Abstract
Functional investigation of the proposed dehydratase domain of ATX, a 6-methylsalicylic acid synthase from Aspergillus terreus, revealed that the domain is not involved in dehydration of the β-hydroxytriketide intermediate tethered on the acyl carrier protein but catalyzes thioester hydrolysis to release the product from the acyl carrier protein. Thus, we renamed this domain the thioester hydrolase (TH) domain. The intermediate bound to the TH domain of mutant H972A formed in the presence of NADPH was released as 6-methylsalicylic acid by both the intact ATX and by THID (a 541-amino acid region containing TH domain and its downstream) protein, in trans. Furthermore, THID showed a catalytic activity to hydrolyze a model substrate, 6-methylsalicylic acid-N-acetylcysteamine. The TH domain is the first example of a product-releasing domain that is located in the middle of a multidomain iterative type I polyketide synthase. Moreover, it is functionally different from serine protease-type thioesterase domains of iterative type I polyketide synthases.
Keywords: Enzyme Catalysis, Enzyme Mechanisms, Fungi, Hydrolases, Metabolism, Protein Domains, 6-Methylsalicylic Acid, 6-Methylsalicylic Acid Synthase, Polyketide, Polyketide Synthase
Introduction
Iterative type I polyketide synthases (iPKSs)3 are large multifunctional enzymes consisting of the minimum catalytic domains for PKS reactions, the β-ketoacyl synthase (KS), acyl transferase (AT) and acyl carrier protein (ACP) domains, and additional domains such as a ketoreductase (KR), dehydratase (DH), enoylreductase (ER), methyltransferase, and thioesterase (TE) domain. Through iterative use of these domains in a programmed manner, each iPKS produces its specific polyketide product. However, little is known how iPKSs control their reactions (1).
6-Methylsalicylic acid synthase (MSAS) was the first PKS to be purified and the first fungal iPKS whose gene was cloned (2–6). A conserved domain search indicated that MSAS consists of KS, AT, DH, KR, and ACP domains (5, 7). These domains of MSAS are thought to catalyze a series of programmed reactions, Claisen condensations, reduction, dehydration, cyclization, and product release (Fig. 1).
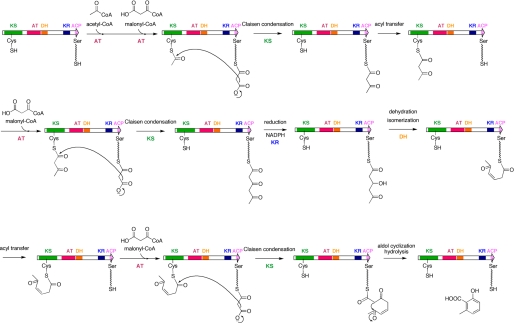
FIGURE 1.
MSAS reaction. MSAS initiates a sequence of reactions by loading of acetyl starter unit and malonyl extender unit. The growing polyketide intermediate is tethered to the phosphopantetheinyl arm (shown with a squiggle line) of the ACP. After the second condensation, the triketide intermediate is reduced by the KR domain, and the β-hydroxyl group generated had been thought to be removed by the DH domain. After the tetraketide formation by another malonyl condensation and aldol cyclization, 6MSA is released from MSAS.
Because MSAS is one of the simplest iPKSs, we have been studying its catalytic mechanism using ATX cloned from Aspergillus terreus (8). We previously carried out the expression of catalytic domain mutants of ATX in yeast. The KS mutant (KSm), AT domain mutant (ATm), and ACP mutant (ACPm) lost 6-methylsalicylic acid (6MSA) production, and the KR domain mutant (KRm) produced triacetic acid lactone as reported (9).
The DH domain of ATX was assigned by comparison of secondary structural elements predicted by PredictProtein with discrete DHs of type II fatty acid synthase (FAS) from Escherichia coli (10). The conserved DH domain motif, HXXXGXXXXP, has been identified in FAS DH, in which the histidine residue is a catalytic residue for dehydration, (11) and the corresponding sequence H972XXXGXXXXP981 was found in ATX. This His972 is crucial for ATX reaction as indicated by our previous result that the ATX H972A mutant (DHm) did not give any product, such as the β-hydroxy triketide (9).
Because MSAS has no apparent TE domain, it has been one of the oldest questions about this enzyme reaction how MSAS releases the final product, 6MSA (12). Type I FASs and modular type I PKSs use TEs for product release, and TE inactivation halts reaction turnover (13, 14). In many nonreducing type NR iPKSs for aromatic compounds, a TE-like domain has an indispensable role to release the product by Claisen-type cyclization, called the Claisen cyclase domain (15). Recent studies on 3-methylorcinaldehyde synthase in Acremonium strictum identified the C terminus reductive domain as functioning to release the product as an aldehyde (16). Involvement of a discrete β-lactamase-type TE was also reported for product release in nonreducing type PKS reactions (17). In some iPKSs, TE domains are involved in macrocyclization and product offloading (18, 19). No releasing domain has been identified among highly reducing type PKSs yet; however, it was proposed that they utilize pyrone ring formation as the driving force to release their products (18, 20) (Fig. 2). Reconstitution of the lovastatin PKS system indicated the involvement of TEs of fatty acid biosynthesis or unrelated PKS pathways (21). In the MSAS reaction, in vitro analysis using the purified recombinant MSAS has indicated that a discrete TE is not involved in the product release (7).
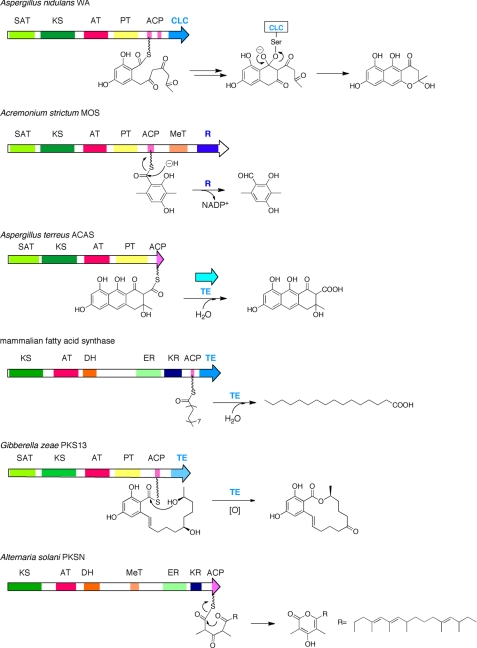
FIGURE 2.
Product-releasing mechanisms in iterative type I PKSs and FAS. CLC, Claisen cyclase domain for releasing YWA1 in WA PKS from Aspergillus nidulans. R, reductive domain for releasing 3-methylorcinaldehyde in 3-methylorcinaldehyde synthase (MOS) from A. strictum. FAS TE, thioesterase domain for releasing product as a free fatty acid. Gibberella zeae PKS13 TE, thioesterase domain for macrocyclization and product release. There is no releasing domain in highly reducing PKSs such as PKSN from Alternaria solani. Discrete thioesterase for releasing atrochrysone carboxylic acid by atrochrysone carboxylic acid synthase (ACAS) from Aspergillus terreus is shown.
Interestingly, the DH motif HXXXGXXXXP is also found in bacterial orsellinic acid synthases (22, 23), although no apparent dehydration is involved in the orsellinic acid synthase reaction. Thus, we previously proposed that (i) the DH domain in MSAS is involved in isomerization of a 2-trans-enoyl intermediate to the 3-cis-enoyl form required for ring cyclization or (ii) the DH domain catalyzes the hydrolytic release of 6MSA tethered as thioester on the MSAS ACP (9). Because based on its predicted secondary structure, the DH domain was presumed to belong to the “hotdog” fold superfamily which includes hydrolases and thioesterases as its main members (24), we decided to examine by in vitro analysis whether or not this domain of ATX (DH-like domain, now renamed TH domain) is involved in product release. In this report, we demonstrated that the DH-like domain of ATX hydrolyzes the thioester bond between tetraketide intermediate and the phosphopantetheinyl arm of ACP to release the product 6MSA and that the 541-amino acid fragment of the DH-like domain region between the AT and KR domain has a thioester-hydrolyzing activity.
EXPERIMENTAL PROCEDURES
Construction of Plasmids
The pESC-atX and pESC-atXDHm plasmids were prepared previously and used as template for amplification of atX, THID (a 541-amino acid region containing TH domain and its downstream) and THm fragments, respectively (9). Full-length atX, THm, THID, and sfp genes were amplified with Phusion High Fidelity DNA polymerase (Finnzymes). To co-express sfp with atX or DHm, pET-atX/sfp and pET-atXDHm/sfp plasmids were constructed by inserting sfp with its ribosomal binding site into the pET-atX and pESC-atXDHm plasmids (supplemental Fig. S4). For attaching a His tag to ATX, THm, and THID, primers with tag-coding sequences were used for gene amplification to construct the pET-atXHis10/sfp, pET-atXDHmHis10/sfp, and pET-THIDHis6/sfp expression plasmids.
Functional Expression of ATX in E. coli
E. coli BL21(DE3)CodonPlus RIPL cells (Stratagene) were transformed with the pET-atX/sfp expression plasmid. The transformant was grown in ATCC 765 medium (25) at 37 °C, 160 rpm until the A600 reached 0.4. After adding 1 mm isopropyl β-d-1-thiogalactopyranoside, the cells were incubated at 30 °C for 24 h. The induction culture was then acidified with 1 m HCl and extracted twice with an equal volume of ethyl acetate. The residue of the extract was dissolved in 20% CH3CN and analyzed by HPLC using a Tosoh DP 8020 pump, PD 8020 photodiode array detector (Tosoh) and an Inertsil ODS-3 column (4.6 × 150 mm) (GL Sciences) with a solvent system of CH3CN containing CH3COOH (1%, solvent B) and H2O containing CH3COOH (1%, solvent A) at a flow rate 0.8 ml min−1. Elution was performed with a linear gradient of solvent A/solvent B from 90:10 to 40:60 over 23 min. 6MSA and triacetic acid lactone were identified at retention time 18.3 min and 6.7 min, respectively.
Purification of His-tagged ATX, THm, and THID
For expression of ATX and THm protein, E. coli ArcticExpress (DE3) RIL (Stratagene) was transformed with pET-atXHis10/sfp or pET-atXDHmHis10/sfp expression plasmid, respectively. The cells were grown in LB liquid medium containing carbenicillin and gentamicin at 30 °C until the A600 reached 0.6. After adding 1 mm isopropyl β-d-1-thiogalactopyranoside, the cells were incubated at 12 °C for 24 h followed by a 3-h incubation at 30 °C for phosphopantetheinylation of ACP by Sfp. THID protein was expressed in BL21(DE3)-CodonPlus RIPL harboring pET-THIDHis6/sfp at 18 °C for 24 h. The cells were harvested and disrupted in lysis buffer (50 mm sodium phosphate buffer, pH 7.5, 0.5 m NaCl, 10% glycerol) by sonication. Then, equilibrated HIS-Select HF nickel affinity gel (Sigma-Aldrich) was added to the lysate. His-tagged proteins were eluted with the elution buffer containing 250 mm imidazole. THID protein was further purified by ion exchange and gel filtration chromatographies on a Mono Q 5/10 GL column (GE Healthcare) and a Superdex-200 HR 10/30 column (GE Healthcare).
In Vitro Analysis of ATX Activity
Incubation of 4 μm purified ATX or THm with 20 μm acetyl-CoA, 40 μm [2-14C]malonyl-CoA, 100 μm NADPH in a total volume of 20 μl was carried out at 25 °C for 2 h. The reaction was quenched with 1 m HCl, and the products were extracted twice with 50 μl of ethyl acetate. The extract residues were separated by thin layer chromatography (TLC) on Silica Gel 60 WF254s plates (Merck) using benzene/acetone/acetic acid (10:2:1, v/v/v) as a mobile phase. After development, TLC plates were analyzed by autoradiography with imaging plate BAS-III and BAS-1500 using Imaging Reader version 1.0 (Fujifilm). 14C-Labeled 6MSA and triacetic acid lactone were detected at RF values of 0.5 and 0.16, respectively.
14C Labeling of THm
ATX and THm were incubated as described above in a total volume of 10 μl. Proteins were separated by SDS-PAGE (10% gel) after reductive denaturation with β-mercaptoethanol and boiling. Then gels were stained with Coomassie Brilliant Blue, dried with gel dryer, and analyzed by autoradiography as described above.
N-terminal Sequencing of Digested THm
Forty μg of THm was digested by 20 units of thrombin at 37 °C overnight and separated with 5–20% gradient gel (Bio-Rad) for N-terminal sequencing of the fragments. The peptides were transferred on Sequi-Blot polyvinylidine difluoride membrane (Bio-Rad), and two fragments were excised from the membrane. Their N-terminal sequences were analyzed with the Procise Sequencing System, model 492cLC (Applied Biosystems).
Alkaline Hydrolysis of THm-intermediate Thioester
Five μm THm was radiolabeled by incubation with 12.5 μm acetyl-CoA, 25 μm [2-14C]malonyl-CoA, and 62.5 μm NADPH in a total volume of 200 μl at 25 °C for 2 h. The reaction mixture was filtrated through PD-10 desalting columns (GE Healthcare) to remove the unreacted substrates. The recovered labeled THm solution was boiled for 5min after adding 1 μl of β-mercaptoethanol and 20 μl of 10 m NaOH (21, 26, 27). The mixture was then acidified with HCl and extracted with 200 μl of ethyl acetate. The extracted product was analyzed by TLC autoradiography as described above.
Enzymatic Hydrolysis of THm-intermediate Thioester
14C-Labeled THm was incubated with 5 μm intact ATX in a total volume of 400 μl at 25 °C for 2 h. The reaction was quenched with HCl, and products were extracted with ethyl acetate and analyzed by TLC autoradiography.
Synthesis of 6MSA N-Acetylcysteamine Thioester (6MSA-SNAC)
Ten mg (0.07 mmol) of 6MSA in 5 ml of ethyl acetate was mixed with 12 mg (0.1 mmol) of N-hydroxysuccinimide (Wako) and 0.1 ml (0.1 mmol) of N-N′-dicyclohexylcarbodiimide (Tokyo Chemical Industry) and the mixture was stirred at room temperature overnight. After removing white precipitates, 12.8 μl (0.12 mmol) of N-acetylcysteamine (Aldrich) was added to the filtrate, and the mixture was stirred at room temperature overnight. 6MSA-SNAC was purified on 50 g of Silica Gel C-200 (Wako) by elution with 50% n-hexane/ethyl acetate to afford 4 mg of colorless oil (supplemental Figs. S5–S8).
Kinetic Analysis of ATX and THID Thioester Hydrolase Activity
The concentration of the enzymes and 6MSA-SNAC and incubation time were varied from 0 to 10 μm, from 0 to 24 mm, from 0 to 240 min, respectively. Reaction mixtures were analyzed directly by HPLC using an Inertsil ODS-3 column (2.0 × 150 mm) (GL Sciences) with 40% of CH3CN containing 0.02% CF3COOH at a flow rate of 0.2 ml min−1. 6MSA-SNAC and 6MSA were identified at retention times 4.3 min and 6.3 min, respectively.
RESULTS
Expression of ATX and DHm
We first tried to express ATX, not only intact ATX but also His10-tagged ATX, at either N or C terminus, in E. coli BL21 with pET vector system. Production of 6MSA was detected in the culture expressing intact ATX and C-terminally tagged ATX, even without phsophopantetheine transferase (Sfp) co-expression, but no 6MSA was detected in cultures expressing N-terminally His-tagged ATX (supplemental Fig. S1). E. coli ArcticExpress(DE3) RIL (Stratagene) was found to be a more suitable host which allowed higher protein expression at lower induction temperature. His10-tagged ATX and DHm were purified by nickel affinity chromatography (supplemental Fig. S2).
The purified ATX showed in vitro activity to synthesize 14C-labeled 6MSA in reaction mixtures containing acetyl-CoA, [2-14C]malonyl-CoA, and NADPH, even without acetyl-CoA. In the absence of NADPH, triacetic acid lactone was formed instead of 6MSA. Phenylmethylsulfonyl fluoride, a serine protease-type thioesterase inhibitor, did not inhibit enzymatic synthesis of 6MSA at all at 1 mm. (data not shown). These results confirmed that ATX by itself can synthesize tetraketide and release 6MSA as the free acid without involvement of serine protease-type thioesterase.
When DHm was incubated in vitro with acetyl-CoA, malonyl-CoA, and NADPH, neither 6MSA nor other products were detected in the reaction mixture. However, DHm produced triacetic acid lactone when NADPH was absent. Thus, it was confirmed that DHm was active to form the triketide intermediate, and the DH-like domain was not involved in triacetic acid lactone release. These results also suggested that DHm could catalyze the formation of a reduced intermediate, which could not be released but is retained on DHm in the presence of NADPH. In other words, the DH-like domain may be involved in product release. Actually, when DHm was incubated with [2-14C]malonyl-CoA and NADPH, 14C labeling of DHm was observed by autoradiography after SDS-PAGE separation, whereas intact ATX was not labeled under the same condition. These results led to the idea that the reduced intermediate formed in the presence of NADPH cannot be released from DHm, but the unreduced triketide intermediate is spontaneously released as triacetic acid lactone. This notion is also supported by the observation that the amount of radioactive intermediate retained on DHm was diminished in incubation without NADPH (Fig. 3).
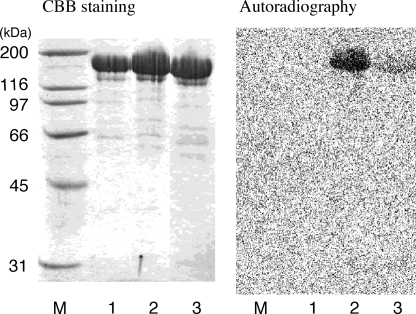
FIGURE 3.
Labeled intermediate bound to DHm. After incubation with acetyl-CoA and [2-14C]malonyl-CoA in the presence of or absence of NADPH, DHm (or intact ATX) was separated by SDS-PAGE and analyzed by autoradiography. M, marker protein; lane 1, ATX; lane 2, DHm + NADPH; lane 3, DHm − NADPH. Left, Coomassie Brilliant Blue staining; right, autoradiograph of the same gel after staining.
Alkaline hydrolysis of 14C-labeled DHm was attempted to identify the possible thioester intermediate on enzyme (21, 26, 27). Release of 14C-labeled 6MSA from the labeled DHm was detected by TLC autoradiography analysis (Fig. 4). This result strongly indicates that 6MSA synthesis proceeds without catalytic dehydration of the β-hydroxy triketide intermediate by DH domain which is therefore not required for tetraketide formation. The subsequent aldol cyclization and aromatization of the tetraketide intermediate then form 6MSA covalently bound to the ACP phosphopantetheine arm as the thioester. Finally, 6MSA is released hydrolytically from ATX by the action of the domain hitherto called DH domain. Thus, we propose to rename this DH-like catalytic domain as the thioester hydrolase (TH) domain and its mutant as THm.
FIGURE 4.

Release of 6MSA bound on DHm by alkaline hydrolysis and enzymatic hydrolysis by ATX. After incubation with acetyl-CoA and [2-14C]malonyl-CoA in the presence of NADPH, labeled DHm (or intact ATX) was subjected to alkaline hydrolysis, and then the released compound was analyzed by TLC and autoradiography. Lane 1, alkaline hydrolysis of 14C-labeled ATX; lane 2, alkaline hydrolysis of 14C-labeled DHm; lane 3, enzymatic hydrolysis of 14C-labeled DHm by ATX.
Because domains on the ATX tetrameric enzyme almost freely interact with each other to reconstitute activity (9), intact ATX was incubated with 14C-labeled THm to examine its releasing activity in trans. As was expected, 14C-labeled 6MSA was released from THm by the active TH domain of intact ATX in the same amount as released by alkaline hydrolysis (Fig. 4).
Identification of the Region Bound to the Intermediate
To determine the region where the labeled intermediate bound to ATX, 14C-labeled THm prepared by incubation with [2-14C]malonyl-CoA and NADPH was digested by thrombin and subjected to SDS-PAGE. Autoradiography of the SDS-PAGE gel showed that two fragments of approximately 20 kDa and 18 kDa were radiolabeled. The N-terminal sequences of these fragments were found to be S1676LAFEADEPLP and K1697AGSASSADAP, respectively, which correspond to sequences upstream of the ACP Ser1761 residue. This result confirms that the intermediate and/or the product 6MSA is retained on the ACP until its release from ATX (Fig. 5).
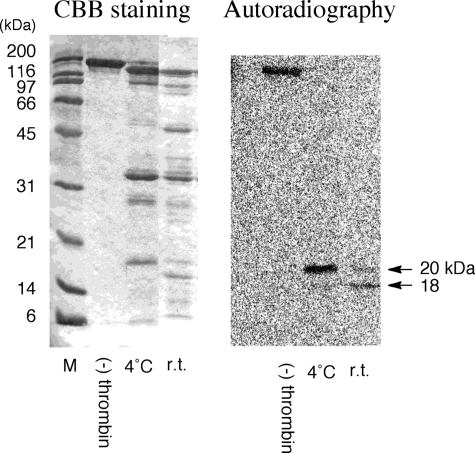
FIGURE 5.
14C-Labeled THm fragments by thrombin digestion. The 14C-labeled THm was subjected to thrombin digestion. After digestion, fragments were separated by SDS-PAGE and analyzed by autoradiography. N-terminal sequences of the labeled fragments were determined by peptide sequencing. Left, Coomassie Brilliant Blue (CBB) staining; right, autoradiograph of the same gel after staining.
Expression of TH Domain Protein
The TH domain of ATX was assigned as a 145-amino acid long region from Gly904 to Thr1048. We then tried to express the TH domain protein to examine its ability to complement to the TH function in the previously reported yeast co-expression system (9). Because co-expression of THm with a KSmATmKRmACPm quadruple mutant containing an active TH domain produced 6MSA, THm complementation by an active TH domain was verified. However, the 145-amino acid long TH domain protein alone could not restore the 6MSA production ability of THm. We have previously reported on the importance of the interdomain region between the TH and KR domains, which may serve as a core sequence to provide the subunit-subunit interaction necessary for active site formation (28). Thus, the Asn897 to Tyr1437 541 amino acid region containing the TH domain and the interdomain region was chosen to express as an independent protein, which was named THID. Complementation of THm by THID was first confirmed in the yeast co-expression system, and then THID was expressed with an N-terminal His6-tag in E. coli. After affinity purification, the THID protein was further purified by ion exchange and gel filtration column chromatography. The purified THID protein gave a major protein band at a position of 61 kDa on SDS-PAGE (supplemental Fig. S3). In vitro MSAS assay confirmed the production of 6MSA by a combination with THm and THID, but no 6MSA was detected in the incubation with either of them alone.
Thioester Hydrolase Activity of ATX and THID
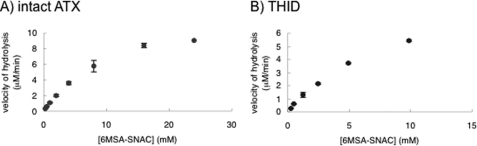
TH activity of ATX and THID was examined using 6MSA-SNAC as a substrate. NAC thioesters have been widely used as an ACP thioester analog. HPLC analysis of the reaction mixture consisting of ATX and 6MSA-SNAC revealed the production of free 6MSA, indicating that ATX itself catalyzes the hydrolysis of the thioester bond of 6MSA-SNAC. When THm was incubated with 6MSA-SNAC, little 6MSA was released. On the other hand, the reaction of 6MSA-SNAC with THID protein, as with ATX, led to the release of free 6MSA. These data indicated that the catalytic role of the TH domain of ATX is to release the MSAS product 6MSA from the PKS. Kinetic analysis showed that the kcat and Km values for 6MSA releasing of ATX were 12.3 mm and 1.4 min−1, respectively, and those of the THID protein were 5.76 mm and 0.75 min−1, respectively (Fig. 6).
FIGURE 6.
Hydrolysis of 6MSA-SNAC thioester by THID. When 6MSA-SNAC was incubated with THID, release of 6MSA was observed in the manner depending on time, enzyme (ATX or THID) amount, and substrate (6MSA-SNAC) concentration. A, kinetic analysis of 6MSA release by intact ATX. B, kinetic analysis of 6MSA release by THID.
DISCUSSION
In this report, we established that the domain of ATX, a MSAS of A. terreus, hitherto called the DH domain, is not involved in dehydration of the β-hydroxy triketide intermediate. Rather, the domain directly hydrolyzes a thioester bond of the tetraketide intermediate tethered to the ACP to release 6MSA. Upon revealing the hidden function of this catalytic domain, this domain was renamed thioester hydrolase (TH) domain. Known product-releasing domains of multidomain PKSs, such as the TE, Claisen cyclase, and R domains, are located at their C termini (18, 29, 30). In some cases, discrete TEs serve for product release of PKSs (17, 21, 31). However, the TH domain of ATX is the first example of a releasing domain located in the middle of a multidomain iPKS. Because ATX is not inactivated by serine-protease inhibitor phenylmethylsulfonyl fluoride and does not have a conserved thioesterase GXSXG motif, His972 of the TH domain is considered to be the catalytic residue to hydrolyze the ACP-bound 6MSA. The importance of a His residue was previously suggested in a report of inactivation experiments using 3-decenoyl-N-acetylcysteamine (3). In this report we confirm the actual role of His972.
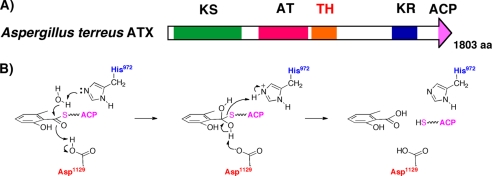
The crystal structure of the DH domain of erythromycin PKS fourth module (EryDH4) has been reported (32). His44 (numbering in EryDH4) of the HXXXGXXXXP motif and Asp206 are proposed to be involved in the dehydration as catalytic residues. Alignment indicates low similarity between ATX TH and EryDH4, but the conserved H972XXXGXXXXP motif and Asp1129 seem to be involved in the TH reaction. The proposed catalytic mechanism of the TH domain is shown in Fig. 7.
FIGURE 7.
TH domain and its role in the MSAS reaction. A, newly assigned architecture of ATX. B, proposed roles of His972 and Asp1129 of the TH domain in releasing 6MSA as an ATX product.
This finding can obviously answer to two questions: (i) how MSAS releases the final product and (ii) why orsellinic acid synthase possesses a DH-like domain. In addition, there was some discussion about the mechanism of formation of the cis double bond necessary for cyclization because the dehydration in FAS forms a trans double bond (33). Now, this matter is resolved as it is shown that there is no DH domain-catalyzed dehydration of the reduced intermediate. Rather, the β-hydroxyl group of the triketide intermediate is retained until the third condensation and cyclization and then eliminated by the driving force toward aromatization.
Intact ATX and the 541-amino acid THID polypeptide showed thioester hydrolysis activity, although the 145-amino acid TH domain region polypeptide did not release the product tethered on THm. Previously, we identified the TH-KR interdomain region which is required for subunit-subunit interaction in ATX (28). The present data also indicate the importance of the interdomain region for the interaction of the TH domain in trans with THm. In nonreducing type PKSs, the region between AT and ACP was assigned to be a product template domain, which is thought to promote the correct intramolecular aldol cyclization of an intermediate and its dehydration (34, 35). In ATX (MSAS), the interdomain region seems to have a different role from that of the product template domain. The mode of subunit-subunit interaction and the mechanism of TH thioester hydrolysis will be examined in further studies such as the three-dimensional structure analysis of THID, product-bound THm, and intact ATX.
Acknowledgment
We thank Prof. Heinz G. Floss (University of Washington) for a critical reading of this manuscript.
This work was supported in part by Grant-in-aid for Scientific Research B 19310139 from the Japan Society for the Promotion of Science (to I. F.) and by a Grant-in-aid for Scientific Research on Priority Areas “Applied Genomics” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to I. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S8.
- iPKS
- iterative type I polyketide synthase
- PKS
- polyketide synthase
- KS
- β-ketoacyl synthase
- KSm
- β-ketoacyl synthase mutant
- ACP
- acyl carrier protein
- ACPm
- acyl carrier protein mutant
- AT
- acyl transferase
- ATm
- acyl transferase mutant
- KR
- ketoreductase
- KRm
- ketoreductase mutant
- DH
- dehydratase
- DHm
- dehydratase mutant
- TE
- thioesterase
- 6MSA
- 6-methylsalicylic acid
- MSAS
- 6-methylsalicylic acid synthase
- FAS
- fatty acid synthase
- TH
- thioester hydrolase
- THID
- a 541-amino acid region containing TH domain and its downstream
- HPLC
- high performance liquid chromatography
- TLC
- thin layer chromatography
- 6MSA-SNAC
- 6-methylsalicylic acid-N-acetylcysteamine
- EryDH4
- DH domain of erythromycin PKS fourth module.
REFERENCES
- 1.Cox R. J. (2007) Org. Biomol. Chem. 5, 2010–2026 [DOI] [PubMed] [Google Scholar]
- 2.Dimroth P., Walter H., Lynen F. (1970) Eur. J. Biochem. 13, 98–110 [DOI] [PubMed] [Google Scholar]
- 3.Scott A. I., Beadling L. C., Georgopapadakou N. H., Subbarayan C. R. (1976) Bioorg. Chem. 3, 238–248 [Google Scholar]
- 4.Dimroth P., Ringelmann E., Lynen F. (1976) Eur. J. Biochem. 68, 591–596 [DOI] [PubMed] [Google Scholar]
- 5.Beck J., Ripka S., Siegner A., Schiltz E., Schweizer E. (1990) Eur. J. Biochem. 192, 487–498 [DOI] [PubMed] [Google Scholar]
- 6.Spencer J. B., Jordan P. M. (1992) Biochem. J. 288, 839–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson M. T., Pohl N. L., Kealey J. T., Khosla C. (1999) Metab. Eng. 1, 180–187 [DOI] [PubMed] [Google Scholar]
- 8.Fujii I., Ono Y., Tada H., Gomi K., Ebizuka Y., Sankawa U. (1996) Mol. Gen. Genet. 253, 1–10 [DOI] [PubMed] [Google Scholar]
- 9.Moriguchi T., Ebizuka Y., Fujii I. (2008) ChemBioChem 9, 1207–1212 [DOI] [PubMed] [Google Scholar]
- 10.Leesong M., Henderson B. S., Gillig J. R., Schwab J. M., Smith J. L. (1996) Structure 4, 253–264 [DOI] [PubMed] [Google Scholar]
- 11.Joshi A. K., Smith S. (1993) J. Biol. Chem. 268, 22508–22513 [PubMed] [Google Scholar]
- 12.Shoolingin-Jordan P. M., Campuzano I. D. G. (1999) in Comprehensive Natural Products Chemistry (Sankawa U. ed.) pp. 345–365, Elsevier, Oxford [Google Scholar]
- 13.Pazirandeh M., Chirala S. S., Wakil S. J. (1991) J. Biol. Chem. 266, 20946–20952 [PubMed] [Google Scholar]
- 14.Kao C. M., Luo G., Cane D. E., Khosla C. (1995) J. Am. Chem. Soc. 117, 9105–9106 [Google Scholar]
- 15.Fujii I., Watanabe A., Sankawa U., Ebizuka Y. (2001) Chem. Biol. 8, 189–197 [DOI] [PubMed] [Google Scholar]
- 16.Bailey A. M., Cox R. J., Harley K., Lazarus C. M., Simpson T. J., Skellam E. (2007) Chem. Commun. 4053–4055 [DOI] [PubMed] [Google Scholar]
- 17.Awakawa T., Yokota K., Funa N., Doi F., Mori N., Watanabe H., Horinouchi S. (2009) Chem. Biol. 16, 613–623 [DOI] [PubMed] [Google Scholar]
- 18.Zhou H., Zhan J., Watanabe K., Xie X., Tang Y. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 6249–6254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M., Zhou H., Wirz M., Tang Y., Boddy C. N. (2009) Biochemisry 48, 6288–6290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujii I., Yoshida N., Shimomaki S., Oikawa H., Ebizuka Y. (2005) Chem. Biol. 12, 1301–1309 [DOI] [PubMed] [Google Scholar]
- 21.Ma S. M., Li J. W.-H., Choi J. W., Zhou H., Lee K. K. M., Moorthie V. A., Xie X., Kealey J. T., Silva N. A. D., Vederas J. C., Tang Y. (2009) Science 326, 589–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaisser S., Trefzer A., Stockert S., Kirschning A., Bechthold A. (1997) J. Bacteriol. 179, 6271–6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahlert J., Shepard E., Lomovskaya N., Zazopoulos E., Staffa A., Bachmann B. O., Huang K., Fonstein L., Czisny A., Whitwam R. E., Farnet C. M., Thorson J. S. (2002) Science 297, 1173–1176 [DOI] [PubMed] [Google Scholar]
- 24.Dillon S. C., Bateman A. (2004) BMC Bioinformatics 5, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kealey J. T., Liu L., Santi D. V., Betlach M. C., Barr P. J. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 505–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh N., Wakil S. J., Stoops J. K. (1984) J. Biol. Chem. 259, 3605–3611 [PubMed] [Google Scholar]
- 27.Castonguay R., He W., Chen A. Y., Khosla C., Cane D. E. (2007) J. Am. Chem. Soc. 129, 13758–13769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriguchi T., Ebizuka Y., Fujii I. (2006) ChemBioChem 7, 1869–1874 [DOI] [PubMed] [Google Scholar]
- 29.Hertweck C. (2009) Angew. Chem. Int. Ed. 48, 4688–4716 [DOI] [PubMed] [Google Scholar]
- 30.Du L., Lou L. (2010) Nat. Prod. Rep. 27, 255–278 [DOI] [PubMed] [Google Scholar]
- 31.Zhang J., Van Lanen S. G., Ju J., Liu W., Dorrestein P. C., Li W., Kelleher N. L., Shen B. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 1460–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keatinge-Clay A. (2008) J. Mol. Biol. 384, 941–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spencer J. B., Jordan P. M. (1992) Biochemistry 31, 9107–9116 [DOI] [PubMed] [Google Scholar]
- 34.Crawford J. M., Dancy B. C. R., Hill E. A., Udwary D. W., Townsend C. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16728–16733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crawford J. M., Thomas P. M., Scheerer J. R., Vagstad A. L., Kelleher N. L., Townsend C. A. (2008) Science 320, 243–246 [DOI] [PMC free article] [PubMed] [Google Scholar]