Abstract
trans-2-Nonenal is an unsaturated aldehyde with an unpleasant greasy and grassy odor endogenously generated during the peroxidation of polyunsaturated fatty acids. 2-Nonenal covalently modified human serum albumin through a reaction in which the aldehyde preferentially reacted with the lysine residues. Modified proteins were immunogenic, and a specific monoclonal antibody (mAb) 27Q4 that cross-reacted with the protein covalently modified with 2-nonenal was raised from mouse. To verify the presence of the protein-bound 2-nonenal in vivo, the mAb 27Q4 against the 2-nonenal-modified keyhole limpet hemocyanin was raised. It was found that a novel 2-nonenal-lysine adduct, cis- and trans-Nϵ-3-[(hept-1-enyl)-4-hexylpyridinium]lysine (HHP-lysine), constitutes an epitope of the antibody. The immunoreactive materials with mAb 27Q4 were detected in the kidney of rats exposed to ferric nitrilotriacetate, an iron chelate that induces free radical-mediated oxidative tissue damage. Using high performance liquid chromatography with on-line electrospray ionization tandem mass spectrometry, we also established a highly sensitive method for detection of the cis- and trans-HHP-lysine and confirmed that the 2-nonenal-lysine adducts were indeed formed during the lipid peroxidation-mediated modification of protein in vitro and in vivo. Furthermore, we examined the involvement of the scavenger receptor lectin-like oxidized low density lipoprotein receptor-1 in the recognition of 2-nonenal-modified proteins and established that the receptor recognized the HHP-lysine adducts as a ligand.
Keywords: Lipid/Oxidation, Lipoprotein/Receptor, Oxygen/Reactive, Protein/Chemical Modification, Protein/Denaturation, Protein/Oxidative Damage
Introduction
Lipid peroxidation in tissue and in tissue fractions represents a degradative process, which is the consequence of the production and propagation of free radical reactions primarily involving membrane polyunsaturated fatty acids, and has been implicated in the pathogenesis of numerous diseases, including atherosclerosis, diabetes, cancer, and rheumatoid arthritis, as well as in drug-associated toxicity, postischemic reoxygenation injury, and aging (1). The peroxidative breakdown of polyunsaturated fatty acids has also been implicated in the pathogenesis of many types of liver injuries and especially in the hepatic damage induced by several toxic substances. The lipid peroxidation leads to the formation of a broad array of different products with diverse and powerful biological activities. Among them are a variety of different aldehydes (2). The primary products of lipid peroxidation, lipid hydroperoxides, can undergo carbon-carbon bond cleavage via alkoxyl radicals in the presence of transition metals giving rise to the formation of short-chain, unesterified aldehydes or a second class of aldehydes still esterified to the parent lipid. These reactive aldehydic intermediates readily form covalent adducts with cellular macromolecules, including protein, leading to the disruption of important cellular functions. The important agents that give rise to the modification of protein may be represented by α,β-unsaturated aldehydic intermediates, such as 2-alkenals, 4-hydroxy-2- alkenals, and 4-oxo-2-alkenals (3, 4).
2-Alkenals represent a group of highly reactive aldehydes containing two electrophilic reaction centers. A partially positive carbon 1 or 3 in such molecules can attack nucleophiles, such as protein. It has been suggested that these aldehydes primarily react with the sulfhydryl group of cysteine, the ϵ-amino group of lysine, and the imidazole group of histidine in the proteins (2, 3). Among the 2-alkenals, 2-nonenal (Fig. 1A) has a characteristically unpleasant greasy and grassy odor. It is also a major contributor to the unpleasant cardboard flavor in aged beer. It was previously shown that 2-nonenal could be formed through lipid peroxidation as a minor product in peroxide- mediated oxidation of high concentrations of linoleic acid hydroperoxide or from liver microsomes treated with ADP/iron in vitro (2). Toyokuni et al. (5) also reported the production of C2–C12 saturated and unsaturated aldehydes, including 2-nonenal, in the kidney of rats exposed to Fe3+-NTA.2 More recently, Haze et al. (6) analyzed the body odor components that adhered to the shirts of the subjects by gas chromatography/mass spectrometry and demonstrated that 2-nonenal is present in increasing amounts in the body odors of persons 40 years or older. They have also suggested that cis-2-nonenal and trans-2-nonenal are formed from the oxidative degradation of polyunsaturated fatty acids, such as palmitoleic acid. However, it is still not clear how 2-nonenal could be formed in vivo. Because of its insolubility in water, 2-nonenal is less reactive with proteins than other 2-alkenals, such as acrolein and crotonaldehyde, and therefore has received relatively little attention as a causative agent for modification of proteins. Only inhibition of enzymes, such as platelet membrane-bound phosphotyrosine phosphatase (7) and liver microsomal glucose-6-phosphatase (8), has been reported.
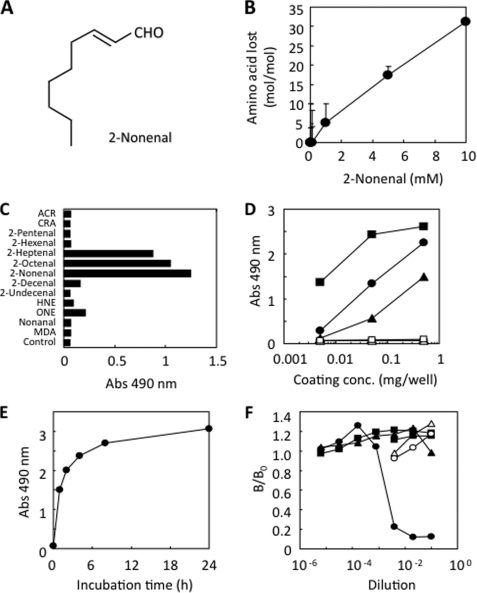
FIGURE 1.
Monoclonal antibody against protein-bound 2-nonenal. A, chemical structure of 2-nonenal. B, loss of lysine residues in protein treated with 2-nonenal. HSA (1 mg/ml) was incubated with 0–10 mm 2-nonenal in 50 mm sodium phosphate buffer, pH 7.2, at 37 °C. C, immunoreactivity of mAb 27Q4 to aldehyde-modified proteins. Affinity of mAb 27Q4 was determined by a direct ELISA using aldehyde-modified BSA as the absorbed antigen. A coating antigen (0.5 μg/well) was prepared by incubating 1 mg of BSA with 1 mm aldehydes in 1 ml of 50 mm sodium phosphate buffer, pH 7.2, for 24 h at 37 °C. ACR, acrolein; CRA, crotonaldehyde; MDA, malondialdehyde; HNE, 4-hydroxy-2-nonenal; ONE, 4-oxo-2-nonenal. D, immunoreactivity of mAb 27Q4 to 2-nonenal-modified proteins. A coating antigen (0.005–0.5 μg/well) was prepared by incubating 1 mg of protein with 1 mm 2-nonenal in 1 ml of 50 mm sodium phosphate buffer, pH 7.2, for 24 h at 37 °C. Proteins are as follows: open square, native lactoglobulin; closed triangle, 2-nonenal-modified HSA; closed circle, 2-nonenal-modified BSA; closed square, 2-nonenal-modified lactoglobulin. E, time-dependent increase in the immunoreactivity of protein treated with 2-nonenal. HSA (1 mg/ml) was incubated with 1 mm 2-nonenal in 50 mm sodium phosphate buffer, pH 7.2, at 37 °C. F, competitive ELISA analysis with the reaction mixtures of amino acid derivatives and 2-nonenal. Competitors were prepared by incubating 50 mm amino acid derivatives, Nα-acetyllysine, Nα-acetylhistidine, or Nα-acetylcysteine, in the presence or absence of 2-nonenal (50 mm), in 50 mm sodium phosphate buffer, pH 7.2, for 24 h at 37 °C. Competitors are as follows: open circle, Nα-acetyllysine; open triangle, Nα-acetylhistidine; open square, Nα-acetylcysteine; closed circle, 2-nonenal/Nα-acetyllysine; closed triangle, 2-nonenal/Nα-acetylhistidine; closed square, 2-nonenal/Nα-acetyllysine.
A structurally diverse protein supergroup called scavenger receptors mediates the cellular uptake of modified lipoproteins. Scavenger receptors are expressed by endothelial cells, macrophages, and smooth muscle cells and mediate recognition, internalization, and physiological responses to a wide range of ligands, including phospholipids, lipoprotein particles, apoptotic cells, and pathogens. Several oxidized low density lipoprotein (LDL) receptors have been identified so far, including SR-A I/II, CD36, SR-BI, FcγRII, lectin-like oxidized LDL receptor (LOX-1), macrosialin, SR expressed by endothelial cells, etc. Among them, LOX-1 is characterized as the major receptor for oxidized LDL in the endothelial cells of large arteries. Its inducible expression (9) might be involved in the oxidized LDL-mediated endothelial dysfunction. LOX-1 exhibits a binding activity for multiple ligands. LOX-1 is capable of interacting with a variety of structurally and functionally distinct ligands, including oxidized LDL, platelets, aged red blood cells, apoptotic cells, advanced glycation end products, heat shock protein 70, bacteria, and phosphatidylserine (10). LOX-1 binds and internalizes a diverse array of macromolecules, although their structures are not always related to each other.
In this study, to understand the mechanism underlying the formation of a covalently modified protein with 2-nonenal in vivo, we raised a monoclonal antibody (mAb) against protein-bound 2-nonenal and identified a novel 2-nonenal-lysine adduct as the epitope. Our immunohistochemical studies demonstrated the localization of the immunoreactive materials in the kidney of rats exposed to Fe3+-NTA, an iron chelate that induces acute renal proximal tubular necrosis, a consequence of free radical-mediated oxidative tissue damage, eventually leading to a high incidence of renal adenocarcinoma in rodents. Importantly, using high performance liquid chromatography with on-line electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS), we also showed evidence that the 2-nonenal-lysine adduct is indeed accumulated during the lipid peroxidation-mediated modification of protein in vitro and in vivo. Furthermore, to evaluate the biological implication of the 2-nonenal modification of protein, we examined the involvement of LOX-1, a member of the scavenger receptor family, in the recognition of the 2-nonenal-lysine adducts.
EXPERIMENTAL PROCEDURES
Materials
trans-2-Nonenal (2-nonenal), Nα-acetyl-l-lysine, and human serum albumin (HSA) were obtained from the Sigma. Acrolein was obtained from Tokyokasei (Tokyo, Japan). Nα-[U-13C6,15N2]Fmoc-Nϵ-boc-lysine (where boc is t-butoxycarbonyl) was obtained from the Cambridge Isotope Laboratories (Andover, MA). The keyhole limpet hemocyanin (KLH) was obtained from Pierce. Ferric nitrate nonahydrate, sodium carbonate, and bovine serum albumin (BSA) were from Wako (Osaka, Japan), and the nitrilotriacetic acid disodium salt was from Nacalai Tesque, Inc. (Kyoto, Japan). The Fe3+-NTA solution was prepared immediately before use by the method described in a previous study (11). The stock solutions of 4-hydroxy-2-nonenal were prepared by the acid treatment (1 mm HCl) of 4-hydroxy-2-nonenal dimethyl acetal, which was synthesized according to the procedure of De Montarby et al. (12). 4-Oxo-2-nonenal was synthesized by the oxidation of 4-hydroxy-2-nonenal dimethyl acetal with pyridinium dichlorochromate, followed by HCl hydrolysis (13). The sodium salt of malondialdehyde was prepared by the Dowex hydrolysis of malondialdehyde bis(diethyl acetal) (14). Other aldehydes were purchased from Wako.
In Vitro Modification of HSA
Modification of the protein by 2-nonenal and its related short-chain aldehydes was performed by incubating HSA (1.0 mg/ml) with 0–10 mm 2-nonenal in 1 ml of 50 mm sodium phosphate buffer, pH 7.2, at 37 °C for 24 h. The Fe2+-catalyzed oxidation of unsaturated fatty acids in the presence of HSA was performed by incubating HSA (1 mg/ml) with 2 mm unsaturated fatty acids in the presence of 50 μm Fe2+ and 1 mm ascorbic acid in 1 ml of 50 mm sodium phosphate buffer, pH 7.2, in atmospheric oxygen at 37 °C. The reaction was terminated by the addition of 1 mm butylated hydroxytoluene and 100 μm diethylenetriaminepentaacetic acid.
In Vitro Peroxidation of LDL
LDL (1.019–1.063 g/ml) was prepared from the plasma of healthy humans by sequential ultracentrifugation and then extensively dialyzed three times against phosphate-buffered saline (PBS, 10 mm sodium phosphate buffer, pH 7.2, containing 150 mm NaCl) containing 0.01% EDTA at 4 °C. LDL used for the oxidative modification by Cu2+ was dialyzed five times against a 1000-fold volume of PBS at 4 °C. The oxidation of LDL was performed by incubating 0.5 mg of LDL with CuSO4 (5 μm) in 1 ml of PBS for 24 h at 37 °C. The reaction was terminated by the addition of 1 mm EDTA and then stored at 4 °C.
Amino Acid Analysis
An aliquot (0.2 ml) of the protein samples (1 mg/ml) incubated for 24 h at 37 °C in the absence or presence of 2-nonenal was treated with 10 mm EDTA (20 μl), 1 n NaOH (20 μl), and 100 mm sodium borohydride (20 μl). After incubation for 1 h at room temperature, 20 μl of 2 n HCl was added to the mixture to stop the reaction, and the mixture was then incubated for 60 min at room temperature after adding 280 μl of 20% trichloroacetic acid. After centrifugation at 5,000 × g for 10 min at 4 °C, the proteins were hydrolyzed in vacuo with 2 ml of 6 n HCl for 24 h at 110 °C. The hydrolysates were then dried and dissolved in sodium citrate buffer, pH 3.15. The amino acid analysis was performed using a JEOL JLC-500 amino acid analyzer equipped with a JEOL LC30-DK20 data analyzing system.
Preparation of Monoclonal Antibody against 2-Nonenal-modified Protein
The immunogen was prepared by incubating the KLH (1.0 mg/ml) with 10 mm 2-nonenal in 3 ml of PBS at 37 °C for 24 h. We immunized the female BALB/c mice (Chubu Kagaku Shizai Co., Ltd., Nagoya, Japan) on day 1 with complete Freund adjuvant and 0.06 mg of immunogen (2-nonenal-modified KLH) and boosted on days 11, 21, and 31 with incomplete Freund adjuvant and 0.02 mg of immunogen by emulsifying and intraperitoneal injection. Titers to 2-nonenal-modified BSA in the immunized mice sera were measured by an enzyme-linked immunosorbent assay (ELISA) (15). Two months after the initial immunization, the immunized mice were given an intraperitoneal boost of 0.06 mg/ml 2-nonenal-modified KLH. Three days later, the spleen cells from the immunized mice were fused with P3/U1 murine myeloma cells in the presence of polyethylene glycol and cultured in hypoxanthine/amethopterin/thymidine selection medium. The culture supernatants of the hybridoma were screened using an ELISA, employing pairs of wells of microtiter plates on which were absorbed 2-nonenal-treated BSA as the antigen (0.5 μg of protein/well). After incubation with 100 μl of the hybridoma supernatants, and with intervening washes with PBS/Tween, the wells were incubated with alkaline phosphatase-conjugated goat anti-mouse IgG, followed by a substrate solution containing 0.5 mg/ml 1,2-phenylenediamine. Hybridoma cells corresponding to the supernatants that were positive on the 2-nonenal-modified BSA and negative on the native BSA were then cloned by limited dilution. After repeated screening, four clones were obtained. Among them, clone 27Q4 showed the most significant recognition of the 2-nonenal-modified BSA. Competitors were prepared by incubating 50 mm amino acid derivatives, Nα-acetyl-l-lysine, Nα-acetylhistidine, or Nα-acetylcysteine, in the presence or absence of 2-nonenal (50 mm), in 50 mm sodium phosphate buffer, pH 7.2, for 24 h at 37 °C.
Isolation and Structural Analysis of Antigenic 2-Nonenal-Lysine Adducts
The reaction mixture (6 ml) contained 200 mm trans-2-nonenal and 200 mm Nα-acetyl-l-lysine in 50 mm sodium phosphate buffer, pH 7.2. After incubation for 72 h at 37 °C, a portion of the reaction mixture was analyzed by a reverse-phase HPLC using a Develosil ODS-HG-5 column (4.6 × 250 mm, Nomura Chemicals) equilibrated in a solution of 5% acetonitrile in 0.01% trifluoroacetic acid. The chromatographic separation was performed by a gradient elution as follows (solvent A was water and solvent B was acetonitrile, both containing 0.01% trifluoroacetic acid): 0–10 min, linear gradient to 40% B; 10–40 min, 40% B; 40–41 min, linear gradient to 100% B; 41–45 min, hold; flow rate = 0.8 ml/min. The isolation and purification of the antigenic adducts (P-1 and P-2) were carried out under the same HPLC conditions and finally obtained 2.3 and 2.9 mg of P-1 and P-2, respectively. Their structures were characterized by LC/MS and 1H and 13C NMR. The LC/MS was measured by a Jasco PlatformII-LC instrument. The LC/MS analysis was performed using a Develosil ODS-HG-5 column (4.6 × 250 mm) eluted with a linear gradient from 100% water containing 0.1% acetic acid to 100% acetonitrile containing 0.1% acetic acid for 60 min at a flow rate of 0.8 ml/min. The elution profiles were monitored by absorbance at 239 nm. The NMR analyses were performed using a Bruker AMX400 (400 MHz) instrument. For P-1, 1H NMR (400 MHz, MeOD): δ 0.85–0.92 (6H, m), 1.25–1.50 (22H, m), 1.66 (2H, m), 1.70–2.08 (7H, m), 2.13 (2H, q), 2.87 (2H, t), 4.36 (1H, q), 4.55 (2H, t), 6.16 (1H, m), 6.51 (1H, d, J = 11.6 Hz), 7.93 (1H, d, J = 6.4 Hz), 8.66 (1H, s), and 8.70 (1H, d, J = 5.4 Hz). For P-2, 1H NMR (MeOD, 600 MHz): δ 0.89–0.94 (6H, m), 1.30–1.52 (11H, m), 1.40–1.52 (2H, m), 1.56 (2H, m), 1.66 (2H, m), 1.70–1.92 (2H, m), 1.96 (3H, s), 2.01–2.06 (2H, m), 2.36 (2H, q), 2.93 (2H, t), 4.39 (1H, q), 4.53 (2H, q), 6.54 (1H, m), 6.66 (1H, d, J = 16.2 Hz), 7.84 (1H, d, J = 6.0 Hz), 8.61 (1H, d, J = 6.0 Hz), 8.91 (1H, s); 13C NMR (150 MHz, MeOD): δ 14.3 (2C), 22.4, 23.4, 23.5 (2C), 29.5, 30.0, 30.2, 31.6, 32.1, 32.5, 32.6, 34.0, 34.2, 53.0, 62.0, 122.4, 129.2, 139.4, 142.2 (2C), 142.5, 160.7, 173.3, and 175.1.
Immunohistochemical Detection of 2-Nonenal Adducts in Vivo
Male Wistar rats (Shizuoka Laboratory Animal Center, Shizuoka, Japan), weighing 130–150 g (6 weeks old), were used. The animals received a single intraperitoneal injection of Fe3+-NTA (15 mg of iron/kg of body weight) or repeated administration as described previously (5). They were sacrificed by decapitation at 0, 1, 6, 24, and 48 h after a single injection or 3 weeks after the repeated administration. Both kidneys of each animal were immediately removed. One of them was fixed in Bouin's solution, embedded in paraffin, cut in 3-mm thick slices, and used for the immunohistochemical analyses by an avidin-biotin complex method with alkaline phosphatase. Briefly, after deparaffinization with xylene and ethanol, normal rabbit serum (Dako; diluted to 1:75) for the inhibition of the nonspecific binding of the secondary antibody, a primary antibody (20 μg/ml), biotin-labeled rabbit anti-mouse IgG serum (Vector Laboratories; diluted to 1:300), and avidin-biotin complex (Vector; diluted to 1:100) were sequentially used. Procedures, using PBS or the IgG fraction (0.5 mg/ml) of the normal mouse serum instead of mAb 27Q4, showed no response or negligible positive responses.
Preparation of Isotope-labeled HPH-lysines
To prepare the [U-13C6,15N2]HHP-lysine, 1 mm Nα-[U-13C6,15N2]Fmoc-Nϵ-Boc-lysine was treated with 1 ml of trifluoroacetic acid for 2 h at room temperature, applied to a C-18 Sep-Pak column, and eluted with 5 ml of methanol. The eluate was evaporated and redissolved in 1 ml of 50 mm sodium phosphate buffer, pH 7.4. The obtained Nα-[U-13C6,15N2]Fmoc-lysine was modified with 1 mm 2-nonenal for 24 h at 37 °C and subsequently treated with 20% piperidine for 2 h at room temperature to remove the Fmoc moiety. The resulting mixture was purified by HPLC, carried out under the same HPLC conditions as already described. LC/ESI/MS/MS analysis with MRM mode of the isotope-labeled HPH-lysines showed that that the standards contained no endogenous (nonlabeled) adducts (supplemental Fig. S1).
LC/ESI//MS/MS
Mass spectrometric analyzes were performed using a Quattro Ultima triple stage quadrupole mass spectrometer (Waters-Micromass, Manchester, UK) equipped with an ESI probe and interfaced with a Shimadzu HPLC system (Shimadzu, Kyoto, Japan). The sample injection volumes of 20 μl each were separated on a Shim-pack VP-ODS 2.0 × 150 mm, 5-μm column (Shimadzu) at a flow rate of 0.3 ml/min. A discontinuous gradient was used by solvent A (H2O containing 0.1% formic acid) with solvent B (acetonitrile) as follows: isocratic elution with 20% solvent B from 0 to 5 min; increasing to 50% solvent B from 5 to 20 min; increasing to 70% solvent B from 20 to 25 min; and then isocratic elution with 70% solvent B from 25 to 30 min. Mass spectrometric analyzes were performed on line using ESI/MS/MS in the positive ion mode with MRM mode (cone potential 35 eV/collision energy 24 eV). The MRM transitions monitored were as follows: [U-13C6,15N2]HHP-lysine, m/z 397 → 261; cis- and trans-HHP-lysine, m/z 389 → 260. l-Glutamate upon reaction with 2-nonenal gave similar MRM transitions but MRM transitions (360 → 260) were detected in the reaction mixture of 2-nonenal/glutamate; however, their elution times were completely different from those of standard HHP-lysines (supplemental Fig. S2). The amount of each HHP-lysine adduct was quantified by the ratio of the peak area of the target adducts and of the HHP-lysine-stable isotope. The HHP-lysine standard concentration was determined by assay of the amino groups using a 2,4,6-trinitrobenzenesulfonic acid assay (16). Briefly, to 30 μl of hydrolyzed HHP-lysine solution was added 30 μl of 4% NaHCO3 and 30 μl of 0.1% 2,4,6-trinitrobenzenesulfonic acid. The solution was allowed to react at 37 °C for 2 h, and 30 μl of 1% SDS and 15 μl of 1 n HCl were then added. The absorbance of the solution was read at 340 nm. The standard curve (using valine as a standard) was linear in the range 0–3.3 nmol of NH2. QuanLynx (version 4.0) software (Waters-Micromass) was used to create standard curves (supplemental Fig. S3) and to calculate the adduct concentrations.
For the LC/ESI/MS/MS analysis of the HHP-lysines in vitro, the protein samples were treated with an equal volume of 20% trichloroacetic acid. After centrifugation at 5,000 × g for 10 min at 4 °C, the proteins were hydrolyzed in vacuo with 2 ml of 6 n HCl for 24 h at 110 °C. The internal standard, [U-13C6,15N2]HHP-lysine, was added to the samples prior to the acid hydrolysis. After the acid hydrolysis, the samples were partially separated with Oasis hydrophilic-lipophilic balance (HLB) cartridges (Waters, Milford, MA). After the sample loading, the HLB cartridges were washed with 2 ml of 50% methanol, and the HHP-lysines were eluted with 2 ml of 80% methanol. The samples were then dried, dissolved in methanol, and subjected to LC/ESI/MS/MS analysis.
For the LC/ESI/MS/MS analyses of the HHP-lysines in vivo, male ddY mice (Shizuoka Laboratory Animal Center, Shizuoka), weighing 25–35 g (6 weeks of age), were used. Animals received a single intraperitoneal injection of Fe3+-NTA (5 mg of iron/kg of body weight). They were sacrificed at 0, 1, 3, 6, and 24 h after the administration. Both kidneys of each animal were immediately removed. The kidneys were homogenized in a Teflon homogenizer in 10 volumes of PBS containing butylated hydroxytoluene (1 mm). The homogenate was centrifuged at 18,000 × g for 15 min, and the precipitates were hydrolyzed in vacuo with 6 n HCl (2 ml) and [U-13C6,15N2]HHP-lysine for 24 h at 110 °C. The hydrolysates were then dried and dissolved in methanol. After the acid hydrolysis, the samples were partially separated with HLB cartridges and subjected to LC/ESI/MS/MS analysis.
DiD-labeled AcLDL Uptake Assays
DiD (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate)-labeled AcLDL was prepared from human LDL according to the methods previously described for 1,1′-dioctadecyl-3, 3,3′,3′-tetramethylindocarbocyanine perchlorate labeling (17). CHO cells that stably expressed CFP-tagged LOX-1 (18) were grown on coverslips. For the cells transiently expressing CFP, CHO cells suspended at a density of 1 × 104 cells/ml were plated on a coverslip 24 h prior to transfection. The cells (40% confluent) were transfected with pECFP-N1 (Clontech) using Lipofectamine reagent according to the manufacturer's protocol and then incubated for 48 h. Cells were treated with each concentration of adducts prepared in F-12 medium without fetal calf serum for 10 min at 37 °C in a 5% CO2 humidified atmosphere, followed by incubation with DiD-AcLDL (0.4 μg/coverslip) for another 15 min. The cells were then fixed with 2% formaldehyde. Each coverslip was inverted onto a glass slide with a spacer, and the cells were examined using a Leica DM IRE2 microscope (Leica Microsystems, Wetzler, Germany) equipped with a ×100, NA 1.4 objective and a Cool SNAP-HG digitalized cooled CCD camera (Roper Scientific, Trenton, NJ) driven by MetaMorph software (Universal Imaging, Downingtown, PA). The expression level of LOX-1 was examined by the CFP fluorescence intensity using an E4 filter with excitation at 436 nm (7-nm bandpass) and a 470-nm long pass emission filter. Images were acquired at 0.2 s exposure time. The DiD-AcLDL uptake was analyzed by the amount of the DiD-derived fluorescence intensity using a Y5 filter, with excitation using a 620 nm (50-nm bandpass) and 700 nm (75-nm bandpass) emission filters. The images were acquired at a 2-s exposure.
Images of over 100 cells from three independent experiments were acquired. After subtracting the background emission level with respect to 436 and 620 nm from the fluorescence image, the fluorescence intensity of each cell was determined from the average pixel value of the whole cell. The expression level of LOX-1 was calculated from the CFP intensity, and the level of uptake of AcLDL was calculated from the DiD intensity (18). For each experimental condition, the level of AcLDL uptake was averaged per expression level of LOX-1.
RESULTS
Monoclonal Antibody against Protein-bound 2-Nonenal
To assess the reactivity of 2-nonenal to proteins, HSA (1 mg/ml) was exposed to 2-nonenal (0–10 mm) under physiological conditions in vitro, and changes in the amino acid composition of the protein were examined by an amino acid analysis. As shown in Fig. 1B, 2-nonenal preferentially reacted with the lysine residues, indicating that 2-nonenal can covalently modify protein spontaneously, and the likely mechanism is a nucleophilic attack by the protein.
To determine whether modification of proteins by 2-nonenal occurs in vivo, we attempted to develop a mAb against 2-nonenal-modified protein. To this end, the keyhole limpet hemocyanin that had been exposed to 2-nonenal was administered to mice at 10-day intervals. During the preparation of the mAb, hybridomas were selected by comparing the reactivities of the culture supernatant to the 2-nonenal-modified BSA. Among the four obtained clones, the clone 27Q4 showed the most distinctive recognition of the 2-nonenal-modified BSA. The specificity of the obtained antibody was characterized. As shown in Fig. 1C, the monoclonal antibody showed a strong immunoreactivity not only with 2-nonenal but also with 2-octenal and 2-heptenal, whereas the unmodified BSA was not recognized by the antibody. We then determined whether the 2-nonenal-modified proteins contained the 27Q4 epitope. The mAb 27Q4 immunoreacted with not only 2-nonenal/BSA, but also with 2-nonenal/HSA and 2-nonenal/lactoglobulin, whereas the unmodified proteins were not recognized by the antibody (Fig. 1D), suggesting that the 2-nonenal-modified proteins contained a common adduct structure recognized by mAb 27Q4. The time course study of formation of the 2-nonenal epitope during the incubation of BSA with 2-nonenal in vitro was determined by direct ELISA using mAb 27Q4. When BSA was incubated with 1 mm 2-nonenal at 37 °C, the immunoreactive materials rapidly increased within 4 h (Fig. 1E). To identify the possible attacking nucleophiles that form antigenic structures, we investigated the reaction of 2-nonenal with individual amino acids likely to form stable antigenic products. We studied lysine, histidine, and cysteine, each protected at the α-amino group as an acetamide, leaving only the side-chain nucleophile as a potential reactant. As shown in Fig. 1F, binding of the 2-nonenal-modified protein to the antibody was hardly inhibited by the reaction mixtures of 2-nonenal/histidine and 2-nonenal/cysteine but was significantly inhibited by the reaction mixture of 2-nonenal/lysine, suggesting that the mAb might recognize a 2-nonenal-lysine adduct as the epitope. None of the original amino acid derivatives, histidine, cysteine, and lysine, cross-reacted with mAb 27Q4.
Isolation and Structural Characterization of the Antigenic 2- Nonenal-Lysine Adducts
Because characterization of the ability of antibodies to recognize specific molecular targets in their native three-dimensional conformation is critical to the use of these reagents, we sought to identify the intrinsic epitope recognized by mAb 27Q4. To identify the 2-nonenal-lysine adduct recognized by mAb 27Q4, the immunoreactivity with the reaction products of 2-nonenal with Nα-acetyllysine was characterized. As shown in Fig. 2A, the ELISA analysis of the HPLC fractions for immunoreactivity with mAb 27Q4 showed that the antibody primarily had an immunoreactivity with two fractions, P-1 and P-2. The LC/MS total ion current analysis of P-2 detected one product, which showed a 242-Da increase in the mass value of the unmodified lysine derivative and gave the [M + H]+ peak at m/z 431.0 (supplemental Fig. S4), suggesting that it was composed of one molecule of Nα-acetyllysine and two molecules of 2-nonenal.
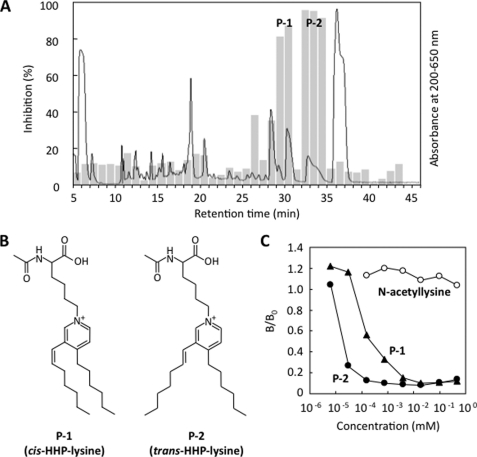
FIGURE 2.
Isolation and structural characterization of the antigenic 2-nonenal-lysine adducts. A, competitive ELISA analysis of HPLC fractions for immunoreactivity with mAb 27Q4. The reaction was performed by incubating 200 mm Nα-acetyllysine with 200 mm 2-nonenal in 1 ml of 50 mm sodium phosphate buffer, pH 7.2, at 37 °C for 24 h. Solid line, profile of UV absorbance at 200–650 nm. Bar, competitive ELISA analysis. B, chemical structures of cis-HHP-lysine and (P-1) and trans-HHP-lysine (P-2). C, competitive ELISA analysis with cis- and trans-HHP-lysine. Competitors are as follows: closed circle, Nα-acetyl-trans-HHP-lysine (P-2); closed triangle, Nα-acetyl-cis-HHP-lysine (P-1); open circle, Nα-acetyllysine.
Because P-2 was a major immunoreactive fraction, we first attempted to identify this product. To characterize the chemical structure, isolation by HPLC on the reverse-phase column was carried out. After purification, the structure of the products was characterized by an NMR analysis. The assignments of protons and carbons were based on the result of the HMBC and distortionless enhancement by polarization transfer experiments. Compared with the 13C NMR and 1H NMR spectra between Nα-acetyllysine and the product (supplemental Figs. S5–S10), the Cb (173.3 ppm), the Cd (175.1 ppm), the Ha proton (1.96 ppm), and the Hc proton (δH 4.39 ppm; δC 53.0 ppm) remained in the 13C NMR and 1H NMR spectra of the product. The correlation spectroscopy spectrum showed correlations between the Hc proton with the He (δH 1.70–1.92 ppm; δC 32.1 ppm) proton, the He proton with the Hf proton (δH 1.40–1.52 ppm; δC 23.4 or 23.5 ppm), the Hf proton with the Hg proton (δH 2.01–2.06 ppm; δC 31.6 ppm), and the Hg proton with the Hh proton (δH 4.53 ppm; δC 62.0 ppm). The 13C NMR spectrum of the product showed five signals (δC 129.2, 139.4, 142.2 (two carbon signals), and 160.7 ppm) in the aromatic carbon region. In the HMBC spectrum, cross-peaks between the δ-proton of the lysine residue, Hh, and carbons (Hh-Ci (142.2 ppm) and Hh-Cm (142.2 ppm)), cross-peaks between Hi (8.61 ppm, J = 6.0 Hz) and carbons (Hi-Ch, Hi-Cj (129.2 ppm) and Hi-Ck (160.7 ppm)), and cross-peaks between Hm (8.91 ppm) with carbons (Hm-Ch, Hm-Ci, Hm-Ck, and Hm-Cl (139.4 ppm)). The coupling constant and HMBC spectrum indicated that Hi and Hj (7.84 ppm, J = 6.0 Hz) are adjacent. Moreover, in the HMBC spectrum, the cross-peaks Hj and carbons (Hj-Ci, Hj-Ck, and Hj-Cl) are observed. The Ck and Cl were missing from the distortionless enhancement by polarization transfer spectrum. This indicates that Ck and Cl are quaternary carbons. These results suggest that the aromatic part of this product seemed to be a pyridinium ring, in which Ck and Cl are substituted. The coupling constant for the vinyl proton (Ht, 6.66 ppm) was J = 16.2 Hz for the trans-proton. In the HMBC spectrum, the cross-peaks between the Ht and carbons (Ht-Ck, Ht-Cl, and Ht-Cm) and cross-peaks between the Hn and carbons (Hn-Cj, Hn-Ck, and Hn-Cl) were observed. These results indicated that the substituted quaternary carbons, Ck and Cl, were adjacent to Cn and Ct, respectively. The residual parts of P-2 were the (E)-1-heptenyl and hexyl groups. The signals at 0.89–0.94 ppm were assigned to Hs and Hz. The correlation spectroscopy experiments were performed to determine the alkyl chain connectivity. With the correlation spectroscopy spectrum, starting from the methyl groups, the spin systems for an (E)-1-heptenyl chain and a hexyl chain were determined. After establishing the connectivities for the alkyl chains, the HMBC experiments were performed. Based on these characteristics, it was determined that P-2 was the novel 2-nonenal-lysine adduct, Nα-acetyl-Nϵ-3-[(hept-1-enyl)-4-hexylpyridinium]lysine (Nα-acetyl-HHP-lysine) (Fig. 2B). The LC/MS total ion current analysis of P-1 detected one product, which showed a 242-Da increase in the mass value of the unmodified lysine derivative and gave the [M + H]+ peak at m/z 430.9 (supplemental Fig. S11), suggesting that P-1 is a cis isomer of Nα-acetyl-HHP-lysine. To confirm the structure of P-1, the NMR experiments were performed (supplemental Figs. S12–S17) and showed that the coupling constant for the vinyl proton (6.51 ppm) was J = 11.6 Hz for the cis proton. Selected ion-current chromatograms obtained from the LC/MS analysis showed that, when 200 mm Nα-acetyllysine was incubated with 200 mm 2-nonenal for 72 h, P-1 and P-2 were formed in the ratio of 1:1.4, indicating that the cis isomer of Nα-acetyl-HHP-lysine can be formed from the trans-2-nonenal-mediated reaction.
As shown in Fig. 2C, the antibody showed a specificity toward both P-1 and P-2 but was rather specific to P-2. Approximately 50 pmol of P-2/well (100 μl) caused a 50% inhibition of the antibody binding to the 2-nonenal-modified protein, whereas at least 100-fold higher concentrations of P-1 were necessary for the same inhibition.
Formation of Immunoreactive Materials with mAb 27Q4 in Vivo
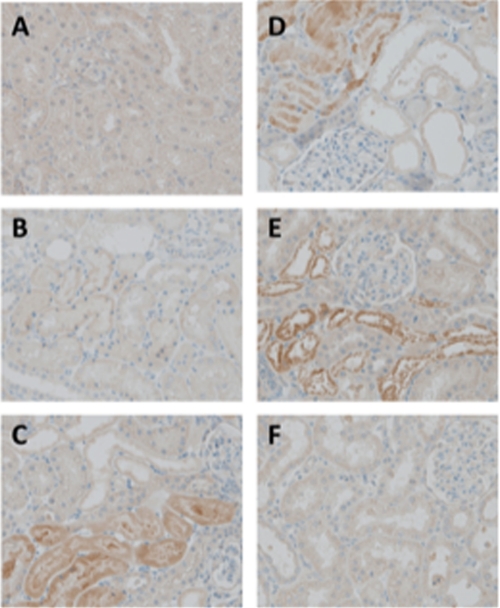
We next sought to explore whether immunoreactive materials with mAb 27Q4 are formed in vivo. For this purpose, we used Fe3+-NTA-treated rats, an established animal model of oxidant injury to the kidney. It has been established that Fe3+-NTA induces acute renal proximal tubular necrosis, a consequence of oxidative tissue damage, that eventually leads to a high incidence of renal adenocarcinoma in rodents (11). Previously, we have shown that the levels of 2-nonenal markedly increase after the administration of Fe3+-NTA (5). In the control rat kidney (Fig. 3A), an almost negligible level of immunoreactivity was observed. The immunoreactivities were found in some of the renal proximal tubular cells 1, 6, 24, and 48 h (Fig. 3, B–E) after the administration of 15 mg of iron/kg of body weight of Fe3+-NTA. No immunoreactivity was observed after the repeated (3 weeks) administrations of Fe3+-NTA (Fig. 3F). These patterns of distribution in the rat kidney are consistent with those of the distribution of membrane lipid peroxidation products and their conjugates with cytosolic proteins, suggesting a correlation between the production of the 2-nonenal/2-alkenals and oxidative stress. Pre-absorption of the antibody with HHP-lysine completely abolished the immunostaining (data not shown).
FIGURE 3.
Immunohistochemistry of renal cortex with mAb 27Q4 (×400). A, untreated control; B, 1 h; C, 6 h; D, 24 h; E, 48 h after single intraperitoneal injection of Fe3+-NTA; F, 3 weeks after repeated intraperitoneal injection of Fe3+-NTA. Refer to “Experimental Procedures” for details.
LC/ESI/MS/MS Analysis of HHP-lysine
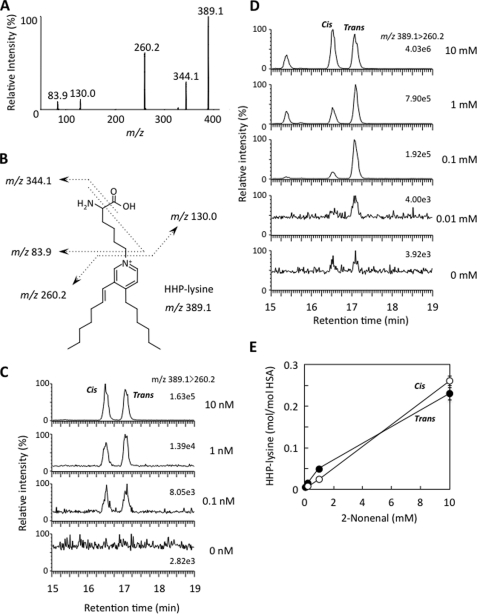
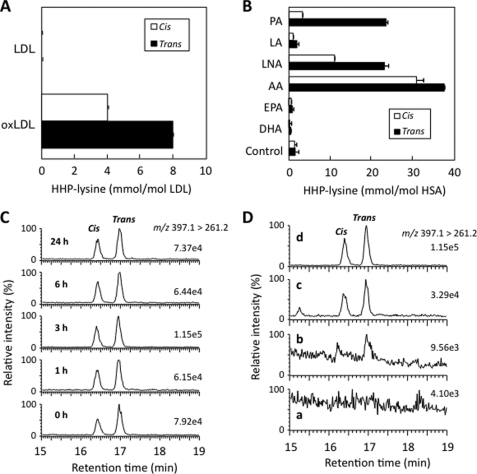
To obtain direct evidence for the formation of the antigenic HHP-lysine adducts in vivo, we first attempted to establish a method for the detection of adducts using LC/ESI/MS/MS. The acid hydrolysis of Nα-acetyl-HHP-lysine gave a single product, which was identified to be the expected de-acetylated product (HHP-lysine) (supplemental Figs. S18 and S19). Collision-induced dissociation of the HHP-lysine produced relevant daughter ions at m/z 344, 260, 130, and 84 (Fig. 4A). These ions were applied to the structure shown in Fig. 4B. The product ions at m/z 84 and 130 were confirmed to be the product ions of a lysine moiety, and the ions at m/z 344 and 389 originated from the HHP-lysine. Fig. 4C demonstrates the LC/ESI/MS/MS analysis of the HHP-lysine (0–10 nm) in the positive ion mode using MRM between the transition from the protonated parent ion [M + H]+ to the characteristic daughter ion (m/z 389.6 → 260.2), allowing detection of both the cis- and trans-HHP-lysines. Chromatograms for the internal standard are shown in supplemental Fig. S20. Using the LC/ESI/MS/MS technique, we attempted to detect HHP-lysine in the 2-nonenal-modified HSA. No adducts were detected in the native HSA, whereas the treatment of HSA with 0–10 mm 2-nonenal in 50 mm sodium phosphate buffer, pH 7.2, for 24 h at 37 °C gave 0.61 and 0.86 mol of the cis- and trans-HHP-lysines, respectively, per mol of protein (Fig. 4, D and E).
FIGURE 4.
LC/ESI/MS/MS analysis of HHP-lysine. A, collision-induced dissociation of the [M + H]+ of trans-HHP-lysine at m/z 389.5 at a collision energy of 25 V. B, proposed structures of individual ions. C, LC/ESI/MS/MS analysis of authentic cis- and trans-HHP-lysines. The ion current tracing of HHP-lysine using LC/ESI/MS/MS with MRM is shown. D, LC/ESI/MS/MS analysis of cis- and trans-HHP-lysines generated in the 2-nonenal-modified HSA. The ion current tracing of cis- and trans-HHP-lysines using LC/ESI/MS/MS with MRM is shown. E, dose-dependent formation of cis- and trans-HHP-lysines in the 2-nonenal-modified HSA. Symbols used are as follows: open circle, cis-HHP-lysine; closed circle, trans-HHP-lysine. D and E, HSA (1.0 mg/ml) was incubated with 2-nonenal (0–10 mm) in 50 mm sodium phosphate buffer, pH 7.2, for 24 h at 37 °C. The native and modified HSA were analyzed by LC/ESI/MS/MS with MRM mode followed by acid hydrolysis.
In Vivo Formation of HHP-lysine via Lipid Peroxidation
It is known that 2-nonenal is generated by the lipid peroxidation of unsaturated fatty acids, such as palmitoleic acid (6). To examine the involvement of the lipid peroxidation during the formation of HHP-lysine, we sought to detect HHP-lysine in the Cu2+-oxidized LDL using LC/ESI/MS/MS. Incubation of LDL with Cu2+ led to the oxidation of the LDL as assessed by the formation of thiobarbituric acid-reactive substances (data not shown). As shown in Fig. 5A, the Cu2+-induced peroxidation of LDL dramatically enhanced the formation of HHP-lysine with the levels of the trans-HHP-lysine exceeding those of the cis-HHP-lysine by 2-fold.
FIGURE 5.
In vivo formation of HHP-lysine via lipid peroxidation. A, LC/ESI/MS/MS analysis of cis- and trans-HHP-lysines in oxidized LDL (oxLDL). LDL (0.5 mg) was incubated with 5 μm Cu2+ in 1 ml of PBS at 37 °C. The native LDL and oxidized LDL were analyzed by LC/ESI/MS/MS with MRM mode followed by acid hydrolysis. B, LC/ESI/MS/MS analysis of cis- and trans-HHP-lysines in protein exposed to lipid peroxidation. The metal-catalyzed oxidation of unsaturated fatty acids in the presence of HSA was performed by incubating HSA (1 mg/ml) with 2 mm unsaturated fatty acids in the presence of 50 μm Fe2+ and 1 mm ascorbic acid in 1 ml of 50 mm sodium phosphate buffer, pH 7.2, in atmospheric oxygen at 37 °C. The native and modified HSA were analyzed by LC/ESI/MS/MS with MRM mode followed by acid hydrolysis. The abbreviations used are as follows: PA, palmitoleic acid; LA, linoleic acid; LNA, γ-linolenic acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid. C, LC/ESI/MS/MS analysis of cis-[U-13C6,15N2]- and trans-[U-13C6,15N2]HHP-lysines in the kidneys of mice exposed to Fe3+-NTA. The ion current tracing of cis-[U-13C6,15N2]- and trans-[U-13C6,15N2]HHP-lysines LC/ESI/MS/MS with MRM is shown. D, LC/ESI/MS/MS analysis of cis- and trans-HHP-lysines in the kidneys of mice exposed to Fe3+-NTA. The ion current tracing of HHP-lysine using LC/ESI/MS/MS with MRM is shown. Panel a, 0 h after administration; panel b, 1 h after administration; panel c, 3 h after administration; panel d, internal standards.
To further examine which fatty acids are involved in the formation of the HHP-lysine, several unsaturated fatty acids were incubated with an iron/ascorbate-mediated free radical generating system in the presence of HSA, and HHP-lysine generated on the protein molecule was analyzed by LC/ESI/MS/MS following acid hydrolysis. As shown in Fig. 5B, the iron/ascorbate-mediated oxidation of palmitoleic acid and ω6-polyunsaturated fatty acids, such as linoleic acid, γ-linolenic acid, and arachidonic acid, in the presence of HSA resulted in a dramatic increase in the levels of HHP-lysine with levels of trans-HHP-lysine exceeding those of cis-HHP-lysine by 3–9-fold.
We then sought to explore whether HHP-lysine is formed in the kidney of animals exposed to Fe3+-NTA in vivo. Kidneys from the untreated and treated animals collected 1 and 3 h after the administration of Fe3+-NTA were analyzed for HHP-lysine using LC/ESI/MS/MS. The MRM transitions monitored were as follows: [U-13C6,15N2]HHP-lysine, m/z 397 → 261; cis- and trans-HHP-lysine, m/z 389 → 260. Multiple distinct MRM channels (parent 389.1 → 344.1, 260.2, 130.0, and 83.9) showed that each of the appropriate transitions showed co-chromatography (supplemental Figs. S21 and S22). In addition, the ratio of the different parent → daughter transitions was also checked (Fig. 5C). As shown in Fig. 5D, the levels of both the cis- and trans-HHP-lysines markedly increased 3 h after the administration of Fe3+-NTA. The yields of the cis- and trans-HHP-lysines at 3 h were 366.5 and 357.8 fmol/g wet tissue weight, respectively.
Recognition of the 2-Nonenal-Lysine Adduct by LOX-1
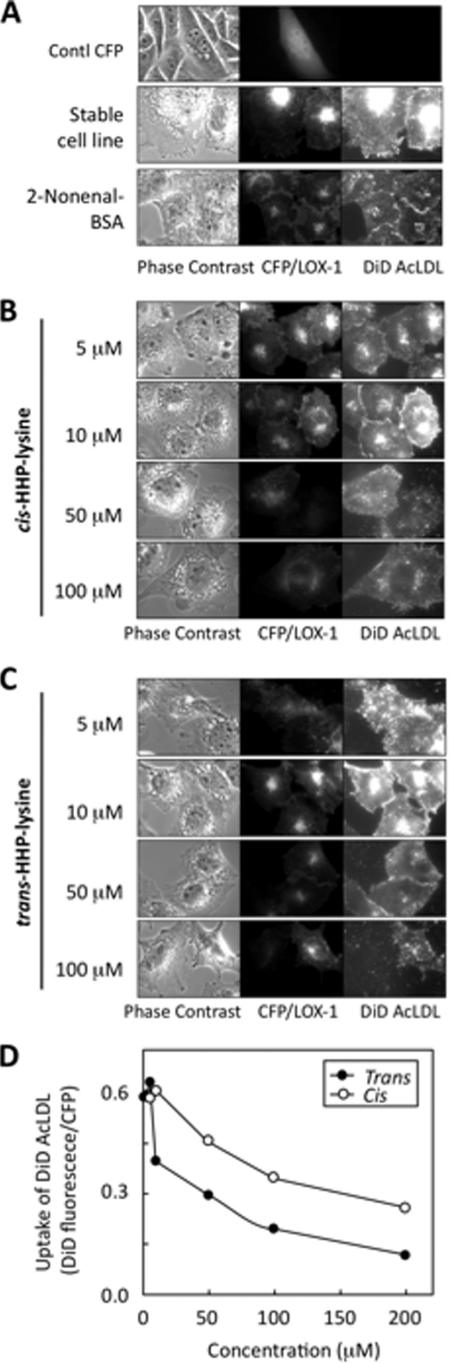
Finally, we evaluated the biological implication of the 2-nonenal modification of protein. To this end, based on the previous findings that a scavenger receptor LOX-1 might be involved in the processing of aldehyde-modified proteins (19) and that, among the aldehyde-treated proteins, the 2-nonenal-modified protein can be very efficiently incorporated into the LOX-1-overexpressing cells,3 we sought to examine if LOX-1 recognizes the 2-nonenal-lysine adduct. AcLDL was used as an alternative ligand to the oxidized LDL, which shows a comparable affinity to LOX-1 (20) to avoid ambiguous effects from variations in the extent of the oxidized LDL oxidation (21, 22). In this assay, the amount of incorporated DiD-labeled AcLDL directly reflected the binding activity of LOX-1, which was confirmed by an independent analysis as described previously (23). The rate of DiD-AcLDL taken up by CHO stably expressing CFP/LOX-1 was assessed by a fluorescence microscope equipped with a cooled CCD camera. In CHO cells transfected with pECFP-N1, the CFP signal was detected in the cytoplasm, and no DiD-AcLDL uptake was detected (Fig. 6A, top). The stable cell line expressed a moderate amount of LOX-1, and a fluorescence derived from DiD-AcLDL was observed (Fig. 6A, middle). The effect of the 2-nonenal-modified BSA upon DiD-AcLDL uptake through LOX-1 was examined in the competition assay to evaluate the LOX-1 recognition of modified proteins. The native BSA failed to show any inhibitory effects (date not shown), whereas the protein modified with 2-nonenal significantly inhibited the uptake of AcLDL (Fig. 6A, bottom). Finally, we evaluated the purified cis- and trans-HHP-lysines for their recognition by LOX-1 in the competition assay, and we observed that they served as a ligand for LOX-1, as evidenced by their ability to effectively compete with AcLDL (Fig. 6, B–D). There was no effect by the 1% DMSO carried from the stock solution of the HHP-lysine (data not shown).
FIGURE 6.
Effect of the 2-nonenal-lysine adducts on ligand uptake by LOX-1. A, CHO cells transfected with vector carrying only CFP (top). LOX-1 expression level and AcLDL uptake ability of stable cell line (middle) is shown. Effect of 2-nonenal-modified BSA (4 μg/ml) on AcLDL uptake (bottom) is shown. B, effect of cis-HHP-lysine (10–100 μm) on AcLDL uptake. C, effect of trans-HHP-lysine (10–100 μm) on AcLDL uptake. Images were acquired using ×100 objective. Panels are as follows: left, phase contrast; middle, CFP/LOX-1 (excitation at 436 nm; exposure time, 0.2 s); right, DiD-AcLDL (excitation at 620 nm; exposure time, 2 s). D, quantitative measurement of fluorescence intensity of each cell. CHO cells stably expressing CFP/LOX-1 were treated with each concentration of adducts and followed by incubation with DiD-AcLDL. The cell-associated fluorescence intensity derived from CFP and DiD (pixel value) was determined as described under “Experimental Procedures.” The specific uptake was expressed as the fluorescence intensity derived from DiD (pixel value) per the fluorescence intensity derived from CFP (pixel value) of each cell. The values represent the means of over 100 cells from three independent experiments.
DISCUSSION
Lipid peroxidation proceeds by a free radical chain reaction mechanism and yields lipid hydroperoxides as major initial reaction products. Subsequently, the decomposition of the lipid hydroperoxides generates a number of breakdown products that display a wide variety of damaging actions. A number of reactive aldehydes derived from lipid peroxidation have been implicated as causative agents in cytotoxic processes initiated by the exposure of biological systems to oxidizing agents (2). On the basis of a large number of reports concerning the detection of lipid peroxidation-specific adducts as biomarkers in human diseases, there is no doubt that the steady-state levels of lipid peroxidation products increase in pathophysiological states associated with oxidative stress. Considerable progress has also recently been made toward understanding the mechanisms of action of lipid peroxidation products. The quantitative and analytical importance of lipid peroxidation-specific adducts has prompted the development of methods to specifically analyze these adducts to understand their chemical nature, formation pathway, and distribution level in vivo.
The results presented herein show that 2-nonenal, a body odorant originating from lipid peroxidation, covalently modifies proteins. A spontaneous chemical reaction was observed in vitro. In addition, we obtained a murine monoclonal antibody, mAb 27Q4, that clearly distinguished the 2-nonenal-modified protein from the native protein. This antibody appeared to be highly specific for the protein-bound 2-alkenals, including 2-heptenal, 2-octenal, and 2-nonenal. Using the antibody, the formation of the 2-nonenal-modified proteins in vivo was tested in the kidney of rats exposed to Fe3+-NTA. The iron chelate was originally used for an experimental model of iron overload (24). Repeated intraperitoneal injections of Fe3+-NTA were reported to induce acute and subacute renal proximal tubular necrosis and a subsequent high incidence (60–92%) of renal adenocarcinoma in male rats and mice (25, 26). A single injection of Fe3+-NTA causes a number of time-dependent morphological alterations in the structure and the function of the renal proximal tubular cells and their mitochondria. During the early stage of injury, typical cellular changes are the loss of the brush border, cytoplasmic vesicles, mitochondrial disorganization, and dense cytoplasmic deposits in the proximal tubular cells. Most of the damaged epithelia show the typical appearance of necrotic cells, and more than half of the proximal tubular cells are gone. It has been suggested that oxidative stress is one of the basic mechanisms of Fe3+-NTA-induced acute renal injury and is closely associated with renal carcinogenesis (27, 28). The present in vivo study has shown that lipid peroxidation generates 2-nonenal covalently bound to proteins in the renal proximal tubules of rats treated with Fe3+-NTA (Fig. 3). To the best of our knowledge, this is the first report of the in vivo formation of protein-bound 2-nonenal/2-alkenals in the target organ of the carcinogenic protocol. It was also striking that the immunoreactivities with mAb 27Q4 were detected even 48 h after the administration of Fe3+-NTA (Fig. 3E). Long retention of this aldehyde may play a role in the Fe3+-NTA-induced renal carcinogenesis.
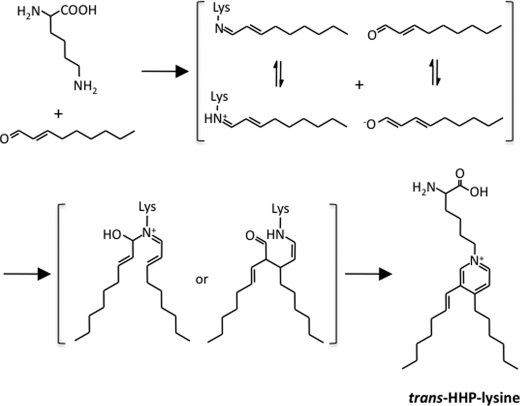
Upon investigation of an antigenic adduct recognized by the antibody (mAb 27Q4), we unexpectedly identified novel lysine-pyridinium adducts, cis- and trans-HHP-lysines. Of interest, the monoclonal antibodies raised against protein-bound 2- alkenals, such as acrolein and crotonaldehyde, recognize pyridinium-containing adducts as the major epitopes (29, 30). It is likely that, due to the placement of a fixed, positive charge on the ϵ-amino group, the pyridinium-containing adducts could be an important immunological epitope generated in 2-alkenal-modified proteins. The formation of the 3,4-substituted pyridinium adducts has also been reported to be a dominant pathway for modification of the primary amine with 2-alkenals, such as 2-hexenal and 2-octenal (31, 32). Based on these studies, HHP-lysine is likely to be formed through the formation of the 2-nonenal-lysine Schiff base (Fig. 7). After the formation of the Schiff base adduct, the C3 position of the initial Schiff base may be attacked by the C2 of the enolate anion from the second aldehyde, followed by dehydration and cyclization to form the isomeric pyridinium adduct. On the other hand, trans-cis isomerization of α,β-unsaturated compounds is known to be catalyzed by amines (addition-elimination). Thus, the isomerization required for the formation of cis-HHP-lysine may occur at the stage of the free 2-nonenal or following Schiff base formation.
FIGURE 7.
Proposed mechanism for the formation of HHP-lysine.
Due to the fact that the core structures of the pyridinium-containing lysine adducts are resistant to the conventional acid hydrolysis of proteins, we established a highly sensitive method for the detection of cis- and trans-HHP-lysine using LC/ESI/MS/MS. In vitro studies of the detection of HHP-lysine demonstrated that the 2-nonenal-lysine adducts were generated in the oxidized LDL (Fig. 5A). In addition, substantial amounts of the 2-nonenal-lysine adducts, mainly the trans-HHP-lysine, were detected in the metal-catalyzed peroxidation of unsaturated fatty acids in the presence of protein (Fig. 5B). These data suggest that covalent modification of proteins by 2-nonenal, generating the pyridinium-containing lysine adducts, could have diagnostic applications. Because oxidative degradation of unsaturated fatty acids, accelerated by lipid peroxides, may be involved in the formation of 2-nonenal, resulting in deterioration of the body odors for the middle-aged and the elderly (6), plasma or red blood cell levels of a long-lived 2-nonenal adduct could provide a measure of past exposure and aid in the management of one's health condition. Such a reporter function would be analogous to the use of glycated hemoglobin as a marker of past hyperglycemia and a guide to the management of diabetes.
It should also be noted that peroxidation of not only palmitoleic acid, but also ω6-polyunsaturated fatty acids, such as linoleic acid, γ-linolenic acid, and arachidonic acid, in the presence of HSA generated HHP-lysine. 2-Nonenal was previously identified as an unpleasant greasy and grassy odor component endogenously generated during the peroxidation of palmitoleic acid (6). However, until this study, bona fide unsaturated fatty acids responsible for the formation of 2-nonenal have remained unidentified. Therefore, this study has established that ω6-polyunsaturated fatty acids also represent an excellent source of 2-nonenal. Although the mechanism of the formation of 2-nonenal during lipid peroxidation has not yet been experimentally resolved, there may be no doubt that 2-nonenal could be ubiquitously generated under oxidative stress.
Scavenger receptors have been shown to bind aldehyde-modified proteins (33–37). These receptors are thought to provide a mechanism for the clearance of these modified proteins from the circulation through a number of cell types. Indeed, a previous study has shown that endothelial cells can bind and degrade an aldehyde-modified protein (38). In this study, to determine whether the 2-nonenal-specific epitopes enter cells though scavenger receptors, we selected LOX-1 and performed inhibition studies. We showed that BSA incubated with 2-nonenal significantly inhibited the LOX-1-mediated uptake of AcLDL (Figs. 6 and 7). In addition, we directly assessed whether HHP-lysine might serve as a ligand and observed that both the cis- and trans-HHP-lysines competed for the uptake of AcLDL. These data suggested that LOX-1 might serve as a receptor for the 2-nonenal-lysine adducts generated on oxidized LDL molecules. Data from the mesenteric vein injection of an aldehyde-modified protein also suggest that LOX-1 may be involved to a certain degree in these processes (38). Thus, it is likely that LOX-1 is involved in the recognition of 2-nonenal-modified proteins. However, the possibility still exists for other receptors to be involved in the processing of 2-nonenal-modified proteins.
In summary, to assess the formation of 2-nonenal generation under oxidative stress in vivo, we raised a new murine monoclonal antibody, mAb 27Q4, against the 2-nonenal-modified KLH. The model system provides a detailed structural characterization of the epitopes, the cis- and trans-HHP-lysines, formed during the reaction of the lysine side-chain amino groups with 2-nonenal. We proved that the immunoreactive materials with mAb 27Q4 were indeed generated in an animal model of oxidative stress in vivo. In addition, using LC/ESI/MS/MS, the biological levels of the 2-nonenal-lysine adduct in vitro and in vivo were accurately estimated. Finally, we examined the involvement of the scavenger receptor LOX-1 in the recognition of 2-nonenal-modified proteins and established that the receptor recognized the HHP-lysine adducts as a ligand. The present results not only offer structural insights into protein modification by lipid peroxidation products but also provide a platform for the chemical analysis of protein-bound aldehydes in vitro and in vivo.
This work was supported by a grant-in-aid for scientific research on innovative areas (research in a proposed research area), from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to K. U.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S22.
S. Machida and K. Uchida, manuscript in preparation.
- Fe3+-NTA
- ferric nitrilotriacetate
- AcLDL
- acetylated LDL
- BSA
- bovine serum albumin
- DiD
- 1,1′-dioctadecyl-3, 3,3′,3′-tetramethylindodicarbocyanine perchlorate
- ELISA
- enzyme-linked immunosorbent assay
- HHP-lysine
- Nϵ-3-[(hept-1-enyl)-4-hexylpyridinium]lysine
- HMBC
- 1H-detected multiple-bond connectivity
- KLH
- keyhole limpet hemocyanin
- LDL
- low density lipoprotein
- LOX-1
- lectin-like oxidized LDL receptor
- HSA
- human serum albumin
- LC/ESI/MS/MS
- high performance liquid chromatography with on-line electrospray ionization tandem mass spectrometry
- mAb
- monoclonal antibody
- MRM
- multiple reaction monitoring
- PBS
- phosphate-buffered saline
- HPLC
- high pressure liquid chromatography
- CFP
- cyan fluorescent protein
- CHO
- Chinese hamster ovary
- Fmoc
- N-(9-fluorenyl)methoxycarbonyl
- HSA
- human serum albumin.
REFERENCES
- 1.Halliwell B., Gutteridge J. M. (1989) Free Radicals in Biology and Medicine, 2nd Ed., Clarendon Press, Oxford [Google Scholar]
- 2.Esterbauer H., Schaur R. J., Zollner H. (1991) Free Radic. Biol. Med. 11, 81–128 [DOI] [PubMed] [Google Scholar]
- 3.Uchida K. (2000) Free Radic. Biol. Med. 28, 1685–1696 [DOI] [PubMed] [Google Scholar]
- 4.Marnett L. J., Riggins J. N., West J. D. (2003) J. Clin. Invest. 111, 583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toyokuni S., Luo X. P., Tanaka T., Uchida K., Hiai H., Lehotay D. C. (1997) Free Radic. Biol. Med. 22, 1019–1027 [DOI] [PubMed] [Google Scholar]
- 6.Haze S., Gozu Y., Nakamura S., Kohno Y., Sawano K., Ohta H., Yamazaki K. (2001) J. Invest. Dermatol. 116, 520–524 [DOI] [PubMed] [Google Scholar]
- 7.Hernández-Hernández A., Sánchez-Yagüe J., Martín-Valmaseda E. M., Llanillo M. (1999) Free Radic. Biol. Med. 26, 1218–1230 [DOI] [PubMed] [Google Scholar]
- 8.Ohyashiki T., Kamata K., Takeuchi M., Matsui K. (1995) J. Biochem. 118, 508–514 [DOI] [PubMed] [Google Scholar]
- 9.Sawamura T., Kume N., Aoyama T., Moriwaki H., Hoshikawa H., Aiba Y., Tanaka T., Miwa S., Katsura Y., Kita T., Masaki T. (1997) Nature 386, 73–77 [DOI] [PubMed] [Google Scholar]
- 10.Chen X. P., Zhang T. T., Du G. H. (2007) Cardiovasc. Drug Rev. 25, 146–161 [DOI] [PubMed] [Google Scholar]
- 11.Toyokuni S., Uchida K., Okamoto K., Hattori-Nakakuki Y., Hiai H., Stadtman E. R. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 2616–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Montarby L., Mosset P., Gree R. (1988) Tetrahedron Lett. 29, 3937–3940 [Google Scholar]
- 13.Rindgen D., Nakajima M., Wehrli S., Xu K., Blair I. A. (1999) Chem. Res. Toxicol. 12, 1195–1204 [DOI] [PubMed] [Google Scholar]
- 14.Marnett L. J., Tuttle M. A. (1980) Cancer Res. 40, 276–282 [PubMed] [Google Scholar]
- 15.Toyoda K., Nagae R., Akagawa M., Ishino K., Shibata T., Ito S., Shibata N., Yamamoto T., Kobayashi M., Takasaki Y., Matsuda T., Uchida K. (2007) J. Biol. Chem. 282, 25769–25778 [DOI] [PubMed] [Google Scholar]
- 16.Habeeb A. F. (1966) Anal. Biochem. 14, 328–336 [DOI] [PubMed] [Google Scholar]
- 17.Stephan Z. F., Yurachek E. C. (1993) J. Lipid Res. 34, 325–330 [PubMed] [Google Scholar]
- 18.Matsunaga S., Xie Q., Kumano M., Niimi S., Sekizawa K., Sakakibara Y., Komba S., Machida S. (2007) Exp. Cell Res. 313, 1203–1214 [DOI] [PubMed] [Google Scholar]
- 19.Duryee M. J., Klassen L. W., Freeman T. L., Willis M. S., Tuma D. J., Thiele G. M. (2003) Biochem. Pharmacol. 66, 1045–1054 [DOI] [PubMed] [Google Scholar]
- 20.Shi X., Niimi S., Ohtani T., Machida S. (2001) J. Cell Sci. 114, 1273–1282 [DOI] [PubMed] [Google Scholar]
- 21.Lougheed M., Steinbrecher U. P. (1996) J. Biol. Chem. 271, 11798–11805 [DOI] [PubMed] [Google Scholar]
- 22.Yoshida H., Kondratenko N., Green S., Steinberg D., Quehenberger O. (1998) Biochem. J. 334, 9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen M., Nagase M., Fujita T., Narumiya S., Masaki T., Sawamura T. (2001) Biochem. Biophys. Res. Commun. 287, 962–968 [DOI] [PubMed] [Google Scholar]
- 24.Awai M., Narasaki M., Yamanoi Y., Seno S. (1979) Am. J. Pathol. 95, 663–673 [PMC free article] [PubMed] [Google Scholar]
- 25.Ebina Y., Okada S., Hamazaki S., Ogino F., Li J. L., Midorikawa O. (1986) J. Natl. Cancer Inst. 76, 107–113 [PubMed] [Google Scholar]
- 26.Li J. L., Okada S., Hamazaki S., Ebina Y., Midorikawa O. (1987) Cancer Res. 47, 1867–1869 [PubMed] [Google Scholar]
- 27.Toyokuni S. (1996) Free Radic. Biol. Med. 20, 553–566 [DOI] [PubMed] [Google Scholar]
- 28.Toyokuni S. (2009) Cancer Sci. 100, 9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furuhata A., Ishii T., Kumazawa S., Yamada T., Nakayama T., Uchida K. (2003) J. Biol. Chem. 278, 48658–48665 [DOI] [PubMed] [Google Scholar]
- 30.Ichihashi K., Osawa T., Toyokuni S., Uchida K. (2001) J. Biol. Chem. 276, 23903–23913 [DOI] [PubMed] [Google Scholar]
- 31.Baker A., Zídek L., Wiesler D., Chmelík J., Pagel M., Novotny M. V. (1998) Chem. Res. Toxicol. 11, 730–740 [DOI] [PubMed] [Google Scholar]
- 32.Alaiz M., Barragan S. (1995) Chem. Phys. Lipids 77, 217–223 [DOI] [PubMed] [Google Scholar]
- 33.Brown M. S., Basu S. K., Falck J. R., Ho Y. K., Goldstein J. L. (1980) J. Supramol. Struct. 13, 67–81 [DOI] [PubMed] [Google Scholar]
- 34.Horiuchi S., Murakami M., Takata K., Morino Y. (1986) J. Biol. Chem. 261, 4962–4966 [PubMed] [Google Scholar]
- 35.Takata K., Horiuchi S., Araki N., Shiga M., Saitoh M., Morino Y. (1988) J. Biol. Chem. 263, 14819–14825 [PubMed] [Google Scholar]
- 36.Takata K., Horiuchi S., Araki N., Shiga M., Saitoh M., Morino Y. (1989) Biochim. Biophys. Acta 986, 18–26 [DOI] [PubMed] [Google Scholar]
- 37.Steinbrecher U. P., Lougheed M., Kwan W. C., Dirks M. (1989) J. Biol. Chem. 264, 15216–15223 [PubMed] [Google Scholar]
- 38.Duryee M. J., Freeman T. L., Willis M. S., Hunter C. D., Hamilton B. C., 3rd, Suzuki H., Tuma D. J., Klassen L. W., Thiele G. M. (2005) Mol. Pharmacol. 68, 1423–1430 [DOI] [PubMed] [Google Scholar]