002/cbic.200800074. [DOI] [PubMed] [Google Scholar]