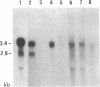
Abstract
The 89-kDa cell surface glycoprotein, P3.58, is detectable on advanced human melanomas in situ but not on benign melanocytes or early melanomas. cDNA cloning of P3.58 from melanoma cells was accomplished by screening a lambda zap expression vector library with monoclonal antibodies produced against the denatured antigen. Nucleotide sequencing of the clones revealed that P3.58 is identical to the intercellular-adhesion molecule 1. No qualitative differences in P3.58 mRNA species could be seen between melanoma cells and hematopoietic cells and no differences in gene organization were observed between peripheral blood leukocytes and melanoma cells. Inspection of the deduced amino acid sequence of P3.58 indicated the presence of the consensus sequence characteristic for complement-binding proteins. The acquisition of this cell-adhesion molecule during the process of tumor progression is speculated to contribute to the development of metastasis in melanoma.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970 Nov;172(5):902–908. doi: 10.1097/00000658-197011000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröcker E. B., Zwadlo G., Holzmann B., Macher E., Sorg C. Inflammatory cell infiltrates in human melanoma at different stages of tumor progression. Int J Cancer. 1988 Apr 15;41(4):562–567. doi: 10.1002/ijc.2910410415. [DOI] [PubMed] [Google Scholar]
- Chung L. P., Bentley D. R., Reid K. B. Molecular cloning and characterization of the cDNA coding for C4b-binding protein, a regulatory protein of the classical pathway of the human complement system. Biochem J. 1985 Aug 15;230(1):133–141. doi: 10.1042/bj2300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Hart I. R. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982 Sep 10;217(4564):998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- Holzmann B., Bröcker E. B., Lehmann J. M., Ruiter D. J., Sorg C., Riethmüller G., Johnson J. P. Tumor progression in human malignant melanoma: five stages defined by their antigenic phenotypes. Int J Cancer. 1987 Apr 15;39(4):466–471. doi: 10.1002/ijc.2910390410. [DOI] [PubMed] [Google Scholar]
- Holzmann B., Johnson J. P., Kaudewitz P., Riethmüller G. In situ analysis of antigens on malignant and benign cells of the melanocyte lineage. Differential expression of two surface molecules, gp75 and p89. J Exp Med. 1985 Feb 1;161(2):366–377. doi: 10.1084/jem.161.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann B., Lehmann J. M., Ziegler-Heitbrock H. W., Funke I., Riethmüller G., Johnson J. P. Glycoprotein P3.58, associated with tumor progression in malignant melanoma, is a novel leukocyte activation antigen. Int J Cancer. 1988 Apr 15;41(4):542–547. doi: 10.1002/ijc.2910410412. [DOI] [PubMed] [Google Scholar]
- Klickstein L. B., Wong W. W., Smith J. A., Weis J. H., Wilson J. G., Fearon D. T. Human C3b/C4b receptor (CR1). Demonstration of long homologous repeating domains that are composed of the short consensus repeats characteristics of C3/C4 binding proteins. J Exp Med. 1987 Apr 1;165(4):1095–1112. doi: 10.1084/jem.165.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen T., Wetsel R. A., Tack B. F. Structural analysis of human complement protein H: homology with C4b binding protein, beta 2-glycoprotein I, and the Ba fragment of B2. J Immunol. 1986 May 1;136(9):3407–3411. [PubMed] [Google Scholar]
- Kürzinger K., Reynolds T., Germain R. N., Davignon D., Martz E., Springer T. A. A novel lymphocyte function-associated antigen (LFA-1): cellular distribution, quantitative expression, and structure. J Immunol. 1981 Aug;127(2):596–602. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Kanehisa M., Krönke M., Peffer N. J., Svetlik P. B., Sullivan M., Greene W. C. Structure of the human interleukin-2 receptor gene. Science. 1985 Nov 8;230(4726):633–639. doi: 10.1126/science.2996141. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Mole J. E., Anderson J. K., Davison E. A., Woods D. E. Complete primary structure for the zymogen of human complement factor B. J Biol Chem. 1984 Mar 25;259(6):3407–3412. [PubMed] [Google Scholar]
- Panneerselvam M., Welt S., Old L. J., Vogel C. W. A molecular mechanism of complement resistance of human melanoma cells. J Immunol. 1986 Apr 1;136(7):2534–2541. [PubMed] [Google Scholar]
- Roth J., Zuber C., Wagner P., Taatjes D. J., Weisgerber C., Heitz P. U., Goridis C., Bitter-Suermann D. Reexpression of poly(sialic acid) units of the neural cell adhesion molecule in Wilms tumor. Proc Natl Acad Sci U S A. 1988 May;85(9):2999–3003. doi: 10.1073/pnas.85.9.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D., Makgoba M. W., Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988 Feb 18;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- te Velde A. A., Keizer G. D., Figdor C. G. Differential function of LFA-1 family molecules (CD11 and CD18) in adhesion of human monocytes to melanoma and endothelial cells. Immunology. 1987 Jul;61(3):261–267. [PMC free article] [PubMed] [Google Scholar]