Abstract
The aims of the present study were to determine the level of oxidative stress and the salient factors leading to the relapse of acute myeloid leukemia (AML). Oxidative stress-related parameters and the expressions of specific genes were monitored in 102 cases of AML during a pretreatment period from a primary status to a relapse status. In addition, age-matched healthy subjects were classified as controls. The activities of adenosine deaminase and xanthine oxidase were higher in the relapse condition, whereas those of glutathione peroxidase, monoamine oxidase, and superoxide dismutase, and the total antioxidant capacity (T-AOC) were lower in the primary condition and in controls. Of particular note, levels of advanced oxidation protein products, malondialdehyde, and 8-hydroxydeoxyguanosine were also significantly higher in relapse patients. Furthermore, real-time PCR with SYBR Green revealed that the expression levels of human thioredoxin (TRX) and indoleamine 2,3-dioxygenase were increased in relapse patients. Pearson correlation analysis revealed that the T-AOC was positively correlated with GSH but negatively correlated with 8-OHdG, TRX, and indoleamine 2,3-dioxygenase. Linear regression showed that a low T-AOC and up-regulated TRX expression were the independent factors correlated with relapse. A strong association between oxidative stress and the incidence of disease relapse was observed, which has potential prognosis implications. These results indicate that oxidative stress is a crucial feature of AML and probably affects the development and relapse of AML.
Keywords: Antioxidant, DNA Damage, Oxidative Stress, Reactive Oxygen Species (ROS), Tumor
Introduction
Oxidative stress is generally defined as an imbalance between the generation of reactive oxygen species (ROS)2 and impaired antioxidant defense systems. It has long been known to be involved in the pathophysiology of cancer (1). ROS produced either endogenously or exogenously can attack lipids, proteins, and nucleic acids simultaneously in living cells. This has led to cells developing various antioxidant defense mechanisms to both prevent the excessive formation of ROS and limit their harmful effects (2). The appropriate redox balance is maintained via the combined action of antioxidant enzymes, among which particularly important roles are played by superoxide dismutase (SOD), glutathione (GSH) peroxidase (GSH-Px), and monoamine oxidase (MAO).
An impaired antioxidant defense system is widely regarded as a cause of oxidative damage accumulation in cells. Various markers of oxidative damage have been identified. Malondialdehyde (MDA), which is one of the most popular markers, was designed to indicate lipid peroxidation (3). Oxidation of amino acid residues such as tyrosine, leading to the formation of dityrosine, protein aggregation, cross-linking, and fragmentation is one type of ROS-mediated protein damage. Proteins that are exquisitely vulnerable to ROS in vivo have been designated as advanced oxidation protein products (AOPP) (4). Measurement of AOPP is especially recommended for monitoring critically ill and cancer patients (5, 6). There are few markers of DNA oxidation in nuclear and mitochondrial DNA. However, in recent years 8-hydroxydeoxyguanosine (8-OHdG) has emerged as a marker of oxidative damage in the pathophysiological processes of cancer (7, 8). It has been shown that this DNA lesion is mutagenic, causing G to T transversions in mammalian cells.
Thioredoxin (TRX) is a 12-kDa thiol reductase that plays an important role in cell proliferation and cancer. As a scavenger of ROS, it can protect against various forms of cellular stress that can cause cell death. TRX functions as an antiapoptosis or cell death factor by inhibiting apoptosis signal-regulating kinase-1, a critical factor in stress-induced cell death, as well as by neutralizing ROS. The TRX system reportedly plays a dominant role in many physiological processes and is also a cell antioxidant (9). Lu et al. (10) recently found that blocking cancer cell DNA replication and repair and inducing oxidative stress by inhibiting both the TRX and GSH systems is an effective cancer chemotherapeutic strategy. Nigro et al. (11) demonstrated that the TRX system is inhibited by 13-hydroxy-15-oxo-zoapatlin, a norkaurane diterpene that was previously shown to possess proapoptotic potential and cause cell-cycle arrest in leukemia cells.
The enzyme indoleamine 2,3-dioxygenase (IDO) is one of the numerous mediators contributing to tumors not being affected by the applicable immune response of the host, and has recently attracted special attention. IDO converts tryptophan (Trp) into kynurenine (Kyn), blocking T-cell activation and inducing immunosuppression. There is mounting evidence that, within the tumor microenvironment, not only tumor cells but also other infiltrating cells such as dendritic cells and monocytes can be sources of IDO (12). Blasts of patients with AML were recently shown to express IDO. The serum Kyn/Trp ratio was found to be elevated in patients with acute myeloid leukemia (AML), suggesting higher IDO activity than in healthy subjects. Moreover, patients with higher Kyn/Trp ratios or high IDO expression exhibited significantly shortened overall and relapse-free survival rates (13, 14).
AML is a malignant neoplasm that is characterized by the clonal proliferation of blood cells within the bone marrow. The dismal survival rates of AML indicate the need for more effective treatment therapies for this condition (15, 16). The cellular and molecular events underlying AML include DNA damage, increased proliferation, deficient cell death, and further genetic instability. There is increasing evidence that the ROS-induced generation of oxidative stress plays a role in leukemia in this multistage process, and interesting results have been reported (17–19). However, few studies have explicitly examined the biochemical involvement of oxidative stress in the relapse of AML. The present study examined the roles of antioxidant enzyme activities, changes in oxidative damage, and expression of specific genes in the relapse of AML.
EXPERIMENTAL PROCEDURES
Patients
All of the patients gave their informed consent to participate (according to the Declaration of Helsinki) after having been fully informed about the purpose of the study between May 2004 and November 2009. They were diagnosed with AML using standard clinical and surface marker criteria at the Second Affiliated Hospital of Xi'an Jiaotong University. Patients with known confusion or who were judged too ill to participate were excluded from the investigation. None of the patients had central nervous system disease, uncontrolled infections, or other malignancies. They were also free of smoking, inflammatory conditions, endocrine dysfunctions, and pathological antecedents. Heparinized blood samples were obtained from 102 patients with AML during a pretreatment period from a primary status to a relapse status. The control subjects comprised 102 age-matched healthy subjects. The investigators at our research laboratory were blinded to the biologic samples. This study was approved by the Xi'an Jiaotong University Ethics and Scientific Committee, and met international standards for patient confidentiality.
Analyses of Oxidative Stress-related Parameters
The pretreatment blood samples were all collected at 8:00 a.m. to avoid diurnal variations in blood components. These blood samples were centrifuged at 3000 × g for 10 min to extract plasma. The total antioxidant capacity (T-AOC) and the activities of SOD, GSH-Px, adenosine deaminase, and xanthine oxidase were measured by chemical colorimetry, and MAO activity was tested by ultraviolet colorimetry. MDA levels were estimated by the thiobarbituric acid reactivity assay. These plasma parameters were assayed using commercially available kits according to the manufacturer's protocol (Nanjing Jiancheng Bioengineering Institute).
Determination of AOPP
Plasma (200 ml) diluted 1:5 in phosphate-buffered saline was placed into a 96-well microtiter plate, and 20 ml of acetic acid was added. In standard wells, 10 ml of 1.16 m potassium iodide (Sigma) was added to 200 ml of chloramine T (Sigma) solution (0–100 mm), followed by 20 ml of acetic acid. The absorbance of the reaction mixture was read immediately at 340 nm on a microplate reader against a blank containing 200 ml of phosphate-buffered saline, 10 ml of potassium iodide, and 20 ml of acetic acid. Finally, AOPP levels were measured by spectrophotometry and calibrated against chloramine T solutions, which absorb at 340 nm in the presence of potassium iodide (20, 21).
Assessment of Oxidative DNA Damage
We collected plasma obtained from whole blood by centrifuging at 2700 × g for 10 min, and then added 50 μl (in duplicate) of each prepared 8-OHdG standard, zero standard, or diluted samples, and 50 μl of monoclonal antibody to precoated wells. The wells were then covered with an adhesive plate sealer and incubated at room temperature for 1 h. Following the sixth aspiration using an automated microplate washer (Bio-Rad), 100 μl of horseradish peroxidase conjugate was added to each well (except the blank), and the plate was then incubated at room temperature for 1 h. Unbound enzyme-labeled secondary antibody was removed by a second washing step. After the addition of 100 μl of tetramethylbenzidine substrate for 15 min in the dark, the color reaction was terminated by adding Stop Solution 2. The 8-OHdG absorbance was measured at 450 nm by a high-throughput universal microplate reader (BMG Labtechnologies). Plasma 8-OHdG levels were assayed quantitatively using a StressXpress DNA damage enzyme-linked immunosorbent assay kit according to the manufacturer's protocol (Stressgen Bioreagents).
SYBR Green Real-time PCR Assay for Gene Expression
Total RNA was extracted from 50 to 100 mg of tissue samples in 1 ml of TRIzol reagent (Invitrogen) and purified using an RNeasy MinElute kit (Qiagen). Cellular RNA was primed with a dT oligonucleotide and reverse transcribed with Superscript II (Promega). The cDNAs thus obtained were tested for integrity by amplification of β-actin transcripts in a 30-cycle PCR. Standard curves were constructed using the mean cycle threshold (CT) value and various concentrations of TRX (ranging from 10−7 to 10−1), resulting in a linear relationship between CT and the logarithm of input DNA. The primers 5′-CCTTCCTGGGCATGGAGTCCTG-3′ (forward) and 5′-GGAGCAATGATCTTGATCTTC-3′ (reverse) were used to amplify a 205-bp fragment of β-actin, primers 5′-TGACGCCTGTGTGAAAGC-3′ (forward) and 5′-AATCAGTGCCTCCAGTTCC-3′ (reverse) were used to amplify a 155-bp fragment of IDO, and primers 5′-TTGGACGCTGCAGGTGATAAAC-3′ (forward) and 5′-GGCATGCATTTGACTTCACACTC-3′ (reverse) were used to amplify a 182-bp fragment of TRX. A total of 20 μl of the reaction sample consisted of 2 μl of cDNA, 10 μl of SYBR Premix Ex TaqII (Takara Bio), 0.4 μl of ROX Reference Dye, 0.8 μl of each primer at 10 μm (Takara Bio), and 6 μl of double-distilled H2O (Takara Bio).
Real-time PCRs were monitored using an ABI GeneAmp 7000 device (Applied Biosystems). Reaction conditions were as follows: 95 °C for 20 s, followed by 40 cycles of 95 °C for 5 s, 55 °C for 20 s, and 72 °C for 31 s. PCRs were performed in triplicate, and the reproducibility of the SYBR Green real-time PCR was assessed by running samples independently on different days.
Statistical Analyses
Variables were analyzed by descriptive statistics to evaluate the clinical characteristics of the cases. All data are reported as mean ± S.D., and were analyzed using one-way analysis of variance to estimate the presence of any statistical differences between the studied samples. Pearson correlations were adopted to note the correlation. Finally, important variables were analyzed by linear regression to identify any independent predictive factors that were correlated with relapse. Two-tailed probability values are listed in the tables, and the level of statistical significance was set at p ≤ 0.05. All statistical analyses were performed using SPSS 13.0 (SPSS, Chicago, IL).
RESULTS
Sociodemographic Variables
Investigations were carried out on 102 patients (58 males, 44 females) suffering from different types of leukemia: M1, myeloblastic leukemia (n = 6); M2, myeloblastic leukemia (n = 29); M3, promyelocytic leukemia (n = 5); M4, myelomonocytic leukemia (n = 20); M5, monocytic leukemia (n = 40); and M7, acute megakaryoblastic leukemia (n = 2). All patients met the recruitment criteria and were aged 38.65 ± 21.21 years (range 17–73 years). When in the primary condition, patients were categorized into group A; on relapse they were recategorized into group B. In addition, they were age matched with 102 healthy subjects, who were classified into group C (control).
Comparison of Plasma Parameters
The levels of oxidative stress were significantly higher in groups A and B than in group C. The T-AOC and concentrations of GSH-Px, SOD, and MAO were lower and the concentrations of AOPP, MDA, and 8-OHdG were higher in group B (p < 0.05). The findings are summarized in Table 1. A particularly significant finding was that the T-AOC decreased significantly, reaching half its initial level at relapse. The mean plasma levels of AOPP and MDA were significantly higher from the initial stage to the relapse stage than in controls.
TABLE 1.
Comparison of plasma parameters in groups A, B, and C
| Item | Group | Mean ± S.D. | F | P |
|---|---|---|---|---|
| Adenosine deaminase | Aa | 35.60 ± 17.24 | 4.03 | 0.01 |
| Bb | 38.11 ± 18.05 | |||
| C | 5.45 ± 7.50 | |||
| Xanthine oxidase | Aa,c | 8.80 ± 1.46 | 7.25 | 0.001 |
| Bb,c | 12.98 ± 2.23 | |||
| C | 5.82 ± 1.35 | |||
| GSH | Aa | 93.68 ± 8.32 | 0.59 | 0.65 |
| Bb | 92.12 ± 24.51 | |||
| C | 373.86 ± 169.79 | |||
| T-AOC | Aa,c | 7.18 ± 2.46 | 9.29 | 0.00 |
| Bb,c | 5.47 ± 2.16 | |||
| C | 15.68 ± 6.05 | |||
| MAO | Ac | 80.54 ± 26.73 | 1.26 | 0.36 |
| Bb | 37.87 ± 53.55 | |||
| C | 85.44 ± 35.49 | |||
| SOD | A | 67.80 ± 19.23 | 0.37 | 0.77 |
| B | 60.72 ± 29.72 | |||
| C | 79.34 ± 21.07 | |||
| A-OPP | A | 20.36 ± 6.86 | 3.63 | 0.08 |
| Bb | 27.82 ± 8.91 | |||
| C | 15.59 ± 7.51 | |||
| MDA | A | 10.23 ± 7.82 | 3.14 | 0.04 |
| B | 11.27 ± 5.15 | |||
| C | 6.52 ± 4.87 | |||
| 8-OHdG | Aa | 115.14 ± 40.19 | 4.02 | 0.01 |
| Bb | 135.20 ± 41.01 | |||
| C | 61.81 ± 31.05 |
a p < 0.05, group A versus group C.
b p < 0.05, group B versus group C.
c p < 0.05, group A versus group B.
TRX and IDO Expression
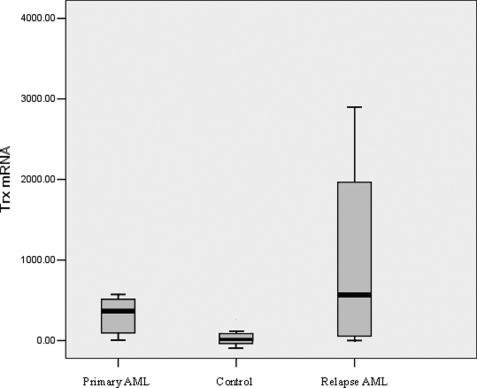
The baseline threshold and CT values were calculated automatically, and the concentration of mRNA was determined by fluorescence detection. Moreover, the expressions of TRX and IDO mRNAs were obtained relative to that of β-actin. The mean TRX/β-actin and IDO/β-actin ratios were 2.82 × 102 and 2.96 × 102, respectively, for primary patients with AML in group A, 7.08 × 102 and 3.35 × 102 for relapse patients in group B, and 1.19 and 0.93 for healthy donors in group C. In addition, the expression levels of TRX mRNA differed significantly among the three groups (Fig. 1).
FIGURE 1.
TRX expression in the different groups.
Pearson Correlation Analysis
The application of Pearson correlation analysis to multiple variables of the 102 cases revealed that the T-AOC was positively correlated with GSH but negatively correlated with 8-OHdG, TRX, and IDO. Moreover, TRX was positively correlated with IDO. These results are demonstrated in Table 2.
TABLE 2.
Pearson correlation analysis with multiple variables
| Variable | T-AOC | AOPP | MDA | 8-OHdG | TRX |
|---|---|---|---|---|---|
| T-AOC | 1.00 | ||||
| AOPP | −0.19 | 1.00 | |||
| MDA | −0.21 | 0.18 | 1.00 | ||
| 8-OHdG | −0.37a | 0.09 | 0.22 | 1.00 | |
| TRX | −0.63b | 0.26 | 0.24 | 0.16 | 1.00 |
| IDO | −0.32a | 0.13 | 0.05 | 0.27 | 0.43a |
a p < 0.05.
b p < 0.01.
Linear Regression Analysis
All patients were mainly treated with standard dose mitoxantrone (4–8 mg/(m2/day), days 1 to 3) and continuous infusion of cytarabine (100 mg/(m2/day), days 1 to 5). The relapse-free survival time (2–53 months) was selected as an independent variable. Linear regression showed that lower T-AOC levels and up-regulated TRX expression were significantly correlated with a reduced relapse-free survival time, and were independent factors for relapse (Table 3).
TABLE 3.
Results of multivariable analysis with linear regression
| Variable | Unstandardized coefficients | Standardized coefficients | t | P |
|---|---|---|---|---|
| T-AOC | −3.122 | −0.558 | −4.988 | 0.000 |
| TRX | 1.245 | 0.368 | 3.288 | 0.002 |
DISCUSSION
Free radicals are crucial to the etiology and progression of several human diseases. Disturbances to oxidative stress metabolism are commonly associated with tumor cells, and the level of oxidative stress among patients increases with disease severity and the accumulation of chromosomal aberrations (22, 23). Leukemias originate from hematopoietic stem cells that lose the capacity to differentiate normally into mature blood cells (24, 25). Although most patients achieve complete remission after chemotherapy, most of them suffer a subsequent leukemia relapse, which is associated with poor long term survival. However, empirical data involving oxidative stress in patients with AML are scarce in the scientific literature, and the complex mechanisms underlying relapse are unclear.
Many experimental and clinical studies have indicated that free radicals are involved in the biochemical mechanisms that underlie hematologic disorders (14–16). Al-Gayyar et al. (26) concluded that leukemia patients produce larger amounts of ROS than nonleukemia patients. Oltra et al. (27) found that lymphocyte SOD and catalase activity is decreased in chronic lymphocytic leukemia, whereas GSH-Px activity is increased. He et al. (28) reported that the plasma activities of SOD and GSH-Px were significantly lower in active acute lymphocytic leukemia patients than in normal control and acute lymphocytic leukemia complete remission patients. Battisti et al. (19) found that MDA levels and serum protein carbonylation were higher in acute lymphocytic leukemia patients than in controls, but that whole blood catalase and SOD activities were reduced in these patients. Mazor et al. (29) concluded that children with acute lymphocytic leukemia had an impaired plasma antioxidant status.
Our investigation revealed alterations of antioxidant enzymes in the plasma of patients with AML. The activities of adenosine deaminase and xanthine oxidase were higher in the relapse condition, whereas the T-AOC and the activities of GSH-Px, MAO, and SOD were lower in the primary and control conditions. The impaired plasma antioxidant status in AML is likely to be associated with increased ROS, as indicated by the decrease in antioxidant activity. Thus, oxidative stress is now recognized as a prominent feature of AML relapse.
The accumulation of ROS in cells damages the resident proteins, lipids, and DNA. AOPP are a characteristic of some diseases and may reflect the stage of the disease (6, 30–32). Kosova et al. (33) concluded that all AOPP, ferrous oxidation in xylenol orange, and MDA levels (which are markers of protein oxidation and lipid hyperoxidation) may induce the development of thyroid cancer, and that these begin to decrease after thyroidectomy. Chang et al. (34) found that serum 8-OHdG, protein carbonyl, and AOPP levels were significantly higher in their colorectal cancer group than in their control group, whereas the activities of antioxidative enzymes were significantly decreased. The findings of our study demonstrate that mean plasma levels of MDA (the lipid peroxidation product) are significantly higher at the relapse stage of AML. Similarly, AOPP levels increase progressively with the evolution of the disease. Importantly, these findings indicate a possible link between decreased levels of antioxidants and increased levels of cell alterations due to oxidative damage, supporting the idea that oxidative stress persists in relapsing AML patients, especially for the M2 and M5 types of leukemia due to their high percentages.
DNA damage, which triggers tumor initiation, can result in cell-cycle arrest, apoptosis, and the destabilization and destruction of the cellular genome. In recent years the biomarker 8-OHdG has become pivotal for measuring the effect of endogenous oxidative damage to DNA and as a factor influencing the initiation and promotion of carcinogenesis (35, 36). Furthermore, the results of clinical studies have suggested that treatment with drugs can protect cells against oxidative damage by enhancing the cellular antioxidant activity and modulating cellular signal pathways (37, 38). Oltra et al. (27) reported that the observed changes in MDA and the degree of DNA damage (as reflected by 8-OHdG levels) were increased in chronic lymphocytic leukemia. The pretherapy levels of urinary 8-OHdG were found to be higher in patients with lymphoma, acute leukemia, and myelodysplastic syndrome than in normal controls (39, 40). In our study, plasma 8-OHdG was also significantly increased from the initial condition to the relapse condition, suggesting that oxidative damage was clearly aggravated after relapse. We also plan to further test if novel mechanisms underlie a causal link between oxidative damage and AML relapse.
An excessive production of ROS not only affects major cellular components including lipids, proteins, and DNA, but also influences the gene expression profile (41). The complexity of the environment and the interindividual variability in cancer susceptibility makes it necessary to perform genetic studies aimed at explaining the physiopathology of relapse. The mammalian TRX system, which comprises TRX, TRX reductase, and NADPH, is the most important thiol system involved in the redox control of signaling and regulatory proteins in apoptosis and cell proliferation. TRX is a redox active protein that regulates the activities of various enzymes, including those that function to counteract oxidative stress within the cell. TRX can also scavenge ROS and directly inhibit proapoptotic proteins (42–44). Human TRX is a putative oncogene that may confer both growth and survival advantages to leukemia cells (10, 11, 45, 46). Furthermore, there is evidence emerging that IDO promotes tumor immune escape by inducing an immunoregulatory or an anergic T-cell phenotype at the systemic level (12). IDO may inhibit T-cell immune responses via down-regulation of Vav1 protein expression and activation (48). Preclinical studies are developing the IDO inhibitor 1-methyltryptophan to block host-mediated immunosuppression and enhance antitumor immunity in the setting of combined chemoimmunotherapy regimens (47).
We sought to understand the pathophysiological effects of AML relapse by examining group differences in specific genes. Our results confirm the presence of a strong correlation between an elevated TRX concentration and the presence of IDO. Moreover, TRX expression tended to increase in the presence of aggressive tumor growth and was significantly correlated with a shorter relapse interval. More importantly, we propose that lower T-AOC and up-regulated TRX are clinically useful parameters for predicting the relapse of acute leukemia, which can be objectively measured and evaluated. However, due to the limited number of cases examined herein, more studies are required to confirm the results.
In conclusion, relapse of AML is a neoplastic condition that is susceptible to alterations in antioxidant defense, oxidative damage, and essential gene alterations. Moreover, the correlation between oxidative stress and the rate of AML relapse is of potential prognostic significance. Our current analysis of oxidative stress is likely to provide insight into the biology of AML and new potential therapeutic targets, which may produce continuous improvement in the relapse rate of acute leukemia.
Acknowledgments
We thank Xing-Mei Cao, Ai-Li He, Yin-Xia Chen, Liu-Jie, Xiao-Rong Ma, Gang Chen, Wan-Hong Zhao, Jian-Li Wang, and Peng-Yu Zhang for technical assistance. We thank the technologists in the Cancer Cytogenetics Laboratory for expert technical assistance. We also thank the patients for their participation.
This work was supported by Shaanxi Natural Science Foundation of China Grant 2007C217.
- ROS
- reactive oxygen species
- 8-OHdG
- 8-hydroxydeoxyguanosine
- AML
- acute myeloid leukemia
- AOPP
- advanced oxidation protein products
- GSH-Px
- glutathione peroxidase
- IDO
- indoleamine 2,3-dioxygenase
- MAO
- monoamine oxidase
- MDA
- malondialdehyde
- SOD
- superoxide dismutase
- T-AOC
- total antioxidant capacity
- TRX
- thioredoxin.
REFERENCES
- 1.Valko M., Rhodes C. J., Moncol J., Izakovic M., Mazur M. (2006) Chem. Biol. Interact. 160, 1–40 [DOI] [PubMed] [Google Scholar]
- 2.Jones D. P. (2008) Am. J. Physiol. Cell Physiol. 295, C849–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanmugam N., Figarola J. L., Li Y., Swiderski P. M., Rahbar S., Natarajan R. (2008) Diabetes 57, 879–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selmeci L., Seres L., Soós P., Székely M., Acsády G. (2008) Acta Physiol. Hung. 95, 209–218 [DOI] [PubMed] [Google Scholar]
- 5.Selmeci L., Seres L., Antal M., Lukács J., Regöly-Mérei A., Acsády G. (2005) Clin. Chem. Lab. Med. 43, 294–297 [DOI] [PubMed] [Google Scholar]
- 6.Chandramathi S., Suresh K., Anita Z. B., Kuppusamy U. R. (2009) J. Cancer Res. Clin Oncol. 135, 319–323 [DOI] [PubMed] [Google Scholar]
- 7.Valko M., Izakovic M., Mazur M., Rhodes C. J., Telser J. (2004) Mol. Cell. Biochem. 266, 37–56 [DOI] [PubMed] [Google Scholar]
- 8.Wei Y. C., Zhou F. L., He D. L., Bai J. R., Ding H., Wang X. Y., Nan K. J. (2009) Int. J. Neuropsychopharmacol. 12, 1089–1096 [DOI] [PubMed] [Google Scholar]
- 9.Koháryová M., Kolárová M. (2008) Gen. Physiol. Biophys. 27, 71–84 [PubMed] [Google Scholar]
- 10.Lu J., Chew E. H., Holmgren A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 12288–12293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nigro P., Dal Piaz F., Gallotta D., De Tommasi N., Belisario M. A. (2008) Free Radic. Biol. Med. 45, 875–884 [DOI] [PubMed] [Google Scholar]
- 12.Zamanakou M., Germenis A. E., Karanikas V. (2007) Immunol. Lett. 111, 69–75 [DOI] [PubMed] [Google Scholar]
- 13.Chamuleau M. E., van de Loosdrecht A. A., Hess C. J., Janssen J. J., Zevenbergen A., Delwel R., Valk P. J., Löwenberg B., Ossenkoppele G. J. (2008) Haematologica 93, 1894–1898 [DOI] [PubMed] [Google Scholar]
- 14.Corm S., Berthon C., Imbenotte M., Biggio V., Lhermitte M., Dupont C., Briche I., Quesnel B. (2009) Leuk. Res. 33, 490–494 [DOI] [PubMed] [Google Scholar]
- 15.Smits E. L., Berneman Z. N., Van Tendeloo V. F. (2009) Oncologist 14, 240–252 [DOI] [PubMed] [Google Scholar]
- 16.Marbello L., Ricci F., Nosari A. M., Turrini M., Nador G., Nichelatti M., Tedeschi A., Vismara E., Morra E. (2008) Leuk. Res. 32, 1221–1227 [DOI] [PubMed] [Google Scholar]
- 17.Zhou F., Zhang W., Wei Y., Zhou D., Su Z., Meng X., Hui L., Tian W. (2007) Leuk. Res. 31, 387–393 [DOI] [PubMed] [Google Scholar]
- 18.Austin C. (2009) Leuk. Res. 33, 1297. [DOI] [PubMed] [Google Scholar]
- 19.Battisti V., Maders L. D., Bagatini M. D., Santos K. F., Spanevello R. M., Maldonado P. A., Brulé A. O., Araújo Mdo C., Schetinger M. R., Morsch V. M. (2008) Clin. Biochem. 41, 511–518 [DOI] [PubMed] [Google Scholar]
- 20.Boulanger E., Moranne O., Wautier M. P., Witko-Sarsat V., Descamps-Latscha B., Kandoussi A., Grossin N., Wautier J. L. (2006) Perit. Dial. Int. 26, 207–212 [PubMed] [Google Scholar]
- 21.Witko V., Nguyen A. T., Descamps-Latscha B. (1992) J. Clin. Lab. Anal. 6, 47–53 [DOI] [PubMed] [Google Scholar]
- 22.Voso M. T., Hohaus S., Guidi F., Fabiani E., D'Alò F., Groner S., Späth D., Doehner K., Leone G., Doehner H., Schlenk R. F. (2008) Leukemia 22, 1685–1691 [DOI] [PubMed] [Google Scholar]
- 23.Koptyra M., Cramer K., Slupianek A., Richardson C., Skorski T. (2008) Leukemia 22, 1969–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorantes-Acosta E., Chávez-González A., Santos J. I., Medina-Sanson A., Mayani H. (2008) Pediatr. Blood Cancer 51, 741–746 [DOI] [PubMed] [Google Scholar]
- 25.Montesinos J. J., Sánchez-Valle E., Flores-Figueroa E., Martínez-Jaramillo G., Flores-Guzmán P., Miranda-Peralta E., Gutiérrez-Romero M., Mayani H. (2006) Leuk. Lymphoma 47, 1379–1386 [DOI] [PubMed] [Google Scholar]
- 26.Al-Gayyar M. M., Eissa L. A., Rabie A. M., El-Gayar A. M. (2007) J. Pharm. Pharmacol. 59, 409–417 [DOI] [PubMed] [Google Scholar]
- 27.Oltra A. M., Carbonell F., Tormos C., Iradi A., Sáez G. T. (2001) Free Radic. Biol. Med. 30, 1286–1292 [DOI] [PubMed] [Google Scholar]
- 28.He Y. L., Cao L. Z., Yang J., Yang M. H., Xu W. Q., Xie M., Shi Z. (2009) Zhongguo Dang Dai Er Ke Za Zhi 11, 88–92 [PubMed] [Google Scholar]
- 29.Mazor D., Abucoider A., Meyerstein N., Kapelushnik J. (2008) Pediatr. Blood Cancer 51, 613–615 [DOI] [PubMed] [Google Scholar]
- 30.Furuya R., Kumagai H., Odamaki M., Takahashi M., Miyaki A., Hishida A. (2009) Nephron Clin. Pract. 112, c255–c261 [DOI] [PubMed] [Google Scholar]
- 31.Marsche G., Frank S., Hrzenjak A., Holzer M., Dirnberger S., Wadsack C., Scharnagl H., Stojakovic T., Heinemann A., Oettl K. (2009) Circ. Res. 104, 750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noyan T., Guducuoglu H., Ilhan M. (2009) Yonsei Med. J. 50, 677–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosova F., Cetin B., Akinci M., Aslan S., Ari Z., Sepici A., Altan N., Çetin A. (2007) Ann. Surg. Oncol. 14, 2616–2620 [DOI] [PubMed] [Google Scholar]
- 34.Chang D., Wang F., Zhao Y. S., Pan H. Z. (2008) Biomed. Environ. Sci. 21, 286–289 [DOI] [PubMed] [Google Scholar]
- 35.Hwang E. S., Kim G. H. (2007) Toxicology 229, 1–10 [DOI] [PubMed] [Google Scholar]
- 36.Valavanidis A., Vlachogianni T., Fiotakis C. (2009) J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 27, 120–139 [DOI] [PubMed] [Google Scholar]
- 37.Liu Y., Adachi M., Zhao S., Hareyama M., Koong A. C., Luo D., Rando T. A., Imai K., Shinomura Y. (2009) Cell Death Differ. 16, 847–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pennington J. D., Wang T. J., Nguyen P., Sun L., Bisht K., Smart D., Gius D. (2005) Drug Resist. Updat. 8, 322–330 [DOI] [PubMed] [Google Scholar]
- 39.Yang Y., Tian Y., Yan C., Jin X., Tang J., Shen X. (2009) Environ. Toxicol. 24, 446–452 [DOI] [PubMed] [Google Scholar]
- 40.Honda M., Yamada Y., Tomonaga M., Ichinose H., Kamihira S. (2000) Leuk. Res. 24, 461–468 [DOI] [PubMed] [Google Scholar]
- 41.Rodrigues M. S., Reddy M. M., Sattler M. (2008) Antioxid. Redox Signal. 10, 1813–1848 [DOI] [PubMed] [Google Scholar]
- 42.Tonissen K. F., Di Trapani G. (2009) Mol. Nutr. Food Res. 53, 87–103 [DOI] [PubMed] [Google Scholar]
- 43.Rao A. K., Ziegler Y. S., McLeod I. X., Yates J. R., Nardulli A. M. (2009) J. Mol. Endocrinol. 43, 251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofer S., Rosenhagen C., Nakamura H., Yodoi J., Bopp C., Zimmermann J. B., Goebel M., Schemmer P., Hoffmann K., Schulze-Osthoff K., Breitkreutz R., Weigand M. A. (2009) Crit. Care Med. 37, 2155–2159 [DOI] [PubMed] [Google Scholar]
- 45.Erkeland S. J., Palande K. K., Valkhof M., Gits J., Danen-van Oorschot A., Touw I. P. (2009) Leuk. Res. 33, 1367–1371 [DOI] [PubMed] [Google Scholar]
- 46.Oh Y. K., Lee T. B., Choi C. H. (2004) Biochem. Biophys. Res. Commun. 319, 41–45 [DOI] [PubMed] [Google Scholar]
- 47.Hou D. Y., Muller A. J., Sharma M. D., DuHadaway J., Banerjee T., Johnson M., Mellor A. L., Prendergast G. C., Munn D. H. (2007) Cancer Res. 67, 792–801 [DOI] [PubMed] [Google Scholar]
- 48.Li R., Wei F., Yu J., Li H., Ren X., Hao X. (2009) Cancer Biol. Ther. 8, 1402–1408 [DOI] [PubMed] [Google Scholar]