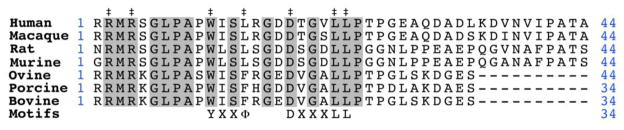Summary
Recent advances in imaging techniques along with more powerful in vitro and in vivo models of receptor-mediated ligand transport are facilitating advances in our understanding of how cells efficiently direct receptors and their cargo to target destinations within the cytoplasm and at the plasma membrane. Specifically, light and 3D electron microscopy studies to examine the trafficking behavior of the neonatal Fc receptor (FcRn), a transport receptor for immunoglobulin G (IgG), have given us new insights into the dynamic interplay between structural components of the cytosolic trafficking machinery, its protein regulators, and the receptors it directs to various locations within the cell. These studies build upon previous biochemical characterizations of FcRn transport and are allowing us to begin formulation of a more complete model for the intracellular trafficking of receptor-ligand complexes.
Introduction
Immunoglobulins are transported across epithelial cell barriers by specialized Fc receptors as a part of adaptive immune processes. Over the past several decades, experiments using these Fc receptors as model systems have greatly improved our understanding not only of receptor-mediated ligand transport, but also of general mechanisms for intracellular protein trafficking. One such Fc receptor, the neonatal Fc receptor (FcRn), mediates the passive acquisition of humoral immunity in early pre- or post-natal mammals by transferring maternal immunoglobulin G (IgG) to the fetus or suckling newborn [1–3]. In addition, FcRn serves to extend the serum half-life of IgG in adult mammals by protecting it from a default degradative pathway in vascular endothelial cells [4,5] and hematopoetic cell types [6,7]. While the ability of FcRn to recycle and transcytose IgG bidirectionally in non-polarized and polarized and cell models has been well documented [8–11], the specific mechanism(s) by which endocytosed IgG is sorted from other vesicular cargo and directed through a progression of endosomal compartments ultimately leading to the plasma membrane for fusion and exocytosis have been poorly understood. Recent advances in techniques for the visualization of FcRn and FcRn-ligand complexes has allowed for new insights into the mechanisms that shape and regulate the process of FcRn-mediated trafficking. A combination of light-, live-cell-, and electron microscopy (EM) experiments of recent interest will be described here. A deeper understanding of how FcRn properly targets itself and its ligand to their proper target destinations within the cells has broad implications in understanding the biology of FcRn and IgG transport and regulation, as well as in our understanding of receptor trafficking as a whole, which underlies a large number of vital cellular processes.
Polarized epithelial barriers and transcytosis
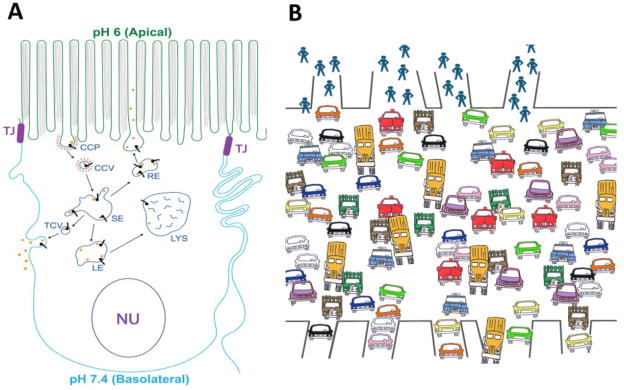
Epithelial cell barriers serve as a means for complex organisms to distinguish, both spatially and functionally, between the external environment and the underlying tissue. Polarized epithelial cells are joined to one another by a structured, oligomeric transmembrane protein complex known as the tight junction (TJ) [12]. This structure confers the cell’s ability to selectively allow the passage of materials—such as ions, small molecules, peptides, lipids, and proteins—either by passive or active (receptor-mediated) transport mechanisms [13–15]. TJs prevent the contents of the membranes above and below the junction from mixing, thus allowing these respective membrane domains to maintain distinct protein and lipid compositions. The two membrane faces created as a result of this physical separation are referred to as the apical (Ap) surface, which faces the external surface of the mucosae or the lumen of an organ, and the basolateral (BL) surface, which faces the serosal surface or bloodstream [12] (Figure 1). The apical and basolateral surfaces of polarized cells are connected to one another via a multivesicular transport pathway called transcytosis. Transcytosis is mediated by transmembrane receptors that originate on either the apical or basolateral cell surface and transport cargo to the opposite plasma membrane (Figure 1). This process requires that receptor-bound cargo be internalized into the endosomal membrane system and trafficked to its target destination as determined by motifs within the cytoplasmic tail of the receptor (for review, see [16]).
Figure 1.
(A) Schematic diagram showing canonical transcytotic and recycling pathways in polarized epithelial cells. Receptors (such as FcRn) at the cell surface bind their cognate ligands (such as IgG) and are internalized into clathrin-coated pits (CCPs), which pinch off from the plasma membrane to form clathrin-coated vescicles (CCVs). After uncoating these vesicles fuse with the endosomal network and enter the sorting endosome (SE) from which they can follow multiple routes including transcytosis via transcytotic vesicles (TCVs), return to the cell surface via the recycling endosome (RE), or degradation by way of the late endosome (LE) and lysosome (LYS). Lysosomal accumulation of IgG is most likely an off-pathway process that occurs in the presence of excess IgG. TJ: Tight junction. Nu: Nucleus. (B) Cartoon depiction of the “Freeway Problem”. Multiple travelers (receptor-ligand complexes) must navigate to multiple target destinations by moving about a crowded freeway (the cytoplasm) in various vehicles (endosomes). How the travelers are directed to the appropriate vehicles and subsequently navigated to their destinations is akin to the problem of intracellular receptor trafficking.
The endosomes of a cell are membrane-bound compartments that serve as the staging ground for intracellular trafficking and protein sorting [17] (Figure 1). The vast numbers of endosomes within the cell are classified into different subpopulations that are associated with different functions (e.g., recycling, transcytosis, or degradation), and that maintain their identities based on unique combinations of traffic-regulating membrane proteins associated with them [18–20]. Only after receptors and their bound cargo have entered endosomes can the processes of receptor sorting and trafficking be performed through a series of interactions between the receptor and the various membrane proteins, adaptor proteins, and cytoskeletal components that comprise the intracellular trafficking machinery [16]. It is this step-wise progression through the intracellular trafficking itinerary that allows for transport to be precisely regulated by the cell.
FcRn-mediated transport of IgG during transcytosis and recycling
Adult mammals have the capacity to generate antibodies against environmental antigens that are encountered during their lifetime. Alternatively, pre-formed antibodies from one individual can be transferred to another and confer immediate immunity to the antigen. Because the immune-rendered individual does not produce the antibodies themselves, this is refereed to as passive immunity [21].
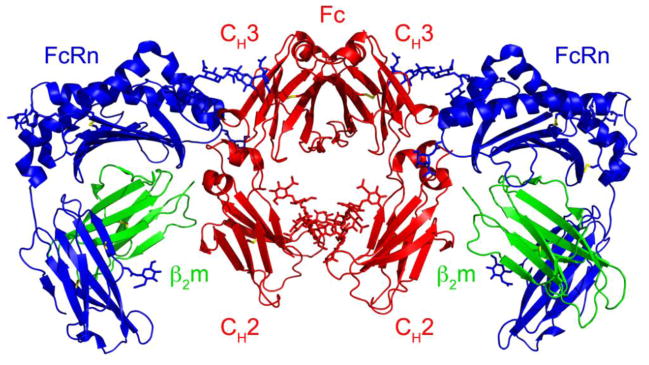
Passive acquisition of antibodies is particularly important in the case of rodents and several other mammals whose young are unable to produce their own IgG for up to two weeks post-natally [21], despite being exposed to the external environment during this time. To compensate, these animals acquire passive immunity during early life via IgG transferred from the mother. IgG-rich colostrum is ingested and passes through the intestine where it is transferred across the polarized epithelial cell lining into the bloodstream. This transfer is mediated by FcRn. FcRn is a heterodimeric type I transmembrane glycoprotein consisting of a membrane-bound heavy chain that is non-covalently associated with a light chain, β2-microglobulin (β2m), which also binds to class I MHC heavy chains [1,22] (Figure 2). High-resolution crystal structures of the ectodomains of human and rat FcRn, as well as a complex structure of rat FcRn in complex with Fc have been obtained [23–26]. FcRn is structurally similar to class I MHC molecules, although the counterpart of the MHC peptide-binding groove is narrowed and non-functional in FcRn, and binding to Fc is mediated in a manner that bears no resemblance to MHC interactions with T cell or other immune receptors.
Figure 2.
Ribbon diagram of the rat FcRn/Fc structure. FcRn forms a 2:1 complex with Fc and IgG molecules (i.e., two FcRn molecules bind to a single homodimeric Fc). FcRn (a heterodimer consisting of a heavy chain (blue) and β2-microglobulin (β2m; green) light chain) binds to Fc (red) at the junction between the CH2 and CH3 domains. In this orientation, the Fab arms of an intact IgG would project down from CH2 domains.
FcRn binds to the Fc region of IgG (Figure 2) with nanomolar affinity at pH ≤ 6.0, but exhibits no binding at the basic pH of blood (pH 7.4) [27]. This pH-dependent binding helps to promote the efficient unidirectional transfer of IgG from the acidic environment of the intestinal lumen to the bloodstream of the neonatal rodent. During gestation in humans and non-human primates maternal IgG is transferred to the fetus via FcRn-mediated transcytosis across placental syncitiotrophoblast cells to provide the fetus with immunity to antigens encountered by the mother [28]. The discovery that FcRn is expressed in the human fetal small intestine [29] suggests that maternal IgG in ingested amniotic fluid could also be delivered to the fetal circulation by transport across intestinal epithelial cells, consistent with a common mechanism for passive immunization of human and rodent offspring. Unlike rodents, humans and non-human primates develop the capacity to produce serum IgG within weeks of birth, thus, while pre-natal IgG transfer is likely needed to confer protection from environmental antigens during the first few weeks of independent life, post-natal transfer of maternal IgG does not occur [21,30].
FcRn also plays an integral role in maintaining high serum levels of IgG. It was hypothesized in the 1950s that IgG has a long half-life relative to other serum proteins because it was protected from degradation by a receptor [21,31]. It was later shown that FcRn serves as the IgG protection receptor by rescuing IgG that has been passively internalized via pinocytosis by vascular endothelial cells from a default catabolic pathway [32–34]. Similar to the mechanism of materno-fetal transfer in during primate gestation, FcRn binds to pinocytosed IgG in the acidic environment of the endosomes. However in this setting FcRn then recycles the IgG back to the cell surface where the IgG can dissociate and re-enter the circulation (Figure 1).
Cellular regulators of FcRn trafficking
The cytoplasmic tail of all species of FcRn contains canonical motifs associated with endocytosis and post-synthetic membrane targeting; a WXXφ motif (where φ is a bulky hydrophobic amino acid), instead of the canonical YXXφ motif, and an acidic dileucine motif (DXXXLL) [11] (Figure 3). These motifs are known from previous biochemical and structural data to bind to specific subunits of the AP-2 clathrin adaptor complex; a key mediator of clathrin-dependent endocytosis [35,36] and have also been shown to play key roles in the transcytosis and basolateral targeting of rat FcRn [37].
Figure 3.
Alignment of the cytoplasmic tail regions from multiple species of FcRn. Conserved residues are marked by gray boxes. Residues that have been experimentally demonstrated to effect receptor transport or trafficking are marked by a double dagger. Regions that correspond to canonical intracellular trafficking motifs are indicated on the bottom row.
One of the larger classes of traffic-regulating proteins is the Rab family of small GTPases. These small (~ 25 kDa) proteins exist in either the inactive GDP-bound or the active GTP-bound state (for reviews see [38,39]). Rab proteins are soluble cytoplasmic proteins that are prenylated in a regulated fashion by the enzyme Rab geranylgeranyl transferase (RabGG transferase), which in turn allows them to be inserted into endosomal membranes [40]. The transient association of Rab proteins with endosomal membranes allows the recruitment of other effector molecules that in turn regulate endosome targeting and fusion [28]. A combination of recent studies using fluorescence microscopy [41] and EM [42] have shown that FcRn is colocalized with endosomes positive for Rab4, Rab5, Rab7, Rab9, and Rab11. Rab5 is a known marker for early endosomes, Rabs 7 and 9 are markers for late endosomes and lysosomes [43], and Rabs 4 and 11 are associated with recycling from sorting endosomes [38,44]. Fluorescence microscopy studies of the interplay between human FcRn (hFcRn) and Rabs 4 and 11 found that while FcRn is present in sorting endosomes that are Rab4-positive and Rab11-positive, tubular carriers that emanate from these sorting endosomes are gradually depleted of Rab4 such that only Rab11 is found on FcRn-positive tubules that fuse with the plasma membrane [41]. Similar studies on late endosomal/lysosomal maturation showed that hFcRn present in late endosomes of endothelial cells is transferred to lysosomes via tubular extensions that transiently fuse with lysosomes, rather than through bulk transfer by means of full fusion, and that late endosomes contain both Rab5 and Rab7, perhaps indicating that FcRn within these compartments is committed to neither the recycling (Rab5-associated) nor lysosomal (Rab7-associated) fate until the time of exit from these structures [45]. The idea that certain sorting structures within the cell are functionally diverse has been supported by recent evidence that FcRn in polarized MDCK cells exits from recycling endosomes to enter either transcytotic or recycling pathways [46], and that Rab25 is required for the transcytotic pathway whereas Rab11, though dispensable for transcytosis, is required for recycling to the basolateral membrane only [46]. Taken together, these data suggest that while specific endosomal subpopulations retain their identities by association with a unique combination of membrane-bound regulator and/or effector molecules these associated proteins can facilitate the sorting and transfer of receptor-ligand complexes with multiple fates from a single endosome or cluster of “identical” endosomes. More recent evidence shows that the hFcRn cytoplasmic tail can bind to the cytoplasmic regulatory protein calmodulin and that this interaction can be abrogated by mutation of two conserved arginine residues in the membrane-proximal region of the cytoplasmic tail (Figure 3) [47]. These mutations reduce both receptor stability (as determined by pulse-chase labeling experiments) and transcytosis of the receptor in epithelial MDCK cells [47]. Whether calmodulin binding represents a direct signal to recruit other effector molecules to endosomes carrying FcRn and FcRn-IgG complexes or acts as a negative regulator by masking interaction sites within the FcRn cytoplasmic tail remains unknown.
Light microscopy studies of FcRn-mediated recycling of IgG
Live-cell fluorescence microscopy had been used to examine events that occur during FcRn-mediated recycling of human IgG (hIgG) in endothelial cells. For example, visualization of FcRn-GFP transfected cells incubated with fluorescently-labeled wild-type hIgG and hIgG bearing a mutation that disrupts binding to FcRn (H435A) showed that wild-type hIgG exited spherical sorting endosomes in tubules that were FcRn-positive, whereas the non-FcRn-binding H435A IgG mutant accumulated in spherical endosomes and was ultimately detected in lysosomes [9], consistent with a model whereby FcRn salvages IgG by physically carrying it along a recycling pathway that terminates at the plasma membrane rather than the default degradative pathway that terminates in the lysosomes. Additionally, recombinant Fc proteins that bear only one FcRn binding site have been shown to have significantly shorter serum persistence in mice [48,49], and greater lysosomal accumulation in transfected Madin-Darby canine kidney cells with concomitant reduction in their transcytotic and recycling efficiencies [10] as compared to their wild-type counterparts with two FcRn binding sites, suggesting that avidity effects conferring high apparent affinity to the FcRn-IgG interaction are necessary for efficient recycling.
Recent work using advanced light microscopy techniques has allowed the visualization of FcRn and FcRn-ligand complexes moving within and between different endosomal subpopulations during intracellular trafficking. The release of FcRn-bound IgG from the recycling surface has also been visualized using total-internal reflection microscopy (TIRFM) in which fluorophores present in a narrow plane of the plasma membrane are excited with laser light (for review of TIRFM, see [50]). In studies using this technique to visualize FcRn trafficking events leading to exocytosis in living endothelial cells two types of events were observed; full-release events in which FcRn-bound IgG quickly and completely exited the membrane fusion site, and “prolonged release” events in which IgG remained associated with the fused vesicle and was released slowly over several minutes [51]. Combining TIRFM with conventional epifluorescence imaging in one setup allows the simultaneous acquisition of more than one focal plane (termed multifocal plane microscopy, or MUM) [52,53]. In MUM studies examining the post-endocytotic fate of FcRn:IgG-containing vesicles, it was observed that two types of endosomal fusion events occur after vesicles pinched off from the plasma-membrane; “direct” fusion in which vesicles fused with the endosomal network immediately following endocytosis, and “indirect” fusion in which newly internalized vesicles move about the cytoplasm for a varied amount of time prior to endosomal fusion [54].
Electron tomography studies of FcRn-mediated transcytosis of IgG
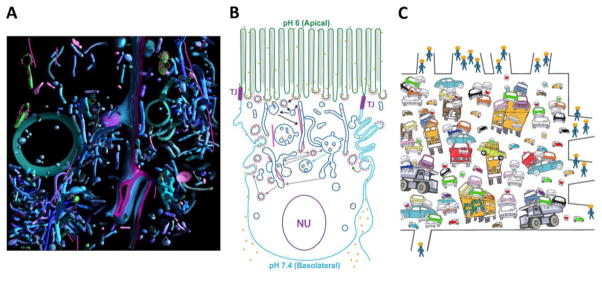
Electron tomography is an EM technique used to obtain three-dimensional (3D) data from a series of two-dimensional projection images [55–58]. A resolution of ~4 nm, at least two orders of magnitude higher than conventional light microscopy, can often be attained from cellular samples [55–58]. To visualize FcRn-mediated ligand transport within an in vivo setting, rat Fc molecules were chemically coupled to 1.4 nm Nanogold clusters via the cysteine residues of the Fc hinge, and gold-labeled Fc (Au-Fc) was fed to neonatal rats to allow FcRn-mediated uptake of the labeled ligand in the intestine, mimicking the transfer of maternal IgG across the intestine of the newborn [59,60]. Electron tomography performed on harvested intestinal samples revealed a complex picture of entangled tubular and irregular Au-Fc-containing endosomes in different regions of the cell [42] (Figure 4A,B). Most of the Fc-containing vesicles, including multivesicular bodies (Figure 4A,B), were identified as early and/or recycling vesicles in immunolabeling studies using antibodies against endosomal markers, which revealed a plethora of morphologies for endosomes bearing early, recycling, and late endosomal markers.
Figure 4.
Multiple pathways for FcRn-mediated transport observed by EM tomography. (A) Segmented model from a 3D reconstruction of gold-labeled Fc (Au-Fc) in endosomes of a neonatal rat intestinal cell (reproduced from Supplementary Figure S11b in [42]). Color coding for structures: tubular and irregular vesicles (blue shades); microtubules (pink rods); lysosomes (violet); rough endoplasmic reticulum (green with scarlet ribosomes); mitochondria (bright green); lateral membranes between adjacent cells (purple and blue); clathrin coats (small red spheres around blue vesicles/buds); Au-Fc (gold spheres). Note that groups of tubular and irregular endosomes sometimes form entangled masses. Only a small number of these endosomes are associated with microtubules, suggesting that these entangled networks may move as a cohesive unit. (B) Schematic representation of observations from EM tomography studies of Au-Fc transport across neonatal rat intestine by FcRn (adapted from Figure 5h in [42]). Gold dots indicate the presence of Au-Fc in the observed structures. Red dashes indicate the presence of clathrin coats. Pink rods are microtubules, which were often associated with tubular endosomes containing Au-Fc. (C) Suggested solution to the “Freeway Problem”. Travelers (receptor-ligand complexes) can take multiple routes to their target destinations, with vehicles (endosomes) often moving in groups rather than as individual entities.
One of the more surprising observations from the electron tomography studies was the presence of clathrin-coated vesicles containing Au-Fc that appeared to be fusing with the lateral plasma membrane [42]. While clathrin is classically believed to be involved in endocytosis via coated pits and some intracellular trafficking functions, its involvement in exocytosis had not generally been considered a possibility because it was believed that the clathrin coats on trafficking vesicles are shed prior to membrane fusion [61–63]. However, two lines of evidence suggested that these clathrin-coated structures could not be endocytosing Au-Fc. First, the pH in the lateral intracellular space (LIS) should not be permissive for FcRn binding, as this space is contiguous with the circulation (pH 7.4). Second, the concentration of Au-Fc in the blood of the rat should not be high enough to permit binding to FcRn and should furthermore be competed away by the high concentration of unlabeled, endogenous IgG in the circulation. For these reasons the authors hypothesized that clathrin-coated vesicles containing FcRn-ligand complexes can partially uncoat and fuse with the target plasma membrane [42]. Subsequently, the vesicle may fully uncoat, releasing the FcRn molecules into the target membrane and allowing for its bound cargo to dissociate into the extracellular space. Alternatively, the partially clathrin-coated vesicle may remain at the membrane, allowing diffusion of ligand through the opening of the partially fused vesicle in a process akin to so-called “kiss-and-run” [64] or “prolonged release” [51] before pinching back off and transporting the free receptor back to the apical surface to acquire more ligand. The later model has the advantage of conserving the energy that would be required to fully uncoat the vesicle (an ATP-dependent process) and to reassemble the endocytic machinery required to return ligand-free FcRn back to the apical surface for subsequent round of transcytosis.
Conclusions
Taken together, recent light microscopy and EM studies of FcRn-mediated transport demonstrate the dynamic interplay of multiple effector molecules required for proper trafficking of FcRn-IgG complexes. They also raise new questions about the precise nature of FcRn-effector interactions within the cytoplasm and whether these interactions are modulated or altered when FcRn is bound or free of its IgG ligand. One immediate question posed by these studies is how FcRn, one of many trafficking receptors within the cell, can precisely navigate the complex endosomal network while carrying cargo to its proper target destinations. By analogy, one can ask how a group of travelers on a busy freeway can determine which vehicles will carry them to their destinations while navigating within and around other traffic and travelers with the same requirement (Figure 4C). A comparison of the recent data from light microscopy and EM tomography supports the notion that FcRn-IgG complexes can take multiple routes to their target destinations and that trafficking, although precisely regulated by signals from the receptor itself and the cytosolic trafficking machinery, may be a flexible and forgiving process. This may not be surprising given the chaotic and highly dense environment in which trafficking takes place – i.e., the cytoplasm.
While exciting advances in our understanding of FcRn trafficking and function have come in recent years due to the development of new techniques to visualize FcRn-ligand complexes during transport, a variety of questions remain. Significant progress is likely to be made in the coming years by combining existing techniques while further developing them. Specifically, studies which combine high resolution, yet static, data offered by EM tomography with the dynamic, yet lower resolution, data from live-cell imaging experiments off great potential to further our understanding of the intricate and pervasive process of receptor-mediated ligand transport.
Acknowledgments
We thank Marta Murphy and Ron Diskin for help making figures. Studies of FcRn are supported in PJB’s laboratory by the National Institutes of Health (2 R37 AI041239-06A1). DBT is a full-time employee of Genentech, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and suggested further reading
• Papers of interest
•• Papers of special interest
- 1.Simister NE, Rees AR. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol. 1985;15:733–738. doi: 10.1002/eji.1830150718. [DOI] [PubMed] [Google Scholar]
- 2.Simister NE, Story CM. Human placental Fc receptors and the transmission of antibodies from mother to fetus. J Reprod Immunol. 1997;37:1–23. doi: 10.1016/s0165-0378(97)00068-5. [DOI] [PubMed] [Google Scholar]
- 3.Simister NE, Story CM, Chen HL, Hunt JS. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol. 1996;26:1527–1531. doi: 10.1002/eji.1830260718. [DOI] [PubMed] [Google Scholar]
- 4.Simister NE, Jacobowitz Israel E, Ahouse JC, Story CM. New functions of the MHC class I-related Fc receptor, FcRn. Biochem Soc Trans. 1997;25:481–486. doi: 10.1042/bst0250481. [DOI] [PubMed] [Google Scholar]
- 5.Telleman P, Junghans RP. The role of the Brambell receptor (FcRB) in liver: protection of endocytosed immunoglobulin G (IgG) from catabolism in hepatocytes rather than transport of IgG to bile. Immunology. 2000;100:245–251. doi: 10.1046/j.1365-2567.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akilesh S, Christianson GJ, Roopenian DC, Shaw AS. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol. 2007;179:4580–4588. doi: 10.4049/jimmunol.179.7.4580. [DOI] [PubMed] [Google Scholar]
- 7.Montoyo HP, Vaccaro C, Hafner M, Ober RJ, Mueller W, Ward ES. Conditional deletion of the MHC class I-related receptor FcRn reveals the sites of IgG homeostasis in mice. Proc Natl Acad Sci U S A. 2009;106:2788–2793. doi: 10.1073/pnas.0810796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claypool SM, Dickinson BL, Wagner JS, Johansen FE, Venu N, Borawski JA, Lencer WI, Blumberg RS. Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fcgamma-receptor. Mol Biol Cell. 2004;15:1746–1759. doi: 10.1091/mbc.E03-11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J Immunol. 2004;172:2021–2029. doi: 10.4049/jimmunol.172.4.2021. [DOI] [PubMed] [Google Scholar]
- 10.Tesar DB, Tiangco NE, Bjorkman PJ. Ligand valency affects transcytosis, recycling and intracellular trafficking mediated by the neonatal Fc receptor. Traffic. 2006;7:1127–1142. doi: 10.1111/j.1600-0854.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, Simister NE. Tryptophan- and dileucine-based endocytosis signals in the neonatal Fc receptor. J Biol Chem. 2001;276:5240–5247. doi: 10.1074/jbc.M006684200. [DOI] [PubMed] [Google Scholar]
- 12.Simons K, Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi J, Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv Drug Deliv Rev. 2005;57:815–855. doi: 10.1016/j.addr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 16••.Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. Excellent review of transcytosis covering a wide variety of in vitro and in vivo systems. [DOI] [PubMed] [Google Scholar]
- 17.Sachse M, Ramm G, Strous G, Klumperman J. Endosomes: multipurpose designs for integrating housekeeping and specialized tasks. Histochem Cell Biol. 2002;117:91–104. doi: 10.1007/s00418-001-0348-0. [DOI] [PubMed] [Google Scholar]
- 18.Perret E, Lakkaraju A, Deborde S, Schreiner R, Rodriguez-Boulan E. Evolving endosomes: how many varieties and why? Curr Opin Cell Biol. 2005;17:423–434. doi: 10.1016/j.ceb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Piper RC, Luzio JP. Late endosomes: sorting and partitioning in multivesicular bodies. Traffic. 2001;2:612–621. doi: 10.1034/j.1600-0854.2001.20904.x. [DOI] [PubMed] [Google Scholar]
- 20.van Ijzendoorn SC. Recycling endosomes. J Cell Sci. 2006;119:1679–1681. doi: 10.1242/jcs.02948. [DOI] [PubMed] [Google Scholar]
- 21.Brambell FWR. The transmission of passive immunity from mother to young. Amsterdam, New York: North-Holland Pub. Co.; American Elsevier; 1970. [Google Scholar]
- 22.Rodewald R, Kraehenbuhl JP. Receptor-mediated transport of IgG. J Cell Biol. 1984;99:159s–164s. doi: 10.1083/jcb.99.1.159s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burmeister WP, Gastinel LN, Simister NE, Blum ML, Bjorkman PJ. Crystal structure at 2.2 A resolution of the MHC-related neonatal Fc receptor. Nature. 1994;372:336–343. doi: 10.1038/372336a0. [DOI] [PubMed] [Google Scholar]
- 24.Burmeister WP, Huber AH, Bjorkman PJ. Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature. 1994;372:379–383. doi: 10.1038/372379a0. [DOI] [PubMed] [Google Scholar]
- 25••.Martin WL, West AP, Jr, Gan L, Bjorkman PJ. Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: mechanism of pH-dependent binding. Mol Cell. 2001;7:867–877. doi: 10.1016/s1097-2765(01)00230-1. High-resolution crystal structure of an FcRn-ligand complex. Identified Fc histidines involved in pH-dependent salt bridges at the FcRn-Fc interface responsible for the pH dependence of the FcRn-IgG interaction. [DOI] [PubMed] [Google Scholar]
- 26.West AP, Jr, Bjorkman PJ. Crystal structure and immunoglobulin G binding properties of the human major histocompatibility complex-related Fc receptor(,) Biochemistry. 2000;39:9698–9708. doi: 10.1021/bi000749m. [DOI] [PubMed] [Google Scholar]
- 27.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 28.Firan M, Bawdon R, Radu C, Ober RJ, Eaken D, Antohe F, Ghetie V, Ward ES. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans. Int Immunol. 2001;13:993–1002. doi: 10.1093/intimm/13.8.993. [DOI] [PubMed] [Google Scholar]
- 29.Shah U, Dickinson BL, Blumberg RS, Simister NE, Lencer WI, Walker WA. Distribution of the IgG Fc receptor, FcRn, in the human fetal intestine. Pediatr Res. 2003;53:295–301. doi: 10.1203/01.PDR.0000047663.81816.E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oxelius VA, Svenningsen NW. IgG subclass concentrations in preterm neonates. Acta Paediatr Scand. 1984;73:626–630. doi: 10.1111/j.1651-2227.1984.tb09986.x. [DOI] [PubMed] [Google Scholar]
- 31.Brambell FW, Halliday R, Morris IG. Interference by human and bovine serum and serum protein fractions with the absorption of antibodies by suckling rats and mice. Proc R Soc Lond B Biol Sci. 1958;149:1–11. doi: 10.1098/rspb.1958.0046. [DOI] [PubMed] [Google Scholar]
- 32••.Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol. 1996;26:690–696. doi: 10.1002/eji.1830260327. Along with references [33] and [34] establishes that the neonatal Fc receptor (FcRn) and the serum IgG protection receptor are identical. [DOI] [PubMed] [Google Scholar]
- 33••.Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE. Increased clearance of IgG in mice that lack beta 2-microglobulin: possible protective role of FcRn. Immunology. 1996;89:573–578. doi: 10.1046/j.1365-2567.1996.d01-775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wernick NL, Haucke V, Simister NE. Recognition of the tryptophan-based endocytosis signal in the neonatal Fc Receptor by the mu subunit of adaptor protein-2. J Biol Chem. 2005;280:7309–7316. doi: 10.1074/jbc.M410752200. [DOI] [PubMed] [Google Scholar]
- 36.Kelly BT, McCoy AJ, Spate K, Miller SE, Evans PR, Honing S, Owen DJ. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature. 2008;456:976–979. doi: 10.1038/nature07422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton EE, Wu Z, Simister NE. Characterization of basolateral-targeting signals in the neonatal Fc receptor. J Cell Sci. 2005;118:2461–2469. doi: 10.1242/jcs.02367. [DOI] [PubMed] [Google Scholar]
- 38.Deneka M, Neeft M, van der Sluijs P. Regulation of membrane transport by rab GTPases. Crit Rev Biochem Mol Biol. 2003;38:121–142. doi: 10.1080/713609214. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. J Cell Sci. 2007;120:3905–3910. doi: 10.1242/jcs.015909. [DOI] [PubMed] [Google Scholar]
- 40.Pereira-Leal JB, Hume AN, Seabra MC. Prenylation of Rab GTPases: molecular mechanisms and involvement in genetic disease. FEBS Lett. 2001;498:197–200. doi: 10.1016/s0014-5793(01)02483-8. [DOI] [PubMed] [Google Scholar]
- 41••.Ward ES, Martinez C, Vaccaro C, Zhou J, Tang Q, Ober RJ. From sorting endosomes to exocytosis: association of Rab4 and Rab11 GTPases with the Fc receptor, FcRn, during recycling. Mol Biol Cell. 2005;16:2028–2038. doi: 10.1091/mbc.E04-08-0735. Interesting paper using live-cell imaging to observe the dynamic associations between FcRn-positive endosomes and Rab GTPases during recycling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.He W, Ladinsky MS, Huey-Tubman KE, Jensen GJ, McIntosh JR, Bjorkman PJ. FcRn-mediated antibody transport across epithelial cells revealed by electron tomography. Nature. 2008;455:542–546. doi: 10.1038/nature07255. Electron tomography was used to visualize FcRn-mediated ligand trafficking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soldati T, Rancano C, Geissler H, Pfeffer SR. Rab7 and Rab9 are recruited onto late endosomes by biochemically distinguishable processes. J Biol Chem. 1995;270:25541–25548. doi: 10.1074/jbc.270.43.25541. [DOI] [PubMed] [Google Scholar]
- 44.Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Gan Z, Ram S, Vaccaro C, Ober RJ, Ward ES. Analyses of the Recycling Receptor, FcRn, in Live Cells Reveal Novel Pathways for Lysosomal Delivery. Traffic. 2009;10:600–614. doi: 10.1111/j.1600-0854.2009.00887.x. Live-cell imaging was used to monitor trafficking of FcRn from sorting endosomes to lysosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzaban S, Massol RH, Yen E, Hamman W, Frank SR, Lapierre LA, Hansen SH, Goldenring JR, Blumberg RS, Lencer WI. The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity. J Cell Biol. 2009;185:673–684. doi: 10.1083/jcb.200809122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickinson BL, Claypool SM, D’Angelo JA, Aiken ML, Venu N, Yen EH, Wagner JS, Borawski JA, Pierce AT, Hershberg R, et al. Ca2+-dependent Calmodulin Binding to FcRn Affects Immunoglobulin G Transport in the Transcytotic Pathway. Mol Biol Cell. 2008;19:414–423. doi: 10.1091/mbc.E07-07-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim JK, Tsen MF, Ghetie V, Ward ES. Catabolism of the murine IgG1 molecule: evidence that both CH2-CH3 domain interfaces are required for persistence of IgG1 in the circulation of mice. Scand J Immunol. 1994;40:457–465. doi: 10.1111/j.1365-3083.1994.tb03488.x. [DOI] [PubMed] [Google Scholar]
- 49.Kim JK, Tsen MF, Ghetie V, Ward ES. Localization of the site of the murine IgG1 molecule that is involved in binding to the murine intestinal Fc receptor. Eur J Immunol. 1994;24:2429–2434. doi: 10.1002/eji.1830241025. [DOI] [PubMed] [Google Scholar]
- 50.Schneckenburger H. Total internal reflection fluorescence microscopy: technical innovations and novel applications. Curr Opin Biotechnol. 2005;16:13–18. doi: 10.1016/j.copbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Ober RJ, Martinez C, Lai X, Zhou J, Ward ES. Exocytosis of IgG as mediated by the receptor, FcRn: an analysis at the single-molecule level. Proc Natl Acad Sci U S A. 2004;101:11076–11081. doi: 10.1073/pnas.0402970101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52••.Prabhat P, Gan Z, Chao J, Ram S, Vaccaro C, Gibbons S, Ober RJ, Ward ES. Elucidation of intracellular recycling pathways leading to exocytosis of the Fc receptor, FcRn, by using multifocal plane microscopy. Proc Natl Acad Sci U S A. 2007;104:5889–5894. doi: 10.1073/pnas.0700337104. Interesting study using MUM to observe FcRn exocytosis in living cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prabhat P, Ram S, Ward ES, Ober RJ. Simultaneous imaging of different focal planes in fluorescence microscopy for the study of cellular dynamics in three dimensions. IEEE Trans Nanobioscience. 2004;3:237–242. doi: 10.1109/tnb.2004.837899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ram S, Prabhat P, Chao J, Ward ES, Ober RJ. High accuracy 3D quantum dot tracking with multifocal plane microscopy for the study of fast intracellular dynamics in live cells. Biophys J. 2008;95:6025–6043. doi: 10.1529/biophysj.108.140392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donohoe BS, Mogelsvang S, Staehelin LA. Electron tomography of ER, Golgi and related membrane systems. Methods. 2006;39:154–162. doi: 10.1016/j.ymeth.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 56.Frey TG, Perkins GA, Ellisman MH. Electron tomography of membrane-bound cellular organelles. Annu Rev Biophys Biomol Struct. 2006;35:199–224. doi: 10.1146/annurev.biophys.35.040405.102039. [DOI] [PubMed] [Google Scholar]
- 57.Subramaniam S. Bridging the imaging gap: visualizing subcellular architecture with electron tomography. Curr Opin Microbiol. 2005;8:316–322. doi: 10.1016/j.mib.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Hoenger A, McIntosh JR. Probing the macromolecular organization of cells by electron tomography. Curr Opin Cell Biol. 2009;21:89–96. doi: 10.1016/j.ceb.2008.12.003. Excellent review of cellular electron tomography. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He W, Kivork C, Machinani S, Morphew MK, Gail AM, Tesar DB, Tiangco NE, McIntosh JR, Bjorkman PJ. A freeze substitution fixation-based gold enlarging technique for EM studies of endocytosed Nanogold-labeled molecules. J Struct Biol. 2007;160:103–113. doi: 10.1016/j.jsb.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He Y, Jensen GJ, Bjorkman PJ. Nanogold as a specific marker for electron cryotomography. Microsc Microanal. 2009;15:183–188. doi: 10.1017/S1431927609090424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Altstiel L, Branton D. Fusion of coated vesicles with lysosomes: measurement with a fluorescence assay. Cell. 1983;32:921–929. doi: 10.1016/0092-8674(83)90077-6. [DOI] [PubMed] [Google Scholar]
- 62.Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- 63.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 64.Fesce R, Grohovaz F, Valtorta F, Meldolesi J. Neurotransmitter release: fusion or ‘kiss-and-run’? Trends Cell Biol. 1994;4:1–4. doi: 10.1016/0962-8924(94)90025-6. [DOI] [PubMed] [Google Scholar]