Abstract
Objectives: To determine the prevalence of the pathogenic strain of Neisseria meningitidis in contacts of patients with meningococcal disease, and to determine which contact groups are likely to be carriers and warrant chemoprophylaxis.
Design: Population based study.
Setting: Norwegian county of Telemark.
Subjects: 1535 primary contacts of 48 patients with meningococcal disease, and 78 secondary contacts.
Interventions: Carriers of the pathogenic strain were treated with rifampicin. All household members and kissing contacts under 15 years of age were treated with oral penicillin. Contacts were taught to recognise the symptoms of meningococcal disease.
Results: In 27 of 48 cases investigated, contacts carrying the pathogenic strain of N meningitidis were found. A total of 42 such contacts were identified. Contacts were stratified into three classes according to the assumed closeness of contact with patients. In class 1 (household members and kissing contacts) the prevalence of the pathogenic strain was 12.4% (95% confidence interval 5.5% to 19.3%). In classes 2 and 3 the prevalence was 1.9% (0.9% to 3.4%) and 1.6% (0.14% to 3.1%).
Conclusions: There is a high rate of carriage of the pathogenic strain of N meningitidis in patients’ household members and kissing contacts, and this supports the practice of giving chemoprophylaxis to these contacts. The prevalence of carriage among other contacts is 2-3 times that found in the general population (0.7%); the benefits of chemoprophylaxis to these contacts may be marginal.
Key messages
Contacts of patients with meningococcal disease have a 12.4% (95% confidence interval 5.5% to 19.3%) risk of carrying the pathogenic meningococcus if they are kissing contacts or household members
The risk of carriage of the pathogenic strain for two groups of contacts less close than household members or kissing contacts is 1.9% (0.9% to 3.4%) and 1.6% (0.14% to 3.1%)
Introduction
Contacts of patients with meningococcal disease have an increased risk of contracting the disease (relative risk for household members between 1000 and 4000).1–3 When meningococcal disease occurs, often carriers of the pathogenic strain of Neisseria meningitidis can be found in the patient’s contacts.4–6 These carriers may develop the disease or the bacterium may spread from person to person eventually causing disease in someone without apparent link with the first patient. The frequency of secondary or associated cases has been reported as 0.5%.7 However, estimates may be higher if the time interval is extended7 or if epidemiological studies using sensitive identification techniques for bacterial strains are applied.5,8,9 One study found 22 (9.5%) associated cases among 220 cases of meningococcal disease in Norway during 1994-96 using such methods.10
To prevent the spread of meningococcal infection, the World Health Organisation and the health authorities of most countries recommend that close contacts should receive chemoprophylaxis to eradicate the pathogenic strain.11–14 However, it may be difficult to define who is a close contact, and still more difficult to define who should be excluded from this definition. Therefore chemoprophylaxis is often given to more contacts than is needed.15 In Norway, before 1970, liberal sulphonamide chemoprophylaxis was practised. However, the emergence of a virulent clone of N meningitidis that was resistant to sulphonamide16 led the Norwegian authorities to abandon chemoprophylaxis for fear of further resistance problems. Instead, household members under 15 years of age are assumed to have meningococcal disease and are treated with penicillin orally for 1 week.17
Since November 1987 we have run the Telemark meningococcal project in which rifampicin prophylaxis is targeted to carriers of the pathogenic strains of N meningitidis identified by DNA fingerprinting of nasopharyngeal meningococci. Secondary cases have not been observed.5 We used data from this project to address the questions: “Who is most likely to carry the pathogenic strain of N meningitidis after a case of meningococcal disease?” and “To whom should chemoprophylaxis be restricted?”
Subjects and methods
The Telemark project
The detailed organisation of this project has been described previously.5 After isolation of meningococci from a patient specimen the local health officer is alerted, who then collects throat samples from members of the patient’s household before initiating penicillin treatment to those under 15 years of age.17 Parents accompanying the patient to hospital are often sampled by the hospital staff.
Based on information from people who know the patient a list of close contacts is drawn up; throat samples are collected from these contacts on the same or next day. Simultaneously, the local community is informed about the disease in open meetings. When a pathogenic strain is found, rifampicin prophylaxis (600 mg twice daily for two days; children <12 years of age 10 mg/kg) is given and throat samples collected from the contact’s household and kissing contacts (secondary contacts).
For each contact a standard questionnaire is completed for personal data, type of contact with the patient, and symptoms of respiratory disease.
Collection of throat samples
—Both tonsils and the posterior pharyngeal surface were sampled with a cotton swab, plated immediately on GC agar base (Mast Diagnostics, Merseyside, UK) supplemented with haematin, 1% IsoVitalex (BBL, Cockeysville, MD,USA), vancomycin (3 mg/l), and colistin (7.5 mg/l), and incubated at 37°C in 10% carbon dioxide within two hours of sampling. Wherever possible, sampling was done by two of the authors (BK and YT).
Identification of the pathogenic strain
—Contacts carrying a meningococcus with a chromosomal DNA fingerprint identical to that of the patient isolate were identified as previously described18 and were defined as carrying the pathogenic strain (fig).
Statistical methods
Confidence intervals for the prevalence of the pathogenic strain were calculated as follows:
The parameter of interest is the probability, p, of carrying the pathogenic strain.
m is the number of patients diagnosed. Assume that patient i has nij contacts in class j, and that each of them has probability pij of carrying a pathogenic strain. The number, Xij, of the nij contacts carrying the pathogenic strain is a binomial (nijpij; i=1,2,...m; j= I, II, III).
Then pj, the probability of carriage for each of the three contact classes, is estimated by the weighted average of the pij’s:
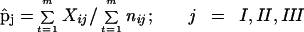 |
1 |
which has a statistical mean pj and a statistical variance estimated by
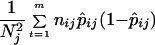 |
2 |
Since the nij’s and Xij’s are small, the p̂ij’s are estimated by
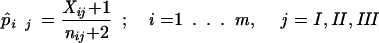 |
Thus an approximate 95% confidence interval for pj is given by
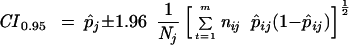 |
Results
Disease characteristics
—From 1 November 1987 to 1 December 1996 there were 48 cases (cases 3-50) of meningococcal disease in the county of Telemark, Norway, verified bacteriologically (table 1). Thirty isolates were serogroup B, 14 were serogroup C, three were serogroup Y, and one was serogroup W135. Twenty four of the patients were under 4 years of age. The remaining cases were distributed in the age groups 5-12 years (six cases), 13-18 years (nine), 19-60 years (five), and >60 years (four).
Table 1.
Details of 48 patients who presented with meningococcal disease in Telemark, Norway from 1 November 1987 to 1 December 1996
| Age (years) | Serogroup
|
No of patients | No of patients with contacts carrying pathogenic strain | |||
|---|---|---|---|---|---|---|
| B | C | Y | W135 | |||
| 0-4 | 16 | 8 | — | — | 24 | 14 |
| 5-12 | 4 | 1 | 1 | — | 6 | 5 |
| 13-18 | 3 | 4 | 2 | — | 9 | 5 |
| 19-60 | 5 | — | — | — | 5 | 1 |
| >60 | 2 | 1 | — | 1 | 4 | 2 |
| Total | 30 | 14 | 3 | 1 | 48 | 27 |
Classification of contacts into groups and classes
—We collected throat specimens from 1535 close contacts (primary contacts) of the patients, and from 78 secondary contacts who were household members or kissing contacts of primary contacts found to carry the pathogenic strain. All contacts approached consented to sampling. The primary contacts were divided into 16 contact groups and further organised into three classes according to the degree of contact with the patient (tables 2 to 4). Class 1 consists of household members and kissing contacts, the groups of contacts assumed to have the closest contact with the patient. Class 2 contacts are considered to have closer contact with the patient than class 3 contacts. Secondary contacts were placed in a separate group, group 18 (table 5).
Table 2.
Bacteriological findings in class 1 contacts (household members and kissing contacts)
| Group | Contact | Total No | No (%) of carriers | No (%) of carriers with pathogenic strain |
|---|---|---|---|---|
| 1 | Father | 37 | 9 (24) | 5 (14) |
| 2 | Mother | 37 | 9 (24) | 6 (16) |
| 3 | Sister | 21 | 2 (10) | 1 (5) |
| 4 | Brother | 29 | 6 (21) | 4 (14) |
| 5 | Kissing | 3 | 1 (33) | 1 (33) |
| 6 | Others | 18 | 9 (50) | 1 (6) |
| All groups | — | 145 | 36 (25) | 18 (12) |
Table 4.
Bacteriological findings in class 3 contacts
| Group | Contacts | Total No | No (%) of carriers | No (%) of carriers with pathogenic strain |
|---|---|---|---|---|
| 13 | Classmates | 286 | 51 (18) | 4 (1) |
| 14 | Children at same nursery | 220 | 9 (4) | 2 (1) |
| 15 | Teachers | 44 | 2 (5) | 0 |
| 16 | Others* | 264 | 31 (12) | 7 (3) |
| All groups | — | 814 | 93 (11.4) | 13 (1.6) |
Neighbours, pupils at same school but not in patient’s class, nurses, and doctors. Contacts sampled only when they had been in close contact with patient during past two weeks before patient became ill.
Table 5.
Contacts carrying pathogenic strain. Age in years unless stated otherwise
| Outbreak No | Sex (age) of patient | Serogroup | Contacts carrying pathogenic strain
|
||
|---|---|---|---|---|---|
| Relation to patient (sex) | Age | Contact group | |||
| 03 | Male (5) | B | Employee daycare centre (male) | 26 | 10 |
| Mother | 35 | 2 | |||
| 04 | Female (11) | B | Father | 35 | 1 |
| 05 | Male (2) | B | Brother | 6 months | 4 |
| Mother | 25 | 2 | |||
| 09 | Female (3) | C | Neighbour (female) | 12 | 16 |
| Playmate (male) | 4 | 16 | |||
| Playmate (male) | 4 | 16 | |||
| Neighbour (female) | 1 | 16 | |||
| Neighbour (female) | 2 | 16 | |||
| Neighbour (male) | 16 | 16 | |||
| 12 | Male (5 months) | B | Father | 28 | 1 |
| 13 | Male (4) | B | Brother | 16 | 4 |
| Mother | 37 | 2 | |||
| 14 | Male (11) | Y | Playmate (male) | 10 | 9 |
| Neighbour (male) | 7 | 16 | |||
| 17 | Female (18) | C | Friend (male) | 19 | 9 |
| Classmate (male) | 19 | 13 | |||
| 18 | Male (1) | C | Mother | 34 | 2 |
| Brother | 4 | 4 | |||
| Home help (female) | 23 | 11 | |||
| 20 | Female (77) | W135 | Daughter | 45 | 12 |
| 21 | Male (39) | B | Kissing contact (male) | 44 | 6 |
| 22 | Male (17) | B | Classmate (male) | 13 | 13 |
| 25 | Female (8 months) | B | Childminder (male) | 24 | 11 |
| Secondary contact (female) | 25 | 18 | |||
| 26 | Male (1) | B | Grandmother | 41 | 7 |
| 27 | Male (18) | C | Friend (male) | 18 | 9 |
| 29 | Male (2) | B | Father | 48 | 1 |
| 30 | Male (18) | B | Best friend (male) | 20 | 9 |
| 36 | Female (1) | B | Grandmother | 56 | 7 |
| 38 | Male (16) | Y | Classmate 1 (male) | 16 | 13 |
| Classmate 2 (male) | 16 | 13 | |||
| Kissing contact of 1 (female) | 15 | 18 | |||
| Kissing contact of 2 (female) | 15 | 18 | |||
| 39 | Male (5 months) | B | Father | 24 | 1 |
| 41 | Male (1) | B | Father | 28 | 1 |
| 43 | Male (4) | C | Mother | 36 | 2 |
| 44 | Male (3) | B | Brother | 8 | 4 |
| Uncle | 35 | 12 | |||
| 46 | Male (11) | B | Best friend (male) | 8 | 9 |
| 47 | Male (15) | B | Kissing contact (female) | 14 | 5 |
| 48 | Male (4) | B | Mother | 35 | 2 |
| Sister | 6 | 3 | |||
| 50 | Female (6) | B | Child 1 (male) | 6 | 14 |
| Brother of child 1 | 10 | 18 | |||
Carrier rate
—Among 1535 primary contacts, 234 meningococcal carriers were found. Of these, 42 carried the pathogenic strain. Thirty six of the 145 class 1 contacts carried meningococci. The pathogenic strain was found in 18 (12.4%: 95% confidence interval 5.5% to 19.3%) of these contacts: 6/37 (16.2%) mothers, 5/37 (13.5%) fathers, 4/29 (13.8%) brothers, 1/21 (4.8%) sisters, 1/18 (5.5%) other household members, and 1/3 (33.3%) kissing contacts. Of 576 class 2 contacts, 105 (18.2%) carried meningococci. The pathogenic strain was found in 11 (1.9%: 0.9% to 3.4%). Of 814 class 3 contacts, 93 (11.4%) carried meningococci. The pathogenic strain was found in 13 (1.6%: 0.14% to 3.1%) of these contacts. Of 78 secondary contacts, 20 (25.6%) carried meningococci. The pathogenic strain was found in four (5.1%) of these contacts.
The pathogenic strain was found in the primary contacts of 27 of the 48 patients, the number of carriers varying between 1 and 6 (table 5). The pathogenic strain was found more often in contacts in the 5-12 years age group than in the other age groups (table 1).
Discussion
In most countries the use of chemoprophylaxis is recommended to prevent secondary disease in close contacts of patients with meningococcal disease.11–14 In a few other countries, including Norway, chemoprophylaxis is not recommended, but household members under 15 years of age are treated with penicillin orally.17 Neither approach has been evaluated in controlled studies.
When a case of meningococcal disease occurs, many people may fulfill accepted criteria for receiving chemoprophylaxis,11–14 and chemoprophylaxis may be prescribed in excess of what is needed.14 High consumption of chemoprophylactic agents may select bacterial resistance, which in meningococci may be associated with virulence.19 Chemoprophylactic agents may also kill non-virulent meningococci and other bacteria that stimulate an immune response against the meningococci.20 Chemoprophylaxis should therefore be restricted to those who are likely to carry the pathogenic strain.
Our study shows that only 42 (2.7%) of 1535 close contacts carried the pathogenic strain of N meningitidis. General use of chemoprophylaxis in all these contacts therefore seems excessive. Sensitive and rapid techniques for identification of the pathogenic strain8,9 allow targeting of chemoprophylaxis to carriers, but have not yet been widely applied as most laboratories lack the technology and resources to perform these tests. In most cases, therefore, the decision of whether to give chemoprophylaxis must be made on the basis of closeness of contact with the patient.
Our study shows that the risk of carriage of the pathogenic strain is highest (12.4%, 95% confidence interval 5.5% to 19.3%) in household members and kissing contacts. Household members have a high relative risk of meningococcal disease (1000-40001–3) and the use of chemoprophylaxis in this group therefore seems well justified. Contacts outside this group, most of whom qualified for chemoprophylaxis according to accepted criteria,11–14 had a considerably lower prevalence of carriage of the pathogenic strain (class 2, 1.9%, 0.9% to 3.4%; class 3, 1.6%, 0.14% to 3.1%). This is higher than the 0.7% prevalence in the general population, during periods of low disease incidence,18,21 but not dramatically so. Should these contacts receive chemoprophylaxis? Our results do not support this practice. However, the relative risk of meningococcal disease is over 1000 for household members, although our results indicate that the carriage rate (12.4%) is only 18 times higher than that found in the general population (0.7%) in other studies. The relative risk of meningococcal disease is therefore not a simple function of the prevalence of the pathogenic strain. Another way to view the problem would be to ask whether the prevalence of the pathogenic strain approaches that needed to initiate epidemic disease. It has been suggested that a high rate of carriage is a prerequisite for epidemic disease,22 but the threshold is not known, and in any case the prevalence will vary from case to case. We therefore feel that the choice of whether to give chemoprophylaxis to contacts outside the patient’s household and kissing contacts should be made on an individual basis, taking into account: other cases in the vicinity or other reasons to suspect an outbreak in the community; a high incidence of influenza or other respiratory infection that may predispose contacts to meningococcal disease and mask the symptoms of early infection; and other predisposing factors. If, however, an isolated case occurs in an otherwise healthy community, we believe that a conservative approach to chemoprophylaxis is justified.
Our study illustrates the need for better understanding of the relation between carrier rate and risk of secondary disease. A controlled study comparing different chemoprophylaxis strategies would in our view be of considerable help. The following strategies should be considered: (a) chemoprophylaxis according to standard recommendations, (b) chemoprophylaxis given only to household members and kissing contacts, and (c) chemoprophylaxis given to household members, kissing contacts, and other close contacts who are found to carry the pathogenic strain. Cost benefit analysis and studies of the prevalence of the pathogenic strain would enhance the value of such a study.
It has been argued that throat swabbing underestimates the true rate of meningococcal carriage.23 Low levels of bacteria in the sample, loss of viability under transport, and variable sampling techniques can all influence the measured carrier rate, but sampling and transport are probably the more important factors.24 We have addressed this problem by attempting to confine sampling to two well trained members of our staff, by plating samples directly after collection, and by sampling the throat, which has been reported to have 100% sensitivity relative to other sampling sites.25 A minority of the samples were collected by hospital and clinic staff and transported before plating. These are almost exclusively samples collected from members of the patient’s household—that is, the group where we found the highest rates of carriage. Serious underestimation of carriage might be expected to lead to secondary cases among the contacts we sampled. None of the 1535 primary and 78 secondary contacts contracted meningococcal disease. We therefore do not think that sampling problems seriously affect this study.
Figure.
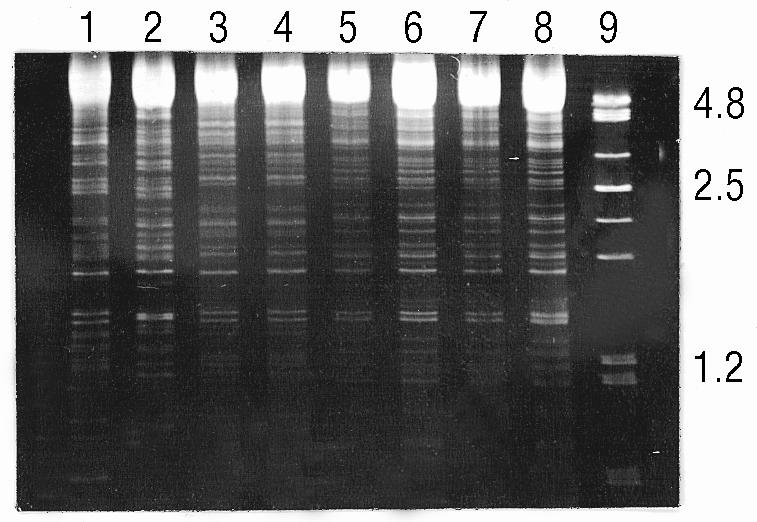
Chromosomal DNA fingerprints of meningococcal isolates from patient (lane 3) and contacts (lanes 1, 2, and 4-8 ). The size marker (lane 9) is a PstI digest of lambda DNA (sizes in kb). Isolate from patient’s kissing contact (lane 4) identical with that of patient
Table 3.
Bacteriological findings in class 2 contacts
| Group | Contacts | Total No | No (%) of carriers | No (%) of carriers with pathogenic strain |
|---|---|---|---|---|
| 7 | Grandparents | 41 | 6 (15) | 2 (5) |
| 8 | Others* | 37 | 5 (14) | 0 |
| 9 | Playmates, close friends | 249 | 50 (20) | 5 (2) |
| 10 | Nursery employees | 60 | 5 (9) | 1 (2) |
| 11 | Childminders, babysitters, home helps | 5 | 2 (40) | 1 (20) |
| 12 | Other family† | 184 | 37 (20) | 2 (1) |
| Total | 576 | 105 (18.2) | 11 (1.9) |
Playmates’ family and childminders’ children.
Parents, children, and siblings outside the patient’s household, and cousins, fathers in law, mothers in law, sons in law, and daughters in law.
Footnotes
Funding: This project is a part of A/S Telelab’s internally funded research programme.
Conflict of interest: None.
References
- 1.De Wals P, Hertoghe L, Borlee-Grimee I, De Maeyer-Cleempoel S, Reginster-Haneuse G, Dachy A, et al. Meningococcal disease in Belgium. Secondary attack rate among household, day-care nursery and pre-elementary school contacts. J Infect. 1981;3(suppl 1):53–61S. doi: 10.1016/s0163-4453(81)80009-6. [DOI] [PubMed] [Google Scholar]
- 2.Meningococcal Disease Surveillance Group. Meningococcal disease. Secondary attack rate and chemoprophylaxis in the United States, 1974. JAMA. 1976;235:261–265. [PubMed] [Google Scholar]
- 3.Olcén P, Kjellander J, Danielsson D, Lindquist BL. Epidemiology of Neisseria meningitidis: prevalence and symptoms from the upper respiratory tract in family members to patients with meningococcal disease. Scand J Infect Dis. 1981;13:105–109. doi: 10.3109/inf.1981.13.issue-2.05. [DOI] [PubMed] [Google Scholar]
- 4.Munford RS, Taunay AE, de Morais JS, Fraser DW, Feldman RA. Spread of meningococcal infection within household. Lancet. 1974;i:1275–1278. doi: 10.1016/s0140-6736(74)90022-1. [DOI] [PubMed] [Google Scholar]
- 5.Kristiansen BE, Tveten Y, Ask E, Rerten T, Knapskog A-B, Steen-Johnsen J, et al. Preventing secondary cases of meningococcal disease by identifying and eradicating disease-causing strains in close contacts of patients. Scand J Infect Dis. 1992;24:165–173. doi: 10.3109/00365549209052608. [DOI] [PubMed] [Google Scholar]
- 6.Cartwright KAV, Stuart JM, Robinson PM. Meningococcal carriage in close contacts of cases. Epidemiol Infect. 1991;106:133–141. doi: 10.1017/s0950268800056491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooke RPD, Riordan T, Jones DM, Painter MJ. Secondary cases of meningococcal infection among close family and household contacts in England and Wales, 1984-7. BMJ. 1989;298:555–558. doi: 10.1136/bmj.298.6673.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristiansen BE, Fermér C, Jenkins A, Ask E, Swedberg G, Sköld O. PCR amplicon restriction endonuclease analysis of the chromosomal dhps gene of Neisseria meningitidis: a method for studying the spread of the disease-causing strain in contacts of patients with meningococcal disease. J Clin Microbiol. 1995;33:1174–1179. doi: 10.1128/jcm.33.5.1174-1179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woods CR, Koeuth T, Estabrook MM, Lupski JR. Rapid determination of outbreak-related strains of Neisseria meningitidis by repetitive element-based polymerase chain reaction genotyping. J Infect Dis. 1996;174:760–767. doi: 10.1093/infdis/174.4.760. [DOI] [PubMed] [Google Scholar]
- 10.Caugant DA, Frøholm LO, Høiby EA, Blystad H, Lystad A. [abstract]. Improved surveillance of meningococcal disease in Norway by continual connection of the epidemiological and bacteriological data. Annual conference of the Institute of Public Health, Oslo, Norway, 1996. Oslo: Institute of Public Health.
- 11.Public Health Laboratory Service. Control of meningococcal disease: guidance for consultants in communicable disease control. Communicable disease report. PHLS Communicable Disease Surveillance Centre: London, 8 Dec, 1995.
- 12.Recommendation of the immunization practices advisory committee meningococcal vaccines. Centre for Disease Control. www.cdc.gov/ (1 April, 1996).
- 13.Benenson AS. Control of communicable diseases manual, 16th ed. Washington DC: American Public Health Association; 1995. [Google Scholar]
- 14.Emerging and other communicable diseases.Meningococcal meningitis fact sheet (No 105). Geneva: World Health Organisation. www.who.ch/programmes/emc/csmfacts.htm (Aug 3, 1996).
- 15.Pearson N, Gunnell DJ, Dunn C, Beswick T, Hill A, Ley B. Antibiotic prophylaxis for bacterial meningitis: overuse and uncertain efficacy. J Public Health Med. 1995;17:455–458. [PubMed] [Google Scholar]
- 16.Bøvre K, Gedde-Dahl TW. Epidemiological patterns of meningococcal disease in Norway 1975-1979. Natl Inst Public Health Ann, Oslo. 1980;3:9–22. [PubMed] [Google Scholar]
- 17.Høiby EA, Moe PJ, Lystad A, Frøholm LO. Phenoxymethylpenicillin treatment of household contacts of meningococcal disease patients. Antonie van Leeuwenhoek J Microbiol. 1986;52:255–257. [Google Scholar]
- 18.Kristiansen BE, Lind KW, Mevold K, Sørensen B, Frøholm LO, Bryn K, et al. Meningococcal carriage: studies of bacterial phenotypic and genomic characteristics and of human antibody levels. J Clin Microbiol. 1988;26:1988–1992. doi: 10.1128/jcm.26.10.1988-1992.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen BM. Mortality in meningococcal infections. Scand J Infect Dis. 1978;10:277–282. doi: 10.3109/inf.1978.10.issue-4.04. [DOI] [PubMed] [Google Scholar]
- 20.Reller LB, McGregor RR, Beaty HN. Bactericidal antibody after colonization with Neisseria meningitidis. J Infect Dis. 1973;127:56–62. doi: 10.1093/infdis/127.1.56. [DOI] [PubMed] [Google Scholar]
- 21.Caugant DA, Høiby EA, Magnus P, Scheel O, Hoel T, Bjune G, et al. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J Clin Microbiol. 1994;32:323–330. doi: 10.1128/jcm.32.2.323-330.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Achtman M. Global epidemiology. In: Cartwright K, editor. Meningococcal disease. Chichester: John Wiley; 1995. pp. 159–176. [Google Scholar]
- 23.Cartwright K, Kroll S. Optimising the investigation of meningococcal disease. BMJ. 1997;315:757–758. doi: 10.1136/bmj.315.7111.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cartwright K. Meningococcal carriage and disease. In: Cartwright K, editor. Meningococcal disease. Chichester: John Wiley; 1995. pp. 115–146. [Google Scholar]
- 25.Olcen P, Kjellander J, Danielsson D, Lindquist BL. Culture diagnosis of meningococcal carriers: yield from different sites and influence of storage in transport medium. J Clin Pathol. 1979;32:1222–1225. doi: 10.1136/jcp.32.12.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]


