Abstract
Yeast filamentous growth is a stress response to conditions of nitrogen deprivation, wherein yeast colonies form pseudohyphal filaments of elongated and connected cells. As proteins mediating adhesion and transport are required for this growth transition, we expect that the protein complement at the yeast cell periphery plays a critical and tightly regulated role in pseudohyphal filamentation. To identify proteins differentially abundant at the yeast cell periphery during pseudohyphal growth, we generated quantitative proteomic profiles of plasma membrane protein preparations under conditions of vegetative growth and filamentation. By isobaric tags for relative and absolute quantification chemistry and two-dimensional liquid chromatography-tandem mass spectrometry, we profiled 2463 peptides and 356 proteins, identifying 11 differentially abundant proteins that localize to the yeast cell periphery. This protein set includes Ylr414cp, herein renamed Pun1p, a previously uncharacterized protein localized to the plasma membrane compartment of Can1. Pun1p abundance is doubled under conditions of nitrogen stress, and deletion of PUN1 abolishes filamentous growth in haploids and diploids; pun1Δ mutants are noninvasive, lack surface-spread filamentation, grow slowly, and exhibit impaired cell adhesion. Conversely, overexpression of PUN1 results in exaggerated cell elongation under conditions of nitrogen stress. PUN1 contributes to yeast nitrogen signaling, as pun1Δ mutants misregulate amino acid biosynthetic genes during nitrogen stress. By chromatin immunoprecipitation and reverse transcription-PCR, we find that the filamentous growth factor Mss11p directly binds the PUN1 promoter and regulates its transcription. In total, this study provides the first profile of differential protein abundance during pseudohyphal growth, identifying a previously uncharacterized membrane compartment of Can1 protein required for wild-type nitrogen signaling and filamentous growth.
Keywords: Proteomics, Yeast, Yeast Genetics, Yeast Metabolism, Yeast Transcription
Introduction
Under conditions of nitrogen stress, certain strains of Saccharomyces cerevisiae implement a dramatic change in growth form characterized by the development of multicellular pseudohyphal filaments (1–4). During pseudohyphal growth, yeast cells delay in G2/M, resulting in an extended period of apically polarized growth and an elongated cell morphology (5–7). The yeast cells also exhibit an altered pattern of budding in which daughter cells emerge from mother cells predominantly opposite the birth end, as opposed to the bipolar pattern of bud emergence observed in diploid cells under conditions of vegetative growth (8). Perhaps most strikingly, filamentous yeast cells remain physically connected after cell division (1). The resulting pseudohyphal filaments adhere to and invade growth substrates, such as agar (9–11). Thought to be a foraging mechanism, yeast filamentous growth has been studied extensively as a model for related hyphal growth transitions in the opportunistic human pathogen Candida albicans, wherein these growth transitions are required for virulence (12).
In S. cerevisiae, at least three signaling pathways regulate the initiation and maintenance of pseudohyphal growth as follows: 1) the cyclic AMP-protein kinase A (PKA)2 pathway; 2) the Kss1p mitogen-activated protein kinase (MAPK) cascade; and 3) a pathway involving the 5′-AMP-activated protein kinase Snf1p. During filamentous growth, nitrogen stress results in activation of the GTP-binding protein Ras2p, which acts upstream of both the PKA and MAPK signaling modules (13–15). Ras2p stimulates the adenylate cyclase Cyr1p, and the resulting increase in cAMP activates Tpk2p, the catalytic subunit isoform of PKA (16, 17). Ras2p also acts through the G-protein Cdc42p and the p21-activated kinase Ste20p to initiate the MAPK cascade of Ste11p, Ste7p, and the MAPK Kss1p (2, 18–22). The Snf1p kinase is required for pseudohyphal growth and for growth on secondary carbon sources (23–25). Snf1p is regulated by the kinase Sak1p during nitrogen signaling, and Snf1p pairs with its Gal83p β-subunit isoform to activate expression of downstream targets that enable pseudohyphal growth (26, 27). Recent genomic studies have identified additional signaling pathways and gene sets that contribute to yeast pseudohyphal growth (28–31), collectively indicating an extensive regulatory network controlling gene and protein activity during the filamentous growth transition.
Filamentous growth signaling networks target a broad set of proteins, including a subset of proteins at the yeast cell periphery (31–35). In particular, proteins at the plasma membrane and cell wall contribute to the pseudohyphal growth response by mediating cell-cell and cell-substrate adhesion, nutrient transport, and receptor-linked signaling events (1, 36–38). For example, the cell surface mannoprotein Muc1p/Flo11p is required for pseudohyphal growth, invasive growth, and biofilm formation in filamentous strains of S. cerevisiae (37, 39–41). MUC1 expression is subject to extensive regulatory control; the PKA, Kss1p MAPK, and Snf1p pathways described above all regulate MUC1 transcription, and epigenetic silencing through the histone deacetylase Hda1p further controls MUC1 expression (34, 42, 43). Muc1p contributes to cell-substrate adhesion in vivo, exhibits homotypic adhesion in vitro, and is the only flocculin family member expressed in filamentous strains of S. cerevisiae (37, 44); however, additional cell wall proteins contribute to cell adhesion (45) and are expected to play essential roles during the pseudohyphal growth response. In addition, membrane transporters such as Mep2p and the amino acid transporter homolog Ssy1p regulate filamentous growth (33, 46). Thus, proteins at the yeast cell periphery play critical roles in pseudohyphal growth, but the extent to which protein composition at the plasma membrane and cell wall is differentially regulated during filamentation is largely unknown. In particular, it is not known if the protein profile at the yeast cell periphery is significantly altered during filamentous growth or if the differentially abundant proteins contribute to the filamentous growth phenotype.
Here, we employ quantitative proteomics to profile changes in protein abundance at the yeast cell periphery during pseudohyphal growth. For this study, we generated plasma membrane protein preparations from yeast colonies under vegetative and filamentous growth conditions and analyzed these samples by mass spectrometry using iTRAQ reagents. The plasma membrane was chosen as the focus of this study for the following three reasons: 1) plasma membrane proteins are known to be important for pseudohyphal growth; 2) plasma membrane protein preparation will also carry along some cell wall proteins; and 3) a significant complement of plasma membrane proteins should be amenable to extraction through a single biochemical protocol, whereas multiple protocols would be needed to isolate a similarly large complement of cell wall proteins. Our analysis identified a set of proteins differentially abundant during pseudohyphal growth, revealing, in particular, an integral component of the plasma membrane compartment of Can1 (MCC), Ylr414cp, that we find to be essential for the pseudohyphal growth response. Ylr414cp, herein renamed Pun1p, exhibits increased abundance under conditions of nitrogen stress and is required for wild-type filamentous growth and cell adhesion. PUN1 is subject to transcriptional regulation by the filamentous growth factors Mss11p and Flo8p; Mss11p directly regulates PUN1 expression under conditions of nitrogen stress, whereas Flo8p acts indirectly. In total, this work presents an informative data set of proteins that change abundance during pseudohyphal growth, while also identifying an essential, but previously unstudied, role for PUN1 in the molecular events enabling the yeast filamentous growth transition.
MATERIALS AND METHODS
Yeast Strains and Growth Conditions
All yeast strains were generated in the Σ1278b background. The genotype of the haploid Y825 strain is MATa ura3-52 leu2Δ0; the genotype of the diploid Y825/6 strain is ura3-52/ura3–52 leu2Δ0/leu2Δ0 (30, 31). Haploid deletion mutants were constructed in strain Y825 by homologous recombination using the PCR-based strategy of Baudin et al. (47). Haploid mutants were independently constructed and mated to generate homozygous diploids. YLR414C was chromosomally tagged with the Venus variant of yellow fluorescent protein in Y825/6 (48). Integrated alleles were generated by standard protocols using the vYFP-KanMX6 cassette from pBS7 (Yeast Resource Center, University of Washington). For co-localization analysis, MCC component genes were amplified by PCR; each open reading frame (without stop codon) was amplified along with 1 kb of upstream genomic DNA for introduction into pDEST-mCherry (49). Upon cloning, the PCR product creates a translational fusion between the 3′-end of the gene and mCherry. YLR414C was also chromosomally tagged with three copies of the hemagglutinin (HA) epitope in Y825/6 for protein quantification by Western blotting. The HA tag was integrated at the 3′-end of YLR414C using the KanMX6 selection cassette from plasmid pFA6a-3HAKanMX6 (50). For chromatin immunoprecipitation, MSS11 and FLO8 were chromosomally tagged with protein A in Y825.
Yeast strains were propagated on rich YPD medium or synthetic medium as described (51). Haploid filamentous growth was induced in standard medium (0.17% yeast nitrogen base (YNB) without amino acids and ammonia, 2% glucose, and 5 mm ammonium sulfate) supplemented with 1% (v/v) butanol or on SLAD plates (0.17% yeast nitrogen base without amino acids and ammonium sulfate, 2% glucose, 50 μm ammonium sulfate, with essential amino acids) plus 1% (v/v) butanol (52). Diploid filamentous growth was induced in liquid low nitrogen medium (0.17% YNB without amino acids and ammonia, 2% glucose and 50 μm ammonium sulfate) or on SLAD plates. Invasive growth was assayed on YPD medium.
Plasma Membrane Protein Preparations
Plasma membrane proteins preparations were generated by cell fractionation on Renografin density gradients according to the protocol described in Chang (53). For these preparations, ∼17 A600 units of yeast cells were harvested from plates (SLAD and standard medium) for each density gradient. Cells were subsequently washed in Tris/EDTA buffer and treated with a mixture of protease inhibitors immediately prior to cell lysis by vortexing with glass beads. After vortexing, the supernatant was reserved, and any remaining unbroken cells were removed by brief centrifugation at 500 × g. The lysate was mixed with an equal volume of Renografin-76, Renocal-76, or Renografin-60 (Bracco Diagnostics, Inc., Princeton, NJ) and placed at the bottom of an SW 50.1 centrifuge tube. The lysate/Renografin mixture was overlaid with 2 volumes each of 34, 30, 26, and 22% Renografin prior to overnight centrifugation at 40,000 rpm. Following centrifugation, fractions from the top of the gradient were collected; the fractions were diluted with Tris/EDTA buffer, and Renografin was removed from these samples by centrifugation in an ultracentrifuge at 100,000 × g for 1 h. Subsequently, the distribution of the plasma membrane marker Pma1p in the fractions was analyzed by Western blotting with antibody directed against Pma1p.
Protein Isobaric Tagging and Mass Spectrometry
Protein extracts from selected fractions containing high levels of Pma1p were labeled with iTRAQ reagents (Applied Biosystems) essentially as described in Keshamouni et al. (54). Protein samples (∼50–100 μg) were reduced with 2.5 mm tris-(2-carboxyethyl)phosphine at 60 °C for 1 h, and cysteine residues were blocked with 10 mm methyl methane thiosulfate at room temperature for 15 min. Proteins were precipitated with acetone overnight at −20 °C, and pellets were collected by centrifugation, washed in cold 90% acetone, and air-dried. Proteins were digested with trypsin overnight at 40 °C. Tris was removed from the tryptic digests prior to isobaric labeling with iTRAQ reagents; subsequently, iTRAQ reagent was added directly to the protein digest, and the mixture was incubated at room temperature for 1 h. The vegetative growth samples (nitrogen sufficiency) were labeled in duplicate using mass 114 and 115 isobaric iTRAQ tags; tryptic peptide pools from the filamentous growth samples (low nitrogen medium) were labeled in duplicate using mass 116 and 117 isobaric iTRAQ tags. After quenching, the labeled peptide pools were mixed 1:1:1:1 for subsequent strong cation exchange (SCX) fractionation and reversed-phase liquid chromatography (LC).
For SCX separation, the combined samples were fractionated on two SCX spin columns run in parallel. Peptides were eluted using 50-μl volumes of KCl in a stepwise gradient from 25 to 350 mm. Seven salt fractions were used, and paired eluates were combined and taken to dryness. Peptides in SCX fractions were subsequently separated by C18 nano-LC using an 1100 Series nano high pressure liquid chromatography equipped with μWPS autosampler, 2/10 microvalve, MWD UV detector (214 nm), and Micro-FC fraction collector/spotter (Agilent). Each SCX salt fraction was reconstituted in 43 μl of 0.1% trifluoroacetic acid in water, and 40 μl was subsequently injected onto a C18 cartridge (Zorbax300SB, 3.5 μm, 150 × 0.1 mm; Agilent) and desalted with solvent C (CH3CN:H2O:trifluoroacetic acid, 5:95:0.1). The enrichment column was placed ahead of a C18 column (Zorbax300SB, 3.5 μm, Agilent) equilibrated with solvent A (CH3CN:H2O:trifluoroacetic acid, 6.5:93.5:0.1), and peptides were eluted with a gradient of solvent B (CH3CN:H2O:trifluoroacetic acid, 90:10:0.1) from 6.5 to 50% solvent B over 90 min at a flow rate of 0.4 μl/min. Column effluent was mixed with matrix delivered with a PHD200 infusion pump (Harvard Apparatus). Fractions were spotted at 30-s intervals onto a stainless steel matrix-assisted laser desorption ionization target plate (192 wells/plate, Applied Biosystems).
Mass spectra were acquired on an Applied Biosystems model 4700 Proteomics Analyzer (TOF/TOF) using 4000 Series Explorer software. Peptides were identified using the MASCOT search engine (Matrix Science) run using GPS Explorer. Each mass spectrometry spectrum was searched against a protein sequence data base (NCBI nonredundant data base), resulting in a set of tryptic peptide matches with confidence values. MASCOT searches were run allowing for one missed cleavage, precursor error tolerance of 0.7 Da, and mass spectrometry fragment tolerance of 0.3 Da. Signature ion peak areas at 114.1, 115.1, 116.1, and 117.1 m/z were extracted from the 4700 raw data using the ABI 4700 software. To quantify differences between the vegetative growth and filamentous growth samples, raw peak heights were normalized by matching the quantiles of the distributions of the 115, 116, and 117 measurements to the quantiles of the 114 measurements using a monotone piece-wise linear function (55). After normalization, the four peak area measurements exhibited similar statistics in mean, variance, and quartiles.
Yeast Filamentous Growth Assays
Invasive growth of haploid deletion mutants was determined by the standard plate-washing assay (1); mid-log phase cultures were spotted onto YPD plates and incubated for 5 days at 30 °C, and surface cells were subsequently washed off the plate under a gentle stream of water. Colony morphology of deletion mutants was observed by streaking mid-log cultures grown in YPD onto SLAD plates (diploid strains) or SLAD supplemented with 1% (v/v) butanol (haploid strains) and incubating at 30 °C for 10 days.
Yeast Microscopy
For fluorescence microscopy, overnight cultures were diluted to an absorbance (A600) of 0.1 in synthetic medium lacking leucine. Yeast cells grown to an A600 of 0.6 were used for co-localization analysis. For assays of filamentous growth, cells were grown overnight, diluted to an A600 of 0.1, and grown in standard medium or inducing conditions as required (30). Filamentous growth in haploid strains was induced by inoculating diluted cultures into standard growth medium supplemented with 1% butanol for 4 h at 30 °C. Diploid filamentous strains were induced as follows: overnight cultures were centrifuged, washed, and inoculated at an A600 of 0.1 in low nitrogen medium at 30 °C for 4 h before observation. All microscopy was performed on an upright Nikon Eclipse 80i microscope with a CoolSNAP ES2 CCD (Photometrics, Tucson, AZ); images were acquired using the MetaMorph software package (Molecular Devices).
DNA Microarray Analysis
RNA isolation, target preparation, hybridization, and data analysis were performed as described previously (29). The GeneChip Yeast Genome S98 array (Affymetrix, Santa Clara, CA) was used in all experiments. All microarray experiments were performed in triplicate with independent biological replicates. Gene annotations were obtained from the Saccharomyces Genome Data Base (SGD). Gene Ontology (GO) Term Finder and GO Slim Mapper tools in SGD were used to assign the genes in GO-based categories, and over-represented GO terms were identified in each data set. Hierarchical cluster analysis was performed using Gene Cluster 3.0, and the data were visualized with Java Tree View, essentially as described in Juan and Huang (56). The transcriptional profiling data described here are accessible through accession number GSE20351 at NCBI, Gene Expression Omnibus (www.ncbi.nlm.nih.gov).
RT-PCR and Chromatin Immunoprecipitation
RNA samples were treated with DNase I (Invitrogen) as per the manufacturer's instructions. First-strand cDNAs were synthesized from total RNA using the Superscript II kit as recommended by the manufacturer (Invitrogen). Quantitative real time PCR was performed in triplicate using the SYBR Green PCR master mix (Applied Biosystems). Data were normalized to an internal control, and the ΔΔCT method was used to obtain relative expression levels for each gene.
Chromatin immunoprecipitation assays were performed essentially as described previously (28). Briefly, the overnight culture was used to inoculate 50 ml of YPD medium to an A600 of 0.2. The cultures were allowed to recover from stationary phase until an A600 of 0.4 was reached. One percent (v/v) of butanol was then added to induce filamentous growth. Cells were cross-linked by formaldehyde treatment and lysed using lysis buffer (50 mm HEPES-KOH, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, and 0.1% sodium deoxycholate, 0.1% SDS, 2 mm phenylmethylsulfonyl fluoride). Protein A-tagged proteins were immunoprecipitated with IgG-Sepharose (Amersham Biosciences). DNA fragments from the immunoprecipitated samples were amplified using the primers indicated in supplemental Table S1.
RESULTS
Identifying Proteins at the Yeast Cell Periphery during Pseudohyphal Growth by Mass Spectrometry
Selected proteins at the yeast cell periphery (i.e. cell adhesion proteins and some transporters) contribute to the filamentous growth response and are known targets of filamentous growth regulators; however, the broad scope of plasma membrane and cell wall proteins that enable filamentous growth is unknown, as is the degree to which these proteins are differentially regulated during the filamentous growth transition. As a substantive step toward addressing these unknowns, we implemented a mass spectrometry-based approach enabling the identification of differentially abundant proteins at the yeast plasma membrane during pseudohyphal growth.
For this study, we used a wild-type diploid strain derived from the Σ1278b genetic background; unlike most standard laboratory strains of S. cerevisiae, Σ1278b undergoes an extensive and easily controlled transition to pseudohyphal growth and is the preferred genetic background for filamentous growth studies (1, 57). A diploid strain was selected for this analysis to yield the full pseudohyphal growth response, surface filamentation and invasive growth on solid medium under conditions of nitrogen stress. This strain was plated on standard growth medium (vegetative growth conditions) and on low nitrogen medium (filamentous growth conditions). Colonies were harvested from both sets of plates, and plasmid membrane proteins were prepared from each harvested culture (see under “Materials and Methods”). The plasma membrane protein preparations were analyzed by mass spectrometry, using amine-specific isobaric tags for relative and absolute quantification (iTRAQ), followed by two-dimensional liquid chromatography-mass spectrometry. By the isobaric tagging approach, samples were labeled with four independent reagents of the same mass that yield four unique reporter ions (m/z = 114–117) upon fragmentation in mass spectrometry; the resulting reporters can be used to quantify the relative abundance of the different samples in the multiplexed set (58, 59). As indicated in Fig. 1A, we labeled two plasma membrane protein preparations from harvested cells grown under normal vegetative growth conditions with iTRAQ reagents 114 and 115, respectively, and two protein preparations from cells under filamentous growth conditions with reagents 116 and 117. Following mass spectrometry, we determined the relative abundance of proteins in the vegetative and filamentous growth samples according to established protocols detailed under “Materials and Methods.”
FIGURE 1.
Mass spectrometric analysis of yeast plasma membrane protein preparations under vegetative and filamentous growth conditions. A, overview of steps in generating yeast plasma membrane protein preparations for isobaric labeling and mass spectrometry (MS). For this analysis, a diploid strain was grown on solid medium to enable the full pseudohyphal response prior to plasma membrane protein extraction and mass spectrometry. WT, wild type; LC MALDI-TOF, liquid chromatography matrix-assisted laser desorption ionization time-of-flight. B, summary of data from iTRAQ-based mass spectrometry (Mass Spec). In total, 356 proteins were profiled, and within this set, proteins that localize to the indicated regions at the cell periphery are tallied in the rectangular inset. C, percentage of each total protein complement identified in this study is presented. Total protein numbers from the yeast proteome as a whole are indicated on the right, and the bar indicates the fraction of that total identified by mass spectrometry. For example, of 15 known proteins localized to membrane rafts, 53% (8) were profiled in this study. Mito, mitochondria; ER, endoplasmic reticulum. D, GO terms statistically enriched in the set of proteins were identified by mass spectrometry, with GO accession numbers and p values listed. This indicates that the set of 356 identified proteins is functionally heterogeneous and that the plasma membrane fractionation protocol was effective in enriching for plasma membrane proteins.
In total, we analyzed 3968 peptides from the plasma membrane preparations and determined the identity of 2463 peptides, corresponding to 356 proteins (Fig. 1B). The plasma membrane preparations were indeed enriched for proteins at the yeast cell periphery; by mass spectrometry, we identified 65 plasma membrane proteins and 19 additional proteins that localize to the yeast cell wall, cell periphery, or bud tip (Fig. 1B). Our mass spectrometry profiles encompass 24% of all known yeast plasma membrane proteins and 53% of known membrane raft proteins (Fig. 1C). This enrichment for plasma membrane proteins is statistically significant; the probability that this set of plasma membrane proteins was identified by chance is 1.73 × 10−21 (Fig. 1D). The complete mass spectrometry data set may be downloaded from ProteomeCommons.
In full, these data provide profiles for ∼270 additional proteins that do not localize to the yeast cell periphery; these proteins were carried over in the plasma membrane preparations because of biochemical similarities to plasma membrane proteins (e.g. other membrane proteins). Although this full data set is extremely informative for our understanding of the regulatory changes underlying the pseudohyphal growth transition, the focus of this study centered specifically upon changes in protein abundance at the plasma membrane and yeast cell periphery.
A Set of Differentially Abundant Proteins at the Yeast Cell Periphery during Pseudohyphal Growth
As indicated in Table 1, we identified 11 proteins with reported localization to the yeast cell periphery exhibiting increased or decreased abundance during pseudohyphal growth. Seven of these identified proteins (Fen2p, Gap1p, Hxt5p, Mep2p, Pma1p, Pma2p, and Yck2p) mediate transport functions (GO:0006810); the statistical probability that this degree of enrichment occurs by chance is 5.97 × 10−3, with a false discovery rate of 0.06%. We do not expect that the increases in protein abundance reported here are due strictly to the increased length of plasma membrane present in elongated yeast cells undergoing pseudohyphal growth. From our analysis, yeast cell circumference increases ∼1.3-fold or less on average in cells exhibiting the elongated morphology characteristic of pseudohyphal growth, i.e. a phenotype present in only a subpopulation of cells under nitrogen stress in a filamentous strain of S. cerevisiae. This level of increase in plasma membrane content is insufficient to account for the 2.0-fold changes in abundance exhibited by the proteins listed in Table 1. Thus, we expect that the observed changes in abundance result from differential regulation of these gene products (either at transcription, translation, or post-translationally) during pseudohyphal growth, suggesting potential functional relevance for these proteins in the filamentous growth transition.
TABLE 1.
Proteins at the yeast cell periphery that are differentially abundant during pseudohyphal growth
| Protein | Description | Mean ratio PHG/normala | Total ion C.I.b |
|---|---|---|---|
| % | |||
| Increased abundance | |||
| Ape2 | Aminopeptidase YscII; may have a role in obtaining leucine from dipeptide substrates; subpopulation of enzyme (50% of total) localized to cell wall-bounded periplasmic space | 2.2 | 100 |
| Gap1 | General amino acid permease; induction and subcellular localization regulated in response to the available nitrogen source; localized to the plasma membrane in the absence of an optimal nitrogen source | 2.6 | 100 |
| Hxt5 | Hexose transporter with moderate affinity for glucose; localized to the plasma membrane | 2.0 | 100 |
| Mep2 | Ammonium permease involved in regulation of pseudohyphal growth; subject to nitrogen catabolite repression regulation; localized to the plasma membrane | 2.6 | 100 |
| Phm7 | Protein of unknown function; expression is regulated by phosphate levels; localized to the cell periphery and vacuole | 2.0 | 100 |
| Pma1 | Essential cation-transporting ATPase; major regulator of cytoplasmic pH and plasma membrane potential; localized to the plasma membrane | 2.1 | 100 |
| Pma2 | Plasma membrane isoform of Pma1p; nonessential; major regulator of pH and plasma membrane potential | 2.2 | 100 |
| Yck2 | Palmitoylated casein kinase I isoform; functions with Yck1p in morphogenesis, septin assembly, and endocytic trafficking; localized to the plasma membrane | 2.0 | 100 |
| Ylr414c | Protein of unknown function; localizes in punctate patches (MCC domain) in the plasma membrane | 2.1 | 99.99 |
| Decreased abundance | |||
| Cof1 | Cofilin; promotes actin filament depolarization in a pH-dependent manner; localized to actin cortical patches | 0.3 | 100 |
| Fen2 | Plasma membrane pantothenate symporter; confers sensitivity to the antifungal agent fenpropimorph | 0.1 | 98.59 |
a Mean of 116:114 and 117:114 iTRAQ ratios indicating change of abundance for a given protein under conditions of nitrogen stress (pseudohyphal growth) as compared with normal growth conditions (vegetative growth).
b Total ion confidence interval (C.I.). Proteins are considered significant with a confidence interval greater than 95% and increase/decrease in abundance rounded to 2.0-fold or greater.
To consider the functional significance of these proteins during pseudohyphal growth, we generated homozygous diploid deletion mutants of each corresponding gene in the filamentous Σ1278b genetic background and assayed each mutant for defects in filamentation. By our analysis, deletion of YCK2, HXT5, APE2, PMA2, and GAP2 results in approximately wild-type levels of diploid pseudohyphal growth (Fig. 2A); invasive growth for all tested mutants mirrors surface filamentation (images not shown). PMA1, COF1, FEN2, and PHM7 were not tested; deletion of PMA1 and COF1 is lethal in both filamentous and nonfilamentous strains of yeast, and the homozygous diploid fen2Δ mutant is viable but very sick. We were unable to construct a homozygous diploid mutant deleted for PHM7, as we could not recover viable haploid deletion strains for mating. In total, two deletion mutants yielded clear filamentous growth defects. As shown in Fig. 2A, strains deleted for MEP2 and YLR414C, respectively, exhibit defects in surface-spread pseudohyphal growth on solid low nitrogen medium. Both genes are nonessential under vegetative growth conditions. MEP2 encodes ammonium permease, a known regulator of pseudohyphal growth (33); however, YLR414C encodes a functionally uncharacterized plasma membrane protein, and its contributions to the pseudohyphal growth response have not been studied previously.
FIGURE 2.
Phenotypic analysis of YLR414C. A, colony morphology of homozygous diploid mutants deleted for the indicated genes from Table 1. Yeast cells were grown on low ammonium medium (SLAD), and surface filamentation was assessed qualitatively. As indicated, ylr414cΔ and mep2Δ mutants exhibit defects in pseudohyphal growth. B, haploid strain deleted for YLR414C exhibits reduced surface filamentation, a loss of elongated cell morphology; C, reduced invasive growth, with the latter phenotype assessed by washing the plate surface under a gentle stream of water (see under “Materials and Methods”). Filamentous growth was induced in the haploid strain on low nitrogen plates supplemented with 1% butanol; cells from these colonies were scraped into a suspension in identical liquid media for analysis of cell morphology by differential interference contrast microscopy. D, haploid strain deleted for YLR414C sediments more slowly than a corresponding wild-type (WT) strain and haploid muc1Δ mutant. Dashed lines indicate levels of cells in test tube cultures. Normalized cultures of wild-type, ylr414cΔ, and muc1Δ cells were initially mixed and allowed to settle for 1 h prior to imaging. To quantify the observed changes in cell sedimentation, a 1-ml aliquot was withdrawn from the upper half of each test tube culture for determination of cell density (OD600). Results from three measurements of independent biological replicates are shown to the right, and increased cell density indicates decreased cell sedimentation. E, homozygous diploid strain deleted for ylr414c exhibits a growth defect relative to wild type. As shown, the indicated cultures were normalized to A600 3.0 and serially diluted 5-fold onto normal (left) and low nitrogen (right) media. Concentrations at which differential growth is evident are boxed in red. The observed defect is more pronounced under conditions of nitrogen stress but evident under normal growth conditions in the filamentous Σ1278b strain as well.
Phenotypic Analysis of YLR414C
To further define the YLR414C-mediated filamentous growth phenotype, we assayed a haploid ylr414cΔ mutant for surface-spread filamentation, cell morphology, and invasive growth. The filamentous growth response in yeast is affected by ploidy, as haploid cells grown under low nitrogen conditions exhibit less surface-spread filamentation than isogenic diploids (60). Haploid surface filamentation can be exaggerated upon addition of butanol to solid low nitrogen medium, and the resulting filamentous growth phenotype is dependent upon the Kss1p-MAPK pathway as described classically for diploids (52). Deletion of YLR414C in a haploid strain of the filamentous Σ1278b background results in loss of surface filamentation on low nitrogen medium supplemented with 1% butanol (Fig. 2B). Cells scraped from ylr414cΔ colonies grown under these conditions exhibit a more rounded morphology than haploid wild-type cells (Fig. 2B), and haploid ylr414cΔ is defective in invasive growth on rich medium (Fig. 2C). Thus, YLR414C is required for haploid and diploid filamentous growth and, because of its increased abundance under conditions of nitrogen stress, is renamed here PUN1 (for plasma membrane protein up-regulated during nitrogen stress).
A haploid mutant in the filamentous Σ1278b background deleted for YLR414C/PUN1 sediments more slowly than a wild-type strain of the same genetic background (Fig. 2D). The pun1Δ mutant also sediments more slowly than a strain of the same background deleted for the cell surface flocculin MUC1 under identical growth conditions. In the Σ1278b strain, MUC1 is the only flocculin expressed, and consequently, the Σ1278b strain does not flocculate relative to other nonfilamentous laboratory strains of S. cerevisiae (44). Flocculation is calcium-dependent cell-cell aggregation, and here, we are not strictly assaying for flocculation but rather the ability of yeast cells to settle at the bottom of a test tube. This process is dependent upon cell-cell adhesion; thus, PUN1 is required, presumably indirectly, for wild-type cell-cell adhesion. PUN1 also affects yeast cell growth, with homozygous diploid pun1Δ mutants exhibiting a pronounced growth defect under conditions of nitrogen stress and a mild growth defect under normal growth conditions (Fig. 2E). This growth defect does not account for the observed filamentous growth phenotype, however, as the pun1Δ mutant is capable of forming colonies. In total, from this phenotypic analysis, PUN1 is required for wild-type filamentous growth, cell growth, and cell-cell adhesion.
MCC Domain and Yeast Pseudohyphal Growth
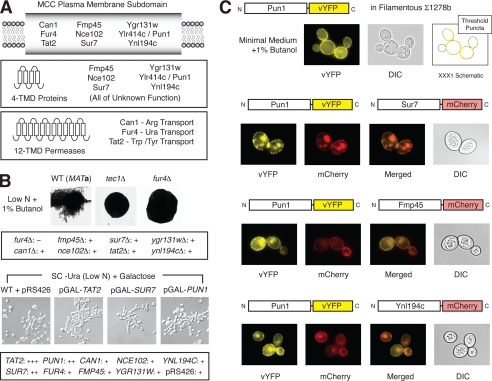
Pun1p localizes to the plasma MCC. The MCC is an ergosterol-rich domain estimated to be ∼300 nm in diameter, corresponding to sites of furrow-like invaginations of the plasma membrane (61–64). The MCC contains the arginine transporter Can1p, the uracil permease Fur4p, and the tryptophan/tyrosine permease Tat2p (62); the tetra span proteins Sur7p, Fmp45p, and Ynl194cp are also localized to the MCC. Grossmann et al. (62) screened the yeast proteome for proteins that co-localize with Sur7p and identified additional integral MCC components, including Ylr414cp/Pun1p. In total, the MCC is now known to contain nine proteins illustrated in Fig. 3A as follows: three transporters with 12 predicted transmembrane domains each and six tetraspan proteins of unknown function. To date, the functional significance of the MCC is unknown, although it may serve a protective function in protein turnover (62).
FIGURE 3.
Phenotypic analysis and localization of MCC proteins under filamentous growth conditions. A, diagrammatic overview of the MCC and its resident proteins. The MCC contains nine integral membrane proteins with either 4 or 12 predicted transmembrane domains (TMDs) as shown. B, haploid deletion mutants were constructed in the filamentous Σ1278b background for all genes encoding plasma membrane proteins localized to the MCC. Colony morphology was assessed qualitatively under conditions that induce filamentous growth; results are summarized in the rectangular inset (“−” indicates reduced filamentous growth and “+” indicates wild-type (WT) surface filamentation), and an image of the surface filamentation defect in fur4Δ is shown. The nonfilamentous tec1Δ mutant is shown for comparison. Images of colony morphology and invasive growth phenotypes for the additional MCC deletion mutants are provided in supplemental Fig. S1. Cell morphology phenotypes upon gene overexpression are indicated in the lower panel. For this study, plasmids carrying MCC component genes under transcriptional control of a galactose-inducible promoter were introduced into a diploid derivative of the filamentous Σ1278b strain, and cell morphology was assessed upon galactose induction under conditions that induce filamentous growth. Images are shown for TAT2, SUR7, and PUN1, and results are summarized below (“+++” and “++” indicate exaggerated filamentous growth). The wild-type strain with empty vector pRS426 serves as a control. Images of the remaining overexpression mutants are provided in supplemental Fig. S2A, and the invasive growth phenotype of TAT2 is shown in supplemental Fig. S2B. C, localization of Pun1p-vYFP in the Σ1278b strain under conditions that induce filamentous growth. The localization of Pun1p and the other MCC component proteins is unchanged under filamentous growth conditions. Pun1p-vYFP localizes in punctate patches at the plasma membrane, consistent with the MCC domain, and co-localizes with Sur7p-mCherry, Fmp45-mCherry, and Ynl194cp-mCherry under filamentous growth conditions. vYFP, variant of yellow fluorescent protein; DIC, differential interference contrast.
To determine whether additional MCC proteins function in the yeast pseudohyphal growth response, we generated single deletion and overexpression mutants of each gene encoding an MCC protein in a derivative of the filamentous Σ1278b genetic background. Each mutant was assayed for colony morphology under conditions that induce filamentous growth in a haploid strain of yeast, and the results from this analysis are presented in Fig. 3B. Deletion of FUR4 results in loss of surface filamentation; the remaining MCC gene deletion mutants, other than the PUN1 mutant presented in Fig. 2, exhibit wild-type filamentous growth properties. Invasive growth phenotypes mirror surface filamentation phenotypes, and corresponding images are shown in supplemental Fig. S1. To assay filamentous growth phenotypes resulting from gene overexpression, plasmid-based MCC genes were expressed from the galactose-inducible GAL1 promoter in a diploid derivative of Σ1278b on minimal medium with galactose as a carbon source. Overexpression of TAT2 resulted in exaggerated cell elongation and invasive growth under conditions of nitrogen stress, although overexpression of SUR7 and PUN1 allowed for exaggerated cell elongation but only enabled wild-type invasive growth. Overexpression of the remaining MCC genes yielded wild-type cell and colony morphology (supplemental Fig. S2). Surface filamentation is reduced in the presence of galactose and was not assayed in this analysis (52). Collectively, these phenotypic studies do not indicate an overall function for the MCC during pseudohyphal growth, but rather highlight the importance of specific MCC transporters and components (e.g. Pun1p) in enabling the wild-type filamentous growth response.
In nonfilamentous strains of yeast, Pun1p localizes in the MCC domain; however, this localization has not been studied in a filamentous yeast background. To determine the localization of Pun1p in the filamentous Σ1278b strain, we generated an integrated PUN1-vYFP fusion, yielding an endogenously expressed Pun1p-vYFP chimera (Fig. 3C). Under conditions of filamentous growth, the Pun1p-vYFP fusion concentrated in punctate patches around the cell periphery, consistent with its localization in the MCC. Furthermore, the Pun1p-vYFP chimera co-localizes with the integral MCC proteins Sur7p, Fmp45p, and Ynl194cp, with each MCC partner visualized as a carboxyl-terminal mCherry fusion. Thus, the MCC is very likely intact during pseudohyphal growth and houses Pun1p in both filamentous and nonfilamentous strains of yeast.
Profiling the Transcriptional Response upon Deletion of PUN1
To identify the molecular basis of the requirement for PUN1 in the pseudohyphal transition, we generated DNA microarray-based transcriptional profiles from a pun1Δ mutant. As Pun1p is not a transcription factor, any changes in transcript abundance upon deletion of PUN1 will result from indirect effects; however, the observed changes will nonetheless provide a detailed readout of cellular processes and pathways affected by PUN1 function. For this study, we used a homozygous diploid strain in the filamentous Σ1278b background deleted for PUN1; a wild-type diploid strain of the same background served as the control. Both strains were grown under conditions of low nitrogen, and RNA was extracted from three biological replicates of each strain after identical culturing (Fig. 4A). The RNA samples were analyzed using DNA microarrays as described under “Materials and Methods.”
FIGURE 4.
Transcriptional profiling of pun1Δ. A, overview of DNA microarray studies to profile transcriptional changes in a homozygous diploid strain deleted for PUN1 under conditions that induce filamentous growth. B, set of 48 genes exhibiting decreased transcript abundance in pun1Δ is statistically enriched for genes involved in amino acid biosynthesis; the corresponding GO accession number and p value is provided, along with the fold-change, t-statistic, and p value for each gene. C, set of 82 genes with increased transcript abundance in pun1Δ is statistically enriched for genes encoding proteins that localize to the plasma membrane, as shown. The color code for the heat maps is shown at the bottom right. WT, wild type.
In total, 82 genes exhibited increased transcript abundance upon deletion of PUN1, although 48 genes yielded decreased mRNA levels. Because the pun1Δ mutant is defective in filamentous growth, the observed transcriptional profile represents, in part, a comparison between a nonfilamentous and filamentous state, even though both the wild-type strain and deletion mutant were grown under conditions of nitrogen stress. In particular, the gene set exhibiting decreased transcript abundance upon deletion of PUN1 was significantly enriched (p value of 6.5 × 10−15) for genes contributing to amino acid biosynthesis (Fig. 4B). The converse effect, up-regulation of amino acid biosynthetic genes, is a known response to conditions of nitrogen stress in yeast. Gasch et al. (65) profiled transcriptional changes in response to nitrogen stress in a nonfilamentous strain of yeast and found increased transcript levels for genes involved in amino acid biosynthesis. Specifically, of the 20 genes exhibiting decreased mRNA abundance in pun1Δ, 15 (ARG1, ARG3, ARG4, ARG5,6, BAT1, CPA2, HIS1, HIS4, LEU1, LEU2, LYS1, MET6, MET13, and STR3) exhibited increased transcript levels after an identical period of nitrogen stress in the Gasch data set. The probability that this level of difference occurs by chance is small (p value of 4.0 × 10−8 by Student's t test), and the correlation between the two data sets in respect to this subset of 20 genes is −0.25, indicating a mild degree of negative correlation. Similarly, by microarray analysis of transcriptional changes underlying the transition to low nitrogen conditions in a filamentous strain of yeast (29), we observed increased mRNA levels for 10 of these same genes (ARG1, ARG3, ARG4, ARG5,6, CPA2, LEU1, LEU2, LYS1, and MET13). Thus, in the absence of PUN1, a subset of genes involved in amino acid biosynthesis exhibit decreased transcript abundance, a response distinct from and approximately opposite the transcriptional changes observed in a wild-type strain during nitrogen stress.
Transcripts exhibiting increased abundance upon deletion of PUN1 are statistically enriched for genes that encode plasma membrane proteins (p value of 2.2 × 10−4). As indicated in Fig. 4C, this gene set is heterogeneous in function and includes several genes involved in the yeast cell response to mating pheromone (STE18, FUS1, FUS3, STE4, GPA1, and STE3). Deletion of a cell wall or plasma membrane protein often elicits a compensatory increase in the abundance of related proteins, and we expect that the transcriptional effect observed here is an example of this response. The increase in FUS3 mRNA upon PUN1 deletion is extremely large; however, we have no evidence to indicate a direct functional link between the genes. FUS3 is the pheromone-responsive MAPK at the foot of the MAPK pathway mediating mating in yeast (21). Because many components of the yeast pheromone response pathway exhibit increased mRNA levels in the pun1Δ mutant, it is possible that the very strong increase in FUS3 mRNA represents the cumulative output resulting from increases in transcript levels of upstream genes within this pathway. Further studies will be needed to determine the mechanism by which PUN1 impacts the yeast mating pathway.
Collectively, deletion of PUN1 results in transcriptional changes that are atypical for cells under conditions of nitrogen stress. These transcriptional effects are consistent with the absence of filamentous growth in the pun1Δ mutant, and the microarray profiles do not indicate a wild-type response to nitrogen stress despite the growth in low nitrogen media. Thus, PUN1 contributes to the wild-type cellular response to nitrogen stress through signaling pathways that regulate the expression of genes involved in amino acid biosynthesis.
Transcription Factor Mss11p Directly Regulates PUN1 Expression
By mass spectrometry, the abundance of Pun1p is increased ∼2-fold under conditions of nitrogen stress in a filamentous strain of S. cerevisiae. To validate this finding, we generated an allele of PUN1 encoding three copies of the HA epitope at its 3′-end and analyzed the abundance of this Pun1p-HA fusion by Western blotting. As shown in Fig. 5A, Western analysis indicates a 1.8-fold increase in Pun1p-HA abundance under conditions of nitrogen stress, confirming the mass spectrometry result.
FIGURE 5.
Regulated PUN1 expression. A, Western blot of Pun1p tagged with three copies of the HA epitope under normal and low nitrogen (N) conditions in the filamentous Σ1278b background. Densitometry was used to quantify Pun1p abundance relative to the Pgk1p control; resulting values were made relative to Pun1p levels under normal nitrogen and are indicated in the bar below the Western blot. This analysis confirms our mass spectrometry result that the abundance of Pun1p is approximately doubled (increased 1.8-fold by Western analysis) under conditions of nitrogen stress. B, RT-PCR analysis of PUN1 mRNA levels in strains deleted for key filamentous growth transcription factors. PUN1 mRNA levels in each mutant were set relative to wild-type (WT) levels, and the relative expression levels with standard deviation are indicated below the bar graph. All RT-PCR assays were performed on cells grown under conditions that induce filamentous growth (standard growth medium supplemented with 1% butanol for 6 h). Strains deleted for FLO8 and MSS11 exhibited decreased levels of PUN1 mRNA. C, chromatin (Chr.) immunoprecipitation of protein A-tagged Flo8p and Mss11p. Oligonucleotide primers for the amplification of DNA fragments for chromatin immunoprecipitation are listed in supplemental Table S1; corresponding DNA fragments are represented graphically here, with the fragment from the MUC1 promoter serving as a positive control and the indicated region of coding sequence from KSS1 serving as a negative control. Results from an untagged wild-type strain are provided as a control. PCR results from the input samples and immunoprecipitated samples are shown (gel images to the left and quantified PCR product to the right for immunoprecipitated Flo8p-ProA and Mss11p-ProA). Error bars indicate standard deviation. As indicated, protein A-tagged Mss11p binds to fragments 3 and 4 upstream of PUN1. D, diagram summarizing the transcriptional regulation of PUN1 and pathway context of Flo8p and Mss11p.
Consistent with this increase in Pun1p abundance, PUN1 transcript levels are also increased under similar growth conditions in the filamentous Σ1278b strain of yeast. DNA microarray analysis (29) identified an increase in PUN1/YLR414C mRNA levels in a wild-type strain of Σ1278b after 2 h of growth in low nitrogen media. To determine whether this increase in transcript abundance is dependent upon filamentous growth regulators, we assayed PUN1 mRNA levels by RT-PCR in strains deleted for the key pseudohyphal growth transcription factors STE12, TEC1, FLO8, and MSS11 (Fig. 5B). Ste12p and Tec1p act downstream of the filamentous growth MAPK pathway (66, 67), and deletion of the corresponding genes, both singly (ste12Δ, tec1Δ) and in combination (ste12Δ/tec1Δ), does not significantly affect PUN1 mRNA levels. However, mutants of the Σ1278b background singly deleted for MSS11 and FLO8 exhibit strongly reduced levels of PUN1 transcript. Mss11p is a filamentous growth transcription factor that by genetic studies is acted upon by both the MAPK and PKA pathways (68); the Flo8p transcription factor acts downstream of the filamentous growth PKA pathway (17, 69). After 6 h of growth under conditions that induce filamentous growth, PUN1 mRNA abundance in mss11Δ and flo8Δ mutants is decreased to levels approximately one-eighth that of a corresponding wild-type strain.
By genetic epistasis studies, overexpression of PUN1 does not rescue filamentous growth defects of the MAPK pathway transcription factors STE12 and TEC1; however, PUN1 overexpression also fails to rescue filamentous growth defects in strains deleted for FLO8 and MSS11 (supplemental Fig. S3). This likely reflects the fact that these transcription factors target hundreds of genes and that expression of PUN1 is insufficient to enable the full pseudohyphal growth response. Thus, genetic epistasis studies are uninformative as a means to place PUN1 in the context of a signaling pathway.
Consequently, to determine whether Mss11p and/or Flo8p directly regulates PUN1 transcription, we assayed PUN1 promoter occupancy by chromatin immunoprecipitation (Fig. 5C). Flo8p does not bind the PUN1 promoter in a region encompassing 831 bp upstream of the gene coding sequence. Mss11p, however, does bind the PUN1 promoter in a region of genomic DNA between 435 and 831 bp upstream of its start codon. As a positive control, both protein A-tagged Mss11p and Flo8p recognize the MUC1 promoter in our assay, consistent with published results (34, 70). Thus, Flo8p regulates PUN1 transcription indirectly, although Mss11p directly binds the PUN1 promoter.
DISCUSSION
In this paper, we applied mass spectrometry to identify changes in protein abundance during yeast pseudohyphal growth. Our analysis focused on plasma membrane protein preparations from a filamentous strain of S. cerevisiae under vegetative growth conditions and under conditions of nitrogen stress. By iTRAQ-based mass spectrometry, we identified 11 proteins that are differentially abundant under conditions of nitrogen stress and that have been localized previously to the yeast cell periphery. In particular, our work identified the gene PUN1 (formerly YLR414C). Pun1p is a component of the plasma membrane MCC subdomain, but its function and regulation were otherwise uncharacterized prior to this study. PUN1 is required for surface-spread and invasive filamentous growth in both haploids and diploids; its deletion also affects cell sedimentation, presumably by affecting cell-cell adhesion. Overexpression of PUN1 results in exaggerated cell elongation, although invasive growth properties are not appreciably increased over wild type. By RT-PCR and chromatin immunoprecipitation, the transcription factor Mss11p binds to the PUN1 promoter and regulates its transcription, indicating a filamentous growth pathway context for PUN1. In total, this work profiles changes in protein abundance at the yeast cell periphery during filamentous growth and identifies a previously uncharacterized gene that is required for the wild-type yeast filamentous response to nitrogen stress.
Changes in Protein Abundance at the Yeast Cell Periphery
By mass spectrometry of plasma membrane protein preparations from cells undergoing vegetative growth and from cells grown under conditions that induce filamentation, we find that changes in protein abundance at the yeast cell periphery may not be widespread. Admittedly, our analysis is not comprehensive for plasma membrane proteins, and we sampled an even smaller population of cell wall proteins; however, we did profile ∼25% of all annotated yeast plasma membrane proteins (65 proteins analyzed of 272 total) and only identified 8 proteins (12%) that are differentially abundant under conditions of nitrogen stress in the filamentous Σ1278b strain. We also identified three additional proteins with known localization at the yeast cell periphery that changed abundance; thus, in total, 98 proteins at the yeast cell periphery were analyzed in this study, and 11 exhibited changes in protein abundance (∼11%). In contrast, whole genome microarray studies indicate that ∼37% of the genome as a whole exhibits differential transcript abundance during a 2-hour exposure to nitrogen stress in the same genetic background used in this study (29). Thus, the yeast cell periphery may not undergo a comprehensive makeover in protein composition during filamentous growth, emphasizing the importance of those proteins that do change abundance as potentially critical mediators of cellular processes during the yeast filamentous response to nitrogen stress.
This set of differentially abundant proteins at the yeast cell periphery is significantly enriched for transport proteins, including permeases for ammonium, general amino acids, cations, and hexose. Although the specific contributions of each transport protein during filamentous growth is unknown, the differential abundance of these proteins is conceptually consistent with established roles for transporters in sensing and responding to environmental growth conditions. For example, the ammonium permease Mep2p is a known sensor of ammonium availability, exhibiting both transport functions and a regulatory role affecting the filamentous growth MAPK pathway through a signaling link that has yet to be determined (71). The differential abundance of transporters under conditions that induce filamentous growth suggests that these proteins are likely to be important regulatory targets during filamentation, either transcriptionally or post-transcriptionally. Furthermore, our data set emphasizes the point that transport proteins contribute strongly to the initiation and control of filamentous growth, a contribution that is less well studied, but no less important, than that of downstream signaling modules encompassing the filamentous growth MAPK cascade and PKA.
PUN1 Is Essential for Filamentous Growth and Is an Important Component of the Yeast Response to Nitrogen Deprivation and Environmental Stress
Pun1p is a component of the MCC domain and exhibits sequence similarity to proteins in Candida glabrata, Emericella nidulans, and several other fungi. Our data identify an essential role for PUN1 during yeast filamentous growth, as pun1Δ mutants do not undergo surface or invasive filament formation in haploids or diploids. Under conditions that induce filamentous growth, pun1Δ mutants exhibit decreased cell sedimentation, indicating that Pun1p indirectly affects cell-cell adhesion. Mutants deleted for PUN1 also exhibit transcriptional profiles that indicate an absence of filamentous growth and impaired cellular response to conditions of nitrogen stress. The latter phenotype suggests that Pun1p contributes to the mechanism by which yeast cells sense and respond to nitrogen stress. Considered in its full context, however, Pun1p may also be characterized as part of the yeast cell response to environmental stress, rather than as strictly a filamentous growth protein. PUN1 mRNA levels are increased in a nonfilamentous strain of S. cerevisiae under conditions of nitrogen stress (65), and the abundance of PUN1 transcript is also increased upon cell wall damage (72). In addition, pun1Δ mutants have been found to exhibit decreased thermotolerance (73). Thus, Pun1p contributes to the yeast response to nitrogen stress in particular and to the cellular response to environmental stress in general.
Pathway Context and Regulation of PUN1
Our studies identify Mss11p as a direct regulator of PUN1 expression and Flo8p as an indirect regulator. Both transcription factors are required for wild-type yeast filamentous growth; Flo8p is phosphorylated by the Tpk2p subunit of PKA, and Mss11p is thought to play a central role downstream of both the filamentous growth MAPK and PKA pathways (74). From previously published genetic epistasis studies, Mss11p may be acted upon by many filamentous growth factors, including the transcriptional regulators PHD1, NRG1/2, and FLO8 itself (68); thus, in S. cerevisiae, Mss11p is thought to act downstream of Flo8p. In C. albicans, however, Flo8p and Mss11p physically interact with each other and may cooperatively bind target gene promoters (75). Similar results have been found in Saccharomyces diastaticus, where Mss11p and Flo8p bind cooperatively to the sequence TTTGCnGCAAA (n = 97) in the STA1 promoter (76). This sequence is not present in the PUN1 promoter, and no consensus motif for Mss11p DNA binding is available in any organism. Studies in S. cerevisiae have failed to identify binding between Mss11p and Flo8p (68), and our analysis identifies Mss11p binding to the PUN1 promoter but not Flo8p binding. Thus, our results are consistent with existing models in S. cerevisiae, wherein Mss11p acts downstream of Flo8p to regulate transcription of PUN1; these regulatory relationships are presented diagrammatically in Fig. 5D. In contrast, we do not identify a role for the filamentous growth MAPK-regulated transcription factors Ste12p and Tec1p in controlling transcription of PUN1. From our results, we identify PUN1 as a transcriptional target of Mss11p, placing PUN1 downstream of both the MAPK and PKA pathways through Mss11p-mediated transcriptional control.
Pun1p is an integral component of the yeast MCC, and the MCC has been reported to provide a protective area, regulating host protein turnover (62). However, we do not expect that reduced turnover is responsible for the increase in Pun1p abundance under conditions of nitrogen stress for two reasons. First, Pun1p is localized to the MCC under vegetative growth conditions as well as during filamentous growth. Second, PUN1 undergoes changes in transcript abundance consistent with its changes in protein abundance. Collectively, this suggests that PUN1 is regulated transcriptionally and that this transcriptional control likely accounts for the changes in Pun1p abundance.
Considering that Pun1p is a plasma membrane protein required for the wild-type yeast response to nitrogen deprivation, it is tempting to speculate that Pun1p may act as part of the mechanism by which yeast cells sense nitrogen status. Along those lines, Pun1p may function as a transporter, as does Mep2p and all three functionally characterized components of the MCC. However, initial genetic studies have failed to identify a function for PUN1 in amino acid transport,3 and extensive biochemical studies will likely be necessary to identify the molecular function of Pun1p. Nonetheless, this study identifies a previously unappreciated role for Pun1p in the nitrogen stress response during filamentous growth, with effects on surface and invasive filamentation and cell-cell adhesion that are suggestive of Mep2p, Muc1p, and other key proteins in the yeast filamentous growth response.
Acknowledgments
We thank Damian Krysan (University of Rochester) and Robert Fuller (University of Michigan) for providing filamentous yeast strains, and Owen Ryan and Charles Boone (University of Toronto) for providing selected deletion mutants in the Σ1278b background. Mass spectrometry was performed at the Michigan Proteome Consortium in the Andrews laboratory (University of Michigan). We thank John Strahler and Angela Walker for assistance with the generation and analysis of mass spectrometry data. Microarray studies were performed by Joe Washburn at the University of Michigan Microarray Facility.
This work was supported, in whole or in part, by National Institutes of Health Grant 1R21A1084539-01 (to A. K.). This work was also supported by Grant RSG-06-179-01-MBC from the American Cancer Society.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S3.
T. Xu and A. Kumar, unpublished results.
- PKA
- cAMP-dependent protein kinase
- MCC
- membrane compartment of Can1
- iTRAQ
- isobaric tags for relative and absolute quantification
- SCX
- strong cation exchange
- RT
- reverse transcription
- MAPK
- mitogen-activated protein kinase
- HA
- hemagglutinin
- GO
- Gene Ontology
- SLAD
- synthetic low ammonium dextrose medium.
REFERENCES
- 1.Gimeno C. J., Ljungdahl P. O., Styles C. A., Fink G. R. (1992) Cell 68, 1077–1090 [DOI] [PubMed] [Google Scholar]
- 2.Liu H., Styles C. A., Fink G. R. (1993) Science 262, 1741–1744 [DOI] [PubMed] [Google Scholar]
- 3.Chandarlapaty S., Errede B. (1998) Mol. Cell. Biol. 18, 2884–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erdman S., Snyder M. (2001) Genetics 159, 919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn S. H., Acurio A., Kron S. J. (1999) Mol. Biol. Cell 10, 3301–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edgington N. P., Blacketer M. J., Bierwagen T. A., Myers A. M. (1999) Mol. Cell. Biol. 19, 1369–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miled C., Mann C., Faye G. (2001) Mol. Cell. Biol. 21, 3714–3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kron S. J., Styles C. A., Fink G. R. (1994) Mol. Biol. Cell 5, 1003–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H., Styles C. A., Fink G. R. (1996) Genetics 144, 967–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W., Mitchell A. P. (1997) Genetics 145, 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cullen P. J., Sprague G. F. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 13619–13624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo H. J., Köhler J. R., DiDomenico B., Loebenberg D., Cacciapuoti A., Fink G. R. (1997) Cell 90, 939–949 [DOI] [PubMed] [Google Scholar]
- 13.Mösch H. U., Roberts R. L., Fink G. R. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 5352–5356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kübler E., Mösch H. U., Rupp S., Lisanti M. P. (1997) J. Biol. Chem. 272, 20321–20323 [DOI] [PubMed] [Google Scholar]
- 15.Mösch H. U., Kübler E., Krappmann S., Fink G. R., Braus G. H. (1999) Mol. Biol. Cell 10, 1325–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson L. S., Fink G. R. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 13783–13787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan X., Heitman J. (1999) Mol. Cell. Biol. 19, 4874–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peter M., Neiman A. M., Park H. O., van Lohuizen M., Herskowitz I. (1996) EMBO J. 15, 7046–7059 [PMC free article] [PubMed] [Google Scholar]
- 19.Leberer E., Wu C., Leeuw T., Fourest-Lieuvin A., Segall J. E., Thomas D. Y. (1997) EMBO J. 16, 83–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook J. G., Bardwell L., Thorner J. (1997) Nature 390, 85–88 [DOI] [PubMed] [Google Scholar]
- 21.Madhani H. D., Styles C. A., Fink G. R. (1997) Cell 91, 673–684 [DOI] [PubMed] [Google Scholar]
- 22.Abdullah U., Cullen P. J. (2009) Eukaryot. Cell 8, 1362–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlson M., Osmond B. C., Botstein D. (1981) Genetics 98, 25–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celenza J. L., Carlson M. (1984) Mol. Cell. Biol. 4, 49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuchin S., Vyas V. K., Carlson M. (2002) Mol. Cell. Biol. 22, 3994–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vyas V. K., Kuchin S., Berkey C. D., Carlson M. (2003) Mol. Cell. Biol. 23, 1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elbing K., McCartney R. R., Schmidt M. C. (2006) Biochem. J. 393, 797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borneman A. R., Leigh-Bell J. A., Yu H., Bertone P., Gerstein M., Snyder M. (2006) Genes Dev. 20, 435–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma J., Jin R., Jia X., Dobry C. J., Wang L., Reggiori F., Zhu J., Kumar A. (2007) Genetics 177, 205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bharucha N., Ma J., Dobry C. J., Lawson S. K., Yang Z., Kumar A. (2008) Mol. Biol. Cell 19, 2708–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin R., Dobry C. J., McCown P. J., Kumar A. (2008) Mol. Biol. Cell 19, 284–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo W. S., Dranginis A. M. (1996) J. Bacteriol. 178, 7144–7151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorenz M. C., Heitman J. (1998) EMBO J. 17, 1236–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rupp S., Summers E., Lo H. J., Madhani H., Fink G. (1999) EMBO J. 18, 1257–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cullen P. J., Sabbagh W., Jr., Graham E., Irick M. M., van Olden E. K., Neal C., Delrow J., Bardwell L., Sprague G. F., Jr. (2004) Genes Dev. 18, 1695–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorenz M. C., Heitman J. (1998) Genetics 150, 1443–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo B., Styles C. A., Feng Q., Fink G. R. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 12158–12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birkaya B., Maddi A., Joshi J., Free S. J., Cullen P. J. (2009) Eukaryot. Cell 8, 1118–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lo W. S., Dranginis A. M. (1998) Mol. Biol. Cell 9, 161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gagiano M., van Dyk D., Bauer F. F., Lambrechts M. G., Pretorius I. S. (1999) Mol. Microbiol. 31, 103–116 [DOI] [PubMed] [Google Scholar]
- 41.Reynolds T. B., Fink G. R. (2001) Science 291, 878–881 [DOI] [PubMed] [Google Scholar]
- 42.Halme A., Bumgarner S., Styles C., Fink G. R. (2004) Cell 116, 405–415 [DOI] [PubMed] [Google Scholar]
- 43.Voynov V., Verstrepen K. J., Jansen A., Runner V. M., Buratowski S., Fink G. R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 14423–14428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Douglas L. M., Li L., Yang Y., Dranginis A. M. (2007) Eukaryot. Cell 6, 2214–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramsook C., Tan C., Garcia M. C., Fung R., Soybelman G., Henry R., Litewka A., O'Meally S., Otoo H. N., Khalaf R. A., Dranginis A. M., Gaur N. K., Klotz S. A., Rauceo J. M., Jue C. K., Lipke P. N. (2010) Eukaryot. Cell 9, 393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forsberg H., Ljungdahl P. O. (2001) Mol. Cell. Biol. 21, 814–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baudin A., Ozier-Kalogeropoulos O., Denouel A., Lacroute F., Cullin C. (1993) Nucleic Acids Res. 21, 3329–3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagai T., Ibata K., Park E. S., Kubota M., Mikoshiba K., Miyawaki A. (2002) Nat. Biotechnol. 20, 87–90 [DOI] [PubMed] [Google Scholar]
- 49.Ma J., Bharucha N., Dobry C. J., Frisch R. L., Lawson S., Kumar A. (2008) Autophagy 4, 792–800 [DOI] [PubMed] [Google Scholar]
- 50.Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. (1998) Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 51.Guthrie C., Fink G. (1991) Guide to Yeast Genetics and Molecular Biology, pp. 12–17, Academic Press, San Diego [Google Scholar]
- 52.Lorenz M. C., Cutler N. S., Heitman J. (2000) Mol. Biol. Cell 11, 183–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang A. (2002) Methods Enzymol. 351, 339–350 [DOI] [PubMed] [Google Scholar]
- 54.Keshamouni V. G., Jagtap P., Michailidis G., Strahler J. R., Kuick R., Reka A. K., Papoulias P., Krishnapuram R., Srirangam A., Standiford T. J., Andrews P. C., Omenn G. S. (2009) J. Proteome Res. 8, 35–47 [DOI] [PubMed] [Google Scholar]
- 55.Li J. Y., Lescure P. A., Misek D. E., Lai Y. M., Chai B. X., Kuick R., Thompson R. C., Demo R. M., Kurnit D. M., Michailidis G., Hanash S. M., Gantz I. (2002) J. Biol. Chem. 277, 9069–9076 [DOI] [PubMed] [Google Scholar]
- 56.Juan H. F., Huang H. C. (2007) Methods Mol. Biol. 382, 405–416 [DOI] [PubMed] [Google Scholar]
- 57.Grenson M. (1966) Biochim. Biophys. Acta 127, 339–346 [DOI] [PubMed] [Google Scholar]
- 58.Gingras A. C., Aebersold R., Raught B. (2005) J. Physiol. 563, 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ranish J. A., Yi E. C., Leslie D. M., Purvine S. O., Goodlett D. R., Eng J., Aebersold R. (2003) Nat. Genet. 33, 349–355 [DOI] [PubMed] [Google Scholar]
- 60.Gancedo J. M. (2001) FEMS Microbiol. Rev. 25, 107–123 [DOI] [PubMed] [Google Scholar]
- 61.Young M. E., Karpova T. S., Brügger B., Moschenross D. M., Wang G. K., Schneiter R., Wieland F. T., Cooper J. A. (2002) Mol. Cell. Biol. 22, 927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grossmann G., Malinsky J., Stahlschmidt W., Loibl M., Weig-Meckl I., Frommer W. B., Opekarová M., Tanner W. (2008) J. Cell Biol. 183, 1075–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alvarez F. J., Douglas L. M., Rosebrock A., Konopka J. B. (2008) Mol. Biol. Cell 19, 5214–5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strádalová V., Stahlschmidt W., Grossmann G., Blazíková M., Rachel R., Tanner W., Malinsky J. (2009) J. Cell Sci. 122, 2887–2894 [DOI] [PubMed] [Google Scholar]
- 65.Gasch A. P., Spellman P. T., Kao C. M., Carmel-Harel O., Eisen M. B., Storz G., Botstein D., Brown P. O. (2000) Mol. Biol. Cell 11, 4241–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Madhani H. D., Fink G. R. (1997) Science 275, 1314–1317 [DOI] [PubMed] [Google Scholar]
- 67.Bao M. Z., Schwartz M. A., Cantin G. T., Yates J. R., 3rd, Madhani H. D. (2004) Cell 119, 991–1000 [DOI] [PubMed] [Google Scholar]
- 68.van Dyk D., Pretorius I. S., Bauer F. F. (2005) Genetics 169, 91–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kobayashi O., Suda H., Ohtani T., Sone H. (1996) Mol. Gen. Genet. 251, 707–715 [DOI] [PubMed] [Google Scholar]
- 70.Gagiano M., Bester M., van Dyk D., Franken J., Bauer F. F., Pretorius I. S. (2003) Mol. Microbiol. 47, 119–134 [DOI] [PubMed] [Google Scholar]
- 71.Rutherford J. C., Chua G., Hughes T., Cardenas M. E., Heitman J. (2008) Mol. Biol. Cell 19, 3028–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodríguez-Peña J. M., Pérez-Díaz R. M., Alvarez S., Bermejo C., García R., Santiago C., Nombela C., Arroyo J. (2005) Microbiology 151, 2241–2249 [DOI] [PubMed] [Google Scholar]
- 73.Mir S. S., Fiedler D., Cashikar A. G. (2009) Mol. Cell. Biol. 29, 187–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pan X., Heitman J. (2002) Mol. Cell. Biol. 22, 3981–3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su C., Li Y., Lu Y., Chen J. (2009) Eukaryot. Cell 8, 1780–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim T. S., Kim H. Y., Yoon J. H., Kang H. S. (2004) Mol. Cell. Biol. 24, 9542–9556 [DOI] [PMC free article] [PubMed] [Google Scholar]