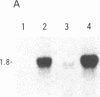
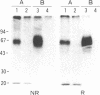
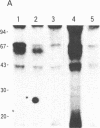
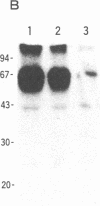
Abstract
Monoclonal antibodies specific for the murine CD2 antigen were identified by an efficient screening method utilizing murine CD2 cDNA transfectants. An unexpected expression of CD2 on murine B cells was revealed by immunofluorescence and immunoprecipitation studies with these monoclonal antibodies and by RNA blot analysis for the murine CD2 transcript.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcover A., Chang H. C., Sayre P. H., Hussey R. E., Reinherz E. L. The T 11 (CD 2) cDNA encodes a transmembrane protein which expresses T 11(1), T 11(2) and T 11(3) epitopes but which does not independently mediate calcium influx: analysis by gene transfer in a baculovirus system. Eur J Immunol. 1988 Mar;18(3):363–367. doi: 10.1002/eji.1830180307. [DOI] [PubMed] [Google Scholar]
- Alcover A., Ramarli D., Richardson N. E., Chang H. C., Reinherz E. L. Functional and molecular aspects of human T lymphocyte activation via T3-Ti and T11 pathways. Immunol Rev. 1987 Feb;95:5–36. doi: 10.1111/j.1600-065x.1987.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Alcover A., Weiss M. J., Daley J. F., Reinherz E. L. The T11 glycoprotein is functionally linked to a calcium channel in precursor and mature T-lineage cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2614–2618. doi: 10.1073/pnas.83.8.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison J. P., Lanier L. L. Structure, function, and serology of the T-cell antigen receptor complex. Annu Rev Immunol. 1987;5:503–540. doi: 10.1146/annurev.iy.05.040187.002443. [DOI] [PubMed] [Google Scholar]
- Anasetti C., Martin P. J., June C. H., Hellstrom K. E., Ledbetter J. A., Rabinovitch P. S., Morishita Y., Hellstrom I., Hansen J. A. Induction of calcium flux and enhancement of cytolytic activity in natural killer cells by cross-linking of the sheep erythrocyte binding protein (CD2) and the Fc-receptor (CD16). J Immunol. 1987 Sep 15;139(6):1772–1779. [PubMed] [Google Scholar]
- Bierer B. E., Barbosa J., Herrmann S., Burakoff S. J. Interaction of CD2 with its ligand, LFA-3, in human T cell proliferation. J Immunol. 1988 May 15;140(10):3358–3363. [PubMed] [Google Scholar]
- Bierer B. E., Peterson A., Barbosa J., Seed B., Burakoff S. J. Expression of the T-cell surface molecule CD2 and an epitope-loss CD2 mutant to define the role of lymphocyte function-associated antigen 3 (LFA-3) in T-cell activation. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1194–1198. doi: 10.1073/pnas.85.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue M. L., Daley J. F., Levine H., Craig K. A., Schlossman S. F. Activation of immature cortical thymocytes through the T11 sheep erythrocyte binding protein. J Immunol. 1987 May 15;138(10):3108–3113. [PubMed] [Google Scholar]
- Bolhuis R. L., Roozemond R. C., van de Griend R. J. Induction and blocking of cytolysis in CD2+, CD3- NK and CD2+, CD3+ cytotoxic T lymphocytes via CD2 50 KD sheep erythrocyte receptor. J Immunol. 1986 Jun 1;136(11):3939–3944. [PubMed] [Google Scholar]
- Breitmeyer J. B., Daley J. F., Levine H. B., Schlossman S. F. The T11 (CD2) molecule is functionally linked to the T3/Ti T cell receptor in the majority of T cells. J Immunol. 1987 Nov 1;139(9):2899–2905. [PubMed] [Google Scholar]
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- Clayton L. K., Sayre P. H., Novotny J., Reinherz E. L. Murine and human T11 (CD2) cDNA sequences suggest a common signal transduction mechanism. Eur J Immunol. 1987 Sep;17(9):1367–1370. doi: 10.1002/eji.1830170922. [DOI] [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Denning S. M., Tuck D. T., Vollger L. W., Springer T. A., Singer K. H., Haynes B. F. Monoclonal antibodies to CD2 and lymphocyte function-associated antigen 3 inhibit human thymic epithelial cell-dependent mature thymocyte activation. J Immunol. 1987 Oct 15;139(8):2573–2578. [PubMed] [Google Scholar]
- Dustin M. L., Sanders M. E., Shaw S., Springer T. A. Purified lymphocyte function-associated antigen 3 binds to CD2 and mediates T lymphocyte adhesion. J Exp Med. 1987 Mar 1;165(3):677–692. doi: 10.1084/jem.165.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverman J., Hunkapiller T., Hood L. A speculative view of the multicomponent nature of T cell antigen recognition. Cell. 1986 May 23;45(4):475–484. doi: 10.1016/0092-8674(86)90279-5. [DOI] [PubMed] [Google Scholar]
- Howard F. D., Ledbetter J. A., Wong J., Bieber C. P., Stinson E. B., Herzenberg L. A. A human T lymphocyte differentiation marker defined by monoclonal antibodies that block E-rosette formation. J Immunol. 1981 Jun;126(6):2117–2122. [PubMed] [Google Scholar]
- Hünig T., Tiefenthaler G., Meyer zum Büschenfelde K. H., Meuer S. C. Alternative pathway activation of T cells by binding of CD2 to its cell-surface ligand. Nature. 1987 Mar 19;326(6110):298–301. doi: 10.1038/326298a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kamoun M., Martin P. J., Hansen J. A., Brown M. A., Siadak A. W., Nowinski R. C. Identification of a human T lymphocyte surface protein associated with the E-rosette receptor. J Exp Med. 1981 Jan 1;153(1):207–212. doi: 10.1084/jem.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A., Summers J. Rat cell line 3y1 and its virogenic polyoma- and sv40- transformed derivatives. Int J Cancer. 1975 Apr 15;15(4):694–706. doi: 10.1002/ijc.2910150419. [DOI] [PubMed] [Google Scholar]
- Kollias G., Evans D. J., Ritter M., Beech J., Morris R., Grosveld F. Ectopic expression of Thy-1 in the kidneys of transgenic mice induces functional and proliferative abnormalities. Cell. 1987 Oct 9;51(1):21–31. doi: 10.1016/0092-8674(87)90006-7. [DOI] [PubMed] [Google Scholar]
- Kollias G., Spanopoulou E., Grosveld F., Ritter M., Beech J., Morris R. Differential regulation of a Thy-1 gene in transgenic mice. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1492–1496. doi: 10.1073/pnas.84.6.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krensky A. M., Sanchez-Madrid F., Robbins E., Nagy J. A., Springer T. A., Burakoff S. J. The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: cell surface antigens associated with CTL-target interactions. J Immunol. 1983 Aug;131(2):611–616. [PubMed] [Google Scholar]
- Lane R. D. A short-duration polyethylene glycol fusion technique for increasing production of monoclonal antibody-secreting hybridomas. J Immunol Methods. 1985 Aug 2;81(2):223–228. doi: 10.1016/0022-1759(85)90207-8. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Grosmaire L. S., Rabinovitch P. S. Crosslinking of surface antigens causes mobilization of intracellular ionized calcium in T lymphocytes. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1384–1388. doi: 10.1073/pnas.84.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linch D. C., Wallace D. L., O'Flynn K. Signal transduction in human T lymphocytes. Immunol Rev. 1987 Feb;95:137–159. doi: 10.1111/j.1600-065x.1987.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Littman D. R. The structure of the CD4 and CD8 genes. Annu Rev Immunol. 1987;5:561–584. doi: 10.1146/annurev.iy.05.040187.003021. [DOI] [PubMed] [Google Scholar]
- Malter J. S., Reed J. C., Kamoun M. Induction and regulation of CD2 mRNA in human lymphocytes. J Immunol. 1988 May 1;140(9):3233–3236. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Moretta A., Poggi A., Olive D., Bottino C., Fortis C., Pantaleo G., Moretta L. Selection and characterization of T-cell variants lacking molecules involved in T-cell activation (T3 T-cell receptor, T44, and T11): analysis of the functional relationship among different pathways of activation. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1654–1658. doi: 10.1073/pnas.84.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flynn K., Knott L. J., Russul-Saib M., Abdul-Gaffar R., Morgan G., Beverley P. C., Linch D. C. CD2 and CD3 antigens mobilize Ca2+ independently. Eur J Immunol. 1986 May;16(5):580–584. doi: 10.1002/eji.1830160521. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Olive D., Poggi A., Kozumbo W. J., Moretta L., Moretta A. Transmembrane signalling via the T11-dependent pathway of human T cell activation. Evidence for the involvement of 1,2-diacylglycerol and inositol phosphates. Eur J Immunol. 1987 Jan;17(1):55–60. doi: 10.1002/eji.1830170110. [DOI] [PubMed] [Google Scholar]
- Plunkett M. L., Sanders M. E., Selvaraj P., Dustin M. L., Springer T. A. Rosetting of activated human T lymphocytes with autologous erythrocytes. Definition of the receptor and ligand molecules as CD2 and lymphocyte function-associated antigen 3 (LFA-3). J Exp Med. 1987 Mar 1;165(3):664–676. doi: 10.1084/jem.165.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramarli D., Fox D. A., Reinherz E. L. Selective inhibition of interleukin 2 gene function following thymocyte antigen/major histocompatibility complex receptor crosslinking: possible thymic selection mechanism. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8598–8602. doi: 10.1073/pnas.84.23.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reem G. H., Carding S., Reinherz E. L. Lymphokine synthesis is induced in human thymocytes by activation of the CD 2 (T11) pathway. J Immunol. 1987 Jul 1;139(1):130–134. [PubMed] [Google Scholar]
- Sanchez-Madrid F., Simon P., Thompson S., Springer T. A. Mapping of antigenic and functional epitopes on the alpha- and beta-subunits of two related mouse glycoproteins involved in cell interactions, LFA-1 and Mac-1. J Exp Med. 1983 Aug 1;158(2):586–602. doi: 10.1084/jem.158.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre P. H., Chang H. C., Hussey R. E., Brown N. R., Richardson N. E., Spagnoli G., Clayton L. K., Reinherz E. L. Molecular cloning and expression of T11 cDNAs reveal a receptor-like structure on human T lymphocytes. Proc Natl Acad Sci U S A. 1987 May;84(9):2941–2945. doi: 10.1073/pnas.84.9.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman W. E., Eriksson E., Dobrow R., Imboden J. B. Inositol trisphosphate is generated by a rat natural killer cell tumor in response to target cells or to crosslinked monoclonal antibody OX-34: possible signaling role for the OX-34 determinant during activation by target cells. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4239–4243. doi: 10.1073/pnas.84.12.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Seki T., Spurr N., Obata F., Goyert S., Goodfellow P., Silver J. The human Thy-1 gene: structure and chromosomal location. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6657–6661. doi: 10.1073/pnas.82.19.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj P., Plunkett M. L., Dustin M., Sanders M. E., Shaw S., Springer T. A. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. 1987 Mar 26-Apr 1Nature. 326(6111):400–403. doi: 10.1038/326400a0. [DOI] [PubMed] [Google Scholar]
- Sewell W. A., Brown M. H., Dunne J., Owen M. J., Crumpton M. J. Molecular cloning of the human T-lymphocyte surface CD2 (T11) antigen. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8718–8722. doi: 10.1073/pnas.83.22.8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell W. A., Brown M. H., Owen M. J., Fink P. J., Kozak C. A., Crumpton M. J. The murine homologue of the T lymphocyte CD2 antigen: molecular cloning, chromosome assignment and cell surface expression. Eur J Immunol. 1987 Jul;17(7):1015–1020. doi: 10.1002/eji.1830170718. [DOI] [PubMed] [Google Scholar]
- Shaw S., Luce G. E., Quinones R., Gress R. E., Springer T. A., Sanders M. E. Two antigen-independent adhesion pathways used by human cytotoxic T-cell clones. Nature. 1986 Sep 18;323(6085):262–264. doi: 10.1038/323262a0. [DOI] [PubMed] [Google Scholar]
- Shaw S., Luce G. E. The lymphocyte function-associated antigen (LFA)-1 and CD2/LFA-3 pathways of antigen-independent human T cell adhesion. J Immunol. 1987 Aug 15;139(4):1037–1045. [PubMed] [Google Scholar]
- Siliciano R. F., Pratt J. C., Schmidt R. E., Ritz J., Reinherz E. L. Activation of cytolytic T lymphocyte and natural killer cell function through the T11 sheep erythrocyte binding protein. Nature. 1985 Oct 3;317(6036):428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Takai Y., Reed M. L., Burakoff S. J., Herrmann S. H. Direct evidence for a receptor-ligand interaction between the T-cell surface antigen CD2 and lymphocyte-function-associated antigen 3. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6864–6868. doi: 10.1073/pnas.84.19.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiefenthaler G., Hünig T., Dustin M. L., Springer T. A., Meuer S. C. Purified lymphocyte function-associated antigen-3 and T11 target structure are active in CD2-mediated T cell stimulation. Eur J Immunol. 1987 Dec;17(12):1847–1850. doi: 10.1002/eji.1830171227. [DOI] [PubMed] [Google Scholar]
- Toyonaga B., Mak T. W. Genes of the T-cell antigen receptor in normal and malignant T cells. Annu Rev Immunol. 1987;5:585–620. doi: 10.1146/annurev.iy.05.040187.003101. [DOI] [PubMed] [Google Scholar]
- Vollger L. W., Tuck D. T., Springer T. A., Haynes B. F., Singer K. H. Thymocyte binding to human thymic epithelial cells is inhibited by monoclonal antibodies to CD-2 and LFA-3 antigens. J Immunol. 1987 Jan 15;138(2):358–363. [PubMed] [Google Scholar]
- Wallner B. P., Frey A. Z., Tizard R., Mattaliano R. J., Hession C., Sanders M. E., Dustin M. L., Springer T. A. Primary structure of lymphocyte function-associated antigen 3 (LFA-3). The ligand of the T lymphocyte CD2 glycoprotein. J Exp Med. 1987 Oct 1;166(4):923–932. doi: 10.1084/jem.166.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware C. F., Sanchez-Madrid F., Krensky A. M., Burakoff S. J., Strominger J. L., Springer T. A. Human lymphocyte function associated antigen-1 (LFA-1): identification of multiple antigenic epitopes and their relationship to CTL-mediated cytotoxicity. J Immunol. 1983 Sep;131(3):1182–1188. [PubMed] [Google Scholar]
- Yagita H., Masuko T., Hashimoto Y. Inhibition of tumor cell growth in vitro by murine monoclonal antibodies that recognize a proliferation-associated cell surface antigen system in rats and humans. Cancer Res. 1986 Mar;46(3):1478–1484. [PubMed] [Google Scholar]
- Yagita H., Okumura K., Nakauchi H. Molecular cloning of the murine homologue of CD2. Homology of the molecule to its human counterpart T11. J Immunol. 1988 Feb 15;140(4):1321–1326. [PubMed] [Google Scholar]