Abstract
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer disease (AD). There is considerable consensus that the increased production and/or aggregation of α-synuclein (α-syn) plays a central role in the pathogenesis of PD and related synucleinopathies. Current therapeutic strategies for treating PD offer mainly transient symptomatic relief and aim at the restitution of dopamine levels to counterbalance the loss of dopaminergic neurons. Therefore, the identification and development of drug-like molecules that block α-synuclein aggregation and prevent the loss of dopaminergic neurons are desperately needed to treat or slow the progression of PD. Here, we show that entacapone and tolcapone are potent inhibitors of α-syn and β-amyloid (Aβ) oligomerization and fibrillogenesis, and they also protect against extracellular toxicity induced by the aggregation of both proteins. Comparison of the anti-aggregation properties of entacapone and tolcapone with the effect of five other catechol-containing compounds, dopamine, pyrogallol, gallic acid, caffeic acid, and quercetin on the oligomerization and fibrillization of α-syn and Aβ, demonstrate that the catechol moiety is essential for the anti-amyloidogenic activity. Our findings present the first characterization of the anti-amyloidogenic properties of tolcapone and entacapone against both α-synuclein and Aβ42 and highlight the potential of this class of nitro-catechol compounds as anti-amyloidogenic agents. Their inhibitory properties, mode of action, and structural properties suggest that they constitute promising lead compounds for further optimization.
Keywords: Diseases/Alzheimer Disease, Diseases/Amyloid, Methods/Electron Microscopy, Methods/Fluorescence, Methods/NMR, P/Drug Interactions, COMT Inhibitors, Catechols, Parkinson Disease
Introduction
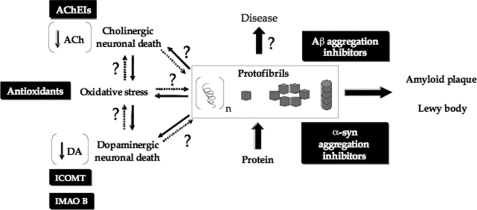
Parkinson disease (PD)2 is the second most common neurodegenerative disorder after Alzheimer disease (AD), affecting nearly 1–2% of the population 65 years and older. A characteristic early pathological change associated with PD is the selective loss of dopaminergic neurons of the substantia nigra pars compacta and other areas of the brain resulting in the degeneration of the nigro-striatal tract and loss of dopamine (DA) (1). Current therapeutic strategies for treating PD offer mainly transient symptomatic relief by aiming to restore the loss of dopamine by “dopamine replacement therapy.” This is accomplished through the administration of levodopa (l-DOPA), a direct precursor of DA and other drugs that increase the lifetime of DA by slowing its metabolism. Catechol O-methyltransferase inhibitors (ICOMT), monoamine oxidase B inhibitors (IMAO B), and peripheral aromatic l-amino acid decarboxylase inhibitors (IAADC) are used as adjunctive medications to l-DOPA to slow DA degradation and increase the availability of brain DA (Scheme 1) (2).
SCHEME 1.
Working model illustrating that protein aggregation, i.e. α-syn in PD and Aβ in AD, has a central role in the generation of the cascade of events that result in neurodegeneration and disease.
Neuropathologically, PD is characterized by the formation of intraneuronal Lewy bodies and Lewy neuritis consisting primarily of fibrillar aggregates of α-synuclein (α-syn), a 14-kDa “natively unfolded” cytosolic protein (3). Accumulating evidence from genetics, animal models, and biochemical and biophysical studies suggests that α-syn aggregation is a toxic event that plays a central role in the initiation and/or progression of PD (4). Mutations or increased expression of α-syn are associated with early onset familial forms of PD (5–7). Overexpression of wild type and disease-associated mutants enhances α-syn aggregation and toxicity in several animal and cellular models of synucleinopathies (8–10). In vitro studies have consistently shown that disease-associated mutations accelerate and enhance α-syn oligomerization (A30P, A53T, and E46K) and/or fibrillization (A53T and E46K) (11–14).
Despite numerous advances in our understanding of the molecular mechanism of α-syn aggregation and toxicity in vitro and in vivo, we still have limited knowledge about the following: 1) the normal physiological function of α-syn; 2) the relative contribution of α-syn aggregation to the pathogenesis of PD; 3) the identity of the toxic α-syn species; and 4) the exact mechanism by which α-syn contributes to the loss of dopaminergic neurons and PD pathology (15–17). The lack of animal models that represent the full spectrum of PD in humans and the absence of effective drugs capable of blocking α-syn aggregation at different stages on the amyloid formation pathway have contributed significantly to this knowledge gap and slow the pace of drug discovery in PD and related disorders.
The ability to block protein aggregation using small molecules provides a unique opportunity to investigate the link between α-syn aggregation and toxicity by assessing the functional and cellular consequences of blocking aggregation at different stages on the amyloid formation pathway. Initial efforts aimed at blocking or reversing protein aggregation focused on preventing amyloid formation or disruption of preformed fibrils as a means of promoting their clearance. Several classes of small molecules and short peptides have been reported as inhibitors or modulators of α-syn and amyloid-β (Aβ) fibrillization in vitro, including polyphenols such as catecholamines, DA, and other catechols (18–22). Dopamine agonists and monoamine oxidase B inhibitors, currently used as anti-parkinsonian agents, were also reported to destabilize preformed fibrils (23, 24).
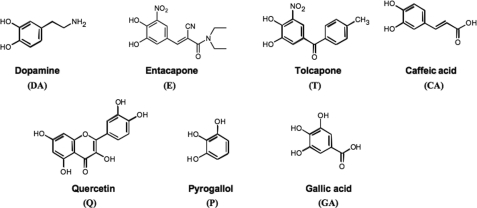
The aim of this work was to evaluate the inhibitory potency of anti-Parkinsonian drugs toward the oligomerization, fibrillogenesis, and toxicity of monomeric and oligomeric forms of α-syn. These molecules include two nitrocatechols, i.e. entacapone (E) and tolcapone (T) (25) (Fig. 1), that have never been specifically tested for their anti-amyloidogenic properties. Here, we were report that E and T are potent inhibitors of α-syn and Aβ42 oligomerization and fibrillogenesis and protect against extracellular toxicity induced by the aggregation of both proteins in PC12 cells. To determine the relative contribution of the catechol moiety in these compounds, we compared their effect to four other natural polyphenols (quercetin (Q), caffeic acid (CA), gallic acid (GA), and pyrogallol (P)) known for their antioxidant properties (26–29) and dopamine (DA), a well known inhibitor of the fibrillization of α-syn and Aβ. Our studies present the first characterization of the anti-amyloidogenic properties of T and E against both α-syn and Aβ42 fibrillization and provide new insights into the mechanism of action of catechol-containing compounds on the amyloid formation pathway of both proteins.
FIGURE 1.
Chemical structure of compounds examined as inhibitors of human WT α-syn and Aβ42 fibril formation.
EXPERIMENTAL PROCEDURES
Thioflavin T, gallic acid, quercetin, and MTT were purchased from Sigma. Dimethyl sulfoxide (DMSO) of microbiological quality, dopamine, caffeic acid, and pyrogallol were from Fluka (Buchs, Switzerland). Entacapone and tolcapone were kindly donated by Prof. Alberto Gasco (Dipartimento della Scienza e Tecnologia del Farmaco, University of Turin, Italy). Dulbecco's modified Eagle's medium, heat-inactivated horse serum, fetal bovine serum, gentamicin, insulin, NuPAGE 4–12% BisTris gel, goat anti-mouse AlexaFluor 680, and SilverXpress silver staining kit were from Invitrogen. β-Amyloid monoclonal antibody 6E10 was from Covance and nitrocellulose membranes from Protran. All chemicals were of analytical grade, and all solutions were prepared in autoclaved distillate water. Compounds solutions were prepared in 100% DMSO (10 mm). Fresh solutions were prepared by diluting stock solutions in water to achieve a final amount of co-solvent less than 1% in the reaction mixtures.
Expression and Purification of α-Synuclein
Human wild type (WT) α-syn was expressed as described previously (12). Cells were harvested, resuspended in buffer, and lysed. The supernatant was saved, concentrated, and loaded onto a Superdex 200 size exclusion column. α-Syn containing fractions were combined, lyophilized, and stored at −20 °C until use.
Preparation and Characterization of Aβ42 Low Molecular Weight (LMW) and Protofibrils (PF)
Aβ42 was synthesized and purified by Dr. James I. Elliot at Yale University (New Haven, CT). Monomeric Aβ42 stock solutions were prepared by dissolving the peptide in 6 m guanidine HCl at a concentration of 1 mg/ml and centrifuged at 8600 rpm for 5 min. LMW and PF Aβ42 stock solutions were prepared by dissolving the peptide in 5% DMSO, 2 m Tris base, pH 7.6. The mixture was subjected to low speed centrifugation at 6000 rpm for 5 min. The supernatant of Aβ42 containing monomeric, LMW, and PF was loaded onto a gel filtration column (Superdex 75 HR 10/30 Amersham Biosciences) previously equilibrated with 10 mm Tris buffer, pH 7.4 (30). The fractions were analyzed by SDS-PAGE, and protein concentration was determined by UV absorbance at 280 nm in 10-mm path length cuvettes using the theoretical molar extinction coefficient at 280 nm for Aβ42 (1490 m−1 cm−1) (31). All Aβ42 stock solutions were diluted with Tris buffer, pH 7.4, to a final peptide concentration of 10 μm. Samples of Aβ42 were incubated at 37 °C in 1.5-ml polypropylene sterile tubes, with and without different concentrations of inhibitors, at molar ratios of Aβ42/inhibitor of 1:0.5 and 1:2.
Preparation of α-Syn and Aβ42 Seeds
The seeds were prepared by incubation of α-syn peptide solution (20 mm Tris buffer, 150 mm NaCl, pH 7.4) and Aβ42 peptide solution (5% DMSO, 2 m Tris buffer, pH 7.6), at 37 °C under agitation for 3 days. The fibrils were then mechanically fragmented to yield narrow distribution of smaller fibrillar structures (100–300 nm long) by ultrasonication on ice using SONICS Vibra CellTM equipped with a fine tip (20 5-s pulses, amplitude of 40, and output of 6 watts). The sonicated fibrils were diluted in 10 mm Tris buffer, pH 7.4. Seeds and monomeric α-syn or Aβ42 were incubated for 4 h at 37 °C with continuous shaking, with and without inhibitors, in polystyrene black 384-well plates (Nunc).
Fibrillization Studies
Purified, lyophilized α-syn was dissolved in 20 mm Tris buffer, 150 mm NaCl. The samples of α-syn at a concentration of 100 μm (as estimated by spectroscopy) were incubated at 37 °C in 1.5-ml sterile polypropylene tubes, with continuous shaking, in the absence and presence of inhibitors, at a molar ratio of α-syn/inhibitors of 1:1, 1:0.5, 1:0.1, and 1:0.01. Aliquots (10 μl) of the α-syn incubations (final protein concentration of 10 μm), previously incubated at 37 °C with and without compounds, were added to 80 μl of 50 μm glycine buffer, pH 8.5, and 10 μl of solution of 100 μm ThT. Aliquots of 80 μl of 10 μm Aβ42 solutions (LMW or PF), previously incubated at 37 °C in the absence and presence of compounds, were added to 10 μl of 100 μm ThT and 10 μl of 50 μm glycine buffer, pH 8.5.
The time course of α-syn and Aβ42 fibrillization was measured by the ThT fluorescence assay. Fluorescence measurements were carried out with a spectrofluorometer (AnalystTM AD 96-384, Bucher Biotec AG, Basel) at 25 °C using polystyrene black 384-well plates. The excitation wavelength was set to 450 nm, and emission was monitored at 485 nm. All measurements were done in triplicate by performing three identical experiments.
Seeding Polymerization Assay
The polymerization of soluble α-syn with or without α-syn fibrils added as seeds and Aβ42 with or without Aβ42 seeds was assayed as described elsewhere (32). Nunc 384 fluorescence plates were filled with monomeric and seed protein solutions. Compounds of interest (or Tris buffer containing 1% DMSO for the control) were finally added to the reaction mixture. The plate was incubated at 37 °C for 3 h under agitation. The kinetics and extent of fibrillization was monitored using the standard ThT binding assay as described above. The assay was run in triplicate by processing three identical plates.
Electron Microscopy Analysis of Fibril Formation
Aβ and α-syn fibril formation was monitored by transmission electron microscopy (TEM). Samples for TEM analysis were prepared by placing 10 μl of the sample solution on Formvar-carbon copper grid for 1 min before removing the excess solution. The grid was washed with 2 drops of distillated water and 1 drop of uranyl acetate before staining with 1% of fresh uranyl acetate for 30 s. The grids were thoroughly examined to get an overall evaluation of the structures present in the sample. Specimens were inspected at 80 kV using a Philip CIME 12 electron microscope. Digitized photographs were recorded with a slow scan CCD camera (Gatan, model 679).
NMR Spectroscopy
NMR spectra were acquired on a Bruker Avance III 600 MHz NMR spectrometer at 15 °C. Measurements were performed using 60 μm 15N-labeled α-syn in 20 mm Tris, 150 mm NaCl, pH 7.4. Two-dimensional 1H-15N heteronuclear single quantum coherence (HSQC) spectra were recorded using 256 × 1024 complex data points in the F1 and F2 dimensions, with a relaxation delay of 1.0 s (33). Sixty four scans per increment were recorded for each spectrum. Spectral widths were 1612.9 and 7211.5 Hz in the 15N and 1H dimensions, respectively.
Spectra were processed with Topspin 1.3 (Bruker Biospin) and NMRPipe (34). Visualization and manipulation were performed using the public domain graphics program Sparky 3 (34). Resonance assignments had been previously obtained (35). The addition of 1% DMSO and a small change in temperature did not interfere with transfer of resonance assignment.
Two-dimensional 1H-15N HSQC spectra were recorded to monitor chemical shift changes induced by the presence of three compounds (E, Q, and T). Compounds were freshly dissolved in DMSO to high concentration and added to the α-syn sample. The concentration of DMSO did not exceed 3% (v/v). Measurements were performed for molar ratios of 1:0.5, 1:2, and 1:10 α-syn/ligand. For each titration step, a reference spectrum was obtained by addition of the same amount of DMSO only. Both intensity and chemical shift differences were analyzed with respect to the DMSO reference spectrum. Mean weighted 1H-15N chemical shift differences were calculated according to Δav = {((Δδ2HN + Δδ2N/25))}/2 (33, 35, 36).
PC12 Preparation and Toxicity Studies
The rat adrenal gland pheochromocytoma cell line, PC12, was grown at 37 °C in 5% of CO2 in Dulbecco's modified Eagle's medium supplemented with 6% heat-inactivated horse serum, 6% of fetal bovine serum, and 50 μg/ml gentamicin. Exponentially growing PC12 cells (5·104 cells per well) were plated in 96-well tissue culture plates (Falcon) in media supplemented with 2 μm insulin and 50 μg/ml gentamicin. The cells were co-treated with Aβ42 crude preparation (40 μm), and the compounds (40 μm) at different concentrations (5, 10, 20, and 40 μm) for 24 h at 37 °C and in 5% of CO2. The peptide and the compounds were added directly to the Dulbecco's modified Eagle's medium supplement with 2 μm insulin and 50 μg/ml gentamicin. Given the slow rate of spontaneous α-syn fibrillization in vitro, the α-syn toxicity studies were performed using preaggregated proteins formed in the presence and absence of inhibitors. Briefly, the α-syn protein was incubated alone for 72 h at 37 °C under agitation conditions alone or in the presence of the inhibitors at a different molar ratio. At this point, α-syn or the α-syn/inhibitor mixtures were added directly to the cell culture medium (Dulbecco's modified Eagle's medium supplemented with 2 μm insulin and 50 μg/ml gentamicin) and incubated with PC12 for 24 h at 37 °C in 5% of CO2. The viability of PC12 cells was evaluated by thiazolyl blue MTT (37) and luminescent cells assays. The MTT assay is based on the conversion of tetrazolium salt to formazan (blue compound), a reaction that only occurs in viable cells because the chemical reaction is carried only by mitochondrial dehydrogenases. MTT was added at final concentration of 0.5 mg/ml for 2 h at 37 °C, and the formed crystals were dissolved using 100 μl of solubilization buffer containing 30% SDS and 70% isopropyl alcohol in water. The absorbance was determined at 570 nm using a microplate reader. The luminescent cell viability assay is a sensitive method of determining the number of viable cells in culture based on quantitation of the ATP present, an indicator of metabolically active cells. The assay procedure involves adding the single reagent (Promega CellTiter-Glo® reagent) directly to cultured cells. The light emission, measured by Safire Instrument, is directly proportional to the ATP produced. All quantitative data are presented as means ± S.E. Statistical analysis between different treatments was calculated by using one way analysis of variance followed by post hoc comparison through Bonferroni's test. A value of p > 0.05 was considered statistically significant.
RESULTS
Inhibition of α-Syn Fibrillization by Entacapone, Tolcapone, and Related Catechols
α-Syn fibril formation is a concentration-dependent process and occurs readily in vitro only under conditions that combine high protein concentrations (100–200 μm) and mechanical agitation to accelerate the process (supplemental Fig. 1). We evaluated the fibrillization of human WT α-syn alone under agitation in the range of 50–200 μm, and a 100 μm protein concentration was chosen as the optimal concentration to assess the inhibitory activity of the compounds (Fig. 1). Under these conditions, α-syn fibrillization was complete after 72–96 h of incubation at 37 °C.
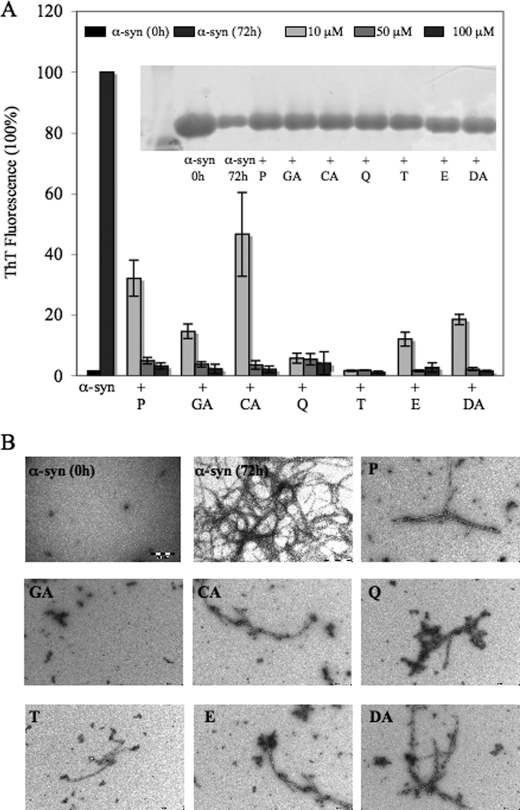
To investigate the influence of the two nitrocatechols, entacapone and tolcapone, and the four natural compounds, quercetin, CA, GA, and pyrogallol on α-syn aggregation, we monitored the fibrillization of α-syn in the absence and presence of each compound in the concentration range of 1–100 μm using ThT fluorescence, SDS-PAGE analysis of soluble protein, and TEM as a function of time. DA was chosen as a reference compound and was tested under the same working conditions. As shown in Fig. 2, after 72 h of incubation, all compounds tested abolished α-syn fibril formation at concentrations of 50 and 100 μm. Compounds E, GA, and Q exhibited strong inhibition of α-syn aggregation even at concentrations of 10 μm, with compound T demonstrating the greatest inhibition potency (Fig. 2A). More than 70–80% reduction of the ThT fluorescence signal was observed in all samples containing ≥10 μm of compounds.
FIGURE 2.
Compounds abolish α-syn fibril formation and increase the soluble forms of α-syn after incubation for 72 h. A, samples of 100 μm α-syn were incubated at 37 °C, with continuous shaking, with and without compounds, at molar ratios of α-syn/inhibitor of 1:0.1, 1:0.5, and 1:1. The time course of protein fibrillization was measured by ThT fluorescence assay after incubation for 72 h. The bar graph represents the amount of fibril formation in absence and in presence of the compounds P, GA, CA, Q, T, E, and DA. The figure shows means of three independent experiments ± S.D. (n = 6). The inhibitory effect of the compounds on α-syn aggregation was evaluated by SDS-PAGE. Samples of α-syn (100 μm) were incubated with and without compounds, at molar ratios of α-syn/inhibitor of 1:1. The bands represent the amount of monomeric form of α-syn before incubation and after incubation for 72 h in the absence and in the presence of the compounds. B, electron micrographs of negatively stained quaternary structures deposited from solutions of α-syn before incubation and after 72 h incubation at 37 °C in the absence and in the presence of 100 μm compound. Scale bar, 200 nm.
The ThT results were confirmed by TEM, which demonstrated the absence of significant amounts of amyloid fibrils in α-syn samples incubated with the compounds at 100 μm concentrations (Fig. 2B). After 72 h, WT α-syn alone formed extensive fibrillar structures with an average diameter of 35 nm. In the samples containing 100 μm of E, T, Q, CA, GA, and P, the number of fibrils was significantly reduced, and spherical oligomers and short sheared fibrils were observed instead. Aggregates formed in the presence of the various compounds were morphologically distinct from those formed by WT α-syn.
To further confirm the inhibitory effect observed by ThT and TEM assays, we quantified the amount of remaining soluble protein by SDS-PAGE analysis of the supernatant after removal of fibrils and insoluble materials by centrifugation. The sample containing α-syn alone showed a reduction in band intensity corresponding to approximately >70–80% loss of soluble α-syn after 72 h of incubation at 37 °C, suggesting that the majority of soluble α-syn has been converted into insoluble fibrils under these conditions. In contrast, all samples containing the compounds (100 μm) showed levels of soluble protein that correspond to the inhibitory effect reported by ThT and TEM. At 1:1 molar ratio, more than ∼60% of the starting protein remained in solution after 72 h of incubation (Fig. 2A).
Inhibition of the Seeding Capacity of Fibrillar α-Syn
The process of amyloid fibril formation follows a nucleation-dependent polymerization mechanism that is characterized by an initial lag time phase (nucleation phase), followed by an exponential growth phase (polymerization phase) and a final plateau (equilibrium phase) (38). The spontaneous breakage of fibrils into smaller aggregates or their disassociation by small molecules or chaperones is believed to contribute to the spreading and acceleration of amyloid formation in vivo via a seeding mechanism (39, 40). This is supported by in vitro and in vivo studies demonstrating that amyloid fibril formation is accelerated by the addition of preformed aggregates (41, 42), which act as seeds that nucleate fibril formation and growth. In other words, the addition of seeds eliminates the lag phase associated with fibril formation. Therefore, blocking the seeding capacity of preformed fibrils is an attractive strategy for slowing amyloid formation and disease progression in PD and related disorders. For this purpose, the ability of the compounds to inhibit the seeding capacity of α-syn fibrils was investigated.
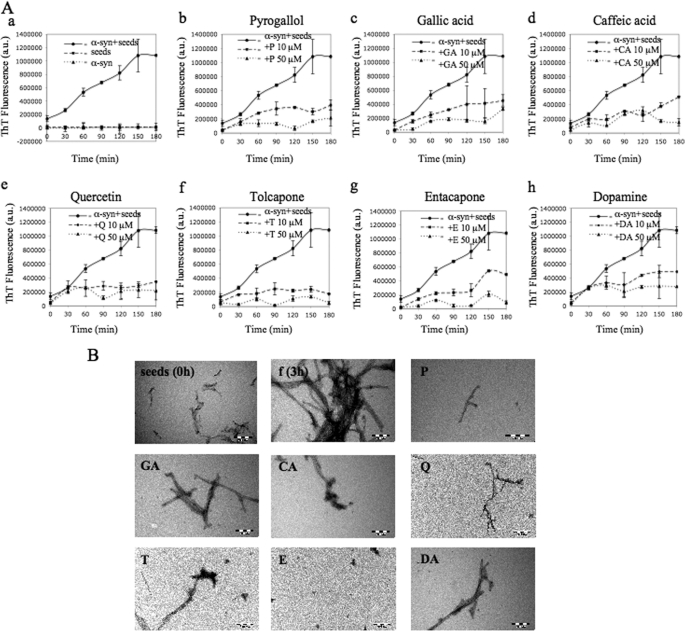
To produce α-syn seeds, mature α-syn fibrils were mechanically disrupted by sonication to yield a narrow distribution of short fibrils. As expected, addition of small amounts of seeds abolished the lag phase and accelerated α-syn fibrillization (Fig. 3A). To determine the relative potency of the compounds toward blocking the seeding capacity of α-syn fibrils, 10 and 50 μm of each compound was added to a solution of freshly prepared monomeric α-syn. Fibrillar seeds at 2 μm final concentration were then added, and the kinetics of fibrillization was monitored by ThT fluorescence. In the absence of compounds, fibrillization proceeds immediately to yield a dense network of amyloid fibrils. The fibrillization reaction was complete within 3 h as opposed to 72–96 h in the absence of the seeds. At 50 μm concentration of E, T, Q, P, and CA (Fig. 3A, filled triangles), the seeding capacity of short α-syn fibrils was abolished by >90%. However, only Q and T exhibited a similar potency at lower concentrations (10 μm). The remaining compounds E, P, CA, GA, and DA still showed greater than 60–75% inhibition of seeded fibril growth at this concentration. These findings were confirmed by TEM, which demonstrated the presence of predominantly spherical structures and short isolated fibrillar assemblies (Fig. 3B) in seeded samples containing 50 μm of the compounds compared with extensive fibril formation in samples containing only α-syn.
FIGURE 3.
Compounds have an inhibitory effect on the α-syn seeding polymerization. A, samples of monomeric α-syn (100 μm) were incubated with the seeds (2 μm) at 37 °C, with continuous shaking, without (panel a) and with (panels b–h) 10 and 50 μm of compounds P, GA, CA, Q, T, E, and DA. The time course of protein fibrillization was measured every 30 min by ThT fluorescence assay for 3 h. B, electron micrographs of negatively stained quaternary structures deposited from solutions of seeds and α-syn + seeds after 3 h of incubation in the absence and in the presence of the compounds (50 μm). Scale bar represents 200 nm.
Entacapone, Tolcapone, and Related Catechols Do Not Bind to Monomeric α-Syn
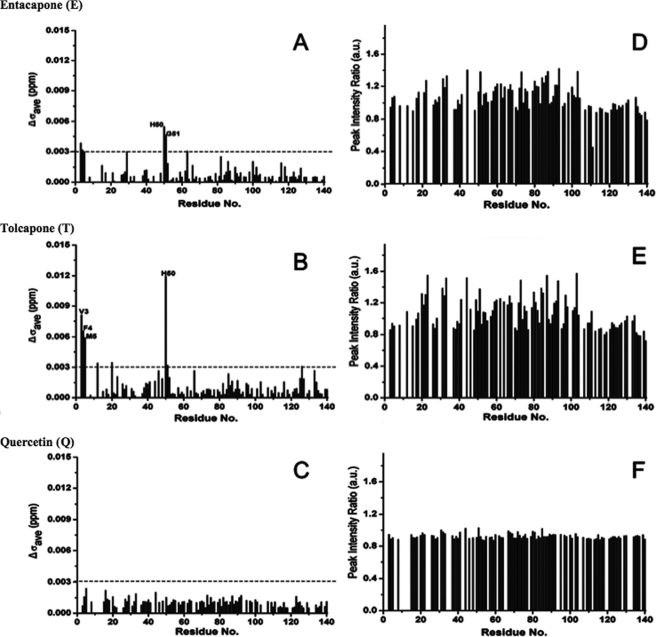
Binding of E, T, and Q to monomeric α-syn was probed using NMR spectroscopy. NMR signals of backbone amides constitute excellent probes of complex formation providing maps of interaction interfaces (43). We monitored the position and intensity of the NMR signals of α-syn in the presence of E, T, and Q for molar ratios up to 1:10 α-syn:compound. In the case of Q, only a 1:2 ratio was reached due to its lower solubility. No significant chemical shift changes were observed for any of the compounds, with the exception of very minor chemical shift changes for His-50 and some N-terminal residues (Fig. 4, A–C). As the very small chemical shift changes observed for His-50 and the two to three N-terminal residues are most likely due to slight changes in pH, the NMR data suggest that there is no direct interaction of the compounds with the backbone of monomeric α-syn.
FIGURE 4.
Analysis of compound binding to monomeric α-syn by NMR spectroscopy. Changes in individual cross-peak positions (A–C) and intensities (D–F) of backbone 15N-1H resonances of α-syn (60 μm) in two-dimensional 1H-15N HSQC spectra in the presence of compounds E (A and D), T (B and E), and Q (C and F). For compounds E and F, molar ratios of 1:10 α-syn/compound were used. Compound Q is less soluble, and only the 1:2 α-syn/compound ratio could be measured. Horizontal lines indicate the average variation of chemical shifts observed for α-syn from sample to sample due to slightly different buffer conditions.
Besides the position of NMR signals, their intensity is very sensitive to changes in the conformational properties of a protein as well as its chemical environment. For example, signal broadening indicates increased chemical exchange. When we compared the intensity of NMR signals in two-dimensional 1H-15N HSQC spectra of α-syn in the free state and in the presence of the compounds E and T, residues in the C-terminal domain showed a different response than those of residues 20–105 (Fig. 4, D–F). NMR signal intensities in the C-terminal domain in the presence of E and T were within 10–15% of the values in the free state. In contrast, a large number of residues in the N-terminal domain of α-syn, in particular in the non-amyloid β-component region, showed an increase of up to 30% in NMR signal intensity in the presence of E and T when compared with the DMSO control spectrum. The increased NMR signal intensities point to an increase in the backbone flexibility of these residues or to a reduced amide proton exchange. Interestingly, a similar increase in NMR signal intensities was observed for residues 22–93 of α-syn in the presence of polyamines, which bind to the C terminus of monomeric α-syn (44).
All Compounds Protect PC12 against α-Syn-induced Cell Death
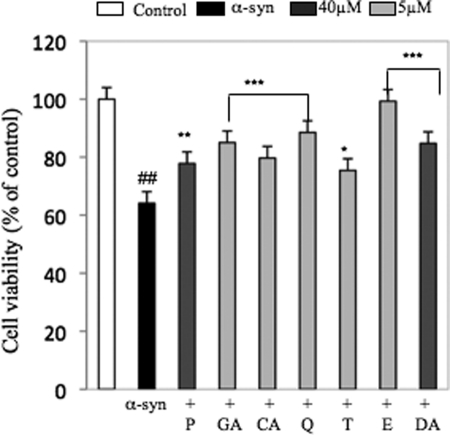
To investigate the effect of the E and T and related compounds in Fig. 1 against α-syn-induced extracellular toxicity on the PC12 cells, we used a preparation of α-syn that was incubated in the presence and/or absence of compounds for 72 °C at 37 °C under agitating conditions. The α-syn/compound mixtures were then added to the cell culture media, and cell viability was evaluated using the MTT assay. The treatment with the preaggregated α-syn (40 μm) showed a reduction of cellular viability by ∼40% (Fig. 5). α-Syn samples, which were incubated with inhibitors, showed a significant increase of cell viability in the range of 10–30%. Interestingly, E was found to be the most active compound, with a protective effect close to 100%. These results suggest a direct correlation between the effect of these compounds on the fibrillization of α-syn and protection against α-syn-induced extracellular toxicity, which may be linked to the ability of the compounds to block the formation of the toxic entity or processes.
FIGURE 5.
Protective effect of the compounds against α-syn-induced toxicity in PC12 cells. PC12 cells were treated with preincubated α-syn (40 μm) alone or co-incubated with 5 μm GA, CA, Q, T, and E and 40 μm P and DA. The cellular viability was evaluated by MTT assay, and the data were expressed as percentage of the control (nontreated cells). The control treatment is set to 100%. Bars are means ± S.E. We used # to compare the data to the control and * with respect to the treatments with α-syn. ##, p < 0.001; ***, p < 0.001; **, p < 0.01; *, p < 0.05.
Entacapone, Tolcapone, and Related Catechols Inhibit the Conversion of LMW Aβ42 into Fibrils
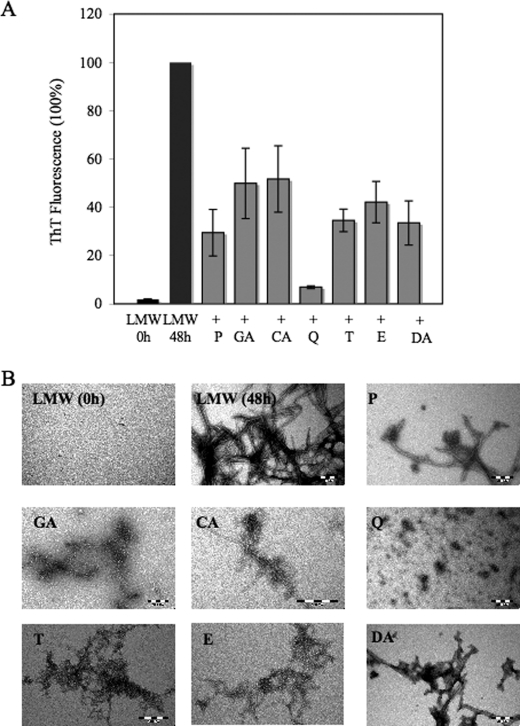
To assess the inhibitory specificity of the compounds toward α-syn fibrillation, we investigated their capacity to inhibit the fibrillization of monomeric LMW and PF β42, which was prepared freshly by size exclusion chromatography, as described previously (30). Fresh monomeric Aβ42 solutions were incubated (37 °C), with and without compounds, at molar ratios compound, Aβ42 of 0.5:1 and 2:1 for 24 and 48 h. In the presence of 5 (data not shown) and 20 μm compounds (Fig. 6A), Aβ42 fibrillization was decreased by ≥50% after 48 h of incubation. Q was found to be the most potent compound, showing greater than 80% inhibition of Aβ42 fibrillization at 5–20 μm, whereas the remaining compounds showed inhibition in the range of 60–70% at higher molar ratios (2:1, compound/Aβ42). These findings indicated that these compounds act by one of the following mechanisms: 1) by stabilizing monomeric Aβ; 2) by kinetic stabilization aggregation intermediates that precede mature fibril formation; or 3) by altering the aggregation properties of Aβ42 such that ThT negative large aggregates are formed.
FIGURE 6.
Compounds inhibit LMW Aβ42 fibril formation. A, samples of LMW Aβ42 were incubated at 37 °C with and without compounds, at molar ratios of Aβ42/inhibitor of 1:2. The time course of protein fibrillization was measured by ThT fluorescence assay. The bar graph represents the amount of fibril formation in the absence and presence of the compounds P, GA, CA, Q, T, E, and DA. The samples containing the compounds showed a decrease of the ThT fluorescence signal after 48 h of incubation. The figure shows means of three independent experiments ± S.D. (n = 6). B, electron micrographs of negatively stained quaternary structures deposited from solutions of LMW Aβ42 (10 μm) before and after 48 h of incubation at 37 °C in the absence and in the presence of 20 μm of the compounds listed. Scale bar, 200 nm.
To determine their mode of action, the samples were analyzed by electron microscopy. TEM images of LMW Aβ42 following incubation for 48 h in the presence of compounds indicate E and T stabilize distinct aggregate morphologies compared with the other compounds (Fig. 6B). Negatively stained TEM images of LMW alone revealed amyloid fibrils (diameter of 30 nm) in the sample after 48 h of incubation (Fig. 6B). Aβ42 solutions containing 20 μm E and T revealed predominantly large networks of PF-like structures and the absence of mature fibrils. In comparison with E and T, the other compounds P, GA, CA, and DA appeared to exert different effects on Aβ fibrillization. GA and CA were shown to stabilize smaller PF structures and result in the formation network of amorphous aggregates, after 48 h of incubation at 37 °C. When Q was added to the sample containing Aβ42 LMW, we observed the formation of predominantly LMW and PF species.
Entacapone, Tolcapone, and Related Catechols Inhibit the Conversion of Aβ42 Protofibrils into Mature Fibrils in a Specific and Concentration-dependent Manner
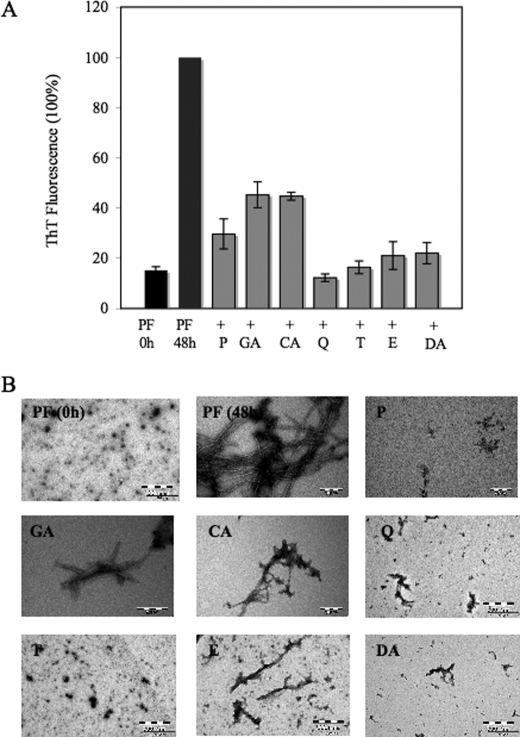
To test our hypothesis and determine whether these compounds act by targeting intermediates on the amyloid pathway, we evaluated their capacity to block the conversion of PFs into mature amyloid fibrils. PFs are metastable oligomeric intermediates, which have been observed during the in vitro fibrillization of Aβ and almost all other amyloidogenic proteins (45). During the last decade, mounting evidence from in vivo and in vitro studies point toward early aggregation intermediates, including PFs, as the major cytotoxic species responsible for triggering neurodegeneration in AD, PD, prion diseases, and other related diseases (46). Freshly isolated PFs were co-incubated with 5 and 20 μm of compounds, and the aggregation was monitored by ThT fluorescence and TEM after 24 h (data not shown) and 48 h at 37 °C. After 48 h, the samples containing PFs alone showed an increase in ThT signal consistent with a conversion of the PFs into mature fibrils (Fig. 7A). When PFs were incubated with 20 μm GA and CA the increase in ThT fluorescence observed with PFs alone was reduced by ∼50%. More than 70% reduction in the ThT signal was observed in the samples containing 20 μm P, E, and DA suggesting that these compounds are more effective at inhibiting the PF to fibril conversion. However, co-incubation of PF with 20 μm Q or T resulted in >80–90% inhibition, with Q being the most potent inhibitor of PF growth and fibrillization in the series. As expected, TEM analysis of the samples containing PFs alone after 48 h of aggregation showed dense networks of fibrils. In the presence of Q, T, E, and DA, mainly PF-like structures, similar to those present at the starting conditions, were observed, in addition to only some isolated short fibrils (Fig. 7B). Short fibrils were observed in the presence of GA and CA after 48 h (Fig. 7B), which is consistent with the higher ThT signal in these samples (Fig. 7A).
FIGURE 7.
Compounds prevent the conversion of Aβ42 protofibrils into mature fibrils in a specific and concentration-dependent manner. A, samples of PF Aβ42 stock solutions were prepared by dissolving the peptide in 5% DMSO, 2 m Tris base, pH 7.6. Samples of Aβ42 were incubated at 37 °C with and without compounds, at molar ratios of Aβ42/inhibitor of 1:2. The time course of protein fibrillization was measured by ThT fluorescence assay. The bar graph represents the amount of fibril formation in the absence and presence of the compounds P, GA, CA, Q, T, E, and DA. The samples containing the compounds showed a decrease of the ThT fluorescence signal after 48 h of incubation. The figure shows means of three independent experiments ± S.D. (n = 6). B, electron micrographs of negatively stained quaternary structures deposited from solutions of PF Aβ42 (10 μm) before incubation and after 48 h of incubation at 37 °C in the absence and presence of 20 μm of the compounds listed. Scale bar, 200 nm.
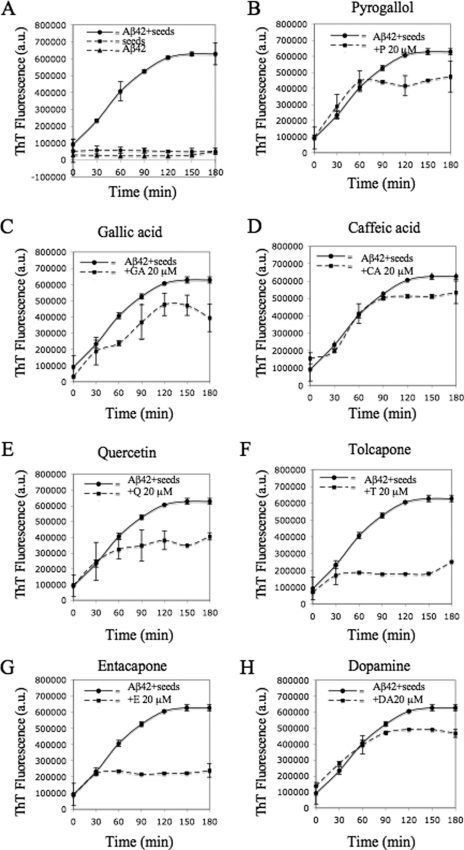
Only Entacapone and Tolcapone Are Effective in Inhibiting the Seeding Capacity of Aβ42
To determine whether E, T, and related compounds can block Aβ42 aggregation at a later stage of the fibrillization process, i.e. fibril growth, the capacity of the compounds to interfere with fibril elongation and the seeding capacity of Aβ42 fibrils was evaluated using the seeding polymerization assay described above. Fibrillar seeds of Aβ42 were prepared by fragmenting preformed and purified fibrils by sonication. The freshly prepared fibril seeds were added to a fresh monomeric solution of Aβ42 and co-incubated with each of the compounds (20 μm). The kinetics of fibrillization was followed over a period of 3 h with ThT, and the structures of the final aggregates were characterized by TEM. Among all the compounds, only E, T, and Q showed significant reduction in fibril growth and inhibition of the seeding capacity by Aβ42 fragmented fibrils (Fig. 8). Analysis of these samples by TEM revealed predominantly short fibrillar structures resembling the fibrillar seeds, consistent with the ThT results (data not shown). These results further confirm that E, T, and Q interfere with the elongation and growth of Aβ42 fibrils possibly via direct interactions with either Aβ42 seeds or monomers or both. After 3 h of incubation, no inhibition was observed in the samples containing P and CA. Moreover, addition of GA and DA to the mixtures containing Aβ42 monomers and seeds did not affect the rate of fibrillization and resulted in only slight reduction of the ThT fluorescence signal (Fig. 8).
FIGURE 8.
Aβ42 monomeric seeding polymerization assays revealed that Q, E, and T have an inhibitory effect on the kinetics. Samples of monomeric Aβ42 (10 μm) were incubated with the seeds (2 μm) at 37 °C, with continuous shaking, without (A) and with (B–H) 20 μm of compounds P, GA, CA, Q, T, E, and DA. The time course of protein fibrillization was measured every 30 min by ThT fluorescence assay for 3 h.
Protection against Aβ42-induced Toxicity in PC12 Cells
Previous studies reported that natural polyphenols like Q, GA, and CA are neuroprotective against Aβ toxicity. The effects of Q and GA have been studied against Aβ42 in primary cultures (47–49) and in vivo models of AD (50). Recently, the protective role of CA on Aβ-induced toxicity in PC12 cells was also described (51). We sought to determine whether there is a correlation between the inhibitory potency of the polyphenolic compounds on Aβ aggregation and their effect on Aβ-induced toxicity in PC12 cells. The compounds were preincubated at concentrations in the range of 5–40 μm with crude preparation of Aβ42, i.e. preparations containing predominantly LMW and PF Aβ42. These conditions were chosen to mimic the pathological situation in vivo where both the LMW and PF species are populated in the diseased AD brain. After incubation with Aβ42 for 20–30 min, the Aβ/compound mixtures were then added to PC12 in cell culture media. The cellular viability was assessed after 24 h using MTT and luminescent assays (30). All the compounds tested did not increase the mortality of PC12 cells in the absence of Aβ42 even at the highest concentration of inhibitors (40 μm, see supplemental Fig. 2), demonstrating that the compounds do not enhance cell viability on their own over a concentration range of 10–40 μm. The cells were then treated for 24 h either with 40 μm Aβ42 alone or in the presence of 5, 10, 20, and 40 μm of the compounds (supplemental Fig. 2). Exposure of PC12 cells to 40 μm Aβ42 reduced the cellular viability by ∼40 and 50% (Fig. 9) as determined by the MTT assay and ATP release, respectively (supplemental Fig. 3), with respect to the control (untreated cells). At higher concentrations (20 and 40 μm), polyphenols were found to be protective against the Aβ42 toxicity, but the attenuation of the toxicity was less evident when compared with the effect observed at low concentration. However, P and DA showed a strong protective effect at a concentration of 40 μm. At a low concentration (5 μm), E and T induced a significant (∼30%) protection against Aβ-induced toxicity, and at higher concentrations (10, 20, and 40 μm), we found an increase of cellular viability and ATP release of ∼20%. These observations indicate that the protection in the co-treatment of the PC12 cells could be related to a dual activity of the compounds, i.e. their inhibitory activity against Aβ aggregation and protection against Aβ-induced cell toxicity.
FIGURE 9.
Protective effect of the compounds against Aβ42-induced toxicity in PC12 cells. PC12 cells were treated with Aβ42 (40 μm) or co-treated in presence of the compounds P, GA, CA, Q, T, E, and DA. The cells were treated with two different compound concentrations, 5 and 20 μm for 24 h. The cellular viability was evaluated by MTT assay, and the data were expressed as percentage of control (nontreated cells). The control treatment is set to 100%. Error bars are means ± S.E. We used # to compare the data to the control and * with respect to the treatments with Aβ42 crude preparation. ##, p < 0.001; ***, p < 0.001; **, p < 0.01.
DISCUSSION
Converging evidence from various sources, including pathology, genetics, biochemistry, cell biology, and animal models suggests that the aggregation of α-syn in PD and Aβ in AD plays a critical role in the pathogenesis of these complex disorders. Hence, strategies aimed at inhibiting and/or reducing the aggregation and amyloid fibril formation of these proteins represent a viable therapeutic strategy to combat and/or prevent the progression of neurodegeneration in both diseases. Toward identifying potent drug-like aggregation inhibitors, E and T, two catechol O-methyltransferase inhibitors currently approved as adjuncts in the therapy of PD, and five other catechol-containing small molecules (GA, CA, Q, P, and DA) were selected, and their effect on the oligomerization and fibrillization of α-syn and Aβ42 was investigated using ThT fluorescence, TEM, and SDS-PAGE analysis.
Our results demonstrate that all catechol-containing compounds shown in Fig. 1 inhibited α-syn and Aβ fibrillization in vitro and showed protective effects against α-syn and Aβ42-induced toxicity in PC12 cells. At 1:1 protein to compound ratio, all compounds tested showed >90% inhibition of α-syn fibrillization and blocked the growth and seeding capacity of α-syn fibrils. However, at lower protein to compound ratios (10:5 and 10:1), only Q, T, and E showed the strongest inhibition. CA and P were the least effective at lower molar ratios and showed only 40–50% inhibition of α-syn fibril formation.
To probe the specificity of these compounds toward α-syn, we determined their capacity to inhibit the fibrillization of Aβ42 monomers, PFs, and fibrils. All the compounds exhibited reduced (55–75%) inhibitory activity against the fibrillization of LMW and PF Aβ42, with the exception of Q, which resulted in >90% reduction in the fibrillization of Aβ42 fibril formation. When we examined the effect of these compounds on the fibrillization of Aβ42 PFs, we observed that T, E, and DA showed greater inhibition (80–90%), with Q being the most effective compound. By TEM analysis, we did not observe significant differences in the structural properties of the aggregates and fibrils formed among the various compounds tested.
There are several mechanisms by which these compounds could inhibit amyloid formation as follows: 1) their ability to stabilize the native monomeric state of amyloid; 2) target different intermediates on the amyloid pathway and block their conversion to fibrils; and 3) alter the aggregation pathway in favor of nonamyloidogenic aggregates. In the case of α-syn, both SDS-PAGE and TEM analyses demonstrate that the presence of these compounds enhances the solubility of α-syn (Fig. 2). On the other hand, none of the compounds tested were shown to stabilize monomeric Aβ42, instead the efficacy of these compounds appears to be linked to their ability to interfere with Aβ42 aggregation at different intermediate stages on the amyloid pathway (Figs. 6A and 7A). All compounds tested were shown to promote the formation of large nonfibrillar aggregates and/or protofibrillar species as discerned by TEM (Figs. 6B and 7B) and the reduced solubility of Aβ42 in the presence of some compounds, despite the fact that incubation with such compounds results in significant reduction in the ThT signals. Consistent with the TEM data, we failed to detect significant accumulation of monomeric Aβ42 after 96 h of incubation in the presence and absence of compounds. Interestingly, each of the compounds appears to exert very specific effects and shows preference for targeting a different aggregation state on the amyloid pathway. For example, although T and E were equally effective in blocking the fibrillization of Aβ42 LMW and PF and showed strong inhibition of Aβ42 fibril growth and seeding capacity, DA and Q were most effective against the fibrillization of Aβ42 LMW and PF but did not show significant inhibition of Aβ42 fibril growth and seeding capacity. Furthermore, incubation of each compound with Aβ42 resulted in the accumulation of Aβ42 aggregates of distinct size and morphological properties (Figs. 6B and 7B).
Together, these results suggest that the presence of the catechol moiety in these compounds is sufficient to impart on them an anti-amyloidogenic activity against α-syn and Aβ42. However, the TEM and solubility studies demonstrate that the compounds interfere with the fibrillization of Aβ42 and α-syn via distinct mechanisms. This hypothesis is further supported by our findings that all the compounds showed strong inhibition of α-syn fibril growth and seeding capacity, whereas only T and E were effective in blocking the growth and seeding capacity of Aβ42 fibrils. The remaining (P, CA, GA, Q, and DA) compounds showed minimal effect even at 1:1 molar ratio, in contrast to their ability to block the seeding capacity of α-syn under similar conditions. To determine whether the specificity and potency of these compounds are mediated by their interaction with specific sequences and/or structural motifs within these two proteins, we sought to determine which residues interact with the most potent compounds, Q, T, and E, using NMR. These studies did not show any significant chemical shift changes for any of the compounds, with the exception of very minor chemical shift changes for His-50 and some N-terminal residues, suggesting that there is no direct binding of the compounds to the backbone of α-syn in its monomeric state. However, an increase in NMR signal intensity was observed in the presence of E and T for a large number of residues in the N-terminal domain of α-syn, in particular in the non-amyloid β-component region. This is in clear contrast to the action of the polyphenol (−)-epigallocatechin gallate, which binds to the backbone of monomeric α-syn, and Aβ40, which decreases the NMR signal intensity of monomeric α-syn and redirects both α-syn and Aβ40 into unstructured off-pathway oligomers (52). Epigallocatechin gallate targets the polypeptide main chain that is identical in all proteins and easily accessible under unfolded conditions (52). In contrast, ThT fluorescence, SDS-PAGE, and TEM measurements indicate that the COMT inhibitors appear to target a conformational feature of oligomers. Currently, it is not known what this conformational feature is but hydrophobic patches formed in oligomers in a rather unspecific manner could be a potential target. Thus, the COMT inhibitors or their scaffold are potentially more useful lead compounds than epigallocatechin gallate, as they preferentially bind to protein aggregates and not to unfolded polypeptide backbones.
Entacapone, Tolcapone, and Related Catechols Protect against Extracellular α-Syn and Aβ42-induced Toxicity in PC12 Cells
To determine whether the ability of these compounds to block and/or alter the fibrillization pathway of Aβ42 and α-syn fibrillization could translate into protection against Aβ42 and α-syn cellular toxicity, we evaluated their protective effect in the cell culture using different assays. At concentrations that showed significant inhibition of Aβ42 and α-syn fibrillization, all the compounds were shown to protect PC12 cells from Aβ42 and α-syn-induced cytotoxicity, by mechanisms that are directly linked to their ability to modulate the fibrillization of both proteins. These results are in agreement with previous studies. Several small polyphenol molecules were shown to exhibit strong anti-amyloidogenic and neuroprotective properties in vitro and in vivo. Bastianetto et al. (49) reported on the neuroprotective effects of GA and other green and black tea catechin gallate against Aβ40 in neuronal cell cultures. Other groups have also demonstrated that catechol and polyphenol compounds are potent anti-amyloid agents and investigated the chemical and structural properties underlying their potency. However, to the best of our knowledge, the anti-amyloidogenic properties of the two nitrocatechols E and T, already known for other biological activities, i.e. antioxidants and catechol O-methyltransferase, have never been described in literature.
Although there is strong evidence in support of the protofibril hypothesis, the exact mechanisms by which protofibrils cause toxicity and the identity of the toxic species remain unknown. Protofibrils represent a heterogeneous mixture of aggregates of various size and morphologies, some of which are likely to contribute to toxicity. It is noteworthy that all the fibrillization inhibitors reported in the literature, including those that protect against Aβ and α-syn toxicity, exert their effect by acting at an intermediate step along the amyloid pathway, i.e. there are no known small molecule inhibitors that stabilize monomeric Aβ and α-syn. As shown in Figs. 6 and 7, the different compounds we tested appear to stabilize or induce the formation of prefibrillar aggregates of distinct morphologies. Together, these results suggest that these molecules act by altering the structure of the aggregates and diverting toxic intermediates toward off-pathway nontoxic species. Studies from several groups have shown that small molecules, including inositol stereoisomers (53), (−)-epigallocatechin gallate (52), as well as Aβ42-derived peptides (54), were shown to alter the toxic properties of Aβ by stabilizing and/or inducing structural remodeling of protofibrillar and fibrillar aggregates. Resveratrol blocks Aβ toxicity without inhibiting oligomer formation (55). Moreover, recent studies from our group (30) and others (56) suggest that amyloid toxicity requires an on-going fibrillization process, i.e. the presence of protofibrils or fibril is not sufficient to cause toxicity unless these species are undergoing an on-going fibrillization process.
Previous studies with DA and other catecholamines, e.g. apomorphine (57), linked their anti-amyloidogenic properties to their ability to undergo rapid autoxidation in aqueous solution, suggesting that one or more oxidation products is responsible for their inhibitor properties (21). However, the two major products of the catecholamine oxidation, i.e. quinines and aminochromes, are relatively unstable and difficult to isolate. To verify this hypothesis, the 3-methoxytyramine, the major metabolite of dopamine, was tested in the same working conditions and in the presence of the two proteins, i.e. α-syn and Aβ42. As expected, the results obtained confirmed that the methylated derivative of dopamine had no effect on the protein fibrillization process (data not shown). The mechanism by which catechol-containing compounds block protein fibrillization remains controversial. Conway et al. (20) reported that DA stabilizes α-syn PFs by forming a DA-α-syn adduct. More recently, Norris et al. (21) suggested a novel mechanism of action in which the dopaminochrome, the oxidized product of DA, inhibits α-syn fibrillization by interacting with the specific amino acid motif in the C terminus and non-amyloid β-component region of α-syn (23, 40, 58, 59). Given the structural similarity among E, T, and other known anti-amyloidogenic catechol derivatives such as DA and Q, it is plausible to speculate that a shared mechanism may underlie the effectiveness of all these compounds. All seven molecules shown in Fig. 1 have in common the fact that each possesses at least one aromatic ring with catechol moiety.
Relevance to α-Syn Toxicity and Parkinson Disease
Although predominantly a cytosolic protein, several lines of evidence suggest a potential role of extracellular α-syn in mediating α-syn toxicity, Lewy body formation, and the pathogenesis of PD and related synucleinopathies. These lines of evidence include the following: 1) recent studies from different laboratories have shown that some monomeric and/or soluble aggregated forms of α-syn are secreted and can be detected in the blood plasma and cerebrospinal fluids of patients suffering from PD and related synucleinopathies (60–63); 2) the fact that exogenous aggregated forms of α-syn have been shown to induce microglial activation and stimulate the production of reactive oxygen species (64) and pro-inflammatory factors (65) and are toxic to mammalian cells and primary neurons (66–72); 3) studies that have shown that the cellular uptake of extracellular α-syn occurs by passive diffusion (monomers) or via receptor-mediated endocytic pathways (73) (oligomers and protofibrils), depending on the aggregation state of α-syn (74); and 4) the fact that small amounts of extracellular α-syn aggregates can efficiently catalyze the aggregation of intracellular α-syn inclusions. Specifically, Luk et al. (75) demonstrated that α-syn fibrils, prepared from recombinant full-length or truncated α-syn, were uptaken by cells within cultures and act as seeds that catalyzed the aggregation and conversion of soluble intracellular α-syn into Lewy body-like inclusions. Finally, 5) Desplats et al. (76) demonstrated neuron-to-neuron and neuron-to-glia transmission of monomeric and aggregated α-syn species in vivo and in cell cultures. These studies suggest that the release and uptake of monomeric and soluble aggregates of α-syn play a central role in inclusion formation, neuronal cell death, and spreading of α-syn pathology in PD. Therefore, targeting the aggregation of extracellular α-syn and/or promoting their clearance have emerged as viable therapeutic strategies for PD and related synucleinopathies. In this regard, the identification of small molecules that block or reverse the aggregation of extracellular α-syn is desirable. Here, we demonstrated that entacapone, tolcapone, and related catechols inhibit α-syn fibrillization in vitro and prevent the formation of toxic aggregates as discerned by their protection against α-syn induced extracellular toxicity in PC12 cells.
Currently, the most effective treatment for PD continues to be the administration of l-DOPA together with a peripheral l-amino acid decarboxylase inhibitor that is unable to enter the central nervous system. However, the amount of l-DOPA reaching the brain after oral administration is very low (about 5–10%). Furthermore, the subsequent metabolism of l-DOPA by COMT clearly limits its availability in the brain. The two COMT inhibitors E and T, approved as adjuncts in the therapy of PD, increase the availability of l-DOPA for conversion to dopamine in the brain (25) mainly by preventing the extensive metabolism of l-DOPA through O-methylation in the periphery (E and T) and partly in brain (T) (77). However, the potential for the use of T as neuroprotective drug in AD or PD is limited due to the fact that it has been shown to cause severe hepatotoxicity resulting in its withdrawal from the market in many countries leaving entacapone as the only COMT inhibitor presently available in the clinic for the treatment of PD (78). However, recent studies demonstrate that tolcapone can be used with benefit when the liver function is actively monitored (79, 80). Although these two compounds share the same pharmacophore, their pharmacokinetic profiles are remarkably different (81). Indeed, studies with rats have shown that tolcapone has a longer duration of action than entacapone and is both a central and peripheral COMT inhibitor, whereas entacapone is essentially a peripheral inhibitor (82–85). However, the benefits of both entacapone and tolcapone in the l-DOPA treatment of patients suffering of Parkinson disease were proved (86–88).
Conclusions
In summary, our study showed that entacapone and tolcapone are potent inhibitors of α-syn and Aβ oligomerization and fibrillogenesis and protect against extracellular toxicity induced by the aggregation of both proteins. Our results provide additional evidence for the potential of catechols as anti-amyloidogenic agents and demonstrate that entacapone and tolcapone belong to the classes of multifunctional drugs (89) because they can inhibit COMT and act as good antioxidants and as effective inhibitors of protein aggregation (90). Whether the anti-amyloidogenic property of entacapone and tolcapone and the protection against α-syn extracellular toxicity contribute to their clinical benefits and enhanced symptomatic treatment of PD or not remains to be determined. Nonetheless, our findings suggest that the structure of entacapone and tolcapone constitute molecular scaffolds that could guide the development of more potent inhibitors of amyloid formation and toxicity. Chemical modifications of entacapone and tolcapone can be envisaged to optimize its pharmacokinetic profile especially by avoiding hepatotoxicity (91), modeling its peripheral metabolism and increasing its blood-brain barrier permeation.
Acknowledgments
We thank Dr. Sara Butterfield and Dr. Marianne Reist for reviewing the manuscript and offering helpful suggestions. We thank the group at the Interdisciplinary Centre for Electron Microscopy and Nathalie Jordan for technical assistance.
This work was supported by the Swiss Federal Institute of Technology Lausanne, grants from the Swiss National Science Foundation and the Strauss Foundation, and by the Max Planck Society and through Deutsche Forschungsgemeinschaft Heisenberg Scholarship ZW 71/2-1 and 3-1 (to M. Z.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- PD
- Parkinson disease
- Aβ42
- amyloid β-(1–42)
- AD
- Alzheimer disease
- CA
- caffeic acid
- E
- entacapone
- GA
- gallic acid
- HSQC
- heteronuclear single quantum coherence
- l-DOPA
- levodopa
- DA
- dopamine
- P
- pyrogallol
- Q
- quercetin
- α-syn
- α-synuclein
- SEC
- size exclusion chromatography
- TEM
- transmission electron microscopy
- ThT
- thioflavin T
- T
- tolcapone
- WT
- wild type
- COMT
- catechol O-methyltransferase
- PF
- protofibril
- LMW
- low molecular weight
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
REFERENCES
- 1.Recchia A., Debetto P., Negro A., Guidolin D., Skaper S. D., Giusti P. (2004) FASEB J. 18, 617–626 [DOI] [PubMed] [Google Scholar]
- 2.Sommer D. B., Stacy M. A. (2008) Expert Rev. Neurother. 8, 1829–1839 [DOI] [PubMed] [Google Scholar]
- 3.Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997) Nature 388, 839–840 [DOI] [PubMed] [Google Scholar]
- 4.Cookson M. R. (2009) Mol. Neurodegener. 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung K. K., Dawson V. L., Dawson T. M. (2003) J. Neurol. 250, Suppl. 3, III15–24 [DOI] [PubMed] [Google Scholar]
- 6.Li J., Uversky V. N., Fink A. L. (2001) Biochemistry 40, 11604–11613 [DOI] [PubMed] [Google Scholar]
- 7.Zarranz J. J., Alegre J., Gómez-Esteban J. C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atarés B., Llorens V., Gomez Tortosa E., del Ser T., Muñoz D. G., de Yebenes J. G. (2004) Ann. Neurol. 55, 164–173 [DOI] [PubMed] [Google Scholar]
- 8.Chen L., Feany M. B. (2005) Nat. Neurosci. 8, 657–663 [DOI] [PubMed] [Google Scholar]
- 9.Lo Bianco C., Shorter J., Régulier E., Lashuel H., Iwatsubo T., Lindquist S., Aebischer P. (2008) J. Clin. Invest. 118, 3087–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masliah E., Rockenstein E., Veinbergs I., Mallory M., Hashimoto M., Takeda A., Sagara Y., Sisk A., Mucke L. (2000) Science 287, 1265–1269 [DOI] [PubMed] [Google Scholar]
- 11.El-Agnaf O. M., Curran M. D., Wallace A., Middleton D., Murgatroyd C., Curtis A., Perry R., Jaros E. (1998) Neuroreport 9, 3925–3927 [DOI] [PubMed] [Google Scholar]
- 12.Paleologou K. E., Schmid A. W., Rospigliosi C. C., Kim H. Y., Lamberto G. R., Fredenburg R. A., Lansbury P. T., Jr., Fernandez C. O., Eliezer D., Zweckstetter M., Lashuel H. A. (2008) J. Biol. Chem. 283, 16895–16905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conway K. A., Harper J. D., Lansbury P. T., Jr. (2000) Biochemistry 39, 2552–2563 [DOI] [PubMed] [Google Scholar]
- 14.Conway K. A., Lee S. J., Rochet J. C., Ding T. T., Harper J. D., Williamson R. E., Lansbury P. T., Jr. (2000) Ann. N.Y. Acad. Sci. 920, 42–45 [DOI] [PubMed] [Google Scholar]
- 15.Horwich A. (2002) J. Clin. Invest. 110, 1221–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Losic D., Martin L. L., Mechler A., Aguilar M. I., Small D. H. (2006) J. Struct. Biol. 155, 104–110 [DOI] [PubMed] [Google Scholar]
- 17.Morgan C., Colombres M., Nuñez M. T., Inestrosa N. C. (2004) Prog. Neurobiol. 74, 323–349 [DOI] [PubMed] [Google Scholar]
- 18.Cappai R., Leck S. L., Tew D. J., Williamson N. A., Smith D. P., Galatis D., Sharples R. A., Curtain C. C., Ali F. E., Cherny R. A., Culvenor J. G., Bottomley S. P., Masters C. L., Barnham K. J., Hill A. F. (2005) FASEB J. 19, 1377–1379 [DOI] [PubMed] [Google Scholar]
- 19.Li H. T., Lin D. H., Luo X. Y., Zhang F., Ji L. N., Du H. N., Song G. Q., Hu J., Zhou J. W., Hu H. Y. (2005) FEBS J. 272, 3661–3672 [DOI] [PubMed] [Google Scholar]
- 20.Conway K. A., Rochet J. C., Bieganski R. M., Lansbury P. T., Jr. (2001) Science 294, 1346–1349 [DOI] [PubMed] [Google Scholar]
- 21.Norris E. H., Giasson B. I., Hodara R., Xu S., Trojanowski J. Q., Ischiropoulos H., Lee V. M. (2005) J. Biol. Chem. 280, 21212–21219 [DOI] [PubMed] [Google Scholar]
- 22.Zhu M., Rajamani S., Kaylor J., Han S., Zhou F., Fink A. L. (2004) J. Biol. Chem. 279, 26846–26857 [DOI] [PubMed] [Google Scholar]
- 23.Li J., Zhu M., Manning-Bog A. B., Di Monte D. A., Fink A. L. (2004) FASEB J. 18, 962–964 [DOI] [PubMed] [Google Scholar]
- 24.Ono K., Hirohata M., Yamada M. (2007) J. Neurosci. Res. 85, 1547–1557 [DOI] [PubMed] [Google Scholar]
- 25.Bonifácio M. J., Palma P. N., Almeida L., Soares-da-Silva P. (2007) CNS Drug Rev. 13, 352–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson K. S. (2005) J. Agric. Food Chem. 53, 10120–10126 [DOI] [PubMed] [Google Scholar]
- 27.Han Y. H., Kim S. Z., Kim S. H., Park W. H. (2008) Int. J. Mol. Med. 21, 721–730 [PubMed] [Google Scholar]
- 28.Dodo K., Minato T., Noguchi-Yachide T., Suganuma M., Hashimoto Y. (2008) Bioorg. Med. Chem. 16, 7975–7982 [DOI] [PubMed] [Google Scholar]
- 29.Armagan A., Uzar E., Uz E., Yilmaz H. R., Kutluhan S., Koyuncuoglu H. R., Soyupek S., Cam H., Serel T. A. (2008) Hum. Exp. Toxicol. 27, 547–552 [DOI] [PubMed] [Google Scholar]
- 30.Jan A., Gokce O., Luthi-Carter R., Lashuel H. A. (2008) J. Biol. Chem. 283, 28176–28189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pace C. N., Vajdos F., Fee L., Grimsley G., Gray T. (1995) Protein Sci. 4, 2411–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naiki H., Nakakuki K. (1996) Lab. Invest. 74, 374–383 [PubMed] [Google Scholar]
- 33.Grzesiek S., Stahl S. J., Wingfield P. T., Bax A. (1996) Biochemistry 35, 10256–10261 [DOI] [PubMed] [Google Scholar]
- 34.Goddard T. D., Kneller D. G. (2003) SPARKY 3, University of California, San Francisco [Google Scholar]
- 35.Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 36.Bertoncini C. W., Jung Y. S., Fernandez C. O., Hoyer W., Griesinger C., Jovin T. M., Zweckstetter M. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 1430–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doré S., Kar S., Quirion R. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 4772–4777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harper J. D., Wong S. S., Lieber C. M., Lansbury P. T., Jr. (1999) Biochemistry 38, 8972–8980 [DOI] [PubMed] [Google Scholar]
- 39.Grimminger-Marquardt V., Lashuel H. A. (2009) Biopolymers 93, 252–276 [DOI] [PubMed] [Google Scholar]
- 40.Jarrett J. T., Lansbury P. T., Jr. (1993) Cell 73, 1055–1058 [DOI] [PubMed] [Google Scholar]
- 41.Harper J. D., Lansbury P. T., Jr. (1997) Annu. Rev. Biochem. 66, 385–407 [DOI] [PubMed] [Google Scholar]
- 42.Harper J. D., Lieber C. M., Lansbury P. T., Jr. (1997) Chem. Biol. 4, 951–959 [DOI] [PubMed] [Google Scholar]
- 43.Craik D. J., Wilce J. A. (1997) Methods Mol. Biol. 60, 195–232 [DOI] [PubMed] [Google Scholar]
- 44.Fernández C. O., Hoyer W., Zweckstetter M., Jares-Erijman E. A., Subramaniam V., Griesinger C., Jovin T. M. (2004) EMBO J. 23, 2039–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lansbury P. T., Lashuel H. A. (2006) Nature 443, 774–779 [DOI] [PubMed] [Google Scholar]
- 46.Caughey B., Lansbury P. T. (2003) Annu. Rev. Neurosci. 26, 267–298 [DOI] [PubMed] [Google Scholar]
- 47.Ansari M. A., Abdul H. M., Joshi G., Opii W. O., Butterfield D. A. (2009) J. Nutr. Biochem. 20, 269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ban J. Y., Nguyen H. T., Lee H. J., Cho S. O., Ju H. S., Kim J. Y., Bae K., Song K. S., Seong Y. H. (2008) Biol. Pharm. Bull. 31, 149–153 [DOI] [PubMed] [Google Scholar]
- 49.Bastianetto S., Yao Z. X., Papadopoulos V., Quirion R. (2006) Eur. J. Neurosci. 23, 55–64 [DOI] [PubMed] [Google Scholar]
- 50.Wang Y. J., Thomas P., Zhong J. H., Bi F. F., Kosaraju S., Pollard A., Fenech M., Zhou X. F. (2009) Neurotox. Res. 15, 3–14 [DOI] [PubMed] [Google Scholar]
- 51.Sul D., Kim H. S., Lee D., Joo S. S., Hwang K. W., Park S. Y. (2009) Life Sci. 84, 257–262 [DOI] [PubMed] [Google Scholar]
- 52.Ehrnhoefer D. E., Bieschke J., Boeddrich A., Herbst M., Masino L., Lurz R., Engemann S., Pastore A., Wanker E. E. (2008) Nat. Struct. Mol. Biol. 15, 558–566 [DOI] [PubMed] [Google Scholar]
- 53.McLaurin J., Golomb R., Jurewicz A., Antel J. P., Fraser P. E. (2000) J. Biol. Chem. 275, 18495–18502 [DOI] [PubMed] [Google Scholar]
- 54.Fradinger E. A., Monien B. H., Urbanc B., Lomakin A., Tan M., Li H., Spring S. M., Condron M. M., Cruz L., Xie C. W., Benedek G. B., Bitan G. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 14175–14180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng Y., Wang X. P., Yang S. G., Wang Y. J., Zhang X., Du X. T., Sun X. X., Zhao M., Huang L., Liu R. T. (2009) Neurotoxicology 30, 986–995 [DOI] [PubMed] [Google Scholar]
- 56.Wogulis M., Wright S., Cunningham D., Chilcote T., Powell K., Rydel R. E. (2005) J. Neurosci. 25, 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lashuel H. A., Hartley D. M., Balakhaneh D., Aggarwal A., Teichberg S., Callaway D. J. (2002) J. Biol. Chem. 277, 42881–42890 [DOI] [PubMed] [Google Scholar]
- 58.Herrera F. E., Chesi A., Paleologou K. E., Schmid A., Munoz A., Vendruscolo M., Gustincich S., Lashuel H. A., Carloni P. (2008) PLoS One 3, e3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamin G., Munishkina L. A., Karymov M. A., Lyubchenko Y. L., Uversky V. N., Fink A. L. (2005) Biochemistry 44, 9096–9107 [DOI] [PubMed] [Google Scholar]
- 60.Borghi R., Marchese R., Negro A., Marinelli L., Forloni G., Zaccheo D., Abbruzzese G., Tabaton M. (2000) Neurosci. Lett. 287, 65–67 [DOI] [PubMed] [Google Scholar]
- 61.El-Agnaf O. M., Salem S. A., Paleologou K. E., Curran M. D., Gibson M. J., Court J. A., Schlossmacher M. G., Allsop D. (2006) FASEB J. 20, 419–425 [DOI] [PubMed] [Google Scholar]
- 62.Lee H. J., Suk J. E., Bae E. J., Lee J. H., Paik S. R., Lee S. J. (2008) Int. J. Biochem. Cell Biol. 40, 1835–1849 [DOI] [PubMed] [Google Scholar]
- 63.Tokuda T., Salem S. A., Allsop D., Mizuno T., Nakagawa M., Qureshi M. M., Locascio J. J., Schlossmacher M. G., El-Agnaf O. M. (2006) Biochem. Biophys. Res. Commun. 349, 162–166 [DOI] [PubMed] [Google Scholar]
- 64.Zhang W., Wang T., Pei Z., Miller D. S., Wu X., Block M. L., Wilson B., Zhang W., Zhou Y., Hong J. S., Zhang J. (2005) FASEB J. 19, 533–542 [DOI] [PubMed] [Google Scholar]
- 65.Klegeris A., Pelech S., Giasson B. I., Maguire J., Zhang H., McGeer E. G., McGeer P. L. (2008) Neurobiol. Aging 29, 739–752 [DOI] [PubMed] [Google Scholar]
- 66.El-Agnaf O. M., Jakes R., Curran M. D., Middleton D., Ingenito R., Bianchi E., Pessi A., Neill D., Wallace A. (1998) FEBS Lett. 440, 71–75 [DOI] [PubMed] [Google Scholar]
- 67.Albani D., Peverelli E., Rametta R., Batelli S., Veschini L., Negro A., Forloni G. (2004) FASEB J. 18, 1713–1715 [DOI] [PubMed] [Google Scholar]
- 68.Du H. N., Tang L., Luo X. Y., Li H. T., Hu J., Zhou J. W., Hu H. Y. (2003) Biochemistry 42, 8870–8878 [DOI] [PubMed] [Google Scholar]
- 69.Forloni G., Bertani I., Calella A. M., Thaler F., Invernizzi R. (2000) Ann. Neurol. 47, 632–640 [PubMed] [Google Scholar]
- 70.Lee E. N., Cho H. J., Lee C. H., Lee D., Chung K. C., Paik S. R. (2004) Biochemistry 43, 3704–3715 [DOI] [PubMed] [Google Scholar]
- 71.Seo J. H., Rah J. C., Choi S. H., Shin J. K., Min K., Kim H. S., Park C. H., Kim S., Kim E. M., Lee S. H., Lee S., Suh S. W., Suh Y. H. (2002) FASEB J. 16, 1826–1828 [DOI] [PubMed] [Google Scholar]
- 72.Sung J. Y., Kim J., Paik S. R., Park J. H., Ahn Y. S., Chung K. C. (2001) J. Biol. Chem. 276, 27441–27448 [DOI] [PubMed] [Google Scholar]
- 73.Ahn K. J., Paik S. R., Chung K. C., Kim J. (2006) J. Neurochem. 97, 265–279 [DOI] [PubMed] [Google Scholar]
- 74.Lee H. J., Suk J. E., Bae E. J., Lee S. J. (2008) Biochem. Biophys. Res. Commun. 372, 423–428 [DOI] [PubMed] [Google Scholar]
- 75.Luk K. C., Song C., O'Brien P., Stieber A., Branch J. R., Brunden K. R., Trojanowski J. Q., Lee V. M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 20051–20056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Desplats P., Lee H. J., Bae E. J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., Lee S. J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 13010–13015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lapish C. C., Ahn S., Evangelista L. M., So K., Seamans J. K., Phillips A. G. (2009) Psychopharmacology 202, 521–530 [DOI] [PubMed] [Google Scholar]
- 78.Assal F., Spahr L., Hadengue A., Rubbia-Brandt L., Burkhard P. R. (1998) Lancet 352, 958. [DOI] [PubMed] [Google Scholar]
- 79.Antonini A., Abbruzzese G., Barone P., Bonuccelli U., Lopiano L., Onofrj M., Zappia M., Quattrone A. (2008) Neuropsychiatr. Dis. Treat. 4, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Truong D. D. (2009) Clin. Interv. Aging 4, 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Novaroli L., Bouchard Doulakas G., Reist M., Rolando B., Fruttero R., Gasco A., Carrupt P. A. (2006) Helv. Chim. Acta 89, 144–152 [Google Scholar]
- 82.Di Stefano A., Sozio P., Iannitelli A., Cerasa L. S. (2009) Expert Opin. Drug Delivery 6, 389–404 [DOI] [PubMed] [Google Scholar]
- 83.Forsberg M., Lehtonen M., Heikkinen M., Savolainen J., Järvinen T., Männistö P. T. (2003) J. Pharmacol. Exp. Ther. 304, 498–506 [DOI] [PubMed] [Google Scholar]
- 84.Learmonth D. A., Palma P. N., Vieira-Coelho M. A., Soares-da-Silva P. (2004) J. Med. Chem. 47, 6207–6217 [DOI] [PubMed] [Google Scholar]
- 85.Nissinen E., Lindén I. B., Schultz E., Pohto P. (1992) Naunyn-Schmiedebergs Arch. Pharmacol. 346, 262–266 [DOI] [PubMed] [Google Scholar]
- 86.Haefeli W. (2007) J. Neurol. 254, 29–3617278044 [Google Scholar]
- 87.Lees A. J., Ratziu V., Tolosa E., Oertel W. H. (2007) J. Neurolog. Neurosurg. Psychiat. 78, 944–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Damier P., Viallet F., Ziegler M., Bourdeix I., Rerat K. (2008) Eur. J. Neurol. 15, 643–648 [DOI] [PubMed] [Google Scholar]
- 89.Novaroli L., Daina A., Bertolini F., Di Giovanni S., Bravo J., Reist M., Carrupt P. A. (2005) Chimia 59, 315–320 [Google Scholar]
- 90.Bertolini F., Novaroli L., Carrupt P. A., Reist M. (2007) J. Pharmacol. Sci. 96, 2931–2944 [DOI] [PubMed] [Google Scholar]
- 91.Boelsterli U. A., Ho H. K., Zhou S., Leow K. Y. (2006) Curr. Drug Metab. 7, 715–727 [DOI] [PubMed] [Google Scholar]