Abstract
The ability to silence the electrical activity of defined neuronal populations in vivo is dramatically advancing our understanding of brain function. This technology may eventually be useful clinically for treating a variety of neuropathological disorders caused by excessive neuronal activity. Several neuronal silencing methods have been developed, with the bacterial light-activated halorhodopsin and the invertebrate allatostatin-activated G protein-coupled receptor proving the most successful to date. However, both techniques may be difficult to implement clinically due to their requirement for surgically implanted stimulus delivery methods and their use of nonhuman receptors. A third silencing method, an invertebrate glutamate-gated chloride channel receptor (GluClR) activated by ivermectin, solves the stimulus delivery problem as ivermectin is a safe, well tolerated drug that reaches the brain following systemic administration. However, the limitations of this method include poor functional expression, possibly due to the requirement to coexpress two different subunits in individual neurons, and the nonhuman origin of GluClR. Here, we describe the development of a modified human α1 glycine receptor as an improved ivermectin-gated silencing receptor. The crucial development was the identification of a mutation, A288G, which increased ivermectin sensitivity almost 100-fold, rendering it similar to that of GluClR. Glycine sensitivity was eliminated via the F207A mutation. Its large unitary conductance, homomeric expression, and human origin may render the F207A/A288G α1 glycine receptor an improved silencing receptor for neuroscientific and clinical purposes. As all known highly ivermectin-sensitive GluClRs contain an endogenous glycine residue at the corresponding location, this residue appears essential for exquisite ivermectin sensitivity.
Keywords: Chloride Channels, Membrane Proteins, Neuroscience, Neurotransmitter Receptors, Site-directed Mutagenesis, Glycine Receptor
Introduction
Several methods for silencing neuronal electrical activity in behaving animals have been developed that could eventually be useful for treating neurological disorders including Parkinson disease, addiction, epilepsy, depression, and chronic pain states (1, 2). Generally, these techniques involve overexpressing an exogenous receptor in molecularly or spatially defined populations of neurons and applying a specific stimulus to activate this receptor and thereby silence the neurons.
The two most successful strategies to date have proved to be bacterial halorhodopsin and the Drosophila allatostatin receptor. Halorhodopsin is a light-activated inward chloride pump, which, when exogenously expressed in neurons, hyperpolarizes neurons rapidly and reversibly upon the application of intense yellow light (3). Although this has been employed successfully to correlate neuronal activity with behavior (4), it is limited by 1) the requirement for strong overexpression and 2) the necessity to deliver intense light to neurons in vivo. The recent development of more efficient light-driven outward proton pumps may address the first limitation (5).
The alternate approach employs a Drosophila G protein-coupled receptor that is activated by the insect neuropeptide allatostatin (6). When expressed exogenously in mammalian neurons, allatostatin receptor activation activates G protein-coupled inwardly rectifying potassium channels via the direct binding of G protein βγ subunits, thereby producing hyperpolarization and cessation of spiking activity. Although this has been successfully used in a number of laboratories and has been validated in behaving mammals (7–11), it is limited by the requirement to directly inject allatostatin into required brain regions.
The halorhodopsin and allatostatin approaches are not ideal for human therapy due to their use of exogenous nonhuman (even nonvertebrate) receptors and the inconvenience of applying stimuli directly to the target neurons. The second of these issues can be addressed by a third silencing approach, which employs a Caenorhabditis elegans αβ heteromeric glutamate-gated chloride channel receptor (GluClR)3 mutated to abolish sensitivity to glutamate while retaining low nanomolar sensitivity to ivermectin (12, 13). In this approach, ivermectin is injected systemically and crosses the blood-brain barrier to activate a chloride flux in neurons expressing exogenous GluClRs, thereby shunting excitatory impulses. Ivermectin is a well tolerated anti-helminthic that is approved by the FDA for a variety of human parasitic infections (14). However, there are three main limitations with the ivermectin-GluClR method. First, GluClR silencing is slowly reversible, requiring days, as opposed to minutes for allatostatin and milliseconds for light-activated proton pumps. Second, use of this method has not yet spread beyond the originating laboratory (15), possibly due to poor functional expression stemming from the requirement to coexpress two different subunits in target neurons. Finally, as with the other silencing methods described above, GluClR exists only in lower phyla, which does not bode well for its eventual use in human clinical practice.
GluClRs are anionic members of the Cys loop family of ligand-gated ion channels, along with the glycine receptor chloride channel (GlyR) and the GABA type-A receptor chloride channel (GABAAR). GlyRs and GABAARs are also directly activated by ivermectin, although the threshold for activation is ∼100–300 nm (16–18), compared with 1–3 nm for many GluClRs (19–21). GlyRs may hold promise as silencing receptors for several reasons. First, they express efficiently as α subunit homomers, which should result in improved expression in individual neurons. Second, α homomeric GlyRs have a large (90 pS) single channel conductance, severalfold greater than the main conducting states of either GluClRs or GABAARs (22–24). Finally, GlyRs are endogenous to humans, which may alleviate antigenicity concerns associated with clinical use. However, glycine sensitivity must first be abolished and ivermectin sensitivity must be increased by two orders of magnitude to make it comparable with that of GluClRs. Here, we describe the development of a minimally mutated human α1 GlyR that successfully incorporates these characteristics.
EXPERIMENTAL PROCEDURES
Molecular Biology
The human α1, α2, α3, and β GlyR subunit cDNAs were each subcloned into the pcDNA3.1 plasmid vector. Site-directed mutagenesis was performed using the QuikChange mutagenesis kit (Stratagene, La Jolla, CA), and the successful incorporation of mutations was confirmed by DNA sequencing.
HEK-293 Cell Culture and Transfection
HEK-293 cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) containing Serum Supreme (Lonza, Walkersville, MD) and penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO) and split onto coverslips in 3-cm dishes. On the following day, cells were transiently transfected with the relevant GlyR cDNAs via a calcium phosphate method. These cells were also transfected with empty pEGFP vector (Clontech, Mountainview, CA, USA) as a fluorescent transfection marker. In experiments investigating α1β heteromeric GlyRs, α1 and β were transfected in a 1:4 ratio.
Neuron Dissociation, Culture, and Transfection
Experiments were performed on P0–P3 male Wistar rats in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes with approval from the Animal Ethics Committee for the University of Queensland. Animals were anesthetized with isoflurane and decapitated. Brains were immediately removed, and hippocampi were dissected out. Neurons were dissociated by incubation in 0.1% trypsin/EDTA (Invitrogen) for 7 min at 37 °C and were washed in HEPES-buffered minimum essential medium with 0.014% trypsin inhibitor (type I-S from soybean; Sigma-Aldrich). After diluting the cells in neurobasal A medium (Invitrogen) supplemented with 2% B27 (Invitrogen) and 0.5 mm l-glutamine, 2 × 105 cells were added to each well of 4-well dishes (BD Falcon). Each well contained a sterilized glass coverslip treated with poly d-lysine (Invitrogen), on which the cells grew. After 1 h, this medium (conditioned medium) was removed, and 0.5 ml of medium was added, consisting half of conditioned medium and half of neurobasal A, 2% B27, penicillin/streptomycin, 0.5 mm l-glutamine, and 10 ng/ml basic fibroblast growth factor (Invitrogen) (growth medium). After 3 days, cells were washed with phosphate-buffered saline, and 0.5 ml of medium was added, consisting of 20% conditioned medium and 80% fresh growth medium. On days P7–P10, cells were washed in phosphate-buffered saline and incubated for 1 h in fresh growth medium. Transfections were performed after 4–7 days in vitro, with recordings performed 2 days later.
Electrophysiology and Data Analysis
HEK-293 cells on coverslips were placed in a bath and visualized with an inverted fluorescence microscope. Cells expressing GlyRs were identified by green fluorescent protein fluorescence. Patch clamp pipettes were pulled from borosilicate glass tubes (Vitrex, Modulohm, Denmark) on a horizontal puller (P97, Sutter Instruments, Novato, CA) and had tip resistances of 1–2 megohms when filled with the pipette solution which consisted of: 145 mm CsCl, 2 mm CaCl2, 2 mm MgCl2, 10 mm HEPES, and 10 mm EGTA, pH 7.4. Bath solution consisted of 140 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm HEPES, and 10 mm glucose, pH 7.4. After establishment of the whole-cell recording configuration, cells were voltage clamped at −40 mV (unless otherwise indicated), and membrane currents were recorded using an Axon Multiclamp 700B amplifier and pClamp10 software (Molecular Devices, Sunnyvale, CA). Currents were filtered at 500 Hz and digitized at 2 KHz.
Ivermectin, moxidectin, emamectin benzoate, eprinomectin, and picrotoxin were all obtained from Sigma-Aldrich. Doramectin was obtained from Toronto Research Chemicals (North York, ON, Canada). Stocks of 10 mm ivermectin or its analogues, dissolved in dimethyl sulfoxide (Sigma-Aldrich), were prepared every 2 weeks and stored at −20 °C. Picrotoxin stocks were made at 100 mm in dimethyl sulfoxide and kept at −20 °C. Solutions for experiments were prepared from these stocks on the day of recording. Solutions were applied to cells via gravity forced perfusion via parallel microtubules and manual control of this system via a micromanipulator effected solution exchange in ∼ 250 ms. Experiments were conducted at room temperature (19–22 °C).
To establish GlyR sensitivity to ivermectin, current magnitude in response to each ivermectin concentration, I, was normalized to maximal ivermectin-gated current magnitude, Imax. These I/Imax values were averaged, and reported data points represent means ± S.E. from three or more experiments. Curves were fitted with EC50 values and Hill slopes (nH) from individual experiments were calculated with the Hill Equation (3-parameter; Sigmaplot 9.0, Jandel Scientific, San Rafael, CA). EC50 and nH values from individual experiments were averaged and reported as means ± S.E. Glycine dose-response relationships were established in the same way but normalized to maximal glycine-evoked current magnitude. In tables, asterisks denote significant differences (*, p < 0.05; **, p < 0.01; and ***, p < 0.001) by Student's unpaired t test.
Electrophysiological experiments on neurons were performed similarly to those on HEK-293 cells, and only the differences are noted here. Transfected neurons were identified not only by GFP fluorescence but also the presence of depolarization-induced spikes and by the activation of inward currents upon stimulation with 100 μm glutamate or 300 μm glycine. Neurons were voltage clamped at −70 mV. Ivermectin gated conductance changes were recorded in the presence of 400 ms voltage ramps from −90 mV to +30 mV applied at 1-s intervals. The recording of conductance changes was considered necessary as ivermectin activation of large chloride currents in neuronal dendrites could produce substantial local chloride concentration changes that distort peak current magnitudes.
RESULTS
To identify the most appropriate clone on which to base a silencing receptor, we compared the glycine and ivermectin sensitivities of human α1, α1β, α2, and α3 GlyRs. Sample glycine- and ivermectin-gated currents in HEK-293 cells expressing α1 GlyRs are shown in Fig. 1, A and B, respectively. Because ivermectin currents are irreversible (18), we generated ivermectin dose-response relationships by successively applying higher ivermectin concentrations. Averaged glycine and ivermectin dose-response curves for α1, α1β, α2, and α3 GlyRs are shown in Fig. 1, C and D. The parameters of best curve fit for these and all other mutant GlyRs examined in this study are summarized in Table 1. As glycine and ivermectin sensitivity did not vary dramatically among the four wild type (WT) constructs tested, we employed the α1 GlyR (the standard model system for structure-function studies) as a starting point for developing an improved ivermectin-activated silencing receptor.
FIGURE 1.
Glycine and ivermectin sensitivity of WT GlyRs. In this and subsequent figures, filled bars indicate glycine application, and unfilled bars indicate ivermectin application. A, sample responses of cells expressing α1 GlyRs to increasing concentrations of glycine. B, sample responses of cells expressing α1 GlyRs to increasing concentrations of ivermectin. Such experiments were used to establish average dose-response relationships for glycine (C) or ivermectin (D) at α1, α2, and α3 homomeric and α1/β heteromeric GlyRs as indicated. The y axis in D is the same as for C. Averaged curve fit parameters are presented in Table 1.
TABLE 1.
Properties of WT and mutant GlyRs examined in this study
*, p < 0.05; **, p < 0.01; and ***, p < 0.001 relative to corresponding μ1 WT value by unpaired t test. #, agonist sensitivity was too low for EC50 values to be calculated. Indicated current magnitudes were recorded at 10 mm glycine or 30 μm ivermectin.
| Glycine |
Ivermectin |
|||||||
|---|---|---|---|---|---|---|---|---|
| EC50 | nH | Imax | n | EC50 | nH | Imax | n | |
| μm | nA | μm | nA | |||||
| α1 | 40 ± 7 | 2.7 ± 0.2 | 10.4 ± 1.4 | 10 | 1.7 ± 0.4 | 2.4 ± 0.2 | 6.7 ± 0.9 | 9 |
| α1β | 45 ± 11 | 1.6 ± 0.3 | 9.5 ± 1.8 | 4 | 2.4 ± 0.7 | 2.2 ± 0.5 | 4.3 ± 0.7 | 4 |
| α2 | 131 ± 14*** | 1.9 ± 0.4 | 7.5 ± 1.5 | 3 | 4.8 ± 1.0** | 3.0 ± 0.6 | 3.2 ± 0.6 | 3 |
| α3 | 190 ± 33*** | 2.3 ± 0.3 | 6.2 ± 1.0 | 4 | 2.8 ± 0.7 | 1.8 ± 0.3 | 4.0 ± 0.6 | 5 |
| T258S | 3.3 ± 1.5** | 1.0 ± 0.1 | 7.8 ± 1.5 | 4 | 0.086 ± 0.030* | 1.9 ± 0.3 | 4.0 ± 0.6 | 4 |
| F207A | 0.4 ± 0.1# | 7 | 5.4 ± 1.7** | 1.7 ± 0.2 | 11 ± 3 | 3 | ||
| F207A/T258S | 0.7 ± 0.1# | 5 | 1.2 ± 0.2 | 1.4 ± 0.2 | 5.9 ± 0.5 | 5 | ||
| A288T | 45 ± 1 | 2.5 ± 0.5 | 12 ± 2.5 | 4 | 0.7 ± 0.3# | 4 | ||
| A288G | 6.0 ± 1.3* | 0.9 ± 0.3 | 4.8 ± 0.7 | 4 | 0.032 ± 0.008* | 1.8 ± 0.4 | 4.3 ± 0.2 | 4 |
| F207A/A288G | 0.8 ± 0.5# | 4 | 0.019 ± 0.006** | 1.8 ± 0.1 | 5.1 ± 0.4 | 6 | ||
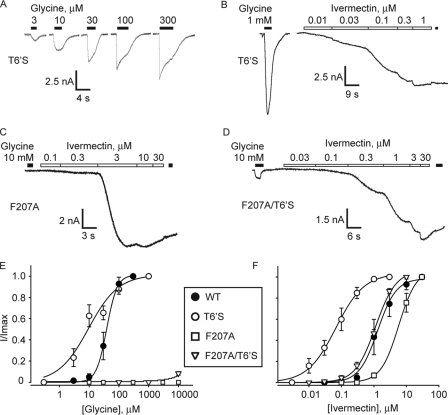
As there are no clues to date concerning the location of the GlyR ivermectin binding site, we generated and screened a cDNA library comprising ∼1600 randomly mutated α1 GlyR clones and searched for mutations that affected ivermectin sensitivity. Library mutation frequency was selected so that each clone contained an average of 1–2 amino acid mutations. The methods for generating this library and screening it in HEK-293 cells for ivermectin sensitivity have recently been described in detail (25). The screen identified one mutation, T258S, that significantly increased GlyR ivermectin sensitivity. This residue is well known to contribute to the lining of the channel pore (26) and to form the pore binding site for several GlyR-active pharmacological modulators (27–30). Electrophysiological analysis in HEK-293 cells confirmed that the T258S mutation significantly increased receptor sensitivity to both glycine (Fig. 2, A and E) (Table 1) and ivermectin (Fig. 2, B and F and Table 1). Indeed, the T258S mutation reduced the ivermectin EC50 from ∼1.7 μm to 90 nm. As the T258S mutant GlyR was activated significantly by 10 nm ivermectin (Fig. 2, B and F), we considered it a potential candidate silencing receptor. We therefore introduced the F207A glycine binding site mutation (31) to eliminate sensitivity to the endogenous agonist glycine. As shown in Fig. 2C, a 10 mm concentration of glycine activated no detectable current in the F207A mutant GlyR, although robust ivermectin-gated currents were observed (Fig. 2C). Unfortunately, this mutant receptor showed a significant decrease in ivermectin sensitivity relative to the WT α1 GlyR (Fig. 2F and Table 1). Thus, it is not surprising that the double mutant T258S/F207A GlyR also exhibited a significantly reduced ivermectin sensitivity relative to the T258S GlyR (Fig. 2, D and F, and Table 1). Indeed, as the ivermectin sensitivity of the double mutant was not significantly different to WT, this construct was discarded as a candidate silencing receptor. Nevertheless, these results imply a long range allosteric coupling between T258S and the ivermectin binding site similar to that previously shown between the residue corresponding to L259S and the acetylcholine binding site of the nicotinic acetylcholine receptor (32).
FIGURE 2.
Glycine and ivermectin sensitivity of T258S and F207A mutant α1 GlyRs. A and B, sample glycine and ivermectin dose-responses in T258S (T6′S) mutant GlyRs. C and D, response to 10 mm glycine and increasing concentrations of ivermectin in single mutant F207A GlyRs and double mutant F207A/T258S GlyRs, as indicated. Points from four or more dose-response experiments on each mutant were averaged to generate glycine (E) and ivermectin (F) dose-response curves for WT, T258S, F207A, and F207A/T258S GlyRs, as indicated. The y axis in F is the same as for E. Averaged curve fit parameters are presented in Table 1.
The random mutant library screen also identified several mutants that decreased or eliminated ivermectin sensitivity. One of these mutations was A288T in the third transmembrane domain. Electrophysiological analysis in HEK-293 cells confirmed that the A288T mutant GlyR retained WT-like sensitivity to glycine but was almost completely insensitive to ivermectin (Fig. 3A and Table 1). As different mutations to Ala288 have contrasting effects on GlyR sensitivity to ethanol (33), we investigated the effects of several different Ala288 mutations on ivermectin sensitivity. Whereas most tested substitutions abolished ivermectin sensitivity (data not shown), the A288G substitution resulted in a dramatic increase in sensitivity to both glycine and ivermectin (Fig. 3, C and D and Table 1).
FIGURE 3.
Glycine and ivermectin sensitivity of α1 GlyRs incorporating mutations to Ala288. A and B, response to 10 mm glycine and increasing concentrations of ivermectin (IVM) at A288T mutant GlyRs and double mutant F207A/A288G GlyRs, as indicated. Points from four or more dose-response experiments on each mutant were averaged to generate glycine (C) and ivermectin (D) dose-response curves for WT, A288G, A288T, and F207A/A288G GlyRs, as indicated. The y axis in C is the same as for D. Averaged curve fit parameters are presented in Table 1. Notably, F207A/A288G confers extreme insensitivity to glycine and extreme sensitivity to ivermectin. Averaged curve fit parameters in C and D are presented in Table 1.
The double mutant F207A/A288G GlyR exhibited a threshold for glycine activation of 1–3 mm (Fig. 3, B and C, see also Fig. 4C below). However, this receptor exhibited robust responses to ivermectin (Fig. 3B). Indeed, as shown in Fig. 3D and Table 1, the ivermectin EC50 of the F207A/A288G mutant GlyR was even lower than that of the A288G mutant GlyR (19 versus 32 nm). As the F207A/A288G GlyR is strongly activated by 10 nm ivermectin, it appears a promising silencing receptor candidate. However, as glycine concentrations can reach 3 mm in the synaptic cleft (34), we attempted to eliminate the remaining glycine responsiveness by introducing the F63A, N102Q, E157D, or K200R ligand-binding domain mutations (31, 35, 36) in addition to the F207A and A288G mutations. However, as each of the triple mutant receptors displayed no response to either 100 mm glycine or 30 μm ivermectin, we concluded they were nonfunctional.
FIGURE 4.
Effect of β subunit incorporation on ivermectin sensitivity of F207A/A288G GlyRs. A, electrophysiological recordings from cells transfected with WT α1 subunits (top) and WT α1 and β subunits (bottom). Filled bar indicates application of 10 μm glycine, and gray bar indicates the superimposed application of 10 μm picrotoxin. Averaged results quantified in the boxed graph (n = 3 cells for α1 homomer and n = 5 for α1/β heteromer) indicate coexpression of α1 and β subunits in functional heteromers. B, sample electrophysiological recordings from cells transfected with F207A/A288G mutant α1 subunits (top) and F207A/A288G mutant α1 and WT β subunits (bottom). Filled bar and arrowheads (from top to bottom) indicate application of 0.1, 1, 10, and 100 mm glycine, and the unfilled bar indicates application of 10 nm ivermectin (IVM) to the same cell. C, mean magnitude of glycine- and ivermectin-activated currents in mutant homomeric α1 GlyRs and heteromeric α1/β GlyRs at the indicated concentrations. Results were averaged from eight cells expressing homomeric GlyRs and 12 cells expressing heteromeric GlyRs, except for 1000 nm ivermectin, where n = 3 for each receptor. There was no significant difference at any agonist concentration. PTX, picrotoxin.
As GlyR β subunit mRNA is widespread in the brain (37), β subunits could potentially oligomerize with the introduced F207A/A288G mutant α1 subunits to modify silencing receptor function. To test for this possibility, we investigated the effect of coexpressing F207A/A288G mutant α1 subunits with WT β subunits in HEK-293 cells. Unfortunately, however, WT α1 and α1β GlyRs have virtually identical sensitivities to ivermectin (Table 1), and picrotoxin, which is used to pharmacologically discriminate between α1 and α1β GlyRs (38), does not inhibit ivermectin-gated currents (18). Thus, a control experiment was performed whereby WT α1 and β subunits were coexpressed and tested for picrotoxin sensitivity. As shown in Fig. 4A, cells transfected with α1 and β subunits generated predominantly heteromeric receptors as evidenced by the characteristic reduction in picrotoxin sensitivity produced by β subunit incorporation. On the same day, using the same β subunit clone and transfection method and cells from the same passage, we cotransfected the F207A/A288G mutant α1 subunit with WT β subunits. As shown in Fig. 4B, responses of cells expressing α1 and α1β subunits to 0.1, 1, 10, and 100 mm glycine and 10 nm ivermectin appeared indistinguishable. As the results presented in Fig. 4C confirm this trend, we conclude that endogenous β subunits do not compromise the functional properties of the F207A/A288G silencing receptor.
As ivermectin analogues vary in their central nervous system side effects, their ability to cross the blood-brain barrier and their pharmacokinetics, ivermectin itself may not necessarily be the optimal analogue to use in vivo in silencing studies. To help in the selection of the most appropriate analogue, we investigated the sensitivity and efficacy of the following commercially available ivermectin analogues at WT and A288G mutant α1 GlyRs: emamectin, eprinomectin, and doramectin. We also tested the structurally related milbemycin derivative moxidectin. We employed the single mutant A288G GlyR in these experiments so that the magnitudes of peak analogue-activated currents could be compared directly with those activated by glycine in the same cell. Averaged dose-response curves for all analogues at WT and A288G mutant GlyRs are shown in Fig. 5, A and B, with parameters of best fit to the Hill equation summarized in Table 2. All compounds behaved as high efficacy agonists, activating maximal currents similar in magnitude to those elicited by glycine in the same cell (data not shown). Table 2 reveals that moxidectin has a 2-fold higher “research index” (EC50 (intended target)/EC50 (undesired)) than ivermectin, with a lower EC50 at the A288G mutant GlyR and a higher EC50 at the WT GlyR. However, the other analogues exhibited higher EC50 values relative to ivermectin at the A288G GlyR (Table 2).
FIGURE 5.
Averaged dose-response curves for ivermectin (IVM), emamectin (EMM), eprinomectin (EPM), doramectin (DRM), and moxidectin (MXD) at WT α1 GlyRs (A) and A288G GlyRs (B). All dose-response relations were averaged from 4–5 cells and averaged curve fit parameters are summarized in Table 2.
TABLE 2.
Effects of ivermectin analogues on WT α1 and A288G mutant α1 GlyRs
#, p < 0.05; ##, p < 0.01; and ###, p < 0.001 relative to corresponding ivermectin value by unpaired t test. *, p < 0.05; **, p < 0.01; and ***, p < 001, relative to corresponding WT value unpaired t test. All results were averaged from n = 4–5 cells.
| WT |
A288G |
|||||
|---|---|---|---|---|---|---|
| EC50 | nH | Imax | EC50 | nH | Imax | |
| μm | nA | μm | nA | |||
| Ivermectin | 1.7 ± 0.4 | 2.4 ± 0.2 | 6.7 ± 0.9 | 0.032 ± 0.008* | 1.76 ± 0.4 | 4.3 ± 0.3 |
| Emamectin | 1.2 ± 0.2 | 2.7 ± 0.5 | 5.4 ± 0.9 | 0.15 ± 0.05**### | 2.9 ± 0.3 | 3.9 ± 0.5 |
| Eprinomectin | 3.4 ± 1.2 | 2.8 ± 0.1 | 6.2 ± 1.1 | 0.14 ± 0.01*## | 2.7 ± 0.8 | 4.7 ± 1.5 |
| Doramectin | 1.9 ± 0.4 | 3.0 ± 0.3 | 4.4 ± 0.7 | 0.08 ± 0.01** | 2.0 ± 0.2* | 2.2 ± 0.6 |
| Moxidectin | 2.3 ± 0.3 | 3.2 ± 0.6 | 4.2 ± 0.8 | 0.025 ± 0.005*** | 3.3 ± 0.8 | 4.4 ± 0.9 |
To test whether the F207A/A288G silencing construct functions effectively in neurons, we transfected cultured rat hippocampal pyramidal neurons with either the F207A/A288G GlyR or the F207A/T258S GlyR as a transfection control. We also investigated nontransfected neurons, which are known to endogenously express GlyRs (39). We applied increasing concentrations of ivermectin while simultaneously applying voltage ramps (see “Experimental Procedures”) at 1-s intervals to record changes in conductance. As shown in the example in Fig. 6A, the ivermectin-mediated conductance increase in untransfected neurons typically exhibited a threshold of ∼1 μm, consistent with that observed in HEK-293 cells. Neurons transfected with F207A/T258S GlyRs unexpectedly conferred neurons with a significantly enhanced sensitivity to ivermectin (Fig. 6B). Finally, as shown in Fig. 6C, in neurons expressing F207A/A288G GlyRs, 10 nm ivermectin activated a large conductance consistent with its effects on the same receptors expressed in HEK-293 cells. Averaged conductances recorded from 4 to 7 neurons expressing each construct are presented in Fig. 6D. We conclude that when expressed in neurons, the F207A/A288G mutant α1 GlyR is potently activated by low nanomolar ivermectin concentrations. This potency compares well with that of the ivermectin-GluClR expressed under similar conditions (13).
FIGURE 6.
Ivermectin-gated conductances in transfected and untransfected neurons. Examples of conductance increases following application of indicated ivermectin concentrations to an untransfected hippocampal pyramidal neuron (A), a hippocampal pyramidal neuron expressing F207A/T258S mutant α1 GlyRs (B), and F207A/A288G mutant α1 GlyRs (C). D, mean maximum ivermectin-activated current magnitudes in transfected and untransfected neurons following the application of indicated concentrations of ivermectin. All values were averaged from four to seven neurons. T6′S, T258S.
DISCUSSION
The silencing receptor developed here exhibits a similar ivermectin sensitivity to the C. elegans αβ heteromeric GluClR (13). Because that receptor is activated by concentrations of ivermectin that reach the brain following systemic injection (15), we expect the F207A/A288G α1 GlyR to respond similarly. However, our human GlyR-based receptor has three main advantages over the GluClR-based silencing receptor. First, it has a larger single channel conductance (22, 23), implying a high inhibitory conductance per expressed receptor. Second, it expresses as a homomer, obviating the requirement to recombinantly express two different cDNAs in each target neuron. Both these measures are likely to result in an improved silencing efficiency in vivo. Finally and perhaps most importantly, the silencing construct described here is derived from a human receptor, making it a promising candidate for clinical applications. Furthermore, as the A288T mutant α1 GlyR exhibits normal WT glycine sensitivity but is insensitive to ivermectin, it should be useful as a control for exogenous receptor expression. With the halorhodopsin, allatostatin, and ivermectin-GluClR approaches, it is currently not possible to control for the expression per se of the silencing construct.
It is conceivable that other ivermectin analogues may be preferable as silencing receptor ligands in terms of their side effect profiles, pharmacokinetics, ability to cross the blood-brain barrier, and silencing receptor potency. Of the analogues investigated here, only moxidectin exhibited a higher potency than ivermectin, although the difference was not statistically significant (Table 2). Moxidectin is a milbemycin, which are aglycones of the avermectins, and these have remarkably fewer side effects than avermectins such as ivermectin (40). Indeed, it is predominantly for this reason that moxidectin is currently being tested in human clinical trials for river blindness (41). Relative to ivermectin, moxidectin exhibits a longer retention time in vivo (40, 42), which is a disadvantage in research experiments where rapid recovery is preferred, but it may be an advantage in many clinical settings. To date, it appears that there has been no comparative study of the ability of moxidectin and ivermectin to cross the blood-brain barrier. However, ivermectin is a potent substrate of P-glycoprotein, which efficiently pumps it out of the brain. Because moxidectin is a poor substrate for P-glycoprotein (43, 44), it is feasible that it may reach higher concentrations in the brain. In this respect, it is of interest to note that moxidectin crosses the blood-brain barrier in two strains of senescence-accelerated mice with particularly high efficiency, although the mechanism remains unknown (45). Given all these considerations, moxidectin may be worthy of consideration as an improved in vivo agonist of the F207A/A288G mutant α1 GlyR.
Finally, it is of interest to consider the mechanism by which A288G increases ivermectin potency. In the GABAAR and GlyR α subunits, this residue lines a water-filled pocket within the receptor transmembrane region at the interface of two adjacent subunits (46). A large body of evidence suggests that it contributes to the binding site for alcohols and intravenous anesthetics at the GlyR and GABAAR (47). In a study that employed a large range of amino acid substitutions at this position, the ability of ethanol to enhance α1 GlyR current magnitude was negatively correlated with side chain volume (33). This was interpreted as evidence either for ethanol being pushed out of its site by increasing side chain volume or for increasing side chain volumes to more effectively emulate the pharmacological effects of ethanol. As stated above, we investigated several substitutions at this position, and only A288G increased ivermectin potency, whereas all larger side chain substitutions (e.g. A288T) severely impaired ivermectin potency. This observation alone is not strong evidence for A288 being an ivermectin binding site, as the mutations could also be disrupting the ability of ivermectin to gate the receptor. Further studies are required to discriminate between these possibilities. However, we note that all GluClRs known to exhibit extremely high ivermectin sensitivity invariably contain glycines at the position corresponding to Ala288 (supplemental Fig. S1). Thus, a glycine residue at this position appears key to the exquisite ivermectin sensitivity of GluClRs. This observation could be of relevance to the understanding of ivermectin tolerance, the development of novel antihelminthics and insecticides, the development of alcohol or anesthetic antagonists, and to improving the ivermectin sensitivity of our silencing construct still further.
Acknowledgments
We thank Nikki Zuvela and Peter Curby for help with neuronal cultures and Drs. Daniel Gilbert and Tim Webb for help with creating and screening the random mutant cDNA library.
This work was supported in part by the Australian Research Council and the National Health and Medical Research Council of Australia (NHMRC).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1 and additional references.
- GluClR
- glutamate-gated chloride channel receptor
- GABAAR
- γ-aminobutyric acid type A receptor
- GlyR
- glycine receptor
- WT
- wild type
- GFP
- green fluorescent protein.
REFERENCES
- 1.Kramer R. H., Fortin D. L., Trauner D. (2009) Curr. Opin. Neurobiol. 19, 544–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo L., Callaway E. M., Svoboda K. (2008) Neuron 57, 634–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang F., Wang L. P., Brauner M., Liewald J. F., Kay K., Watzke N., Wood P. G., Bamberg E., Nagel G., Gottschalk A., Deisseroth K. (2007) Nature 446, 633–639 [DOI] [PubMed] [Google Scholar]
- 4.Arrenberg A. B., Del Bene F., Baier H. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 17968–17973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow B. Y., Han X., Dobry A. S., Qian X., Chuong A. S., Li M., Henninger M. A., Belfort G. M., Lin Y., Monahan P. E., Boyden E. S. (2010) Nature 463, 98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lechner H. A., Lein E. S., Callaway E. M. (2002) J. Neurosci. 22, 5287–5290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan E. M., Yamaguchi Y., Horwitz G. D., Gosgnach S., Lein E. S., Goulding M., Albright T. D., Callaway E. M. (2006) Neuron 51, 157–170 [DOI] [PubMed] [Google Scholar]
- 8.Tan W., Janczewski W. A., Yang P., Shao X. M., Callaway E. M., Feldman J. L. (2008) Nat. Neurosci. 11, 538–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wehr M., Hostick U., Kyweriga M., Tan A., Weible A. P., Wu H., Wu W., Callaway E. M., Kentros C. (2009) J. Neurophysiol. 102, 2554–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Narayan S., Geiman E., Lanuza G. M., Velasquez T., Shanks B., Akay T., Dyck J., Pearson K., Gosgnach S., Fan C. M., Goulding M. (2008) Neuron 60, 84–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y., Won J., Karlsson M. G., Zhou M., Rogerson T., Balaji J., Neve R., Poirazi P., Silva A. J. (2009) Nat. Neurosci. 12, 1438–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li P., Slimko E. M., Lester H. A. (2002) FEBS Lett. 528, 77–82 [DOI] [PubMed] [Google Scholar]
- 13.Slimko E. M., McKinney S., Anderson D. J., Davidson N., Lester H. A. (2002) J Neurosci 22, 7373–7379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omura S. (2008) Int. J. Antimicrob. Agents 31, 91–98 [DOI] [PubMed] [Google Scholar]
- 15.Lerchner W., Xiao C., Nashmi R., Slimko E. M., van Trigt L., Lester H. A., Anderson D. J. (2007) Neuron 54, 35–49 [DOI] [PubMed] [Google Scholar]
- 16.Adelsberger H., Lepier A., Dudel J. (2000) Eur. J. Pharmacol. 394, 163–170 [DOI] [PubMed] [Google Scholar]
- 17.Dawson G. R., Wafford K. A., Smith A., Marshall G. R., Bayley P. J., Schaeffer J. M., Meinke P. T., McKernan R. M. (2000) J. Pharmacol. Exp. Ther. 295, 1051–1060 [PubMed] [Google Scholar]
- 18.Shan Q., Haddrill J. L., Lynch J. W. (2001) J. Biol. Chem. 276, 12556–12564 [DOI] [PubMed] [Google Scholar]
- 19.Cully D. F., Vassilatis D. K., Liu K. K., Paress P. S., Van der Ploeg L. H., Schaeffer J. M., Arena J. P. (1994) Nature 371, 707–711 [DOI] [PubMed] [Google Scholar]
- 20.McCavera S., Rogers A. T., Yates D. M., Woods D. J., Wolstenholme A. J. (2009) Mol. Pharmacol. 75, 1347–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolstenholme A. J., Rogers A. T. (2005) Parasitology 131, S85–95 [DOI] [PubMed] [Google Scholar]
- 22.Bormann J., Rundström N., Betz H., Langosch D. (1993) EMBO J. 12, 3729–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franke C., Hatt H., Dudel J. (1986) Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology 159, 591–609 [DOI] [PubMed] [Google Scholar]
- 24.Macdonald R. L., Olsen R. W. (1994) Annu. Rev. Neurosci. 17, 569–602 [DOI] [PubMed] [Google Scholar]
- 25.Gilbert D. F., Islam R., Lynagh T., Lynch J. W., Webb T. I. (2009) Front Mol. Neurosci. 2, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unwin N. (2005) J. Mol. Biol. 346, 967–989 [DOI] [PubMed] [Google Scholar]
- 27.Chen X., Cromer B., Webb T. I., Yang Z., Hantke J., Harvey R. J., Parker M. W., Lynch J. W. (2009) Neuropharmacology 56, 318–327 [DOI] [PubMed] [Google Scholar]
- 28.Hawthorne R., Cromer B. A., Ng H. L., Parker M. W., Lynch J. W. (2006) J. Neurochem. 98, 395–407 [DOI] [PubMed] [Google Scholar]
- 29.Heads J. A., Hawthorne R. L., Lynagh T., Lynch J. W. (2008) J. Neurochem. 105, 1418–1427 [DOI] [PubMed] [Google Scholar]
- 30.Yang Z., Cromer B. A., Harvey R. J., Parker M. W., Lynch J. W. (2007) J. Neurochem. 103, 580–589 [DOI] [PubMed] [Google Scholar]
- 31.Grudzinska J., Schemm R., Haeger S., Nicke A., Schmalzing G., Betz H., Laube B. (2005) Neuron 45, 727–739 [DOI] [PubMed] [Google Scholar]
- 32.Gleitsman K. R., Shanata J. A., Frazier S. J., Lester H. A., Dougherty D. A. (2009) Biophys. J. 96, 3168–3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamakura T., Mihic S. J., Harris R. A. (1999) J. Biol. Chem. 274, 23006–23012 [DOI] [PubMed] [Google Scholar]
- 34.Beato M. (2008) J. Neurosci. 28, 7412–7425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajendra S., Vandenberg R. J., Pierce K. D., Cunningham A. M., French P. W., Barry P. H., Schofield P. R. (1995) EMBO J. 14, 2987–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vafa B., Lewis T. M., Cunningham A. M., Jacques P., Lynch J. W., Schofield P. R. (1999) J. Neurochem. 73, 2158–2166 [PubMed] [Google Scholar]
- 37.Malosio M. L., Marquèze-Pouey B., Kuhse J., Betz H. (1991) EMBO J. 10, 2401–2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pribilla I., Takagi T., Langosch D., Bormann J., Betz H. (1992) EMBO J. 11, 4305–4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keck T., White J. A. (2009) Rev. Neurosci. 20, 13–22 [DOI] [PubMed] [Google Scholar]
- 40.McKellar Q. A., Benchaoui H. A. (1996) J. Vet. Pharmacol. Therapy 19, 331–351 [DOI] [PubMed] [Google Scholar]
- 41.Siva N. (2009) BMJ 339, b2755. [DOI] [PubMed] [Google Scholar]
- 42.Cotreau M. M., Warren S., Ryan J. L., Fleckenstein L., Vanapalli S. R., Brown K. R., Rock D., Chen C. Y., Schwertschlag U. S. (2003) J. Clin. Pharmacol. 43, 1108–1115 [DOI] [PubMed] [Google Scholar]
- 43.Griffith J., Fletcher N., Clemence R., Blanchflower S., Brayden D. J. (2005) J. Vet Pharmacol. Therapy 28, 257–265 [DOI] [PubMed] [Google Scholar]
- 44.Lespine A., Martin S., Dupuy J., Roulet A., Pineau T., Orlowski S., Alvinerie M. (2007) Eur. J. Pharm. Sci. 30, 84–94 [DOI] [PubMed] [Google Scholar]
- 45.Lee V. K., Tiwary A. K., Sharma-Reddy P., Lieber K. A., Taylor D. K., Mook D. M. (2009) Comp. Med. 59, 227–233 [PMC free article] [PubMed] [Google Scholar]
- 46.Bali M., Jansen M., Akabas M. H. (2009) J. Neurosci. 29, 3083–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lobo I. A., Harris R. A. (2005) Int. Rev. Neurobiol. 65, 53–87 [DOI] [PubMed] [Google Scholar]