Abstract
Platelet-derived growth factor-DD (PDGF-DD) is a recently discovered member of the PDGF family. The role of PDGF-DD in pathological angiogenesis and the underlying cellular and molecular mechanisms remain largely unexplored. In this study, using different animal models, we showed that PDGF-DD expression was up-regulated during pathological angiogenesis, and inhibition of PDGF-DD suppressed both choroidal and retinal neovascularization. We also demonstrated a novel mechanism mediating the function of PDGF-DD. PDGF-DD induced glycogen synthase kinase-3β (GSK3β) Ser9 phosphorylation and Tyr216 dephosphorylation in vitro and in vivo, leading to increased cell survival. Consistently, GSK3β activity was required for the antiangiogenic effect of PDGF-DD targeting. Moreover, PDGF-DD regulated the expression of GSK3β and many other genes important for angiogenesis and apoptosis. Thus, we identified PDGF-DD as an important target gene for antiangiogenic therapy due to its pleiotropic effects on vascular and non-vascular cells. PDGF-DD inhibition may offer new therapeutic options to treat neovascular diseases.
Keywords: Apoptosis, Cell Migration, Cytokine, Extracellular Matrix, Fibroblast, Growth Factors, Mouse, Angiogenesis, GSK3β, PDGF-D
Introduction
Although antiangiogenic therapy has shown some therapeutic efficacy (1), the clinical needs are still unmet, and the field is facing many challenges (2). One problem in antiangiogenic therapy is that, while one angiogenic factor is targeted, other angiogenic factors are selectively up-regulated, therefore bypassing the therapeutic effect of the antiangiogenic reagents, which allows the pathological neovascularization to proceed (3). Moreover, mature blood vessels covered by pericytes or smooth muscle cells are more resistant to antiangiogenic therapy and difficult to prune (4–6). Thus, novel antiangiogenic strategies targeting not only vascular endothelial cells, but also pericytes, smooth muscle cells, and other non-vascular cells involved in angiogenesis are highly desired. Furthermore, emerging drug resistance (7) and adverse side effects (8) constitute challenging problems to overcome. Finding new target genes with pleiotropic effects on multiple cell types important for pathological angiogenesis has become an important goal in antiangiogenic therapy.
Ocular neovascular disorders are the major causes of vision impairment or loss. Neovascular age-related macular degeneration is a major reason for blindness in the aged population in the Western society due to outgrowth of new blood vessels from the choroid (9). Despite many angiogenesis inhibitors tested, the progression of this pathology cannot be halted in all the patients, implicating the existence of yet uncovered angiogenic factors involved. Retinopathy of prematurity (ROP),2 one of the most common causes of vision loss in childhood, is caused primarily by the overgrowth of abnormal blood vessels throughout the retina. Currently, there is no satisfying treatment for ROP. Thus, new and effective anti-neovascularization therapies to inhibit pathological angiogenesis are still needed.
PDGF-DD is the fourth member of the PDGF family that binds to and activates its cognate receptor PDGFR-β (10, 11). The biological function of PDGF-DD remains largely to be explored. PDGF-DD protein is produced as a secreted homodimer and needs to be proteolytically processed for receptor binding (10). PDGF-DD is expressed in many different tissues (10, 11) and cells, including vascular fibroblastic adventitial cells (12), artery medial smooth muscle cells (13), and endothelial cells (13). PDGF-DD expression can also be found in the neointima of arteries in chronic allograft nephropathy (14). PDGF-DD plays an important role in epithelial to mesenchymal transition (15). PDGF-DD overexpression induced blood vessel maturation during angiogenesis in the skin and skeletal muscles (16). PDGF-DD has also been shown to play an important role in inflammation, because PDGF-DD overexpression induced macrophage recruitment into the skin and skeletal muscles (16), whereas intracoronary PDGF-D gene transfer enhanced cardiac allograft inflammation (17). Indeed, PDGF-DD is expressed in macrophages and promoted monocyte migration in a dose-dependent manner (18). In addition, PDGF-DD contributes to tumor growth (19–22). PDGF-DD is abundantly expressed in the eye (23). PDGF-DD is produced by human adult retinal pigment epithelial (RPE) cells and induces their proliferation and migration (24). Taken together, PDGF-DD is a potent growth factor with versatile functions in many different biological processes.
In the present work, we utilized different animal models to study the function of PDGF-DD in pathological angiogenesis and the potential of PDGF-DD inhibition in suppressing pathological neovascularization. We found that PDGF-DD expression was up-regulated during pathological angiogenesis. Importantly, PDGF-DD inhibition decreased both choroidal and retinal neovascularization. We further uncovered a novel mechanism underlying the function of PDGF-DD. PDGF-DD regulated glycogen synthase kinase (GSK)-3β phosphorylation and expression in vitro and in vivo, leading to increased cell survival. Thus, PDGF-DD is a critical player in pathological angiogenesis, and PDGF-DD inhibition may provide new therapeutic opportunities to treat neovascular diseases.
EXPERIMENTAL PROCEDURES
Laser-induced CNV Model
All animal experiments were approved by the Animal Care and Use Committee at the NEI/National Institutes of Health (NIH) (animal study protocol NEI-553) and were performed according to the NIH guidelines and regulations. The laser-induced choroidal neovascularization (CNV) model was described previously (25). Mouse PDGF-D shRNA (2 μg/eye, Open Biosystems, catalogue number RMM3981-97056099) was injected intravitreally immediately after laser treatment together with the transfection reagent in vivo-jetPEITM (Polyplus Transfection, New York, NY) according to the manufacturer's instructions. For LiCl (Sigma) treatment, 1 μl/eye of 1 m stock solution was injected intravitreally immediately after laser treatment together with PDGF-D shRNA. The same amount and volume of NaCl was used as a control. The CNV area was analyzed at 1 or 2 weeks after laser treatment using isolectin B4 (IB4, Invitrogen) or hematoxylin & eosin staining. For eye tissue (choroid and retina) isolation, the anterior segment and the vitreous of the eyes were removed. The retina was dissected from the RPE-choroid eye cup. The dissected tissues were put on dry-ice immediately for RNA or protein analysis, or fixed for morphological analysis.
ROP Model
The ROP model was performed as described previously (25, 26). For antiangiogenic treatment, immediately after 5 days in hyperoxia, the mice received intravitreal injection of PDGF-D shRNA (2 μg/eye) together with the transfection reagent in vivo-jetPEITM (Polyplus Transfection) according to the manufacturer's instructions. After another 5 days in normoxia, the retinae were harvested for IB4 (Invitrogen) staining or gene expression analysis. Image analysis was performed using a Carl Zeiss Imager Z1 and the AxioVision software (Zeiss) as described previously (25).
Cell Proliferation/Survival, Migration, Real-time PCR, and Immunofluorescence Staining
Immortalized rat retinal pericyte cells (TR-rPCT) and retinal capillary endothelial cells (TR-iBRB) were cultured as described previously (25, 27). Primary mouse choroidal fibroblasts were isolated and cultured as described (28). The Vybrant® MTT Cell Proliferation Assay Kit (MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Molecular Probes) was performed as described (25, 27). For the migration assay, confluent monolayer growth-arrested cells were wounded using a rubber scraper. The dishes were washed with serum-free medium and incubated for 6 or 16 h in serum-free medium containing the active form of human PDGF-DD recombinant protein (10) or bovine serum albumin (50 ng/ml). Each well was photographed at 10× magnification. Migration percentage corresponds to the ratio of the area of the cells migrated versus the total wound area. Real-time PCR assay and the primers used were described previously (25, 27). Immunofluorescence staining was performed as described previously (25, 27). The antibodies used were: anti-PDGF-DD (10), rabbit anti-mouse collagen IV polyclonal antibody (2150-1470, AbD Serotec), rat anti-mouse Mac3 (BD Pharmingen), anti-smooth muscle cell α-actin (Dako, M0851), and anti-phosphorylated-PDGFR-β (Santa Cruz Biotechnology, sc-16569).
PDGFR-β, Akt, Erk, and GSK3β Phosphorylation/Expression Assay and Western Blot
PDGFR-β activation assay was performed as described previously (10). Briefly, cultured cells were stimulated with the active form of recombinant human PDGF-DD protein (10) at 50 ng/ml for 10 min, and cell lysates were subjected to further analysis. For immunoprecipitation assay, cell lysates were incubated with an anti-PDGFR-β antibody (Santa Cruz Biotechnology) overnight at 4 °C and precipitated with immobilized protein-G (Thermo Scientific). Immunoprecipitated samples were separated on a 10% SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and incubated with an anti-phosphotyrosine antibody (pY99, Santa Cruz Biotechnology). Antibodies used to detect different phosphorylation sites of PDGFR-β were p-PDGFR-β (Tyr716), sc-16569; p-PDGFR-β (Tyr740), sc-17173; p-PDGFR-β (Tyr857), sc-12907; and p-PDGFR-β (Tyr1021), sc-12909 (Santa Cruz Biotechnology). To detect activated and total Akt or Erk, antibodies against phosphorylated Akt (#9271, Cell Signaling Technology), total Akt (#4685, Cell Signaling Technology), phosphorylated Erk (Thr202/Tyr204, Cell Signaling Technology), and total Erk (137F5, Cell Signaling Technology) were used in Western blot assays. For neutralizing antibody experiments, cells were treated with PDGFR-β neutralizing antibody (500 ng/ml, R&D Systems, AF385) for overnight. The cells were then stimulated with PDGF-DD protein as described above in the presence of PDGFR-β neutralizing antibody (500 ng/ml) in serum-free medium. Other antibodies used for Western blot assays were: anti-mouse PDGF-DD (Santa Cruz Biotechnology), monoclonal anti-β-actin conjugated with horseradish peroxidase (Sigma, A-3854), anti-GSK3α/β (R&D, AF2157), and anti-phospho-GSK3α/β (R&D, AF1590).
Protective Effect of PDGF-DD on Primary Choroidal Fibroblasts Expressing Wild-type or Mutant GSK3β
Primary choroidal fibroblast cells were transfected with expression constructs of wild-type (GSK3β-WT) or mutant (GSK3β-A9) GSK3β (kind gifts from Dr. Silvio Gutkind at NIDCR/NIH), using the FuGENE6 transfection kit (Roche Applied Science). Two days after transfection, the active form of the recombinant human PDGF-DD protein (10) (100 ng/ml) or the same amount of bovine serum albumin was added to the medium. After 15 min, H2O2 was added to the medium to a final concentration of 0.5%. After 10 min, the medium was replaced with fresh medium containing MTT (Molecular Probe). MTT assay was performed after 4 h using a commercial kit (Molecular Probe).
Aortic Ring Assay
The aortic ring assay was performed as described (29). Briefly, aortas were excised from wild-type mice. The fatty/connective tissues surrounding the aorta were removed carefully under a surgical microscope. Aortic rings (1 mm in length) were cut and rinsed five times with endothelial basal media (Lonza). Forty-eight-well plates were coated with 150 μl/well basement membrane extract (growth factor-reduced, Cultrex). After gelling at 37 °C for 30 min, an aortic ring was placed on top of the basement membrane extract gel, and another 150 μl of basement membrane extract was added onto it. After 30 min, 500 μl of endothelial basal media containing PDGF-DD (R&D Systems, 500 ng/ml) with or without the differentiation-inducing factor-3 (DIF3, Sigma-Aldrich, 30 μm) was added to each well. On day 14, images of the aortic rings were taken using a phase-contrast microscope equipped with a digital camera (Carl Zeiss, Axiovert-20). Images were converted to binary ones by application of a morphologic low pass filter and threshold transformation using Adobe Photoshop CS3. The number of microvessels/field was counted in four fields surrounding the aortic ring where the highest microvessel density was found. The mean value of microvessel density of the four fields was used for each aortic ring.
Statistics
Two-tailed Student's t test was used for statistical analysis. Differences were considered statistically significant when p < 0.05. The data are represented as mean ± S.E. Assays using cultured cells were performed in triplicates.
RESULTS
Up-regulation of PDGF-DD and PDGFR-β Expression in Choroidal Neovascularization
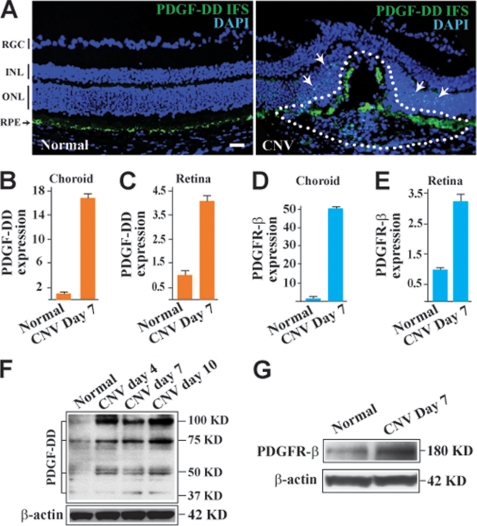
To investigate the potential role of PDGF-DD in pathological angiogenesis, we first checked the expression of PDGF-DD in a mouse model of laser-induced CNV. Immunofluorescence staining (IFS) showed abundant PDGF-DD expression in the eyes with CNV, particularly within the CNV area (Fig. 1A, right, lined area). PDGF-DD expression was also abundant in the retina surrounding the CNV area (Fig. 1A, right, arrows). In normal retinae, PDGF-DD expression was mainly detected in the RPE cells (Fig. 1A, left, arrow). Real-time PCR showed up-regulated expression of PDGF-D and PDGFR-β in the retinae and choroids after induction of CNV (Fig. 1, B–E). Western blot analysis demonstrated higher levels of PDGF-DD protein in the neovascular retinae (Fig. 1F). Several PDGF-DD protein bands were detected, including the full-length (100 kDa) and the differently processed forms (75, 50, and 37 kDa). In addition, Western blot assay detected higher PDGFR-β protein level in the retinae with CNV (Fig. 1G). The increased expression of PDGF-DD and its receptor PDGFR-β during CNV formation indicated a possible role of PDGF-DD in CNV.
FIGURE 1.
PDGF-DD and PDGFR-β expression is up-regulated in CNV. A, IFS displayed abundant PDGF-DD expression (green) within the CNV area (CNV, right, lined), while PDGF-DD expression was detected mainly in the retinal pigment epithelial cell layer (RPE, left, arrow) in normal retina. RGC, retinal ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; and ONL, outer nuclear layer. Blue color, nuclei stained by 4′,6-diamidino-2-phenylindole (DAPI). Scale bar: 50 μm. B and C, real-time PCR showed up-regulated expression of PDGF-D in the choroids (B) and retinae (C) with CNV compared with normal choroids and retinae. Arbitrary units after normalizing against β-actin were used for PDGF-D expression with their normal controls set to 1. D and E, real-time PCR showed up-regulated expression of PDGFR-β in the choroids (D) and retinae (E) with CNV as compared with normal choroids and retinae. Arbitrary units after normalizing against β-actin were used for PDGFR-β expression with their normal controls set to 1. F, Western blot assay showed increased PDGF-DD protein levels in the retinae with CNV using β-actin as a loading control. Full-length (100 kDa) and differentially processed (75, 50, and 37 kDa) PDGF-DD were detected. G, Western blot assay showed an increased PDGFR-β protein level in the retinae with CNV using β-actin as a loading control.
PDGF-DD Inhibition Suppresses Choroidal Neovascularization
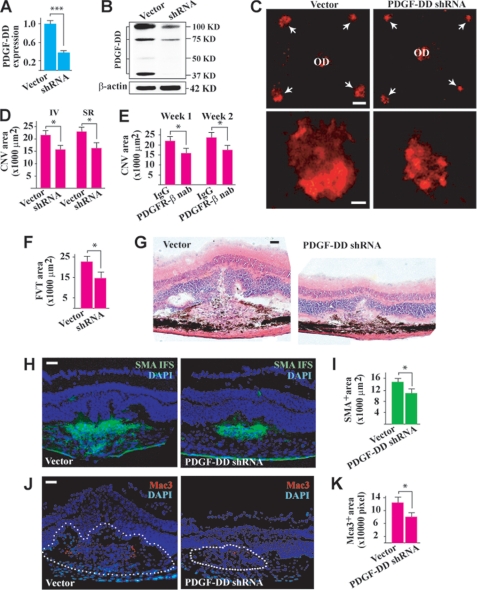
Because PDGF-DD was up-regulated during CNV, we next tested whether PDGF-DD inhibition would affect CNV formation. Intravitreal injection of a PDGF-D shRNA reduced PDGF-D expression level in the retina to ∼37% of the normal level as measured by real-time PCR 2 days after the injection (Fig. 2A, n = 8, p < 0.001). Western blot analysis detected less PDGF-DD protein in the PDGF-D shRNA-treated retinae (Fig. 2B). In the laser-induced CNV model, PDGF-D shRNA reduced CNV formation 1 week after intravitreal or subretinal injection (Fig. 2, C and D, n = 8, p < 0.05, arrows in C). Moreover, intravitreal administration of a PDGFR-β neutralizing antibody decreased CNV area at different time points after laser treatment (Fig. 2E, n = 8, p < 0.05). It is noteworthy that the inhibitory effect of the PDGFR-β neutralizing antibody on CNV formation was comparable to that of PDGF-D shRNA (Fig. 2D, n = 8, p < 0.05), suggesting that PDGF-DD is an important ligand of PDGFR-β that plays a significant role in CNV formation. Histological analysis showed less fibrovascular tissue formation in the PDGF-D shRNA-treated CNV (Fig. 2, F and G, n = 7, p < 0.05). PDGF-D shRNA treatment also decreased edema formation within the CNV area as shown by the reduced hump formation and empty space around the neovascular area (Fig. 2G). Furthermore, PDGF-D shRNA treatment reduced the areas positive for the smooth muscle cell α-actin (vascular smooth muscle cell marker) within the CNVs (Fig. 2, H and I, n = 7, p < 0.01), indicating an effect of PDGF-DD on vascular smooth muscle cells. Macrophages also play an important role in CNV (30). PDGF-D shRNA treatment reduced Mac3+ staining (a macrophage marker) within the CNV areas (Fig. 2, J and K, n = 7, p < 0.05), demonstrating that PDGF-DD inhibition suppressed inflammation during CNV formation.
FIGURE 2.
PDGF-DD inhibition suppressed CNV. A, intravitreal injection of PDGF-D shRNA reduced PDGF-D expression to ∼37% of normal level in the retina 2 days after injection as measured by real-time PCR. Arbitrary unit after normalizing against β-actin was used for gene expression level. B, Western blot assay confirmed that intravitreal injection of PDGF-D shRNA reduced PDGF-DD protein level in the retina. Full-length (100 kDa) and differentially processed (75, 50, and 37 kDa) PDGF-DD were detected. C and D, PDGF-D shRNA treatment reduced CNV formation (arrows in C) 1 week after intravitreal (IV) or subretinal (SR) injection as measured by IB4 staining (red). OD: optic nerve disc. Scale bars in C: top panel, 200 μm; lower panel, 50 μm. E, intravitreal injection of PDGFR-β neutralizing antibody (nab) decreased CNV areas at different time points after treatment. F and G, histological analysis showed less fibrovascular tissue (FVT) formation in the PDGF-D shRNA-treated CNVs as revealed by hematoxylin & eosin staining. There was also less edema formation in the PDGF-D shRNA-treated CNVs as shown by the reduced hump formation and empty space around the neovascular area. H and I, immunofluorescence staining (IFS) showed that PDGF-D shRNA treatment reduced the area positive for smooth muscle cell α-actin (SMA, green, vascular smooth muscle cell marker). Scale bar in H: 50 μm. J and K, IFS showed that PDGF-D shRNA treatment reduced Mac3+ staining (red, macrophage marker) within the CNV areas. Scale bar in J: 50 μm. Blue color in H and J: nuclei stained by 4′,6-diamidino-2-phenylindole (DAPI). *, p < 0.05; ***, p < 0.001.
PDGF-DD Regulates the Expression of Many Proangiogenic and Proapoptotic Genes
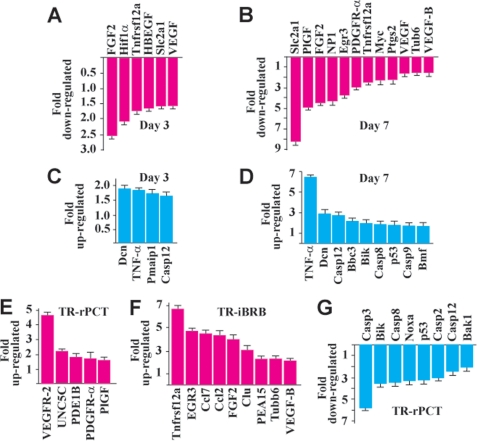
We investigated the effect of PDGF-DD knockdown on the expression of many proangiogenic and proapoptotic genes in the choroids with CNV. Real-time PCR showed decreased expression of many proangiogenic genes in the PDGF-D shRNA-treated choroids with CNV at different time points (Fig. 3, A and B, n = 8). These genes included fibroblast growth factor 2 (FGF2), vascular endothelial growth factor (VEGF), placental growth factor (PlGF), and VEGF-B, which play important roles in CNV (25, 31–34). At day 3 after CNV, which is an early stage of CNV (35), the down-regulation of the proangiogenic genes was moderate (Fig. 3A). However, at day 7 after CNV, which is a peak time of angiogenesis, the down-regulation of the proangiogenic genes was more prominent and involved more genes (Fig. 3B). In addition, PDGF-D shRNA treatment up-regulated the expression of many proapoptotic genes in the choroids with CNV at different time points (Fig. 3, C and D, n = 8). These genes included Dcn and TNF-α, which are inhibitors of PDGF- and PDGFR-β-induced vascular cell proliferation, survival, and migration (36–39). Furthermore, the regulatory effect of PDGF-DD on the expression of the proangiogenic and proapoptotic genes was confirmed in different vascular cells, such as the rat retina-derived vascular pericytes (TR-rPCT) and the rat retina-derived vascular endothelial cells (TR-iBRB, Fig. 3, E–G). Thus, PDGF-DD inhibition suppressed the expression of many proangiogenic genes and up-regulated the expression of many proapoptotic genes that play important roles in angiogenesis.
FIGURE 3.
PDGF-DD regulated expression of proangiogenic and proapoptotic genes. A and B, PDGF-D shRNA treatment down-regulated the expression of many proangiogenic genes in the choroids with CNV at an early stage (A, day 3) and the peak time (B, day 7) of CNV as measured by real-time PCR. C and D, PDGF-D shRNA treatment increased the expression of many proapoptotic genes in the choroids with CNV at an early stage (C, day 3) and the peak time (D, day 7) of CNV as measured by real-time PCR. E and F, PDGF-DD protein treatment up-regulated the expression of many proangiogenic genes in rat retina-derived vascular pericytes (E, TR-rPCT) and in rat retina-derived vascular endothelial cells (F, TR-iBRB). G, PDGF-DD protein treatment inhibited the expression of many proapoptotic genes in the TR-rPCT cells.
PDGF-DD Inhibition Suppresses Retinal Neovascularization
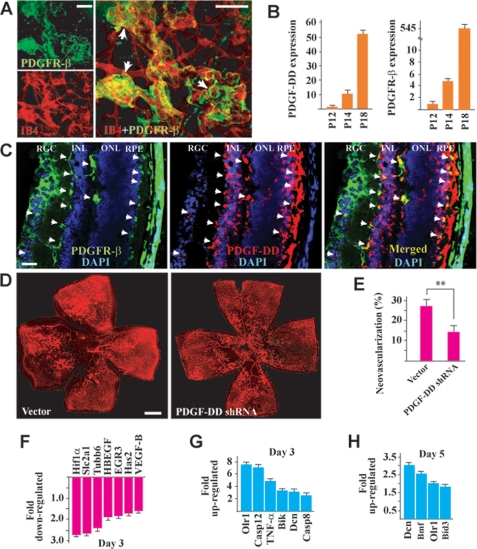
We next performed the ROP mouse model (40, 41) to test whether PDGF-DD inhibition could suppress retinal neovascularization. Immunofluorescence staining showed expression of PDGFR-β, the receptor for PDGF-DD (10), on the neovessels in the retina, with a higher expression level in the neovascular tufts (Fig. 4A, arrows). Real-time PCR showed up-regulated expression of PDGF-D and PDGFR-β during retinal neovascularization (Fig. 4B, n = 7). Immunofluorescence staining of cross-sections of P18 neovascular retinae displayed PDGFR-β expression (Fig. 4C, left, green) in different retinal layers, including the retinal ganglion cell (RGC) layer, the inner nuclear layer (INL, mainly on the cord-like blood vessels indicated by the arrows), and the retinal pigment epithelial cells (RPE, indicated by the arrows). Increased PDGF-DD expression (Fig. 4C, middle, red) was mainly found in the INL and RPE layers. Co-localization of PDGF-DD with PDGFR-β was found in the RGC, INL, and RPE layers (Fig. 4C, right, yellow, indicated by the arrows). Intravitreal injection of PDGF-D shRNA inhibited retinal neovascularization (Fig. 4, D and E, n = 8, p < 0.01). Moreover, real-time PCR showed that upon shRNA-mediated PDGF-D knockdown, the expression of many proangiogenic genes in the neovascular retinae was inhibited (Fig. 4F, n = 8). These genes included Hif1α, Has2, and VEGF-B, which play important roles in retinal neovascularization (25, 42, 43). Furthermore, upon PDGF-D knockdown, the expression of many proapoptotic genes in the neovascular retina was increased at different time points (Fig. 4, G and H, n = 8). These genes included Olr1, an inducer of vascular cell apoptosis (44), TNF-α, and Dcn, inhibitors of PDGF- and PDGFR-β-induced vascular cell migration, proliferation, and survival (36–39, 45). Thus, PDGF-DD inhibition reduced retinal neovascularization and regulated the expression of many proangiogenic and proapoptotic genes in the neovascular retinae.
FIGURE 4.
PDGF-DD inhibition suppressed retinal neovascularization. A, immunofluorescence staining revealed PDGFR-β (green) expression in the neovessels (red, IB4 staining) of the neovascular retina, with a higher expression level in the neovascular tufts (arrows). Scale bars: 100 μm. B, PDGF-D and PDGFR-β expression was up-regulated at days 2 and 6 of retinal neovascularization (postnatal day (P), P14 and P18) compared with that of day 0 (P12) as measured by real-time PCR. Arbitrary unit normalized against β-actin was used for gene expression level. C, immunofluorescence staining of cross-sections of P18 neovascular retina showed that PDGFR-β expression (left, green) was found in different retinal layers, including the RGC layer, the INL (mainly on the cord-like blood vessels indicated by the arrows), and the RPE cells (indicated by arrows). PDGF-DD expression (middle, red) was mainly found in the INL and RPE layers. Co-localization of PDGF-DD with PDGFR-β was found in the RGC, INL, and RPE layers (right, yellow, arrows). Scale bar: 50 μm. D and E, intravitreal injection of a PDGF-D shRNA inhibited retinal neovascularization. Neovessels were visualized by IB4 staining (red). Scale bar: 500 μm. F, PDGF-D shRNA treatment inhibited the expression of many proangiogenic genes in the neovascular retinae as measured by real-time PCR. G and H, PDGF-D shRNA treatment up-regulated the expression of many proapoptotic genes in the neovascular retina at different time points as measured by real-time PCR. *, p < 0.05; **, p < 0.01.
PDGF-DD Promotes Vascular Cell and Fibroblast Proliferation, Survival, and Migration
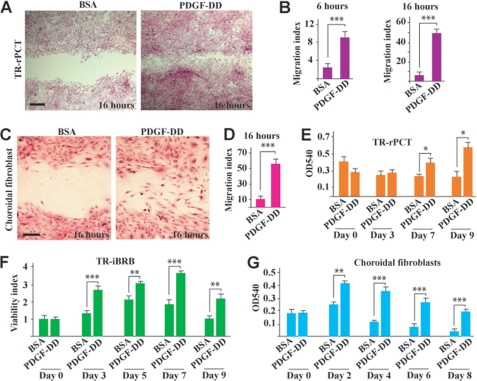
To investigate the cellular targets of PDGF-DD, we studied the effect of PDGF-DD on the migration and proliferation/survival of different vascular and non-vascular cells. In a monolayer cell migration assay, PDGF-DD protein promoted migration of retinal vascular pericytes (TR-rPCT) at different time points (Fig. 5, A and B, n = 6, p < 0.001), and migration of choroidal fibroblasts (Fig. 5, C and D, n = 6, p < 0.001). Moreover, PDGF-DD protein increased proliferation/survival of TR-rPCT cells (Fig. 5E, n = 6, p < 0.05), rat retinal-derived vascular endothelial cells (TR-iBRB) (Fig. 5F, n = 6, p < 0.01 or 0.001), and mouse choroidal fibroblasts (Fig. 5G, n = 5, p < 0.01 or 0.001) at different time points. Thus, PDGF-DD has pleiotropic effects on multiple vascular and non-vascular cells, which play important roles in pathological angiogenesis.
FIGURE 5.
PDGF-DD promoted vascular cell and fibroblast proliferation, survival, and migration. A and B, PDGF-DD protein promoted migration of retinal vascular pericytes (TR-rPCT) at different time points in a monolayer cell migration assay. Scale bar in A: 100 μm. C and D, PDGF-DD protein promoted choroidal fibroblast migration in a monolayer cell migration assay. Scale bar in C: 50 μm. E–G, PDGF-DD protein promoted proliferation/survival of the rat retinal derived vascular pericytes (TR-rPCT, E), rat retinal-derived vascular endothelial cells (TR-iBRB, F), and mouse choroidal fibroblasts (G) at different time points. Viability index in F: arbitrary unit with A540 values at day 0 set to 1. *, p < 0.05; **, p < 0.01; and ***, p < 0.001.
PDGF-DD Activates PDGFR-β, Erk, and Akt and Regulates GSK3β Phosphorylation
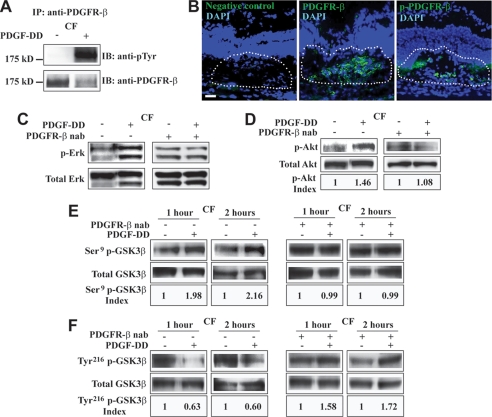
We next investigated whether PDGF-DD could activate its receptor PDGFR-β (10). Immunoprecipitation (IP) followed by immunoblot (IB) showed robust PDGFR-β activation in choroidal fibroblasts (CF) after PDGF-DD protein stimulation (50 ng/ml, Fig. 6A). Western blot assay using antibodies against different phosphorylation sites of PDGFR-β did not reveal significant differences between PDGF-DD- and PDGF-BB-mediated PDGFR-β activation in cultured mouse primary choroidal fibroblasts (supplemental Fig. S1). Immunofluorescence staining displayed the highest PDGFR-β expression in the CNV area (Fig. 6B, middle, green, lined area), where phosphorylated PDGFR-β (p-PDGFR-β) was also detected (Fig. 6B, right, green, lined area). PDGF-DD protein activated Erk and Akt in choroidal fibroblasts (CF, Fig. 6, C and D). The PDGF-DD-induced Erk and Akt activation was abolished by a neutralizing antibody against PDGFR-β (Fig. 6, C and D), demonstrating that the effect of PDGF-DD on ERK and Akt activation was mediated by PDGFR-β. Furthermore, PDGF-DD protein stimulation led to GSK3β Ser9 phosphorylation and Tyr216 dephosphorylation in the CFs at different time points (Fig. 6, E and F, left two panels). The effect of PDGF-DD on GSK3β phosphorylation was abolished by the PDGFR-β neutralizing antibody (Fig. 6, E and F, right two panels), demonstrating that the regulatory effect of PDGF-DD on GSK3β phosphorylation was mediated by PDGFR-β. The baseline phosphorylation levels of Erk, Akt, and GSK3β Ser9 seemed to be increased by PDGFR-β neutralizing antibody compared with those in the absence of the neutralizing antibody (Fig. 6, C–E). We currently cannot explain this exactly. However, we cannot exclude the possibilities of compensatory up-regulation of the intracellular autocrine PDGF receptor pathway, which cannot be inhibited by PDGFR-β neutralizing antibody (46–48), or the absence of the PDGFR activation-induced negative feedback pathways that suppress the activation of Erk and other downstream signals (49–51). In addition, in the presence of PDGFR-β neutralizing antibody, the phosphorylation levels of GSK3β Tyr216 seemed to be increased by PDGF-DD (Fig. 6F). Future work is needed to explain this. However, we cannot exclude the possibility that, under such a specific condition where PDGFR-β was not available to PDGF-DD, PDGF-DD at a relatively high concentration might be forced to interact with other molecules, resulting in increased GSK3β Tyr216 phosphorylation.
FIGURE 6.
PDGF-DD activated PDGFR-β, Erk, Akt, and modulated GSK3β phosphorylation in vitro. A, immunoprecipitation (IP) followed by immunoblot (IB) showed that PDGF-DD protein stimulation led to PDGFR-β activation in mouse choroidal fibroblasts (CF). B, immunofluorescence staining detected abundant PDGFR-β expression (middle, lined area, green), as well as phosphorylated PDGFR-β (p-PDGFR-β, right, green, lined area) within the CNV area. Blue color: 4′,6-diamidino-2-phenylindole (DAPI)-stained nuclei. Scale bar: 50 μm. C and D, PDGF-DD protein induced Erk (C) and Akt (D) activation in mouse choroidal fibroblasts (CF, left penal). A neutralizing antibody against PDGFR-β (PDGFR-β, nab) abolished the effect of PDGF-DD on Erk and Akt activation (right penal). p-Akt index: arbitrary units of densitometry after normalization against total Akt with the controls set to 1. E, PDGF-DD protein stimulation led to GSK3β Ser9 phosphorylation in primary mouse CFs at different time points (left two panels). A PDGFR-β neutralizing antibody (PDGFR-β, nab) abolished the effect of PDGF-DD on GSK3β Ser9 phosphorylation (right two panels). Ser9 p-GSK3β index: arbitrary units of densitometry after normalization against total GSK3β with the controls set to 1. F, PDGF-DD protein induced Tyr216 dephosphorylation in primary mouse CFs at different time points (left two panels). A PDGFR-β neutralizing antibody (PDGFR-β, nab) abolished the effect of PDGF-DD on GSK3β Tyr216 dephosphorylation (right two panels). Tyr216 p-GSK3β index: arbitrary units of densitometry after normalization against total GSK3β with the controls set to 1.
PDGF-DD Regulates GSK3β Phosphorylation and Expression in Vivo
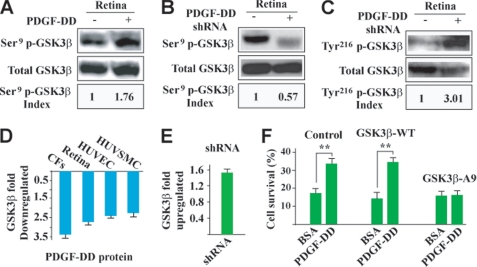
We next tested whether PDGF-DD regulated GSK3β phosphorylation in vivo. Intravitreal injection of PDGF-DD protein increased GSK3β Ser9 phosphorylation in the retinae (Fig. 7A), while intravitreal injection of PDGF-D shRNA decreased GSK3β Ser9 phosphorylation (Fig. 7B). In addition, shRNA-mediated PDGF-D knockdown increased GSK3β Tyr216 phosphorylation in the retinae (Fig. 7C). Moreover, real-time PCR showed down-regulated GSK3β expression in the PDGF-DD-treated vascular and non-vascular cells in vitro, and in the PDGF-DD-treated retina in vivo (Fig. 7D). On the contrary, PDGF-D knockdown by shRNA up-regulated GSK3β expression in the neovascular retinae (Fig. 7E). GSK3β induces vascular cell apoptosis (52). Inhibition of GSK3β by either Akt-dependent GSK3β Ser9 phosphorylation or down-regulating its expression increases vascular cell survival (52, 53). In contrast, phosphorylation of GSK3β Tyr216 increases its apoptotic activity (54). Thus, our data suggested that the survival/anti-apoptotic effect of PDGF-DD may be mediated, at least in part, by regulating GSK3β phosphorylation and expression.
FIGURE 7.
PDGF-DD regulated GSK3β phosphorylation and expression in vivo and protected choroidal fibroblasts by regulating GSK3β phosphorylation. A, intravitreal injection of PDGF-DD protein increased GSK3β Ser9 phosphorylation in mouse retinae. Ser9 p-GSK3β index: arbitrary units of densitometry after normalization against total GSK3β with the control set to 1. B and C, PDGF-D shRNA intravitreal injection decreased GSK3β Ser9 phosphorylation (B) and increased GSK3β Tyr216 phosphorylation in mouse retinae (C). Ser9 (Tyr216) p-GSK3β index: arbitrary units of densitometry after normalization against total GSK3β with the control set to 1. D, PDGF-DD protein down-regulated GSK3β expression in different cells in vitro, and in mouse retinae in vivo. E, PDGF-DD knockdown by shRNA up-regulated GSK3β expression in the retinae with neovascularization as compared with the vector-treated samples. F, the mutant form of human GSK3β, in which the Ser9 was mutated to alanine (GSK3β-A9), was expressed in primary CFs. The wild-type GSK3β (GSK3β-WT)-transfected and non-transfected cells were used as controls. PDGF-DD protein protected CFs from H2O2-induced cell death in the GSK3β-WT-transfected as well as in the non-transfected cells. In the GSK3β-A9-transfected cells, the protective effect of PDGF-DD was abolished. **, p < 0.01.
PDGF-DD Protects Fibroblasts by Regulating GSK3β Phosphorylation
To further verify whether the survival/anti-apoptotic effect of PDGF-DD was achieved by regulating GSK3β phosphorylation, we expressed in primary choroidal fibroblasts a mutant form of human GSK3β, in which Ser9 was mutated to alanine (GSK3β-A9, thus lacking the ability of Ser9 phosphorylation). Wild-type GSK3β (GSK3β-WT)-transfected and non-transfected cells were used as controls. PDGF-DD protein treatment (100 ng/ml) protected the GSK3β-WT-transfected and non-transfected choroidal fibroblasts from H2O2-induced cell death (Fig. 7F, n = 6, p < 0.01). However, in the cells expressing the mutant form GSK3β-A9, the protective effect of PDGF-DD was abolished (Fig. 7F), thus demonstrating that the survival/anti-apoptotic effect of PDGF-DD required GSK3β Ser9 phosphorylation.
GSK3β Activation Diminishes PDGF-DD-induced Angiogenesis
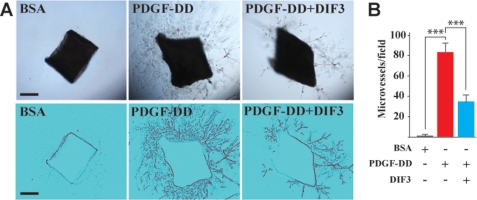
To further determine the relevance of the above findings, we investigated the role of GSK3β in PDGF-DD-induced angiogenesis. For this purpose, we utilized the DIF3, a known GSK3β activator (55, 56), in combination with a mouse aortic ring assay (57, 58). PDGF-DD protein treatment induced vascular cell proliferation, migration, and microvessel formation in the aortic ring assay (Fig. 8, A and B, n = 8, p < 0.001). Co-treatment of the aortic rings with the GSK3β activator DIF3 together with PDGF-DD protein attenuated the PDGF-DD-induced microvessel formation (Fig. 8, A and B, n = 8, p < 0.001), demonstrating that inhibition of GSK3β activity is required for PDGF-DD-induced angiogenesis.
FIGURE 8.
GSK3β activation attenuated PDGF-DD-induced angiogenesis in aortic ring assay. A and B, PDGF-DD protein induced vascular cell proliferation, migration, and microvessel formation in an aortic ring assay (A, middle). Co-treatment of the aortic rings with the GSK3β activator DIF3 with PDGF-DD protein attenuated the PDGF-DD-induced microvessel formation. Lower panel in A: binary images of the aortic rings with branching microvessels. Scale bar: 500 μm; ***, p < 0.001.
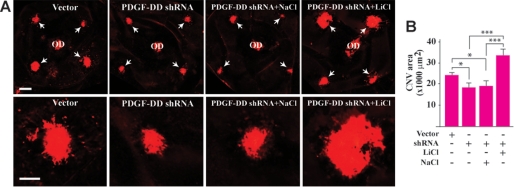
GSK3β Inhibition Abolishes the Antiangiogenic Effect of PDGF-DD Knockdown
To ascertain the importance of GSK3β activity in the reduction of CNV induced by PDGF-DD knockdown, we utilized lithium chloride (LiCl), a known GSK3β inhibitor (59–61), in combination with the laser-induced CNV mouse model. Treatment with PDGF-D shRNA inhibited CNV formation (Fig. 9, A and B, n = 9, p < 0.05). Co-injection of the GSK3β inhibitor LiCl with PDGF-D shRNA abolished the reduction of CNV induced by PDGF-D shRNA (Fig. 9, A and B, n = 9, p < 0.001). NaCl was used as a control and had no effect (Fig. 9, A and B, n = 9, p > 0.05). These data demonstrated that GSK3β activity was required for the antiangiogenic effect of PDGF-DD knockdown.
FIGURE 9.
GSK3β inhibition abolished the antiangiogenic effect of PDGF-DD shRNA. A and B, in the laser-induced CNV model, PDGF-D shRNA treatment inhibited CNV formation. Co-injection of the GSK3β inhibitor, LiCl, abolished the reduction of CNV formation induced by PDGF-D shRNA. Co-injection of NaCl had no effect. Scale bar in the upper panel in A: 200 μm; in the lower panel in A: 100 μm. *, p < 0.05; ***, p < 0.001.
DISCUSSION
In this report, we showed that PDGF-DD expression was up-regulated during pathological angiogenesis, and PDGF-DD inhibition suppressed both choroidal and retinal neovascularization in different animal models. We further provided mechanistic insights underlying the effect of PDGF-DD. In particular, PDGF-DD induced GSK3β Ser9 phosphorylation and Tyr216 dephosphorylation in vitro and in vivo, resulting in increased cell survival. Indeed, GSK3β activity was required for the antiangiogenic effect of PDGF-DD inhibition. In addition, PDGF-DD acted as a potent regulator of the expression of GSK3β and many other proangiogenic and proapoptotic genes. In summary, our data showed that PDGF-DD targeting may have therapeutic values in treating neovascular diseases.
VEGF has been considered to be the key angiogenic factor and the first target gene in antiangiogenic therapy (62), and anti-VEGF therapies have shown efficacies in treating neovascular diseases (63). However, not all patients with neovascular disorders respond to anti-VEGF therapy (64, 65), indicating that other angiogenic molecules or non-VEGF-driven pathways play important roles in the pathogenesis of neovascular diseases. Indeed, it has been shown that VEGF is responsible for ∼50% of the angiogenic provocations in pathological angiogenesis (66). Furthermore, tolerance to anti-VEGF reagents has been observed in the clinic (64). VEGF inhibition may result in different side effects in patients responsive to anti-VEGF therapy (67, 68). These may include neuronal apoptosis (69–71), ultrastructural changes in capillaries (72), mitochondrial disruption in the inner segments of photoreceptors (73), and inflammation (74). In addition, the costs of the current clinically available anti-VEGF reagents are daunting. Thus, new antiangiogenic reagents are still needed. In this study, we found that PDGF-DD expression was up-regulated in both neovascular choroids and retinae, and PDGF-DD knockdown by shRNA suppressed choroidal and retinal neovascularization, demonstrating that PDGF-DD may be a new target molecule in antiangiogenic therapy.
Several mechanisms underlie the antiangiogenic effect of PDGF-DD targeting. First, PDGF-DD is expressed by macrophages (13) and is a potent chemoattractant for them (18). It is known that macrophages play an important role in pathological angiogenesis (75). Because PDGF-DD inhibition reduced macrophage infiltration during CNV formation, the antiangiogenic effect of PDGF-DD targeting may thus be achieved, at least in part, by suppressing inflammation. Second, PDGF-DD promoted migration, proliferation, and survival of multiple cell types, including fibroblasts, vascular pericytes, and endothelial cells, which play important roles in pathological angiogenesis (75, 76). PDGF-DD inhibition thus attenuated the availability of multiple cellular components needed for pathological angiogenesis. In addition, PDGF-DD is a potent regulator of the expression of numerous proangiogenic and proapoptotic genes involved in pathological angiogenesis, such as VEGF, FGF2, PlGF, and VEGF-B, important players in CNV (25, 31–34), Dcn and TNF-α, apoptosis inducers and inhibitors of PDGF-induced vascular cell proliferation, migration, and survival (36–39). Apoptosis occurs in the neovasculature during pathological angiogenesis (77, 78). Induction of apoptosis in the neovasculature has been considered as a promising antiangiogenic approach to inhibit pathological neovascularization (79, 80). Thus, by modulating the expression of many other genes important for angiogenesis, PDGF-DD targeting may result in a broad amplification of an antiangiogenic effect.
The signaling pathways induced by PDGF-DD are poorly understood thus far. We first identified PDGFR-β as the receptor mediating the PDGF-DD-induced Erk and Akt activation, because treatment with PDGFR-β neutralizing antibody abolished the PDGF-DD-induced effects. We further uncovered that GSK3β is an important downstream effector of PDGF-DD. PDGF-DD induced GSK3β Ser9 phosphorylation and GSK3β Tyr216 dephosphorylation in choroidal fibroblasts in vitro and in the retina in vivo. It is known that inhibition of GSK3β activity by Akt-dependant Ser9 phosphorylation or through GSK3β Tyr216 dephosphorylation promotes vascular cell survival (52, 53). Moreover, the involvement of GSK3β in PDGF-DD-induced angiogenesis was confirmed by the aortic ring assay in vitro, and by the laser-induced CNV model in vivo. When the GSK3β activator, DIF3, was added together with PDGF-DD to the aortic rings, the PDGF-DD-induced microvessel formation was impaired. When LiCl, a GSK3β inhibitor, was administered together with PDGF-D shRNA into the mouse vitreous, the antiangiogenic effect of PDGF-D shRNA on CNV formation was abolished. It is noteworthy that, thus far, little is known about the role of GSK3β in pathological angiogenesis, and whether GSK3β is involved in neovessel formation induced by other angiogenic factors. Future studies are needed to verify these roles and to explore further whether activation of GSK3β could be utilized to inhibit pathological angiogenesis.
In summary, using different animal models, we showed that PDGF-DD plays an important role in pathological angiogenesis by affecting multiple cell types, and PDGF-DD inhibition repressed both choroidal and retinal angiogenesis. Mechanistically, we revealed that the effect of PDGF-DD was mediated by regulating GSK3β phosphorylation and expression. Due to its multiple roles in pathological angiogenesis, PDGF-DD inhibition may provide new therapeutic possibilities to treat neovascular diseases.
Acknowledgments
We thank Dr. Silvio Gutkind at the NIH/NIDCR for kindly providing the GSK3β-WT and GSK3β-A9 expression constructs.
This work was supported, in whole or in part, by the National Institutes of Health Intramural Research Program of the NEI. This work was also supported by the Macular Degeneration Research program of the American Health Assistance Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- ROP
- retinopathy of prematurity
- PDGF-DD
- platelet-derived growth factor-DD
- GSK3β
- glycogen synthase kinase 3β
- CNV
- choroidal neovascularization
- RPE
- retinal pigment epithelial
- IB4
- isolectin B4
- TR-rPCT
- rat retinal pericyte
- TR-iBRB
- retinal capillary endothelial cell
- IFS
- immunofluorescence staining
- SMA
- smooth muscle cell α-actin
- CF
- choroidal fibroblast
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- FGF2
- fibroblast growth factor 2
- VEGF
- vascular endothelial growth factor
- PlGF
- placental growth factor
- RGC
- retinal ganglion cell
- INL
- inner nuclear layer
- DIF3
- differentiation-inducing factor-3
- IPL
- inner plexiform layer
- shRNA
- short hairpin RNA
- Erk
- extracellular signal-regulated kinase.
REFERENCES
- 1.Folkman J. (2007) Nat. Rev. Drug Discov. 6, 273–286 [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P., De Smet F., Loges S., Mazzone M. (2009) Nat. Rev. Clin. Oncol. 6, 315–326 [DOI] [PubMed] [Google Scholar]
- 3.Dorrell M. I., Aguilar E., Scheppke L., Barnett F. H., Friedlander M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 967–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmeliet P. (2005) Nature 438, 932–936 [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N., Kerbel R. S. (2005) Nature 438, 967–974 [DOI] [PubMed] [Google Scholar]
- 6.Benjamin L. E., Hemo I., Keshet E. (1998) Development 125, 1591–1598 [DOI] [PubMed] [Google Scholar]
- 7.Shojaei F., Wu X., Malik A. K., Zhong C., Baldwin M. E., Schanz S., Fuh G., Gerber H. P., Ferrara N. (2007) Nat. Biotechnol. 25, 911–920 [DOI] [PubMed] [Google Scholar]
- 8.Maharaj A. S., Walshe T. E., Saint-Geniez M., Venkatesha S., Maldonado A. E., Himes N. C., Matharu K. S., Karumanchi S. A., D'Amore P. A. (2008) J. Exp. Med. 205, 491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambati J., Ambati B. K., Yoo S. H., Ianchulev S., Adamis A. P. (2003) Surv. Ophthalmol. 48, 257–293 [DOI] [PubMed] [Google Scholar]
- 10.Bergsten E., Uutela M., Li X., Pietras K., Ostman A., Heldin C. H., Alitalo K., Eriksson U. (2001) Nat. Cell Biol. 3, 512–516 [DOI] [PubMed] [Google Scholar]
- 11.LaRochelle W. J., Jeffers M., McDonald W. F., Chillakuru R. A., Giese N. A., Lokker N. A., Sullivan C., Boldog F. L., Yang M., Vernet C., Burgess C. E., Fernandes E., Deegler L. L., Rittman B., Shimkets J., Shimkets R. A., Rothberg J. M., Lichenstein H. S. (2001) Nat. Cell Biol. 3, 517–521 [DOI] [PubMed] [Google Scholar]
- 12.Uutela M., Laurén J., Bergsten E., Li X., Horelli-Kuitunen N., Eriksson U., Alitalo K. (2001) Circulation 103, 2242–2247 [DOI] [PubMed] [Google Scholar]
- 13.Karvinen H., Rutanen J., Leppänen O., Lach R., Levonen A. L., Eriksson U., Ylä-Herttuala S. (2009) Eur. J. Clin. Invest. 39, 320–327 [DOI] [PubMed] [Google Scholar]
- 14.Liu G., Changsirikulchai S., Hudkins K. L., Banas M. C., Kowalewska J., Yang X., Wietecha T. A., Volpone J., Gilbertson D. G., Alpers C. E. (2008) Hum. Pathol. 39, 393–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong D., Wang Z., Sarkar S. H., Li Y., Banerjee S., Saliganan A., Kim H. R., Cher M. L., Sarkar F. H. (2008) Stem Cells 26, 1425–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uutela M., Wirzenius M., Paavonen K., Rajantie I., He Y., Karpanen T., Lohela M., Wiig H., Salven P., Pajusola K., Eriksson U., Alitalo K. (2004) Blood 104, 3198–3204 [DOI] [PubMed] [Google Scholar]
- 17.Tuuminen R., Nykänen A. I., Krebs R., Soronen J., Pajusola K., Keränen M. A., Koskinen P. K., Alitalo K., Lemström K. B. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 691–698 [DOI] [PubMed] [Google Scholar]
- 18.Wågsäter D., Zhu C., Björck H. M., Eriksson P. (2009) Atherosclerosis 202, 415–423 [DOI] [PubMed] [Google Scholar]
- 19.Li H., Fredriksson L., Li X., Eriksson U. (2003) Oncogene 22, 1501–1510 [DOI] [PubMed] [Google Scholar]
- 20.Xu L., Tong R., Cochran D. M., Jain R. K. (2005) Cancer Res. 65, 5711–5719 [DOI] [PubMed] [Google Scholar]
- 21.Wang Z., Kong D., Banerjee S., Li Y., Adsay N. V., Abbruzzese J., Sarkar F. H. (2007) Cancer Res. 67, 11377–11385 [DOI] [PubMed] [Google Scholar]
- 22.Heldin C. H., Rubin K., Pietras K., Ostman A. (2004) Nat. Rev. Cancer 4, 806–813 [DOI] [PubMed] [Google Scholar]
- 23.Ray S., Gao C., Wyatt K., Fariss R. N., Bundek A., Zelenka P., Wistow G. (2005) J. Biol. Chem. 280, 8494–8502 [DOI] [PubMed] [Google Scholar]
- 24.Li R., Maminishkis A., Wang F. E., Miller S. S. (2007) Invest. Ophthalmol. Vis. Sci. 48, 5722–5732 [DOI] [PubMed] [Google Scholar]
- 25.Zhang F., Tang Z., Hou X., Lennartsson J., Li Y., Koch A. W., Scotney P., Lee C., Arjunan P., Dong L., Kumar A., Rissanen T. T., Wang B., Nagai N., Fons P., Fariss R., Zhang Y., Wawrousek E., Tansey G., Raber J., Fong G. H., Ding H., Greenberg D. A., Becker K. G., Herbert J. M., Nash A., Yla-Herttuala S., Cao Y., Watts R. J., Li X. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 6152–6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritter M. R., Banin E., Moreno S. K., Aguilar E., Dorrell M. I., Friedlander M. (2006) J. Clin. Invest. 116, 3266–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y., Zhang F., Nagai N., Tang Z., Zhang S., Scotney P., Lennartsson J., Zhu C., Qu Y., Fang C., Hua J., Matsuo O., Fong G. H., Ding H., Cao Y., Becker K. G., Nash A., Heldin C. H., Li X. (2008) J. Clin. Invest. 118, 913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klyve P., Nicolaissen B., Naess O. (1996) Acta Ophthalmol. Scand. 74, 26–30 [DOI] [PubMed] [Google Scholar]
- 29.Kojima T., Chang J. H., Azar D. T. (2007) Am. J. Pathol. 170, 764–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noda K., She H., Nakazawa T., Hisatomi T., Nakao S., Almulki L., Zandi S., Miyahara S., Ito Y., Thomas K. L., Garland R. C., Miller J. W., Gragoudas E. S., Mashima Y., Hafezi-Moghadam A. (2008) FASEB J. 22, 2928–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahl A., Paschek L., Martin G., Feltgen N., Hansen L. L., Agostini H. T. (2009) Graefes Arch. Clin. Exp. Ophthalmol. 247, 767–773 [DOI] [PubMed] [Google Scholar]
- 32.Spilsbury K., Garrett K. L., Shen W. Y., Constable I. J., Rakoczy P. E. (2000) Am. J. Pathol. 157, 135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Julien S., Kreppel F., Beck S., Heiduschka P., Brito V., Schnichels S., Kochanek S., Schraermeyer U. (2008) Mol. Vis. 14, 1358–1372 [PMC free article] [PubMed] [Google Scholar]
- 34.Rakic J. M., Lambert V., Devy L., Luttun A., Carmeliet P., Claes C., Nguyen L., Foidart J. M., Noël A., Munaut C. (2003) Invest. Ophthalmol. Vis. Sci. 44, 3186–3193 [DOI] [PubMed] [Google Scholar]
- 35.Campos M., Amaral J., Becerra S. P., Fariss R. N. (2006) Invest. Ophthalmol. Vis. Sci. 47, 5163–5170 [DOI] [PubMed] [Google Scholar]
- 36.D'Antoni M. L., Torregiani C., Ferraro P., Michoud M. C., Mazer B., Martin J. G., Ludwig M. S. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 294, L764–L771 [DOI] [PubMed] [Google Scholar]
- 37.Fischer J. W., Kinsella M. G., Levkau B., Clowes A. W., Wight T. N. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 777–784 [DOI] [PubMed] [Google Scholar]
- 38.Molander C., Kallin A., Izumi H., Rönnstrand L., Funa K. (2000) Exp. Cell Res. 258, 65–71 [DOI] [PubMed] [Google Scholar]
- 39.Au P. Y., Martin N., Chau H., Moemeni B., Chia M., Liu F. F., Minden M., Yeh W. C. (2005) Oncogene 24, 3196–3205 [DOI] [PubMed] [Google Scholar]
- 40.Economopoulou M., Langer H. F., Celeste A., Orlova V. V., Choi E. Y., Ma M., Vassilopoulos A., Callen E., Deng C., Bassing C. H., Boehm M., Nussenzweig A., Chavakis T. (2009) Nat. Med. 15, 553–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Economopoulou M., Bdeir K., Cines D. B., Fogt F., Bdeir Y., Lubkowski J., Lu W., Preissner K. T., Hammes H. P., Chavakis T. (2005) Blood 106, 3831–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeNiro M., Alsmadi O., Al-Mohanna F. (2009) Exp. Eye Res. 89, 700–717 [DOI] [PubMed] [Google Scholar]
- 43.Mochimaru H., Takahashi E., Tsukamoto N., Miyazaki J., Yaguchi T., Koto T., Kurihara T., Noda K., Ozawa Y., Ishimoto T., Kawakami Y., Tanihara H., Saya H., Ishida S., Tsubota K. (2009) Invest. Ophthalmol. Vis. Sci. 50, 4410–4415 [DOI] [PubMed] [Google Scholar]
- 44.Lu J., Yang J. H., Burns A. R., Chen H. H., Tang D., Walterscheid J. P., Suzuki S., Yang C. Y., Sawamura T., Chen C. H. (2009) Circ. Res. 104, 619–627 [DOI] [PubMed] [Google Scholar]
- 45.Nili N., Cheema A. N., Giordano F. J., Barolet A. W., Babaei S., Hickey R., Eskandarian M. R., Smeets M., Butany J., Pasterkamp G., Strauss B. H. (2003) Am. J. Pathol. 163, 869–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keating M. T., Williams L. T. (1988) Science 239, 914–916 [DOI] [PubMed] [Google Scholar]
- 47.Betsholtz C., Westermark B., Ek B., Heldin C. H. (1984) Cell 39, 447–457 [DOI] [PubMed] [Google Scholar]
- 48.Rosenmüller T., Rydh K., Nånberg E. (2001) J. Cell. Physiol. 188, 369–382 [DOI] [PubMed] [Google Scholar]
- 49.Chang Y., Zhuang D., Zhang C., Hassid A. (2004) Am. J. Physiol. Heart Circ. Physiol. 287, H2201–H2208 [DOI] [PubMed] [Google Scholar]
- 50.Chang Y., Ceacareanu B., Zhuang D., Zhang C., Pu Q., Ceacareanu A. C., Hassid A. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 501–507 [DOI] [PubMed] [Google Scholar]
- 51.Funa K., Uramoto H. (2003) Acta Biochim. Pol. 50, 647–658 [PubMed] [Google Scholar]
- 52.Allard D., Figg N., Bennett M. R., Littlewood T. D. (2008) J. Biol. Chem. 283, 19739–19747 [DOI] [PubMed] [Google Scholar]
- 53.Frame S., Cohen P., Biondi R. M. (2001) Mol. Cell 7, 1321–1327 [DOI] [PubMed] [Google Scholar]
- 54.Liang M. H., Chuang D. M. (2007) J. Biol. Chem. 282, 3904–3917 [DOI] [PubMed] [Google Scholar]
- 55.Takahashi-Yanaga F., Sasaguri T. (2009) J. Pharmacol. Sci. 109, 179–183 [DOI] [PubMed] [Google Scholar]
- 56.Takahashi-Yanaga F., Taba Y., Miwa Y., Kubohara Y., Watanabe Y., Hirata M., Morimoto S., Sasaguri T. (2003) J. Biol. Chem. 278, 9663–9670 [DOI] [PubMed] [Google Scholar]
- 57.Blacher S., Devy L., Burbridge M. F., Roland G., Tucker G., Noël A., Foidart J. M. (2001) Angiogenesis 4, 133–142 [DOI] [PubMed] [Google Scholar]
- 58.Zhu W. H., Nicosia R. F. (2002) Angiogenesis 5, 81–86 [DOI] [PubMed] [Google Scholar]
- 59.Choi S. E., Kang Y., Jang H. J., Shin H. C., Kim H. E., Kim H. S., Kim H. J., Kim D. J., Lee K. W. (2007) J. Vasc. Res. 44, 365–374 [DOI] [PubMed] [Google Scholar]
- 60.Mendes C. T., Mury F. B., de Sá Moreira E., Alberto F. L., Forlenza O. V., Dias-Neto E., Gattaz W. F. (2009) Eur. Arch. Psychiatry Clin. Neurosci. 259, 16–22 [DOI] [PubMed] [Google Scholar]
- 61.Loberg R. D., Vesely E., Brosius F. C., 3rd (2002) J. Biol. Chem. 277, 41667–41673 [DOI] [PubMed] [Google Scholar]
- 62.Ferrara N. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 789–791 [DOI] [PubMed] [Google Scholar]
- 63.Andreoli C. M., Miller J. W. (2007) Curr. Opin. Ophthalmol. 18, 502–508 [DOI] [PubMed] [Google Scholar]
- 64.Rodrigues E. B., Farah M. E., Maia M., Penha F. M., Regatieri C., Melo G. B., Pinheiro M. M., Zanetti C. R. (2009) Prog. Retin. Eye Res. 28, 117–144 [DOI] [PubMed] [Google Scholar]
- 65.Williams A. J., Fekrat S. (2006) Am. J. Ophthalmol. 142, 683–684 [DOI] [PubMed] [Google Scholar]
- 66.Aiello L. P., Pierce E. A., Foley E. D., Takagi H., Chen H., Riddle L., Ferrara N., King G. L., Smith L. E. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 10457–10461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hernandez-Pastor L. J., Ortega A., Garcia-Layana A., Giraldez J. (2008) Am. J. Health Syst. Pharm. 65, 1805–1814 [DOI] [PubMed] [Google Scholar]
- 68.van Wijngaarden P., Qureshi S. H. (2008) Clin. Exp. Optom. 91, 427–437 [DOI] [PubMed] [Google Scholar]
- 69.Nishijima K., Ng Y. S., Zhong L., Bradley J., Schubert W., Jo N., Akita J., Samuelsson S. J., Robinson G. S., Adamis A. P., Shima D. T. (2007) Am. J. Pathol. 171, 53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saint-Geniez M., Maharaj A. S., Walshe T. E., Tucker B. A., Sekiyama E., Kurihara T., Darland D. C., Young M. J., D'Amore P. A. (2008) PloS ONE 3, e3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Avci B., Avci R., Inan U. U., Kaderli B. (2009) Invest. Ophthalmol. Vis. Sci. 50, 3438–3446 [DOI] [PubMed] [Google Scholar]
- 72.Peters S., Heiduschka P., Julien S., Ziemssen F., Fietz H., Bartz-Schmidt K. U., Schraermeyer U. (2007) Am. J. Ophthalmol. 143, 995–1002 [DOI] [PubMed] [Google Scholar]
- 73.Inan U. U., Avci B., Kusbeci T., Kaderli B., Avci R., Temel S. G. (2007) Invest. Ophthalmol. Vis. Sci. 48, 1773–1781 [DOI] [PubMed] [Google Scholar]
- 74.Manzano R. P., Peyman G. A., Khan P., Kivilcim M. (2006) Retina 26, 257–261 [DOI] [PubMed] [Google Scholar]
- 75.Kvanta A. (1995) Curr. Eye Res. 14, 1015–1020 [DOI] [PubMed] [Google Scholar]
- 76.Motiejūnaite R., Kazlauskas A. (2008) Exp. Eye Res. 86, 171–177 [DOI] [PubMed] [Google Scholar]
- 77.Hinton D. R., He S., Lopez P. F. (1998) Arch. Ophthalmol. 116, 203–209 [DOI] [PubMed] [Google Scholar]
- 78.Davies M. H., Stempel A. J., Powers M. R. (2008) Invest. Ophthalmol. Vis. Sci. 49, 4195–4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Semkova I., Fauser S., Lappas A., Smyth N., Kociok N., Kirchhof B., Paulsson M., Poulaki V., Joussen A. M. (2006) FASEB J. 20, 1689–1691 [DOI] [PubMed] [Google Scholar]
- 80.Lima E., Silva R., Kachi S., Akiyama H., Shen J., Aslam S., Yuan Gong Y., Khu N. H., Hatara M. C., Boutaud A., Peterson R., Campochiaro P. A. (2006) J. Cell. Physiol. 208, 161–166 [DOI] [PubMed] [Google Scholar]