Abstract
Class IA phosphoinositide 3-kinase (PI3K) p110 catalytic subunits are activated upon Src homology 2 domain-mediated binding of their p85 regulatory subunits to tyrosine-phosphorylated pYXXM motifs in receptor tyrosine kinases (RTKs) or adaptor proteins. The PI3K pathway is activated by phosphate and tensin homolog (PTEN) loss in most prostate cancers (PCa), but the contribution of upstream RTKs that may be targeted therapeutically has not been assessed. Immunoblotting of p85-associated proteins in serum-starved PTEN-deficient LNCaP and C4-2 PCa cells showed a small set of discrete tyrosine-phosphorylated proteins, but these proteins were not recognized by an anti-pYXXM motif antibody and were not found in PTEN-deficient PC3 PCa cells. LC/MS/MS using label-free proteomics and immunoblotting showed that p85 was associated primarily with p110β and p110δ. An interaction with ErbB3 was also detected but was independent of ErbB3 tyrosine phosphorylation and was not required for basal PI3K activity. Basal tyrosine phosphorylation of p110β and p110δ could be blocked by c-Src inhibitors, but this did not suppress PI3K activity, which was similarly independent of Ras. Basal PI3K activity was mediated by p110β in PC3 cells and by both p110β and p110δ in LNCaP cells, whereas p110α was required for PI3K activation in response to RTK stimulation by heregulin-β1. These findings show that basal PI3K activity in PTEN-deficient PCa cells is RTK-independent and can be mediated by p110β and p110δ. Increased p110β expression in PCa may be required for RTK-independent PI3K pathway activation in adult prostate epithelium with genetic or epigenetic PTEN down-regulation.
Keywords: Akt PKB, Phosphatidylinositol Kinase, Phosphotyrosine, PI 3-Kinase, Receptor Tyrosine Kinase, SH2 Domains, PTEN, Prostate Cancer
Introduction
The class I phosphoinositide 3-kinases (PI3Ks)4 produce phosphatidylinositol 3,4,5-trisphosphate, which recruits proteins containing phosphatidylinositol 3,4,5-trisphosphate-binding pleckstrin homology domains to the plasma membrane (1). This class can be further divided into class IA PI3Ks, which consist of a p85 regulatory subunit (p85α, p85β, or p55γ) and a p110 catalytic subunit (p110α, p110β, or p110δ) and class IB PI3Ks that have a p101 regulatory subunit and a p110γ catalytic subunit. The class IA PI3Ks are generally activated by a subset of receptor tyrosine kinases (RTKs), which undergo tyrosine phosphorylation in response to ligand binding. The class IA PI3Ks are then recruited to the membrane by their p85 regulatory subunits, which contain SH2 domains that bind to pYXXM motifs in tyrosine-phosphorylated RTKs or adaptor proteins that are tyrosine-phosphorylated by RTKs (2). In addition to mediating membrane recruitment, p85 inhibits the catalytic activity of the p110 subunit, and inhibition is relieved upon binding to RTKs or adaptor proteins (3). This PI3K pathway is negatively regulated by phosphate and tensin homolog (PTEN), which functions as a phosphatidylinositol 3,4,5-trisphosphate phosphatase whose loss and subsequent activation of PI3K signaling makes a major contribution to many cancers (4–7). Studies of PTEN gene deletion, mutations, and protein expression have shown that PTEN is lost in a large fraction of metastatic prostate cancers (PCa) and in many aggressive primary PCa and that partial loss of PTEN by genetic or epigenetic mechanisms probably contributes to PCa development (8–12). Studies in mice have confirmed that PTEN loss promotes the development of PCa and that PTEN is haploinsufficient because loss of one allele can drive PCa when combined with other genetic lesions (13–15).
Although PTEN loss appears to be the most common overall genetic mechanism for activation of PI3K in tumors, large scale sequencing efforts have identified activating mutations or amplification of p110α as another common mechanism for PI3K pathway activation (16). Significantly, although activating mutations in p110α can clearly contribute to tumorigenesis, recent studies suggest that PTEN-deficient cancers may be dependent on p110β, although this is not a consistent finding, and the molecular basis for this dependence is not known (17–21). A third mechanism that contributes to increased type IA PI3K signaling in tumors is overexpression or constitutive activation of certain RTKs. Indeed, PI3K activation by mutant EGFR in non-small cell lung cancer and by amplified ErbB2 in breast cancer are major mechanisms by which these RTKs drive tumor growth, and drugs that target these RTKs can suppress PI3K signaling and tumor growth (22, 23).
Therapeutic approaches to block PI3K in advanced PCa have focused on development of direct inhibitors of PI3K or its downstream effectors, in particular Akt. However, there has been little focus on blocking PI3K by targeting upstream RTKs because it has not been clear whether there is a requirement for p85 binding to activated RTKs or adaptor proteins in PTEN-deficient PCa cells. Significantly, a recent study in PTEN-deficient glioblastoma multiforme (GBM) showed that multiple RTKs were activated and required for p85 recruitment and PI3K activation and that PI3K activity could be abrogated through the simultaneous use of multiple RTK inhibitors (24). In this study, we examined whether particular RTKs are functioning as upstream activators of PI3K in PTEN-deficient PCa cells and are thereby potential therapeutic targets. The results demonstrate that basal PI3K activity in these cells is RTK-independent and mediated by p110β and p110δ, whereas PI3K activation in response to RTK stimulation is mediated by p110α.
EXPERIMENTAL PROCEDURES
Antibodies, Reagents, and Cells
Rabbit anti-pPRAS40 (Thr246), anti-PI3K p85-conjugated agarose beads, mouse anti-Tyr(P) (4G10), and 4G10-conjugated agarose beads were from Upstate Biotechnology (Lake Placid, NY). Unconjugated rabbit anti-PI3K p85 polyclonal antibody was kindly provided by Dr. L. Cantley (Beth Israel Deaconess Medical Center, Boston, MA). Mouse anti-ErbB3 (2B5) was from Lab Vision (Fremont, CA). Rabbit polyclonal antibodies against EGF receptor (EGFR), ErbB2/Her2, Akt, pAkt (Ser473), pAkt (Thr308), ERK1/2, phospho-ERK1/2 (Thr202/Tyr204), Ras, and p85 binding motif (pYXXM) were from Cell Signaling (Danvers, MA). Mouse anti-PI3K p110α was from BD Biosciences (San Diego, CA). Rabbit anti-PI3K p110β and p110δ were from Abcam (Cambridge, MA) and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), respectively. Mouse anti-β-tubulin was from Chemicon (Temecula, CA). Rabbit IgG was from Vector Laboratories (Burlingame, CA). Normal mouse serum, protein A, and protein G beads were purchased from Pierce. Horseradish peroxidase-conjugated mouse and rabbit IgG were from Promega (Madison, WI).
PI3K inhibitor LY294002 was from Sigma. Ras inhibitor FTI-277 and Src inhibitor PP2 were obtained from Calbiochem, and another Src inhibitor, dasatinib, was kindly provided by Dr. N. Gray (Dana-Farber Cancer Institute, Boston, MA). Recombinant human heregulin-β1 and EGF were from R & D Systems (Minneapolis, MN). Protease inhibitor mixture tablets (EDTA-free) were from Roche Applied Science. ErbB3 siRNA and control siRNAs were from Dharmacon RNA Technologies (Lafayette, CO). The PTEN-deficient LNCaP, C4-2, and PC3 human prostate cancer cell lines were from ATCC and maintained in RPMI 1640 with 10% fetal bovine serum (FBS).
Immunoprecipitation and Immunoblotting
For anti-p85 immunoprecipitations, LNCaP or C4-2 cells grown in 10-cm plates were serum-starved for 3 days. They were then washed twice with cold 50 mm Tris-HCl buffer (pH 7.5) containing 150 mm NaCl (TBS) and lysed with TBS containing 1% Triton X-100, 1 mm Na3VO4, and a mixture of protease inhibitors. Cell lysates were sonicated for 10 s and centrifuged at 13,000 rpm, 4 °C for 15 min to remove cell debris. Cell lysates were precleared with rabbit IgG (preconjugated to protein A beads) at 4 °C for 45 min with continuous agitation. Cell lysates (1 mg) were mixed with 5 μg of p85 antibody in a final volume of 1 ml and incubated at 4 °C for 2 h with continuous agitation (5 μg of rabbit IgG was used as control). At the end of incubation, 20 μl of protein A beads were added, and samples were incubated at 4 °C for another 2 h with continuous agitation. The samples were then transferred to MicroSpin columns (Amersham Biosciences) with the bottoms deplugged. The beads were washed 6 times (600 μl each) in lysis buffer, followed by twice in TBS. The columns were spun at 1000 rpm for 30 s to remove extra buffer, and the column bottoms were then plugged. The immune complexes were eluted with 10 μl of Laemmli buffer without β-mecaptoethanol at 65 °C for 15 min and spun down at 2,000 rpm for 30 s. The flow-through was mixed with 1 μl of β-mecaptoethanol and boiled for 5 min. Proteins were resolved on 4–12% NuPAGE gels (Invitrogen), followed by membrane transfer and immunoblotting. Briefly, the membranes were blocked with 5% milk in TBS containing 0.2% Tween 20 (TBS/T) at room temperature for 2 h and incubated with primary antibodies at 4 °C overnight. After being washed with TBS/T, the membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h, washed, and developed with enhanced chemiluminescence reagents (PerkinElmer Life Sciences).
Immunoprecipitations with p85 antibody-conjugated agarose beads were carried out similarly. Briefly, 2 mg of cell lysates were mixed with 20 μl of p85 antibody conjugated agarose beads and incubated at 4 °C with continuous agitation overnight. Bead washing, elution, and immunoblotting were performed as described above. Immunoprecipitation with 4G10-conjugated beads was also performed as described above, except that serum-starved LNCaP or PC3 cells were lysed in radioimmune precipitation buffer (50 mm Tris-HCl, pH 8.0 containing 150 mm NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS) with 1 mm EDTA, 1 mm EGTA, 1 mm β-glycerophosphate, 1 mm pyrophosphate, 100 mm sodium fluoride, 1 mm Na3VO4, and a mixture of protease inhibitors.
For sequential immunoprecipitations with ErbB3 and p85 antibodies, LNCaP cells were lysed with TBS containing 1% Triton X-100 and a mixture of protease inhibitors. Cell lysates (1 mg) were immunoprecipitated with 5 μg of mouse anti-ErbB3 antibody or normal mouse serum and 20 μl of protein G beads. The beads were spun down at 1,000 rpm for 3 min and washed as described above. The supernatants were mixed with p85 antibody-conjugated agarose beads, and the immunoprecipitation was carried out as above. To analyze whether the interaction between p85 and RTKs was mediated by the SH2 domain of p85 recognizing pYXXM, LNCaP cells grown in 10-cm plates were serum-starved for 2 days and then treated with EGF (20 ng/ml, 5 min) or HRG-β1 (40 ng/ml, 15 min). Cells were then lysed and subjected to immunoprecipitation with anti-p85 or anti-Tyr(P), followed by blotting with the anti-pYXXM motif antibody.
LC/MS/MS Analysis
LNCaP and PC3 cells on 15-cm plates (20–40 plates) were lysed and subjected to immunoprecipitation with anti-p85, followed by mass spectrometry to identify p85-interacting proteins. Specifically, immunoprecipitates were resolved on 4–12% NuPAGE gels, followed by Coomassie Blue staining. Each lane was cut into multiple fragments, reduced with DTT, alkylated with iodoacetamide, and digested overnight with trypsin at pH 8.3. Tryptic peptides were extracted and then analyzed by data-dependent reversed-phase microcapillary tandem mass spectrometry (LC/MS/MS) using an LTQ two-dimensional linear ion trap mass spectrometer (Thermo Scientific, San Jose, CA) operated in positive ion mode at a flow rate of 250 nl/min. A 75 μm (inner diameter) × 15 μm (inner diameter tip) PicoFrit microcapillary column (New Objective, Woburn, MA) was self-packed with 5-μm C18 resin to 15 cm (length). The column was equilibrated, and peptides were loaded using buffer A (0.1% formic acid, 0.9% acetonitrile, 99% water) and then eluted with a gradient from 5% buffer B (acetonitrile) to 38% B, followed by 95% B for washing. One mass spectrometry survey scan was followed by eight MS/MS scans.
The Sequest algorithm in Proteomics browser software (Thermo Scientific, San Jose, CA) was used for data base searching of all MS/MS spectra against the reversed and concatenated Swiss-Prot protein data base with differential modifications: oxidation (+16 Da) of Met. Peptide sequences were initially accepted if they matched the forward data base and passed the following Sequest browser scoring thresholds: 2+ ions, Xcorr ≥ 2.0, Sf ≥ 0.4, p ≥ 0; 3+ ions, Xcorr ≥ 2.65, Sf ≥ 0.5, p ≥ 5. Peptides with gas phase charges of 1+ and 4+ were generally not accepted as valid due to difficulty of interpretation of such ions. After passing the scoring thresholds, all MS/MS were then manually inspected rigorously to be sure that all b− (fragment ions resulting from amide bond breaks from the peptide's N terminus) and y− ions (fragment ions resulting from amide bond breaks from the peptide's C terminus) aligned with the assigned protein data base sequence. Some digested samples were reanalyzed using an Orbitrap XL mass spectrometer (Thermo Scientific, San Jose, CA) in an attempt to discover additional proteins using a targeted ion MS/MS (TIMM) approach to search for known p85-binding proteins.5 False discovery rates for peptide identifications were calculated to be less than 1.5%.
Quantification of PI3K Subunits
Relative quantification of the regulatory and catalytic subunits of PI3K in LNCaP and PC3 cells was achieved by utilizing a previously published method whereby the average total ion current (TIC) from MS/MS spectra for all peptides identifying each protein subunit was calculated and compared across all subunits (25). The method of quantifying different proteins within a single sample is valid because multiple peptides of varying ionization properties are averaged together, overcoming variability in peptide ionization efficiency. The calculations were processed using in-house developed software called NakedQuant version 1.0 (26). Supplemental Tables S1 and S2 list the peptides and TIC values used in the quantitative analysis.
siRNA Transfections
LNCaP cells (5 × 104/well) were seeded into 24-well plates 2 days before ErbB3 siRNA transfection, and 20 pmol of siRNAs in 2 μl of Lipofectamine were then added to each well. Media were replaced 24 h later, and cells were maintained in RPMI 1640 supplemented with 10% FBS for 24 h, followed by serum starvation for 48 h. Cells were then lysed with 100 μl of radioimmune precipitation buffer containing 1 mm Na3VO4 and a mixture of protease inhibitors. Cell lysates were centrifuged at 13,000 rpm, 4 °C for 15 min to remove cell debris, and 10 μg of total protein from each sample were resolved on 4–12% NuPAGE gels, followed by membrane transfer and immunoblotting. LNCaP cells grown in 10-cm plates were transfected with ErbB3 siRNA similarly to cells plated in 24-well plates, except that 120 pmol of siRNAs in 12 μl of Lipofectamine were used for each plate. After serum starvation for 48 h, cells were lysed with TBS containing 1% Triton X-100 and a mixture of protease inhibitors, followed by immunoprecipitation with p85 antibody and immunoblotting.
Drug Treatments
The dual EGFR/ErbB2 inhibitor lapatinib was used to investigate the effects of EGFR/ErbB2 on the phosphorylation of ErbB3. Serum-starved LNCaP cells grown in 10-cm plates were treated with lapatinib (10 μm) for 6 h. Cells were lysed and subjected to immunoprecipitation with anti-p85 or anti-Tyr(P) antibodies, followed by immunoblotting. To study the effects of Ras, serum-starved LNCaP and PC3 cells plated in 24-well plates were treated with farnesylation inhibitor FTI-277 (20 μm) for 2 days followed by EGF stimulation (20 ng/ml, 5 min). Cells were then lysed with TBS containing 1% Triton X-100 and a mixture of protease inhibitors followed by immunoblotting. To analyze whether phosphorylation of the catalytic p110 subunits was dependent on Src, LNCaP or PC3 cells grown on 10-cm plates were serum-starved for 1–2 days and then treated with either PP2 (10 μm, 2 h) or dasatinib (10 μm, 2 h). Afterward, cells were lysed and immunoprecipitated as above with p85 or 4G10 antibodies and immunoblotted. All experiments were performed at least three times, and representative gels are shown.
RESULTS
p85 Associates with Tyrosine-phosphorylated Proteins in Serum-starved PTEN-deficient PCa Cells
PI3K activity in PTEN intact cells is stimulated by binding of a p85 regulatory subunit SH2 domain to a tyrosine-phosphorylated RTK or adaptor protein, but the extent to which PI3K signaling is dependent on this p85-mediated activation step in PTEN-deficient PCa cells has not been determined. Significantly, each of the available PTEN-deficient PCa cell lines, LNCaP, C4-2 (derived from LNCaP), and PC3, has high levels of basal PI3K pathway activity (as assessed by immunoblotting for pAkt (Thr308 and Ser473) even when cultured in serum-free medium for 2–3 days (Fig. 1A). Nonetheless, PI3K inhibition with LY294002 results in rapid loss of Akt phosphorylation, indicating that continuous PI3K activity is required to maintain downstream signaling even in the absence of PTEN.
FIGURE 1.
p85 association with tyrosine-phosphorylated proteins in serum-starved PTEN-deficient PCa cells. A, PTEN-deficient LNCaP, C4-2, or PC3 cells were cultured for 1–3 days in serum-free medium and then treated for 6 h with PI3K inhibitor LY294002 (LY; 20 μm) or vehicle control (DMSO) (D) or left untreated (−). Lysates (10 μg) were then immunoblotted for pAkt (Thr308 and Ser473). B, serum-starved LNCaP or C4-2 cells (2–3 days) were lysed in TBS plus 1% Triton X-100, immunoprecipitated (IP) with anti-p85 or control rabbit Ig, and then immunoblotted with anti-Tyr(P) antibody (4G10). C, cell lysates (10 μg) from LNCaP or C4-2 cells grown in serum-free medium (SFM) or medium with 10% FBS were immunoblotted with anti-Tyr(P). D and E, LNCaP cells were either maintained in medium with 10% FBS or serum-starved for 2 days. Serum-starved cells were then stimulated with EGF (20 ng/ml, 5 min) or HRG-β1 (40 ng/ml, 15 min). Cell lysates were immunoprecipitated with anti-p85 (D) or anti-Tyr(P) (E), and the immunoprecipitates were blotted with anti-pYXXM. Molecular markers are indicated at the margins.
To address whether this basal PI3K activity is dependent on p85 binding to one or a discrete subset of constitutively activated RTKs or adaptor proteins, we initially determined whether p85 was associated with tyrosine-phosphorylated proteins in serum-starved LNCaP or C4-2 cells. Cells were serum-starved for 3 days, lysates were immunoprecipitated with anti-p85 antibody, and the precipitated proteins were then immunoblotted with an anti-Tyr(P) antibody. This analysis in LNCaP cells revealed two major lower bands that were consistent with the p85 regulatory and p110 catalytic subunits of PI3K (asterisks) and three more slowly migrating bands of ∼190, 150, and 130 kDa (arrows) (Fig. 1B). An identical pattern was observed in C4-2 cells (Fig. 1B). These bands were specific because they were not precipitated by a control rabbit Ig, and they represented only a subset of the total input cellular tyrosine-phosphorylated proteins (Fig. 1C).
The p85 subunit SH2 domains associate with tyrosine-phosphorylated proteins through binding to pYXXM motifs in RTKs and adaptor proteins (2). To determine whether constitutive tyrosine phosphorylation of a specific protein was mediating p85 recruitment directly through SH2 domain binding, we next immunoblotted the p85 immunoprecipitates with a pYXXM motif-specific antibody. Discrete major p85-associated bands at ∼190 kDa were detected by the anti-pYXXM antibody after EGF or heregulin-β1 stimulation, with the band after heregulin-β1 (arrow) being consistent with ErbB3 (which contains six pYXXM motifs) (Fig. 1D). The antibody detected several proteins in the p85 immunoprecipitates from LNCaP cells grown in 10% FBS. A series of weaker bands were observed in cells cultured in serum-free medium, but they did not clearly correspond to the discrete bands between 130 and 190 kDa detected by the anti-Tyr(P) Ab.
As a further sensitive assay to detect proteins containing pYXXM motifs in serum-starved LNCaP cells, whole cell lysates were immunoprecipitated with an anti-Tyr(P) Ab and then immunoblotted with the pYXXM motif Ab. As shown in Fig. 1E, several bands could be detected when cells were grown in 10% FBS, but these were markedly diminished in the serum-starved cells. As expected, stimulation with EGF or heregulin-β1 resulted in a dramatic increase in a band consistent with ErbB3. Taken together, these data suggested that the association between p85 and tyrosine-phosphorylated proteins (detected in Fig. 1B) was not due to direct p85 SH2 domain-mediating binding.
p85-associated Proteins Identified by LC/MS/MS
Although major specific p85-associated proteins in serum-starved LNCaP cells were not detected by the pYXXM motif antibody, the interactions that were weakly detected by this antibody or more readily detected by the Tyr(P) antibody (see Fig. 1B) may nonetheless be physiologically significant in PTEN-deficient cells. Therefore, we next focused on the identification of p85-associated proteins using liquid chromatography/tandem mass spectrometry (LC/MS/MS). Anti-p85 beads were used to carry out large scale immunopurifications from serum-starved LNCaP cells, which were then run on SDS-PAGE. Multiple gel slices corresponding to protein molecular masses from ∼60 to 220 kDa were then subjected to in-gel trypsin digestion, and eluted peptides were identified by LC/MS/MS (Table 1) using a linear ion trap mass spectrometer. As expected, a large fraction of the peptides detected in the ∼100 kDa range were from the α and β isoforms of p85 and the p110 PI3K catalytic subunits. Interestingly, peptide recovery indicated that p110β and p110δ were the major p85-associated p110 isoforms, with much lower levels of p110α (see below).
TABLE 1.
p85-associated proteins identified by LC/MS/MS in LNCaP cells
| Accession | Protein name | Spectral count |
|---|---|---|
| PIK3R2 | Phosphatidylinositol 3-kinase regulatory subunit β | 34 |
| PK3CB | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit β isoform | 25 |
| PK3CD | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit δ isoform | 24 |
| PIK3R1 | Phosphatidylinositol 3-kinase regulatory subunit α | 17 |
| MAP1B | Microtubule-associated protein 1B | 12 |
| EWS | RNA-binding protein EWS | 10 |
| FAS | Fatty acid synthase | 8 |
| MYH10 | Myosin-10 | 8 |
| LIMC1 | LIM and calponin homology domain-containing protein 1 | 6 |
| RFIP1 | Rab11 family-interacting protein 1 | 6 |
| MYH9 | Myosin-9 | 5 |
| MYO6 | Myosin-VI | 5 |
| PK3CA | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit α isoform | 5 |
| TERA | Transitional endoplasmic reticulum ATPase | 5 |
| SYEP | Bifunctional aminoacyl-tRNA synthetase | 4 |
| IF4G1 | Eukaryotic translation initiation factor 4 γ 1 | 4 |
| ILF3 | Interleukin enhancer-binding factor 3 | 4 |
| PNPT1 | Polyribonucleotide nucleotidyltransferase 1, mitochondrial | 4 |
| SFPQ | Splicing factor, proline- and glutamine-rich | 4 |
| SMRC1 | SWI/SNF complex subunit SMARCC1 | 4 |
| DHX9 | ATP-dependent RNA helicase A | 3 |
| RAD50 | DNA repair protein RAD50 | 3 |
| MATR3 | Matrin-3 | 3 |
| TPR | Nucleoprotein TPR | 3 |
| K1967 | Deleted in breast cancer gene 1 protein (DBC.1) (DBC-1) (p30 DBC) | 3 |
| TFR1 | Transferrin receptor protein 1 | 3 |
| SC16A | Protein transport protein Sec16A | 2 |
| ERBB3 | Receptor tyrosine protein kinase ErbB-3 | 2 |
| GAB1a | GRB2-associated binding protein 1 | 2 |
| NUCL | Nucleolin | 1 |
a Identified in a subsequent analysis focused on targeting peptides from known p85-interacting proteins.
Among the p85-associated proteins identified initially by LC/MS/MS, only ErbB3 had been shown previously to mediate p85 SH2 domain binding and PI3K activation (Table 1). To further assess whether there was an interaction between ErbB3 and p85 in serum-starved cells, we carried out immunoblotting of p85 immunoprecipitates with anti-ErbB3, which confirmed the interaction in LNCaP and C4-2 cells (Fig. 2A). On a subsequent LC/MS/MS analysis of the p85 immunoprecipitates using a more sensitive Orbitrap XL mass spectrometer, we also detected GAB1 (Table 1). However, we could not detect GAB1 associated with p85 by immunoblotting (data not shown). Consistent with the LC/MS/MS data, we similarly did not find detectable levels of ErbB2, EGFR, c-MET, insulin-like growth factor receptor, insulin receptor, IRS-2, or IRS-4 (LNCaP cells are IRS-1-deficient) in the p85 immunoprecipitates by immunoblotting (data not shown).
FIGURE 2.
p85 interacts with ErbB3 independently of ErbB3 phosphorylation. A, lysates from serum-starved LNCaP or C4-2 cells (2 days) were immunoprecipitated (IP) with anti-p85 (p85) or control rabbit IgG (Ig), followed by blotting for ErbB3. Input is 1% of the material used for coimmunoprecipitation. B, LNCaP cells maintained in medium with 10% FBS were lysed in TBS plus 1% Triton X-100 and immunoprecipitated with anti-ErbB3 or control Ig, followed by anti-p85. The p85 immunoprecipitates were immunoblotted with anti-Tyr(P) (middle left) or anti-ErbB3 (bottom left). Top left, level of ErbB3 remaining in the anti-ErbB3 or Ig precleared lysates before the p85 immunoprecipitation. Right panels, anti-Tyr(P) (top) or anti-ErbB3 blots of initial anti-ErbB3 or Ig control immunoprecipitates. The arrow indicates a faint band at ∼190 kDa consistent with ErbB3. C and D, LNCaP cells transfected with ErbB3 or control non-targeted siRNA (C), or mock-transfected (M), were maintained in 10% FBS medium for 24 h, followed by serum starvation for 48 h. A portion of each cell lysate (10 μg) was then immunoblotted as indicated (C). The remaining lysates were immunoprecipitated with anti-p85, followed by immunoblotting with anti-ErbB3 (C) or anti-Tyr(P) (D). Molecular markers are indicated at the margins. The arrow indicates the position of proteins around 190 kDa.
p85 Interaction with ErbB3 Is Independent of ErbB3 Phosphorylation and ErbB2 Activity
The ErbB3 polypeptide is 148 kDa, but it is glycosylated and migrates at ∼185 kDa on SDS-PAGE, which suggested that the p85-associated tyrosine phosphorylated band at ∼190 kDa might be ErbB3. To test this hypothesis, we determined whether depleting ErbB3 by immunoprecipitation with anti-ErbB3 would decrease the p85-associated ∼190 kDa tyrosine-phosphorylated band in a subsequent anti-p85 immunoprecipitation. As shown in Fig. 2B (top left), ErbB3 could be substantially depleted from a LNCaP whole cell lysate by an initial immunoprecipitation with anti-ErbB3. The ErbB3 depletion also markedly decreased the amount of ErbB3 that was coprecipitated with p85 (Fig. 2B, bottom left). However, this ErbB3 depletion did not decrease the intensity of the tyrosine-phosphorylated band at ∼190 kDa or other bands that were coprecipitated by anti-p85. Moreover, an anti-Tyr(P) blot of ErbB3 immunoprecipitated by the anti-ErbB3 Ab showed that ErbB3 was not substantially tyrosine-phosphorylated (Fig. 2B, right panels).
This result indicated that ErbB3 was not one of the major tyrosine-phosphorylated proteins associated with p85. However, we could not rule out the possibility that a small pool of heavily tyrosine-phosphorylated ErbB3 was associated with p85 and was not efficiently immunoprecipitated by the anti-ErbB3. Therefore, we next used siRNA to down-regulate ErbB3 expression. As shown in Fig. 2C, total ErbB3 expression was markedly reduced by ErbB3 siRNA versus a control siRNA. ErbB3 associated with p85 was also decreased, although this decrease was less marked than the decrease in total ErbB3, suggesting a relatively high affinity p85-ErbB3 interaction. Importantly, there was again no clear decrease in the p85-associated tyrosine-phosphorylated protein at ∼190 kDa (Fig. 2D). Moreover, there was no evident effect of the ErbB3 siRNA on PI3K activity, as assessed by Akt phosphorylation at Ser473 or Thr308 (Fig. 2C). Taken together, these results demonstrated that p85 was constitutively associated with ErbB3 in LNCaP cells, but this interaction appeared to be independent of ErbB3 phosphorylation and was not clearly required for PI3K activity.
To further address whether ErbB3 phosphorylation made any contribution to the p85-ErbB3 interaction or PI3K activity, we treated LNCaP cells with a dual EGFR/ErbB2 inhibitor (lapatinib) to further suppress any basal tyrosine phosphorylation of ErbB3 mediated by EGFR or ErbB2. Using an anti-Tyr(P) immunoprecipitation, followed by immunoblotting to detect tyrosine phosphorylated proteins, we found that lapatinib (10 μm for 6 h) suppressed the basal tyrosine phosphorylation of both EGFR and ErbB2 in serum-starved LNCaP cells (Fig. 3A). Consistent with the results in Figs. 1 and 2, there was no readily detectable ErbB3 in the anti-Tyr(P) immunoprecipitation. Moreover, there was no detectable effect of lapatinib on PI3K activity, as assessed by Akt phosphorylation (Fig. 3A). Significantly, lapatinib did not decrease the interaction between p85 and ErbB3, further supporting the conclusion that this interaction is independent of ErbB3 phosphorylation by ErbB2 or EGFR (Fig. 3B). Surprisingly, the intensity of the p85-associated band at ∼190 kDa detected by Tyr(P) immunoblotting was selectively decreased by lapatinib, suggesting that it may be an EGFR or ErbB2 substrate that is binding directly or indirectly to p85 (Fig. 3C). In any case, although the identity of this ∼190-kDa protein remains to be determined, it does not appear to contribute to PI3K activity.
FIGURE 3.
PI3K activity and p85-ErbB3 interaction in LNCaP cells are independent of EGFR/ErbB2 activity. Serum-starved LNCaP cells (2 days) on 10-cm plates were treated with EGFR/ErbB-2 dual inhibitor lapatinib (10 μm) or vehicle (DMSO; D) for 6 h. A, cell lysates were immunoprecipitated (IP) with anti-Tyr(P), followed by immunoblotting for EGFR, ErbB2, or ErbB3. Whole cell lysates prior to immunoprecipitation were also immunoblotted as indicated for input EGFR, ErbB2, ErbB3, and Akt and for pAkt. B and C, cell lysates were immunoprecipitated with anti-p85 or rabbit IgG (Ig), followed by immunoblotting for ErbB3 (B) or Tyr(P) (C).
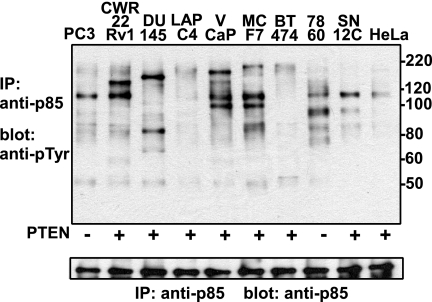
p85 Is Not Associated with Tyrosine-phosphorylated Proteins in PC3 Cells
To further assess the possible importance of p85 membrane recruitment by tyrosine-phosphorylated proteins, we examined p85-associated tyrosine-phosphorylated proteins from PC3 cells (also a PTEN-deficient PCa cell line) and a series of other cell lines in serum-free medium. Significantly, the only major tyrosine-phosphorylated band associated with p85 in PC3 cells was ∼110 kDa, consistent with the PI3K catalytic subunits (Fig. 4). It should be noted that anti-Tyr(P) binding to this ∼110-kDa protein could possibly be nonspecific because the PI3K p110 catalytic subunit, which is tightly associated with p85, is the major protein on these blots. However, it appears to be specific because comparable amounts of p85 were immunoprecipitated from each of the other cell lines, but the ∼110 kDa band was found to be tyrosine-phosphorylated in only a subset of the cells. Tyrosine phosphorylation of this band does not appear to correlate with PTEN status (CWR22, DU145, LAPC4, and VCaP are PCa cell lines with intact PTEN) or cell type. Interestingly, additional major p85-associated tyrosine-phosphorylated bands were detected in other cells, but their identities and significance with respect to PI3K activity remain to be determined. In any case, these results in PC3 cells support the conclusion that SH2 domain-mediated binding of p85 to a tyrosine-phosphorylated RTK or adaptor protein is not required for basal PI3K activity in PTEN-deficient PCa cells.
FIGURE 4.
p85 is not associated with tyrosine-phosphorylated proteins in PC3 cells. Human PCa cell lines PC3, CWR22Rv1, DU145, LAPC4, and VCaP; human breast cancer lines MCF7 and BT474; human renal carcinoma cell lines 786O (PTEN deficient) and SN12C; and a human cervical cancer cell line (HeLa) grown in 10-cm plates were serum-starved for 1 day. Cell lysates were immunoprecipitated (IP) with anti-p85 and immunoblotted with anti-Tyr(P) (top) or anti-p85 (bottom). Molecular markers are indicated at the right.
p110 Phosphorylation Is Mediated by c-Src and Is Not Required for PI3K Activity
Although the functional significance of PI3K p110 tyrosine phosphorylation is not clear, we next considered that it may be contributing to PI3K activity in PTEN-deficient PCa cells. To further assess whether the p85-associated tyrosine-phosphorylated proteins at ∼110 kDa were PI3K catalytic subunits, we first carried out anti-Tyr(P) immunoprecipitations from serum-starved LNCaP and PC3 cells, followed by immunoblotting for each of the p110 isoforms. As shown in Fig. 5A, substantial levels of p110β and lower levels of p110δ were precipitated by the anti-Tyr(P) antibody in both cells, whereas there was no detectable p110α (not shown). It should be noted that p110 in these experiments could possibly be precipitated indirectly through an association with another tyrosine-phosphorylated protein, although the lack of other p85-associated tyrosine-phosphorylated proteins in PC3 cells suggests that it is being precipitated directly.
FIGURE 5.
p110 tyrosine phosphorylation is mediated by c-Src and is not required for PI3K activity. A, serum-starved LNCaP (2 days) and PC3 cells (1 day) were lysed and immunoprecipitated (IP) with anti-Tyr(P), followed by immunoblotting for anti-p110β or -δ. Input is 1% of the material used for the precipitation. B, serum-starved PC3 cells (1 day) were treated with lapatinib or Src inhibitors PP2 or dasatinib for 2 h, all at 10 μm. Cell lysates (1 mg) were then immunoprecipitated with anti-p85 and immunoblotted with anti-Tyr(P). C, serum-starved PC3 cells (1 day) were treated with PP2 or dasatinib at 10 μm for 2 h. Cell lysates were then immunoprecipitated with anti-Tyr(P), followed by immunoblotting with anti-p110β or -δ. Whole cell lysates (10 μg) were immunoblotted with pAkt, pPRAS40, or total Akt. D, serum-starved LNCaP cells (2 days) were treated with lapatinib, PP2, or dasatinib for 2 h, all at 10 μm. Cell lysates (1 mg) were immunoprecipitated with anti-p85 and immunoblotted with anti-Tyr(P). E, serum-starved LNCaP cells (2 days) were treated with PP2 or dasatinib at 10 μm for 2 h. Cell lysates were immunoprecipitated with anti-Tyr(P), followed by blotting with anti-p110β or -δ. Whole cell lysates (10 μg) were immunoblotted with pAkt, pPRAS40, or total Akt. Molecular markers are indicated at the right in B and D.
Lapatinib treatment did not decrease the major ∼110-kDa tyrosine-phosphorylated band that coprecipitates with p85 in PC3 cells, indicating that it was not phosphorylated by EGFR or ErbB2 (Fig. 5B). To identify other tyrosine kinases that may be mediating p110 phosphorylation, we used public data bases (Phosphosite and Scansite, available on the World Wide Web) to determine previously identified sites of tyrosine phosphorylation on the PI3K p110 catalytic subunits and candidate kinases for these sites, which suggested phosphorylation by c-Src or a c-Src family kinase. Strikingly, treatment with c-Src inhibitors (PP2 or dasatinib) resulted in the complete loss of the p85-associated tyrosine-phosphorylated band at 110 kDa in serum-starved PC3 cells (Fig. 5B). Consistent with p110β being the predominant tyrosine-phosphorylated p110 isoform in PC3 cells, Tyr(P) immunoprecipitation, followed by p110 blotting, showed a marked decline in p110β tyrosine phosphorylation (Fig. 5C). However, c-Src inhibition did not clearly suppress Akt phosphorylation or activity, as assessed by phosphorylation of the Akt substrate PRAS40 (Fig. 5C).
As shown in Fig. 3, lapatinib treatment of LNCaP cells resulted in loss of the p85-associated tyrosine-phosphorylated band at ∼190 kDa but did not decrease the bands at ∼110 or ∼130–150 kDa (Fig. 5D). In contrast, PP2 and dasatinib markedly decreased all of the p85-associated tyrosine-phosphorylated bands in LNCaP cells. Consistent with the tyrosine-phosphorylated band at ∼110 kDa in the LNCaP cells being PI3K catalytic subunits, PP2 and dasatinib markedly decreased p110β and p110δ tyrosine phosphorylation (Fig. 5E). However, as observed in PC3 cells, these c-Src inhibitors did not substantially decrease PI3K activity as assessed by Akt phosphorylation and activity (Fig. 5E). These results demonstrate that tyrosine phosphorylation of the PI3K catalytic subunit is mediated by c-Src (or a Src family kinase) but does not contribute to PI3K activity. Moreover, the results further support the conclusion that basal PI3K activity is not dependent on p85 recruitment by an activated RTK or adaptor protein.
PI3K Pathway Activity in PTEN-deficient PCa Cells Is Ras-independent
An alternative mechanism by which RTKs may recruit and activate PI3K is by generating GTP-Ras, which can bind to the p110 subunits via Ras binding domains located carboxyl to the p85 binding domains (27). GTP-Ras has been shown to enhance p110α activity, and an intact p110α-Ras interaction is required for Ras-mediated tumorigenesis, although the importance of GTP-Ras for other p110 isoforms has not been established (28, 29). The LC/MS/MS analysis of p85-associated proteins from LNCaP cells did not reveal an association of Ras, and this was confirmed by immunoblotting of anti-p85 immunoprecipitates with a pan-anti-Ras antibody (data not shown). However, a weak transient interaction could not be excluded. Therefore, to further assess whether Ras was contributing to PI3K pathway activation, we treated serum-starved LNCaP and PC3 cells with a farnesylation inhibitor, FTI-277. Immunoblotting with a pan-anti-Ras Ab confirmed that the drug prevented formation of the more rapidly migrating farnesylated protein (fRas), but there was no effect on PI3K pathway activation in serum-starved LNCaP or PC3 cells (Fig. 6). It should be noted that the drug did not abrogate EGF-mediated ERK activation, indicating either that adequate Ras could still be recruited (farnesylated or independent of farnesylation) or that EGFR was signaling independently of Ras.
FIGURE 6.
PI3K pathway activity in PTEN-deficient PCa cells is Ras-independent. LNCaP (left) or PC3 cells (right) plated into 24-well plates were treated with farnesylation inhibitor FTI-277 (20 or 40 μm; prepared in serum-free medium) for 2 days, and non-drug-treated wells were serum-starved for the same duration. At the end of FTI-277 treatment, cells were either treated with LY294002 (20 μm, 2 h) or stimulated with EGF (20 ng/ml, 5 min). Whole cell lysates (10 μg) were analyzed by immunoblotting for pAkt, pPRAS40, total Akt, ERK1/2, phospho-ERK1/2, Ras, or tubulin. Upper and lower bands corresponding to Ras and farnesylated Ras (fRas) are indicated.
Distinct p110 Isoforms Mediate Basal Versus Growth Factor-stimulated PI3K Activity
It was noteworthy that the LC/MS/MS analysis of p85 associated proteins in LNCaP cells indicated that p110β and p110δ were the major isoforms (Table 1). In addition, a label-free quantitative LC/MS/MS analysis based on the calculated average TIC (25) of all identified peptides corresponding to PI3K protein subunits was utilized to quantify the relative abundance of PI3K regulatory and catalytic subunits in both LNCaP and PC3 cell lines. This analysis for LNCaP was consistent with Table 1 and for PC3 cells also showed lower levels of p110α, although the levels in PC3 were closer to those of p110β and p110δ (Fig. 7A) (see supplemental Tables S1 and S2 for PI3K quantification). Results from immunoblotting LNCaP and PC3 whole cell lysates with isoform-specific p110 Abs were in general agreement with the LC/MS/MS data (Fig. 7B). Moreover, the immunoblotting indicated that p110α levels in LNCaP and PC3 cells were lower than in a small series of breast cancer lines and in PCa cells with intact PTEN (VCaP and CWR22) (Fig. 7B). Interestingly, available cDNA/oligonucleotide microarray data show that p110β and p110δ mRNA, but not p110α mRNA, are increased in PCa versus normal prostate (Fig. 7C) (data not shown) (30, 31). A recent immunohistochemical study similarly found an increase in p110β in PCa versus normal prostate epithelium (32). In contrast, microarray data in GBM, which is also characterized by PTEN loss, shows that p110β is decreased (Fig. 7C) (33).
FIGURE 7.
Expression of PI3K p110 isoforms in PCa cell lines. A, LC/MS/MS analyses of the relative abundance by average TIC of the PI3K subunits p85α and -β and p110α, -β, and -δ in LNCaP and PC3 cells. B, expression levels of p110 isoforms in a variety of cancer cell lines based on immunoblotting. 10 μg of cell lysates from breast cancer lines (MCF-7, MB-MDA-231, BT-474, and SKBR-3) or PCa cell lines (LNCaP, PC3, VCaP, CWR22Rv1, and C4-2) were analyzed by immunoblotting with p110 isoform-specific antibodies. Intensity of each band relative to tubulin is shown. C, p110β and p110δ mRNA levels in normal prostate versus PCa or normal brain versus GBM. Expression data were generated by Oncomine based on results from Vanaja et al. (30) (PCa p110β), Dhanasekaran et al. (31) (PCa p110δ), and Sun et al. (33) (GBM).
Based on these observations, we addressed whether the basal PI3K activity in PC3 and LNCaP cells, which appears to be independent of p85 binding to RTKs or adaptor proteins, was mediated by a particular p110 isoform. Significantly, siRNA-mediated depletion of p110β, but not p110α or p110δ, decreased PI3K activity in serum-starved PC3 cells (Fig. 8A). This result is consistent with a recent study using p110β short hairpin RNA in PC3 cells, which showed that PI3K activity and tumor growth (in vitro and in vivo) were p110β-dependent (17). In contrast, PI3K pathway activation in PC3 cells in response to heregulin-β1 was suppressed by depletion of p110α but not p110β or p110δ (Fig. 8B). In serum-starved LNCaP cells, siRNA targeting both p110β and p110δ (which is expressed at higher levels in LNCaP versus PC3 cells) decreased PI3K activity, whereas p110α siRNA again had no effect (Fig. 8C). However, as in PC3 cells, only the p110α siRNA decreased PI3K pathway activation in response to heregulin-β1 (Fig. 8D). Finally, we addressed whether basal PI3K activity could be further suppressed by simultaneously silencing both p110β and -δ. As shown in Fig. 8, E and F, targeting both p110β and -δ did not appear to be more effective. We presume this reflects an inability to completely silence both p110β and -δ, but it remains possible that p110α can mediate some basal activity in the absence of other catalytic subunits. Taken together, these results indicate that basal PI3K activity in LNCaP and PC3 cells is mediated through p110β and p110δ (in LNCaP) and that this activity is independent of RTK-mediated p85 recruitment.
FIGURE 8.
Distinct p110 isoforms mediate basal versus growth factor-stimulated PI3K activity. PC3 (A) or LNCaP (C) cells in 24-well plates were transfected with siRNAs for each p110 isoform. After serum starvation for 1–2 days, cells were lysed, and 10 μg of cell lysates were subjected to immunoblotting for each p110 isoform, pAkt, total Akt, or β-tubulin. Results are quantified in bar graphs and are representative of 2–3 independent experiments for each cell type (in addition to results in E and F). PC3 (B) or LNCaP (D) transfected with siRNAs for each p110 isoform were serum-starved for 1–2 days, followed by HRG-β1 stimulation (100 ng/ml, 15 min). Cell lysates were then subjected to immunoblotting as indicated. E and F, PC3 or LNCaP cells, respectively, were transfected with siRNAs for each p110 isoform or with both p110β and -δ siRNA and were then analyzed by immunoblotting after serum starvation for 2 days.
DISCUSSION
Activation of class IA PI3Ks by RTKs is mediated through binding of p85 regulatory subunit Src homology 2 domains, recognizing pYXXM motifs, to tyrosine-phosphorylated RTKs or adaptor proteins. PTEN loss enhances and prolongs the PI3K signal, but some level of basal PI3K activity is still required to maintain the activation of downstream targets, such as Akt. This study examined whether the high basal PI3K activity in PTEN-deficient PCa cells was dependent on p85 recruitment by one or a small subset of RTKs, which might then be therapeutic targets. Immunoblotting of p85-associated proteins in serum-starved PTEN-deficient LNCaP and C4-2 PCa cells showed a small set of discrete tyrosine-phosphorylated proteins, but these proteins were not clearly recognized by an anti-pYXXM motif antibody and were not observed in PTEN-deficient PC3 cells. LC/MS/MS analysis of proteins that coimmunoprecipitated with p85 showed that ErbB3 was associated with p85 in serum-starved LNCaP cells, but this interaction was independent of ErbB3 tyrosine phosphorylation and was not required for basal PI3K activity. Using siRNA specific for each p110 isoform, we found that this basal PI3K activity was mediated by p110β in PC3 cells and by both p110β and p110δ in LNCaP cells, whereas p110α was required for PI3K activation in response to RTK stimulation by heregulin-β1. Taken together, these findings indicate that basal PI3K activity in PTEN-deficient PCa cells is RTK-independent and mediated by p110β and p110δ.
The p110β dependence of basal PI3K activity is consistent with a recent study using inducible short hairpin RNA targeting p110α or p110β, which similarly found that PI3K activity in PC3 cells, as well as PTEN-deficient U87MG glioma cells and BT549 breast cancer cells, was dependent on p110β (17). This recent study also showed that p110β depletion decreased PI3K activity and growth in vivo in PC3 xenografts. The in vivo importance of p110β for PI3K activation and growth in PTEN-deficient PCa is further supported by a study showing that loss of p110β but not p110α could decrease Akt activation and development of neoplasia in the anterior prostate of mice with prostate-specific PTEN deletion (although this previous study did not examine the ventral and dorsolateral prostate, which are more closely related to human prostate) (18).
Our finding that PI3K activation in response to heregulin-β1 is dependent on p110α extends previous results showing that p110α is the major isoform activated in response to some other RTKs, such as insulin receptor in liver, although this is not a consistent finding and may be cell type-dependent (18, 21, 34–38). However, the molecular basis for this preferential coupling of RTKs to p110α is not clear, and the extent to which additional RTKs in other cell types are coupled selectively to p110α remains to be determined.
The mechanisms mediating basal p110β and p110δ activation in serum-starved LNCaP cells and p110β activation in PC3 cells are similarly unclear. In contrast to p110α, previous studies indicate that p110β may be activated through binding to G protein-coupled receptors (GPCRs) (38–41). In preliminary studies, we have not observed an effect of pertussis toxin on basal PI3K activity, but this toxin only blocks a subset of GPCRs. Alternatively, p110β may have a modest level of constitutive activity in the absence of RTK or GPCR stimulation that is adequate to maintain PI3K pathway activation in PTEN-deficient cells. In support of this hypothesis, p110β overexpression has been found to mediate the transformation of fibroblasts (42).
In contrast to our findings in PTEN-deficient PCa, a recent study that focused on PTEN-deficient GBM cells found that p85 was associated with multiple activated RTKs and adaptor proteins under serum-starved conditions and that combination therapies targeting multiple RTKs could suppress PI3K activity in PTEN-intact and -deficient tumor cells (24). The RTK dependence for basal PI3K activity suggests that PTEN-deficient GBM cells lack RTK-independent mechanisms for activation of p110β or p110δ or possibly express p110β at too low a level for any constitutive activity to sustain PI3K pathway activation. This apparent biological difference between PTEN-deficient PCa and GBM cell lines may reflect an abundance of peptide growth factors in the microenvironment during GBM development or the absence of pathways mediating RTK-independent p110β or p110δ activation. A biological difference between PTEN-deficient PCa and GBM with respect to PI3K signaling is further supported by gene expression studies, which indicate that p110α is increased relative to p110β in GBM, whereas p110β is increased in PCa (see Fig. 7). However, the extent to which RTKs in GBM are signaling through p110α versus other isoforms remains to be determined.
It is noteworthy that PI3K pathway activation in PCa is frequently due to PTEN loss, with activating mutations in p110α that are found in other cancers being rare in PCa. The reason for this preference is not clear, but it is presumed to reflect nonredundant functions of other PI3K catalytic subunits that are required for prostate carcinogenesis and possibly additional PTEN-regulated pathways distinct from PI3K. In any case, the findings in this study suggest that prostate epithelial cells that have sustained genetic or epigenetic loss of PTEN activity may be dependent initially on p110β (or possibly p110δ) for PI3K pathway activation and positive selection due to a relative lack of growth factors mediating activation of RTKs and p110α in the microenvironment of the fully developed adult human prostate. If this hypothesis is correct, then selective p110β inhibitors or inhibitors of particular GPCRs or other upstream activators of p110β, if present, may be most effective at early stages of PCa development. In contrast, because disease progression is probably associated with increased RTK stimulation and activation of p110α, the extent to which selective inhibition of p110β will be effective in more advanced PCa is not clear and may be dependent on whether it still has a nonredundant downstream function.
This work was supported, in whole or in part, by National Institutes of Health Grants Prostate SPORE P50 CA90381 and P01CA89021 (to S. P. B.). This work was also supported by United States Department of Defense Grant PC073779 (to S. P. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2.
J. Asara, manuscript in preparation.
- PI3K
- phosphoinositide 3-kinase
- RTK
- receptor tyrosine kinase
- PTEN
- phosphate and tensin homolog
- PCa
- prostate cancer(s)
- GBM
- glioblastoma multiforme
- EGF
- epidermal growth factor
- siRNA
- small interfering RNA
- FBS
- fetal bovine serum
- LC
- liquid chromatography
- MS/MS
- tandem mass spectrometry
- TIC
- total ion current
- pAkt
- phospho-Akt
- Ab
- antibody
- EGFR
- epidermal growth factor receptor
- ERK
- extracellular signal-regulated kinase
- GPCR
- G protein-coupled receptor
- SH2
- Src homology 2.
REFERENCES
- 1.Engelman J. A., Luo J., Cantley L. C. (2006) Nat. Rev. Genet. 7, 606–619 [DOI] [PubMed] [Google Scholar]
- 2.Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. (1993) Cell 72, 767–778 [DOI] [PubMed] [Google Scholar]
- 3.Yu J., Zhang Y., McIlroy J., Rordorf-Nikolic T., Orr G. A., Backer J. M. (1998) Mol. Cell. Biol. 18, 1379–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carracedo A., Pandolfi P. P. (2008) Oncogene 27, 5527–5541 [DOI] [PubMed] [Google Scholar]
- 5.Keniry M., Parsons R. (2008) Oncogene 27, 5477–5485 [DOI] [PubMed] [Google Scholar]
- 6.Yuan T. L., Cantley L. C. (2008) Oncogene 27, 5497–5510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao L., Vogt P. K. (2008) Oncogene 27, 5486–5496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairns P., Okami K., Halachmi S., Halachmi N., Esteller M., Herman J. G., Jen J., Isaacs W. B., Bova G. S., Sidransky D. (1997) Cancer Res. 57, 4997–5000 [PubMed] [Google Scholar]
- 9.McMenamin M. E., Soung P., Perera S., Kaplan I., Loda M., Sellers W. R. (1999) Cancer Res. 59, 4291–4296 [PubMed] [Google Scholar]
- 10.Wang S. I., Parsons R., Ittmann M. (1998) Clin. Cancer Res. 4, 811–815 [PubMed] [Google Scholar]
- 11.Rubin M. A., Gerstein A., Reid K., Bostwick D. G., Cheng L., Parsons R., Papadopoulos N. (2000) Hum. Pathol. 31, 504–508 [DOI] [PubMed] [Google Scholar]
- 12.Feilotter H. E., Nagai M. A., Boag A. H., Eng C., Mulligan L. M. (1998) Oncogene 16, 1743–1748 [DOI] [PubMed] [Google Scholar]
- 13.Wang S., Gao J., Lei Q., Rozengurt N., Pritchard C., Jiao J., Thomas G. V., Li G., Roy-Burman P., Nelson P. S., Liu X., Wu H. (2003) Cancer Cell 4, 209–221 [DOI] [PubMed] [Google Scholar]
- 14.Trotman L. C., Niki M., Dotan Z. A., Koutcher J. A., Di Cristofano A., Xiao A., Khoo A. S., Roy-Burman P., Greenberg N. M., Van Dyke T., Cordon-Cardo C., Pandolfi P. P. (2003) PLoS Biol. 1, E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Backman S. A., Ghazarian D., So K., Sanchez O., Wagner K. U., Hennighausen L., Suzuki A., Tsao M. S., Chapman W. B., Stambolic V., Mak T. W. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 1725–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuels Y., Wang Z., Bardelli A., Silliman N., Ptak J., Szabo S., Yan H., Gazdar A., Powell S. M., Riggins G. J., Willson J. K., Markowitz S., Kinzler K. W., Vogelstein B., Velculescu V. E. (2004) Science 304, 554. [DOI] [PubMed] [Google Scholar]
- 17.Wee S., Wiederschain D., Maira S. M., Loo A., Miller C., deBeaumont R., Stegmeier F., Yao Y. M., Lengauer C. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 13057–13062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia S., Liu Z., Zhang S., Liu P., Zhang L., Lee S. H., Zhang J., Signoretti S., Loda M., Roberts T. M., Zhao J. J. (2008) Nature 454, 776–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torbett N. E., Luna-Moran A., Knight Z. A., Houk A., Moasser M., Weiss W., Shokat K. M., Stokoe D. (2008) Biochem. J. 415, 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oda K., Okada J., Timmerman L., Rodriguez-Viciana P., Stokoe D., Shoji K., Taketani Y., Kuramoto H., Knight Z. A., Shokat K. M., McCormick F. (2008) Cancer Res. 68, 8127–8136 [DOI] [PubMed] [Google Scholar]
- 21.Jia S., Roberts T. M., Zhao J. J. (2009) Curr. Opin. Cell Biol. 21, 199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelman J. A., Cantley L. C. (2006) Clin. Cancer Res. 12, 4372s–4376s [DOI] [PubMed] [Google Scholar]
- 23.Engelman J. A., Jänne P. A., Mermel C., Pearlberg J., Mukohara T., Fleet C., Cichowski K., Johnson B. E., Cantley L. C. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3788–3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stommel J. M., Kimmelman A. C., Ying H., Nabioullin R., Ponugoti A. H., Wiedemeyer R., Stegh A. H., Bradner J. E., Ligon K. L., Brennan C., Chin L., DePinho R. A. (2007) Science 318, 287–290 [DOI] [PubMed] [Google Scholar]
- 25.Asara J. M., Christofk H. R., Freimark L. M., Cantley L. C. (2008) Proteomics 8, 994–999 [DOI] [PubMed] [Google Scholar]
- 26.Yang X., Friedman A., Nagpal S., Perrimon N., Asara J. M. (2009) J. Biomol. Tech. 20, 272–277 [PMC free article] [PubMed] [Google Scholar]
- 27.Pacold M. E., Suire S., Perisic O., Lara-Gonzalez S., Davis C. T., Walker E. H., Hawkins P. T., Stephens L., Eccleston J. F., Williams R. L. (2000) Cell 103, 931–943 [DOI] [PubMed] [Google Scholar]
- 28.Gupta S., Ramjaun A. R., Haiko P., Wang Y., Warne P. H., Nicke B., Nye E., Stamp G., Alitalo K., Downward J. (2007) Cell 129, 957–968 [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Viciana P., Sabatier C., McCormick F. (2004) Mol. Cell. Biol. 24, 4943–4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanaja D. K., Cheville J. C., Iturria S. J., Young C. Y. (2003) Cancer Res. 63, 3877–3882 [PubMed] [Google Scholar]
- 31.Dhanasekaran S. M., Dash A., Yu J., Maine I. P., Laxman B., Tomlins S. A., Creighton C. J., Menon A., Rubin M. A., Chinnaiyan A. M. (2005) FASEB J. 19, 243–245 [DOI] [PubMed] [Google Scholar]
- 32.Zhu Q., Youn H., Tang J., Tawfik O., Dennis K., Terranova P. F., Du J., Raynal P., Thrasher J. B., Li B. (2008) Oncogene 27, 4569–4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun L., Hui A. M., Su Q., Vortmeyer A., Kotliarov Y., Pastorino S., Passaniti A., Menon J., Walling J., Bailey R., Rosenblum M., Mikkelsen T., Fine H. A. (2006) Cancer Cell 9, 287–300 [DOI] [PubMed] [Google Scholar]
- 34.Chaussade C., Rewcastle G. W., Kendall J. D., Denny W. A., Cho K., Grønning L. M., Chong M. L., Anagnostou S. H., Jackson S. P., Daniele N., Shepherd P. R. (2007) Biochem. J. 404, 449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foukas L. C., Claret M., Pearce W., Okkenhaug K., Meek S., Peskett E., Sancho S., Smith A. J., Withers D. J., Vanhaesebroeck B. (2006) Nature 441, 366–370 [DOI] [PubMed] [Google Scholar]
- 36.Knight Z. A., Gonzalez B., Feldman M. E., Zunder E. R., Goldenberg D. D., Williams O., Loewith R., Stokoe D., Balla A., Toth B., Balla T., Weiss W. A., Williams R. L., Shokat K. M. (2006) Cell 125, 733–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J. J., Cheng H., Jia S., Wang L., Gjoerup O. V., Mikami A., Roberts T. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16296–16300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guillermet-Guibert J., Bjorklof K., Salpekar A., Gonella C., Ramadani F., Bilancio A., Meek S., Smith A. J., Okkenhaug K., Vanhaesebroeck B. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 8292–8297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciraolo E., Iezzi M., Marone R., Marengo S., Curcio C., Costa C., Azzolino O., Gonella C., Rubinetto C., Wu H., Dastrù W., Martin E. L., Silengo L., Altruda F., Turco E., Lanzetti L., Musiani P., Rückle T., Rommel C., Backer J. M., Forni G., Wymann M. P., Hirsch E. (2008) Sci. Signal. 1, ra3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murga C., Fukuhara S., Gutkind J. S. (2000) J. Biol. Chem. 275, 12069–12073 [DOI] [PubMed] [Google Scholar]
- 41.Hazeki O., Okada T., Kurosu H., Takasuga S., Suzuki T., Katada T. (1998) Life Sci. 62, 1555–1559 [DOI] [PubMed] [Google Scholar]
- 42.Kang S., Denley A., Vanhaesebroeck B., Vogt P. K. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 1289–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]