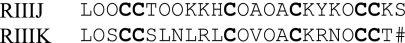Abstract
Conus snail (Conus) venoms are a valuable source of pharmacologically active compounds; some of the peptide toxin families from the snail venoms are known to interact with potassium channels. We report the purification, synthesis, and characterization of κM-conotoxin RIIIJ from the venom of a fish-hunting species, Conus radiatus. This conopeptide, like a previously characterized peptide in the same family, κM-RIIIK, inhibits the homotetrameric human Kv1.2 channels. When tested in Xenopus oocytes, κM-RIIIJ has an order of magnitude higher affinity (IC50 = 33 nm) to Kv1.2 than κM-RIIIK (IC50 = 352 nm). Chimeras of RIIIK and RIIIJ tested on the human Kv1.2 channels revealed that Lys-9 from κM-RIIIJ is a determinant of its higher potency against hKv1.2. However, when compared in a model of ischemia/reperfusion, κM-RIIIK (100 μg/kg of body weight), administered just before reperfusion, significantly reduces the infarct size in rat hearts in vivo without influencing hemodynamics, providing a potential compound for cardioprotective therapeutics. In contrast, κM-RIIIJ does not exert any detectable cardioprotective effect. κM-RIIIJ shows more potency for Kv1.2-Kv1.5 and Kv1.2-Kv1.6 heterodimers than κM-RIIIK, whereas the affinity of κM-RIIIK to Kv1.2-Kv1.7 heterodimeric channels is higher (IC50 = 680 nm) than that of κM-RIIIJ (IC50 = 3.15 μm). Thus, the cardioprotection seems to correlate to antagonism to heteromultimeric channels, involving the Kv1.2 α-subunit rather than antagonism to Kv1.2 homotetramers. Furthermore, κM-RIIIK and κM-RIIIJ provide a valuable set of probes for understanding the underlying mechanism of cardioprotection.
Keywords: Channels/Potassium, Membrane/Channels, Peptides/Chemical Synthesis, Peptides/Interactions, Toxin/Channels, Cardioprotection, Conotoxin
Introduction
Potassium channels are a very diverse group of ion channels. Their diversity arises primarily from the large number of genes encoding the principal K channel2 α-subunits that can be grouped based on their predicted membrane topology into subunits with six, four, and two transmembrane domains (1, 2). Among the six transmembrane domain K channels, voltage-activated K channels comprising 12 subfamilies (from Kv1 through Kv12) are a major class, with each subfamily encoding a set of related subunits. Thus, in mammals, the Kv1 (Shaker) subfamily comprises nine subtypes (Kv1.1–Kv1.9), each of which can be expressed to form a functional homotetrameric channel complex. These Kv1.x subtypes can coassemble with other Kv1.x subunits in various combinations to form a wide variety of heterotetrameric channels as well, contributing to a greater number of functional K channels. In addition, the combination with the accessory subunits results in even greater K channel diversity (3).
K channels are ubiquitous in all living organisms for maintaining membrane potential and modulating the electrical excitability of cells. To investigate the structure and function of K channels, subtype-specific ligands would be highly desirable. However, compared with the enormous diversity of different molecular forms of K channels, the set of known toxins that are targeted with high selectivity to specific K channel subtypes is relatively small.
Conus toxins provide pharmacological tools to distinguish different K channel subtypes. During their evolution, over 700 species of cone snails synthesize and secrete their own repertoire of 100–200 different toxins (conotoxins) (4). The majority of these conotoxins are small, disulfide-rich peptides that can be classified into a number of superfamilies according to their structures. These structurally constrained conotoxins are highly potent and specific biological agents that target a diversity of receptors and ion channels, including sodium channels, K channels, calcium channels, nicotinic acetylcholine receptors, etc. (5, 6).
Several conotoxins that interact with K channels have been identified from the Conus venoms. One conotoxin that was previously identified, κM-conotoxin RIIIK (κM-RIIIK), belongs to the M superfamily of conopeptides. κM-RIIIK was shown to selectively block three different K channels of different phylogenetic origins: the Shaker K channel (from Drosophila), TSha1 K channel (from trout), and the mammalian Kv1.2 channel (7, 8). In this report, the characterization of another member of the κM-conotoxin family, κM-RIIIJ, is described.
Previously, a structurally and genetically unrelated conopeptide belonging to the O superfamily that targets the Kv1 family, κ-conotoxin PVIIA (9), was shown to be cardioprotective in several ischemia/reperfusion animal models (10, 11). In this report, we compare the cardioprotective effects of the two κM-conotoxins in a rodent model of ischemia/reperfusion.
EXPERIMENTAL PROCEDURES
Peptide Purification
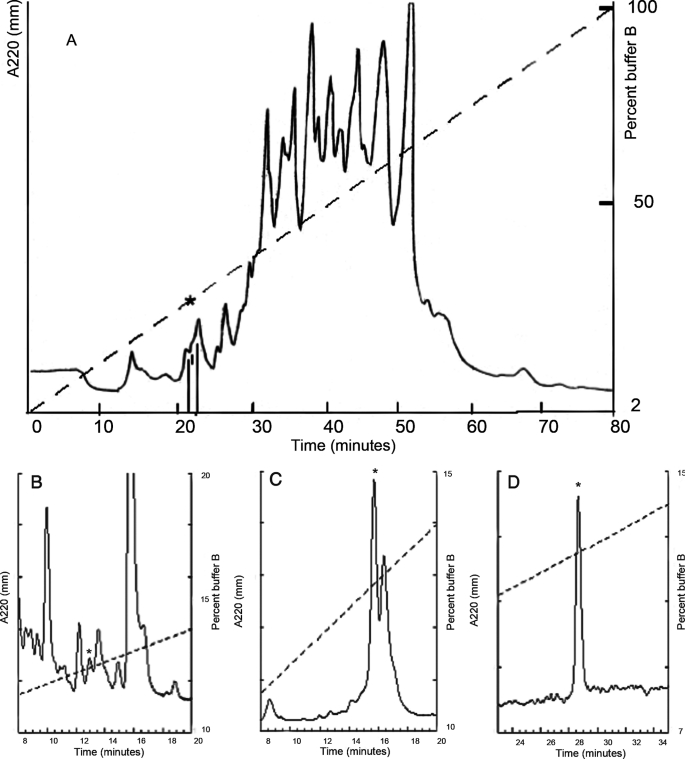
Specimens of Conus radiatus were collected from the Philippines. The venom apparatus was dissected from the snails, and the venom was squeezed out, lyophilized, and stored at −70 °C. Crude venom was extracted as previously described (12). The venom extract was applied to a preparative Vydac C18 HPLC column (22 mm × 250 mm) and eluted using a gradient of 2–100% buffer B (B90, 90% acetonitrile with 0.1% trifluoroacetic acid) at 2% B90/min. An analytical Vydac C18 HPLC column (4.6 mm × 250 mm) with a gradient of 10–15% B90 at 0.2% B90/min (see Fig. 1, B and C) and a gradient of 7–15% B90 at 0.2% B90/min (see Fig. 1D) was used for subsequent fractionations. The same column was used for the coelution experiment with a gradient of 10–15% B90 at 0.2% B90/min.
FIGURE 1.
Isolation of κM-RIIIJ from C. radiatus venom. The asterisk in each HPLC chromatogram indicates the location of the fraction containing the peptide κM-RIIIJ. All of the elution buffers had 0.1% trifluoroacetic acid. A, the venom extract was chromatographed in a preparative Vydac C18 column eluted with 2–100% B90. B, the peak containing κM-RIIIJ in A was subfractionated using an analytical Vydac C18 column and an elution gradient of 10–15% B90 at a flow rate of 0.2%B90/min. C, the peak containing κM-RIIIJ in B was subfractionated using the same gradient as in B. D, the peak containing κM-RIIIJ in C was subfractionated using the gradient of 7–15% B90 at a flow rate of 0.2% B90/min.
Mass Spectrometry
Electrospray ionization-mass spectrometry spectra were measured on a Bruker Esquire 3000 Plus instrument performed at the Salk Institute for Biological Studies (San Diego, CA). All of the spectra were recorded in positive ion mode. Alternatively, MALDI mass spectra were obtained through the Mass Spectrometry and Proteomic Core Facility of the University of Utah.
Amino Acid Sequence Determination
The purified peptide was reduced with 100 mm Tris-HCl (pH 8.5) and 10 mm of dithiothreitol at 65 °C for 30 min. The linearized peptide was purified with an analytical Vydac C18 HPLC column. The linearized peptide was sequenced and analyzed using a PE ABI model 492 Procise sequencer and phenyl isothiocyanate analyzer at the DNA/peptide facility of the University of Utah.
Peptide Synthesis and Confirmation
Peptide chimeras were designed by switching intercysteine sequences or multiple or single amino acid residues between κM-RIIIJ and κM-RIIIK. Peptide synthesis was carried out by DNA/peptide facility of the University of Utah. The oxidative folding of the linear peptides was carried out in the presence of 1 mm GSSG, 1 mm GSH, 0.1 m Tris-Cl (pH 8.7). The homogeneity of the synthetic peptides was verified by MALDI mass spectrometry.
The Inhibitory Effect of κM-RIIIJ and κM-RIIIK on K Channels Expressed in Xenopus Oocytes
Xenopus oocytes were prepared as described previously (13). cRNAs of rat subtype Kv1.1 and human subtypes Kv1.2–Kv1.6, KCNQ2/KCNQ3, and BK subunit (Protinac, Germany) were produced with mMESSAGE mMACHINE (Ambion Inc.). For the coexpression of KCNQ2/KCNQ3, each oocyte was injected with 2.5 ng of each cRNA. For the other subtypes, each oocyte was injected with 5 ng of cRNA. Prior to electrophysiological recording, the vitelline membranes of the oocytes were removed mechanically with fine forceps. Whole cell currents were recorded using a two-electrode voltage-clamp amplifier (OC-725C; Warner Instruments, Hamden, CT) 1–5 days after cRNA injection. The intracellular electrodes were filled with 3 m KCl and had a resistance of ∼0.5 megohm. Current records were low pass filtered at 3 kHz and sampled at 10 kHz. Leak and capacitive currents were corrected online using a P/n method. A 30-μl cylindrical oocyte-recording chamber fabricated from Sylgard (Dow Corning, Midland, MI) was perfused with Ringer's buffer (115 mm NaCl, 2.5 mm KCl, 1.8 mm CaCl2, 10 mm HEPES, 0.1 mg/ml bovine serum albumin, pH 7.4) at a rate of 1 ml/min. Lyophilized κM-RIIIJ, κM-RIIIK, and the chimeras were dissolved in buffer and diluted to 10× final concentration, and 3 μl were added to the bath chamber to achieve a 1× final concentration. Oocytes were held at −90 mV and stepped to each test potential at the step of 10 mV. All of the electrophysiological experiments were performed at room temperature.
Dose-response curves were fit to the equation Y = 100/{1 + ([toxin]/IC50)}, where Y is the relative response (%), [toxin] is the toxin concentration, and IC50 is the concentration of toxin at which 50% of its effect is observed. The data were analyzed by Kaleidagraph 4.0.1 and Prism 3.0.
Generation of Concatenated Kv1.2 Constructs
cDNA from the human Kv1.2, Kv1.5, Kv1.6, and Kv1.7 α-subunits cloned into pSGEM expression vector (14) were concatenated to generate heterodimers of Kv1.2-Kv1.x potassium channels. In brief, the stop codon from hKv1.2 and the initiation of translation codon of the “tail” hKv1.x α-subunit were disrupted by PCR mutagenesis to include compatible restriction sites for α-subunit ligation. The resulting cDNA constructs contain one single reading frame encoding for two α-subunits where Kv1.2 is followed by the tail Kv1.x α-subunit without exogenous linker. The sequences of all the PCR-generated constructs were verified through DNA sequence analysis using the dideoxy chain termination method with dye terminators on an Applied Biosystems 373 DNA sequencer (Applied Biosystems, Weiterstadt, Germany). cDNA constructs were transcribed in vitro with the T7 polymerase (Stratagene), and the rendered capped cRNAs were injected in Xenopus oocytes for electrophysiological analysis as described (15).
In Vivo Infarct Size Determinations
Infarct size in a rat in vivo ischemia/reperfusion model was performed as previously described (16). Male Wistar rats (290–350 g body weight; Charles River, Sulzfeld, Germany) were anesthetized with pentobarbital (90 mg kg−1, intraperitoneally), tracheotomized, and ventilated with room air (tidal volume, 10 ml kg−1; 50 strokes/min) enriched with oxygen to keep arterial oxygen saturation at >95%. The left jugular vein and carotid artery were cannulated to compensate for fluid loss (0.9% NaCl, 1.5 ml kg−1 h−1), to maintain anesthesia (450 μg kg−1 min−1 pentobarbital), and to register arterial blood pressure and heart rate. Core temperature was continuously monitored and maintained at 37.0–37.8 °C. A lateral thoracotomy was performed, the pericardium was opened, and a 6–0 prolene suture was looped under the left descending coronary artery. Cardiac ischemia was induced for 30 min followed by 150 min of reperfusion. After this procedure, the hearts were removed, and the aorta was quickly cannulated. The coronary artery ligature was retied, and the hearts were perfused with black Chinese ink at a constant pressure (100 mm Hg). This was designed to stain the perfused myocardium black, whereas the area at risk remained unstained. The atria and the right ventricle were discarded, and the left ventricle including the septum was cut into slices (1-mm thickness) from apex to base. The slices were then incubated for 30 min at room temperature in 2,3,5-triphenyltetrazolium chloride (1% in 0.1 m phosphate buffer, pH 7.4), which stained viable tissue red and in this way demarcated the pale area of infarct size. The areas of the left ventricle, the area at risk, and the infarct size were quantified by computer-assisted planimetry. Conotoxins κM-RIIIK and κM-RIIIJ (100 μg/kg of body weight) were applied intravenously after 25 min of ischemia corresponding to 5 min before reperfusion. The investigation had been approved by the authorities of the State of Schleswig-Holstein (Germany), and the experiments conformed to the guide for the care and use of laboratory animals published by the United States National Institutes of Health.
Statistics
Infarction experiments with each of the conotoxins were performed in separate studies with individual control groups. The infarct size and the area at risk were compared between treatment and respective control groups by t test; hemodynamic parameters were analyzed by two-way analysis of variance considering time as a grouped parameter. The error level for significance was 0.05.
RESULTS
Peptide Purification and Characterization
An initial fractionation of C. radiatus venom was carried out to identify venom components that inhibited voltage-gated potassium channels belonging to the Kv1 (Shaker) subfamily. A fraction that inhibited the Kv1.2 channels was identified, and this was further fractionated to purify the active venom component to homogeneity (Fig. 1, B–D). The monoisotopic molecular weight of the purified peptide was 2805.84 Da.
The amino acid sequence was determined using Edman methods; the results are shown in Table 1. The sequence and mass determination are consistent with each other. The peptide purified and characterized is homologous to κM-conotoxin RIIIK and is designated κM-RIIIJ. A comparison of the sequences of the two peptides is in Sequence 1, where O indicates four transhydroxyprolines, and # is an amidated C terminus.
TABLE 1.
Sequences, masses of κM-RIIIJ, κM-RIIIK, and chimeras and their effect on human Kv1.2 channels expressed on oocytes
The values represent the means ± S.E. (p < 0.05; n ≥ 4).
| Peptide | Sequence | Mass | IC50 |
|---|---|---|---|
| nm | |||
| RIIIJ | LOOCCTOOKKHCOAOACKYKOCCKS | 2805.2 | 33 ± 6 |
| RIIIK | LOSCCSLNLRLCOVOACKRNOCCT# | 2647.2 | 352 ± 20 |
| RIIIJΔ6–11 | LOOCCSLNLRLCOAOACKYKOCCKS | 2787.3 | 374 ± 57 |
| RIIIJΔ6–8 | LOOCCSLNKKHCOAOACKYKOCCKS | 2792.2 | 67 ± 16 |
| RIIIJΔ9–11 | LOOCCTOOLRLCOAOACKYKOCCKS | 2794.2 | 336 ± 66 |
| RIIIJΔ10–11 | LOOCCTOOKRLCOAOACKYKOCCKS | 2815.3 | 48 ± 4 |
| RIIIKΔ9 | LOSCCSLNKRLCOVOACKRNOCCT# | 2662.2 | 102 ± 7 |
SEQUENCE 1.
Chemical Synthesis and Electrophysiological Characterization of κM-RIIIJ and κM-RIIIK
κM-RIIIJ was synthesized, folded, and characterized by mass spectrometry as described under “Experimental Procedures.” Coelution of the synthetic and native κM-RIIIJ using an analytical Vydac C18 column resulted in a single symmetric peak (data not shown). The effects of κM-RIIIJ on different human K channel subtypes were investigated using the Xenopus oocyte system. κM-RIIIJ blocked the Kv1.2 channel in a dose-dependent manner (Fig. 2); a comparison of dose-response curves for κM-RIIIJ and κM-RIIIK is shown in Fig. 3. κM-RIIIJ has a higher potency for the human Kv1.2 channel (IC50 = 33 ± 6 nm, n = 4) than κM-RIIIK (IC50 = 352 ± 20 nm, n = 4). The block of the human Kv1.2 channels was reversible; the toxins were washed off in 3 min. κM-RIIIJ and κM-RIIIK were also tested on the other K channel subtypes (Table 2), but they both showed very low affinity for the other mammalian K channel subtypes tested.
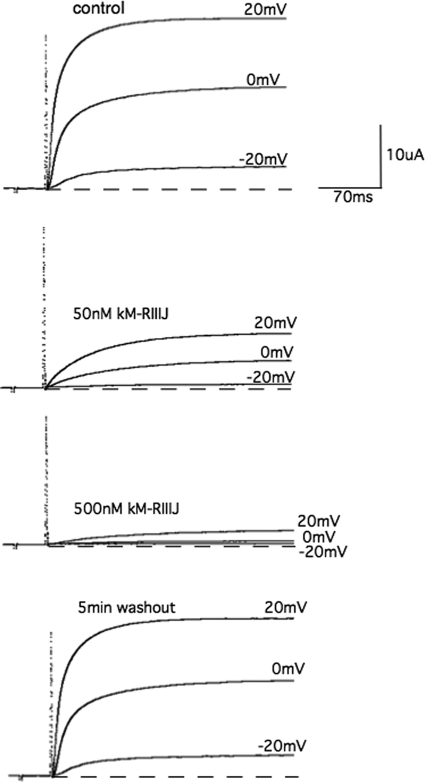
FIGURE 2.
The electrophysiological effect of κM-RIIIJ. Whole cell currents recorded from an oocyte expressing human Kv1.2 channel evoked by test potentials to −20, 0, and 20 mV (top panel) from a holding potential of −90 mV. The addition of 50 and 150 nm of κM-RIIIJ results in the block of the currents with a dose-dependent effect (two middle panels), which are reversible (bottom panel).
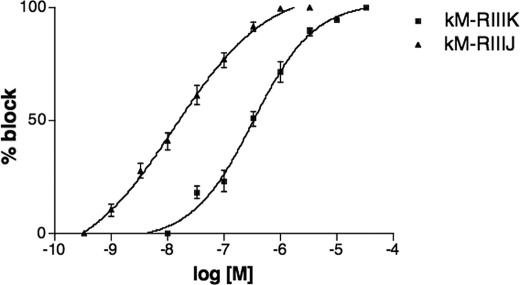
FIGURE 3.
Dose-response curves for the block by κM-RIIIJ (▲) and κM-RIIIK (■). Whole cell currents were recorded from oocytes expressing human Kv1.2 channel evoked by test potentials to 20 mV (n = 3–5). The error bars represent S.E.M.
TABLE 2.
Approximate IC50 (nm) of κM-RIIIJ and κM-RIIIK on different K channel subtypes
NB, not blocked (n ≥ 4).
| κM-RIIIJ | κM-RIIIK | |
|---|---|---|
| nm | nm | |
| Kv1.1 | ∼4,000 | >100,000 |
| Kv1.2 | 33 | 352 |
| Kv1.3 | ∼10,000 | >100,000 |
| Kv1.4 | NB | NB |
| Kv1.5 | ∼70,000 | NB |
| Kv1.6 | ∼8,000 | >100,000 |
| KCNQ2/KCNQ3 | NB | NB |
| BK | NB | NB |
Design of RIIIK and RIIIJ Chimeras and Determination of the Important Residues in κM-RIIIJ
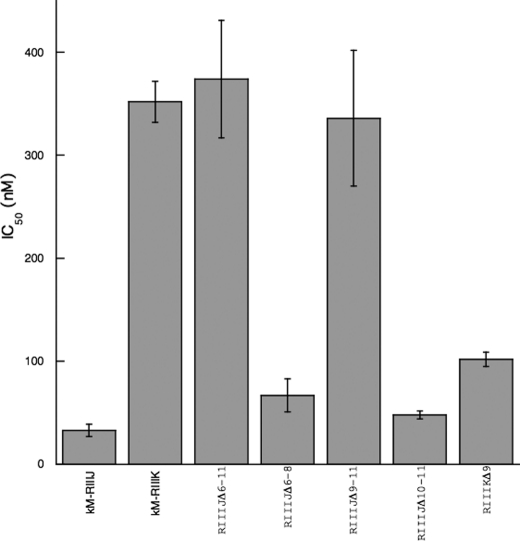
The affinity of κM-RIIIJ for the Kv1.2 subtype is an order of magnitude greater than that of κM-RIIIK (Fig. 3). We investigated what important residues in κM-RIIIJ conferred the greater affinity of this homolog for Kv1.2 channels. Although both peptides belong to the M superfamily, the sequences of κM-RIIIJ and κM-RIIIK within the first intercysteine loop are diverse. Thus, chimeras were synthesized in which the residues in the first intercysteine loops of κM-RIIIJ and κM-RIIIK were switched (Table 1). Upon folding, all chimeras in Table 1 yielded a single major HPLC peak (data not shown); these chimeras were tested on the human Kv1.2 channel expressed in Xenopus oocytes (Fig. 4).
FIGURE 4.
The IC50 of κM-RIIIJ, κM-RIIIK and chimeras on the human Kv1.2 channel expressed in Xenopus oocytes. Whole cell currents were recorded from oocytes expressing human Kv1.2 channel evoked by test potentials to 20 mV (n = 3–5). The error bars represent S.E.M.
The chimera RIIIJΔ6–11 was made in which the entire first intercysteine loop (residues 6–11) of κM-RIIIJ was replaced with that of κM-RIIIK and was tested on the Kv1.2 channel; RIIIJΔ6–11 showed an affinity for Kv1.2 that was significantly lower than κM-RIIIJ; the IC50 was close to that of κM-RIIIK, suggesting that the first intercysteine loop is one major determinant of the higher affinity of κM-RIIIJ. Although the affinity of the chimera RIIIJΔ6–8 for the Kv1.2 channels did not change significantly, the affinity of RIIIJΔ9–11 for Kv1.2 was significantly decreased (to the level of κM-RIIIK), indicating that residues 9–11 in κM-RIIIJ contribute significantly to its higher potency, whereas residues 6–8 do not.
Based on these results, the chimera RIIIJΔ10–11 was synthesized; it showed a similar affinity to κM-RIIIJ, suggesting that Lys-9 is a key determinant. A chimera with the residue Leu-9 in κM-RIIIK substituted with Lys resulted in an increase in the affinity of RIIIKΔ9 to a level closer to that of κM-RIIIJ. We conclude that Lys-9 is an important residue conferring the higher affinity of κM-RIIIJ for Kv1.2 channels.
Cardioprotective Effects of κM-RIIIK and κM-RIIIJ
With κ-conotoxin PVIIA, a peptide that also interacts with K channels, a reduction in infarct size in vivo when administered after the onset of ischemia was previously demonstrated (10, 11). This cardioprotective action of κ-PVIIA led us to investigate whether κM-RIIIK and κM-RIIIJ had similar effects in an in vivo rat ischemia/reperfusion model.
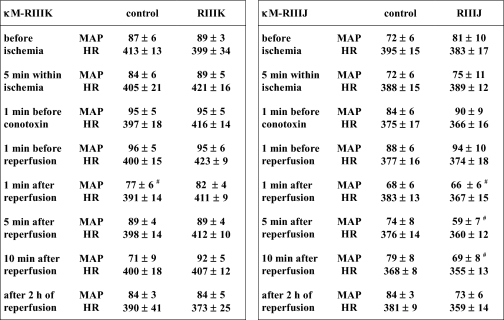
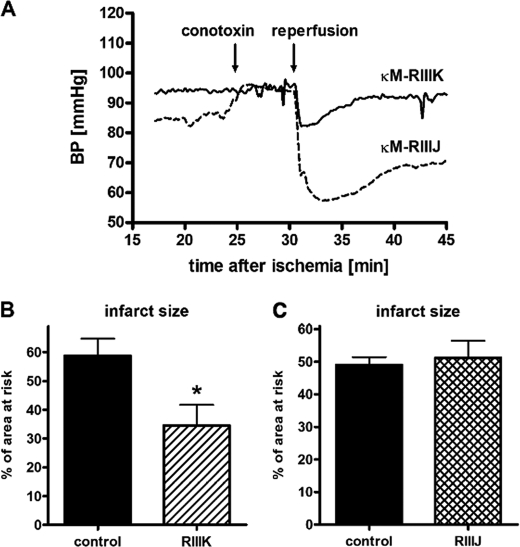
Each peptide was investigated in a separate study employing 30 min of coronary occlusion and 150 min of myocardial reperfusion, during which it was administered as an intravenous bolus 5 min before the start of reperfusion. Over the time course of the experiments, the developed blood pressures showed significant time-related alterations in treatment as well as control groups, whereas heart rates remained constant (Table 3). Application of κM-RIIIK or κM-RIIIJ did not provoke differences in blood pressures or heart rates to the respective time points of the control groups. However, the time course of blood pressures after application of κM-RIIIJ showed a significant reduction in the early phase of reperfusion as compared with basal values before ischemia or drug application, which was not apparent in the control group or after treatment with κM-RIIIK (Table 3). Indeed, the drop of blood pressure occurring with the onset of reperfusion was more pronounced and prolonged after κM-RIIIJ compared with κM-RIIIK (Fig. 5A). Apparently, this influence did not persist throughout the reperfusion period.
TABLE 3.
Rat hemodynamic data
MAP, mean arterial pressure (mm Hg). HR, heart rate (beats/min). The values represent the means ± S.E. No differences between conotoxin-treated and control groups were apparent at any time point. The symbol denotes time-dependent differences as related to the preconotoxin period (n = 5–7, p < 0.05).
FIGURE 5.
A, blood pressure development at the time points of conotoxin application (25 min) and reperfusion (30 min) after treatment with κM-RIIIK or κM-RIIIJ. The average values of mean blood pressures of five to seven experiments are depicted. Application of κM-RIIIJ appeared to cause a slight acute increase in blood pressure that was not statistically verified. However, κM-RIIIJ enhanced the drop of blood pressure upon reperfusion, whereas the blood pressure development after κM-RIIIK was equivalent to that of the control experiments. B and C, infarct size expressed as a percentage of the area at risk in rats treated with κM-RIIIK (B) or with κM-RIIIJ (C) administration 5 min before reperfusion. *, significant difference versus vehicle (p < 0.05). The values represent the means ± S.E.M.
The peptides κM-RIIIK and κM-RIIIJ also exerted distinct effects on the relative infarct size in this rat ischemia/reperfusion model (Fig. 5B). Bolus intravenous injection of κM-RIIIK administered 5 min before reperfusion reduced infarct size from 59 ± 6% of the risk zone in untreated animals to 34 ± 7%, whereas κM-RIIIJ exerted no detectable protection. None of the peptides influenced the size of the area at risk (for κM-RIIIK, control was 50 ± 5% and treated was 42 ± 4%, (n = 9); for κM-RIIIJ, control was 46 ± 4% and treated was 47 ± 5% of left ventricle (n = 5)). These results demonstrate that κM-RIIJ affects the blood pressure at the beginning of reperfusion. Nevertheless only κM-RIIIK exhibited protective effects on cardiac tissue when administered after an ischemic event.
Effects of κM-RIIIK and κM-RIIIJ on Heteromeric Kv1.2 Channel
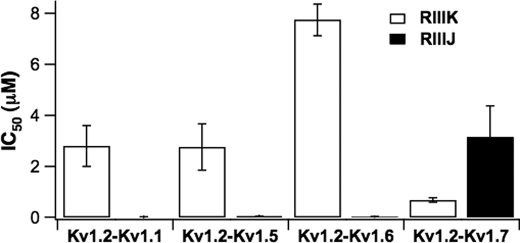
To investigate whether heteromerization with other Kv1 α-subunits would affect the affinity of κM-RIIIK and κM-RIIIJ to Kv1.2 channel; Kv1.2 channels were concatenated with Kv1.1, Kv1.5, Kv1.6, and Kv1.7 to form heterodimers of Kv1.2 and the corresponding Kv1.x subunit. The affinity of the peptides was estimated by two-electrode voltage clamp measurements in the Xenopus expression system. Fig. 6 shows that for Kv1.2-Kv1.1 heterodimers, the affinity of κM-RIIIK was 2.8 ± 0.8 μm, whereas the affinity of κM-RIIIJ was 12 ± 8.5 nm (mean ± S.E.). Similar results were obtained for Kv1.2-Kv1.5 and Kv1.2-Kv1.6 heterodimeric channels. The affinity of κM-RIIIK to those channels remains in the micromolar range (Kv1.2-Kv1.5, 2.76 ± 0.91 μm; Kv1.2-Kv1.6, 7.74 ± 0.63 μm) and much lower compared with κM-RIIIJ, which is in the low nanomolar range (Kv1.2-Kv1.5, 47 ± 3 nm; Kv1.2-Kv1.6, 24 ± 4 nm). These results indicate that the affinity of κM-RIIIK to Kv1.2-Kv1.1, Kv1.2-Kv1.5, and Kv1.2-Kv1.6 channels is about 1 order of magnitude lower compared with homomeric Kv1.2 channels, whereas the affinity of κM-RIIIJ to those dimers is similar compared with homomeric Kv1.2 channels. Surprisingly, the affinity of κM-RIIIK for Kv1.2-Kv1.7 channels is higher (680 ± 80 nm) than the affinity of κM-RIIIJ (3.15 ± 1.22 μm) (Fig. 6), demonstrating that this heteromeric channel containing Kv1.2-Kv1.7 is more sensitive to κM-RIIIK than to κM-RIIIJ.
FIGURE 6.
The IC50 of κM-RIIIJ and κM-RIIIK on concatenated human Kv1.2 channel heterodimers expressed in Xenopus oocytes. Whole cell currents were recorded from oocytes expressing a given channel heterodimer evoked by test potentials to 40 mV from a holding potential of −80 mV. The IC50 values were obtained from the fractional currents (Fc) in the presence of the toxins (IC50 = Fc/1 − Fc * [Tx]) (n = 4–5). The error bars represent S.E.M.
DISCUSSION
This report describes the purification and characterization of κM-conotoxin RIIIJ from the crude venom of C. radiatus, from which a homologous peptide, κM-RIIIK, was previously characterized (8). Both κM-RIIIJ and κM-RIIIK selectively block Kv1.2 expressed in Xenopus oocytes. The discovery of various K channel blockers in the venom of C. radiatus suggests that this species uses a general prey capture strategy of fish-hunting snails in which K channel blockers are an essential component to achieve rapid and rigid immobilization of the prey (17). Although κM-RIIIK and κM-RIIIJ are structurally related, the affinity of κM-RIIIJ for Kv1.2 channels is 1 order of magnitude higher than that of κM-RIIIK. Nevertheless, κM-RIIIK protects against the ischemia/reperfusion-induced heart infarction in rats, whereas κM-RIIIJ does not exert any apparent cardioprotective effect. The affinity of κM-RIIIK to Kv1.2-Kv1.7 heterodimers is higher than that of κM-RIIIJ.
Investigation of Residues Responsible for the More Potent Block by κM-RIIIJ of the Kv1.2 Channel
Both κM-RIIIJ and κM-RIIIK are selective for the Kv1.2 channel expressed in Xenopus oocytes (Table 2). However, the IC50 of κM-RIIIJ for Kv1.2 is 1 order of magnitude lower than that of κM-RIIIK. The characterization of the peptide chimeras made by switching the amino acid residues between κM-RIIIK and κM-RIIIJ revealed that Lys-9 in RIIIJ was important for the higher blocking potency of RIIIJ. It has been shown that κM-RIIIK adopted a motif of a basic ring comprising three positively charged amino acid residues (Arg-10, Lys-18, and Arg-19) and one protonated Leu on the N terminus and, via electrostatic interaction, bonded to the negatively charged backbone carbonyls and the side chains of the acidic residues on the K channels (18, 19). Compared with κM-RIIIK, κM-RIIIJ contains one protonated N-terminal Leu, three positively charged amino acid residues at the corresponding or similar positions (Lys-10, Lys-18, and Lys-20), and additional positive charges (Lys-9, His-11, and Lys-24) that may provide a stronger interaction between the peptide and the K channel pore, thus resulting in a higher affinity for the Kv1.2 channel. However, the introduction of Lys-9 into κM-RIIIK (RIIIKΔ9) did not completely decrease the IC50 of RIIIKΔ9 to the level of κM-RIIIJ, suggesting that other factors are also involved. One such factor could be the different conformations adopted by the side chains of Lys-9 in the presence (κM-RIIIJ) and absence of Hyp (RIIIKΔ9). Hyp residues are believed to permit sharp twisting of a peptide structure and help stabilize peptide conformation (18). Thus, in the presence of Hyps, Lys-9 in κM-RIIIJ may adopt more favorable dihedral angles and less steric hindrance (20–24). Thus, in terms of molecular interactions, the side chain of Lys-9 in RIIIJ could be a good fit for the target K channel through locally optimized interaction of the side chain, whereas Lys-9 in RIIIKΔ9 adopts a less favorable conformation, making it block Kv1.2 with lower affinity than κM-RIIIJ.
Cardioprotection of κM-RIIIK
Ischemia/reperfusion-induced myocardial infarction is one of the leading causes of death. Many efforts have been made to search for the agents that could reduce the extent of myocardial infarction elicited by reperfusion injury. The investigation of the cardioprotective effects of κM-RIIIK and κM-RIIIJ started from the discovery that κ-PVIIA, a conopeptide and a Shaker K channel blocker purified from the venom of Conus purpurascens, was proven to be cardioprotective in rabbits, rats, and dogs when applied after the onset of ischemia but before reperfusion without producing any hemodynamic effects (10, 11). The present study demonstrated a similar activity of the structurally unrelated conotoxin κM-RIIIK, thereby indicating the general potential of Conus venoms for the treatment of ischemia/reperfusion injury. New information on the potential target of conotoxin-related protection is revealed by the respective inactivity of a closely related peptide, κM-RIIIJ.
Because the investigated conotoxins share a common activity as inhibitors of voltage-gated K channels, it may be postulated that enhanced K currents play a deleterious role during postischemic reperfusion. This occurs as a paradox because K cannels will promote the restoration of membrane potential during reperfusion, and special subtypes (e.g. K-ATP) are mandatory for protection caused by preconditioning. However, rapid restoration of ischemic derangements may contribute to reperfusion injury. It has been shown that experimental preservation of intracellular acidosis and contractile dysfunction during the early phase of reperfusion can limit postischemic myocardial damage (25, 26). Because myocyte repolarization will restore cellular excitability and will increase the driving force for sodium and Ca2+ influx, its delay until energetic replenishment of the cardiomyocytes should be beneficial. The slow recovery of blood pressure from postischemic dysfunction in rats treated with κM-RIIIJ may reflect just the consequence of delayed repolarization. In this case, however, the effect of κM-RIIIJ was persistent and may have interfered with complete repolarization and final salvage of the cardiomyocytes. Short duration of action, incomplete efficacy, or lack of hemodynamic activity may even afford the cardioprotective properties of the less potent Kv1.2 blockers, κM-RIIIK and κ-PVIIA (Table 4). Optimized parameters of efficacy and kinetics of therapeutic K channel blockade still need to be established. Plasma half-lives in particular have not been determined and may be short for peptide toxins. This is unlikely to interfere with therapeutic efficacy because the early reperfusion phase is decisive for postischemic injury, so that an intervention during only the first 2 min of reperfusion has been shown to be required and sufficient for an attenuation of reperfusion injury (27). Such short periods of action may even be produced by conotoxins independently of their pharmacokinetic properties, e.g. by prolonged binding to their target structures.
TABLE 4.
Summary of the effects of the conopeptides on the Kv1.2 channel and their cardioprotective actions
Further contributors to the in vivo activities of therapeutic K channel blockade may arise from noncardiac functions of Kv1.2 channels. This channel subtype is highly prevalent in the central nervous system (28) and could be involved in cardiovascular regulation because of its presence in sympathetic neurons and vascular smooth muscle (29, 30). Although direct effects of the applied conotoxins on these tissues cannot be excluded, the absence of conotoxin-related changes in heart rate indicates against an involvement of neuronal reflexes. Similarly, blood pressures showed no differences between the placebo- and the conotoxin-treated animals, so that a prominent vascular influence appears unlikely.
The cardioprotective differences between κ-PVIIA, κM-RIIIK, and κM-RIIIJ raise the question of whether the blockade of Kv1.2 channel can be considered as the common principle of cardioprotection. Although the Kv1.2 channel subunit is expressed in rat heart and all three peptides are Kv1.2 channel blockers (Table 4), the effect of the three conotoxins on the Kv1.2 channel are opposite to their cardioprotective actions. The most potent Kv1.2 channel blocker, κM-RIIIJ, was not cardioprotective, whereas the weaker blockers were (Table 4), suggesting that, physiologically, the homomultimeric Kv1.2 channel does not constitute their common target for protection. However, formation of heteromeric channels or ischemia-related modifications may affect electrophysiological properties as well as the binding selectivities of conotoxins. The Kv1.2 channel subtype has been demonstrated to be phosphorylated during ischemia involving the cardioprotective phosphatidylinositol 3-kinase (31). Such modulation will alter the conductivity of the channel as well as its intracellular distribution (32). Importantly, the channel α-subunit Kv1.2 frequently forms heteromultimers with related subunits and thereby attains strikingly different ligand specificities (33). Our investigation of Kv1.2 heteromers revealed profound differences in the affinity of κM-RIIIK and κM-RIIIJ for heterodimeric constructs with Kv1.1, Kv1.5, and Kv1.6, respectively. Most interestingly, the affinity of κM-RIIIK for heterodimeric Kv1.2-Kv1.7 channels is higher than that of κM-RIIIJ, whereas for all the other heteromeric channels investigated, κM-RIIIK had a much lower affinity compared with κM-RIIIJ. Overall the observed changes in the affinity of κM-RIIIK and κM-RIIIJ to the Kv1.2-containing dimers show that these heteromeric channels do have a different pharmacological profile than homomeric ones. Furthermore, these data underscore the known specificity of conopeptides for their targets. Kv1 channels and especially Kv1.5, Kv1.2, and Kv1.7 are known to be present in heart tissue, but to our knowledge very little is known about the existence and composition of Kv1.2 heterotetramers in the heart. Although further work is needed to clarify the molecular target involved in the cardioprotective effect of κM-RIIIK, our data indicate a heteromeric channel containing Kv1.2 and Kv1.7 as a possible candidate.
An additional underlying mechanism for the cardioprotective action of κM-RIIIK could be the state dependence of the interaction with the target K channel protein. In general, K channel blockers are not anticipated to be cardioprotective, because K channel blockers would depolarize the cell membrane and exacerbate cell excitotoxicity by elevating intracellular calcium level and triggering cell death. However, it has been proven that despite blocking the peak current of K channels, κ-PVIIA was capable of increasing the K current by inhibiting the K channels from progressively entering into a C-type inactivated state under conditions of long-lasting depolarization (34). This discovery is of potential biomedical significance, because when cardiac myocytes are exposed to ischemia/reperfusion, their membrane potential becomes depolarized. The inhibition of the progression into a C-type inactivated state and the resulting enhanced outward K currents would make heart cells repolarized and less excitable. Thus, one possibility is that κM-RIIIK and κ-PVIIA interact with a specific heteromultimeric K channel that contains the Kv1.2 subunit in heart and that an increase of the K current through this K channel target occurs under certain conditions because the peptide inhibits channel progression into a C-type inactivated state. This hypothesis is presently being investigated.
Acknowledgments
K. Tempel is gratefully acknowledged for skillful experimental work. We thank Dr. Tamkun for sharing the rat Kv1.1 clone. We are grateful to Diana Leibeling and Nina Strüver for generating the concatenated Kv1.2 dimers. We thank Dr. M. Sanguinetti and Dr. G. Bulaj for helpful discussion.
This work was supported, in whole or in part, by National Institutes of Health Grant GM48677 (to B. M. O.).
- K channel
- potassium channel
- HPLC
- high pressure liquid chromatography
- MALDI
- matrix-assisted laser desorption ionization.
REFERENCES
- 1.Coetzee W. A., Amarillo Y., Chiu J., Chow A., Lau D., McCormack T., Moreno H., Nadal M. S., Ozaita A., Pountney D., Saganich M., Vega-Saenz de Miera E., Rudy B. (1999) Ann. N.Y. Acad. Sci. 868, 233–285 [DOI] [PubMed] [Google Scholar]
- 2.Jan L. Y., Jan Y. N. (1997) Annu. Rev. Neurosci. 20, 91–123 [DOI] [PubMed] [Google Scholar]
- 3.Gulbis J. M. (2002) Novartis Found. Symp. 245, 127–141 [PubMed] [Google Scholar]
- 4.Röckel D., Korn W., Kohn A. J. (1995) Manual of the Living Conidae, Verlag Christa Hemmen, Wiesbaden, Germany [Google Scholar]
- 5.Olivera B. M. (2006) J. Biol. Chem. 281, 31173–31177 [DOI] [PubMed] [Google Scholar]
- 6.Terlau H., Olivera B. M. (2004) Physiol. Rev. 84, 41–68 [DOI] [PubMed] [Google Scholar]
- 7.Ferber M., Al-Sabi A., Stocker M., Olivera B. M., Terlau H. (2004) Toxicon 43, 915–921 [DOI] [PubMed] [Google Scholar]
- 8.Ferber M., Sporning A., Jeserich G., DeLaCruz R., Watkins M., Olivera B. M., Terlau H. (2003) J. Biol. Chem. 278, 2177–2183 [DOI] [PubMed] [Google Scholar]
- 9.Shon K. J., Stocker M., Terlau H., Stühmer W., Jacobsen R., Walker C., Grilley M., Watkins M., Hillyard D. R., Gray W. R., Olivera B. M. (1998) J. Biol. Chem. 273, 33–38 [DOI] [PubMed] [Google Scholar]
- 10.Lubbers N. L., Campbell T. J., Polakowski J. S., Bulaj G., Layer R. T., Moore J., Gross G. J., Cox B. F. (2005) J. Cardiovasc. Pharmacol. 46, 141–146 [DOI] [PubMed] [Google Scholar]
- 11.Zhang S. J., Yang X. M., Liu G. S., Cohen M. V., Pemberton K., Downey J. M. (2003) J. Cardiovasc. Pharmacol. 42, 764–771 [DOI] [PubMed] [Google Scholar]
- 12.Imperial J. S., Bansal P. S., Alewood P. F., Daly N. L., Craik D. J., Sporning A., Terlau H., López-Vera E., Bandyopadhyay P. K., Olivera B. M. (2006) Biochemistry 45, 8331–8340 [DOI] [PubMed] [Google Scholar]
- 13.Cartier G. E., Yoshikami D., Gray W. R., Luo S., Olivera B. M., McIntosh J. M. (1996) J. Biol. Chem. 271, 7522–7528 [DOI] [PubMed] [Google Scholar]
- 14.Liman E. R., Tytgat J., Hess P. (1992) Neuron 9, 861–871 [DOI] [PubMed] [Google Scholar]
- 15.Finol-Urdaneta R. K., Strüver N., Terlau H. (2006) J. Gen. Phys. 128, 133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfrum S., Richardt G., Dominiak P., Katus H. A., Dendorfer A. (2001) Br. J. Pharmacol. 134, 370–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terlau H., Shon K. J., Grilley M., Stocker M., Stühmer W., Olivera B. M. (1996) Nature 381, 148–151 [DOI] [PubMed] [Google Scholar]
- 18.Al-Sabi A., Lennartz D., Ferber M., Gulyas J., Rivier J. E., Olivera B. M., Carlomagno T., Terlau H. (2004) Biochemistry 43, 8625–8635 [DOI] [PubMed] [Google Scholar]
- 19.Verdier L., Al-Sabi A., Rivier J. E., Olivera B. M., Terlau H., Carlomagno T. (2005) J. Biol. Chem. 280, 21246–21255 [DOI] [PubMed] [Google Scholar]
- 20.Brinckmann J., Kim S., Wu J., Reinhardt D. P., Batmunkh C., Metzen E., Notbohm H., Bank R. A., Krieg T., Hunzelmann N. (2005) Matrix Biol. 24, 459–468 [DOI] [PubMed] [Google Scholar]
- 21.Hill J. M., Alewood P. F., Craik D. J. (1996) Biochemistry 35, 8824–8835 [DOI] [PubMed] [Google Scholar]
- 22.Keizer D. W., West P. J., Lee E. F., Yoshikami D., Olivera B. M., Bulaj G., Norton R. S. (2003) J. Biol. Chem. 278, 46805–46813 [DOI] [PubMed] [Google Scholar]
- 23.Lancelin J. M., Kohda D., Tate S., Yanagawa Y., Abe T., Satake M., Inagaki F. (1991) Biochemistry 30, 6908–6916 [DOI] [PubMed] [Google Scholar]
- 24.Nielsen K. J., Watson M., Adams D. J., Hammarström A. K., Gage P. W., Hill J. M., Craik D. J., Thomas L., Adams D., Alewood P. F., Lewis R. J. (2002) J. Biol. Chem. 277, 27247–27255 [DOI] [PubMed] [Google Scholar]
- 25.Kaplan S. H., Yang H., Gilliam D. E., Shen J., Lemasters J. J., Cascio W. E. (1995) Cardiovasc. Res. 29, 231–238 [PubMed] [Google Scholar]
- 26.Schlüter K. D., Schwartz P., Siegmund B., Piper H. M. (1991) Am. J. Physiol. 261, H416–H423 [DOI] [PubMed] [Google Scholar]
- 27.Cohen M. V., Yang X. M., Downey J. M. (2007) Circulation 115, 1895–1903 [DOI] [PubMed] [Google Scholar]
- 28.Vacher H., Mohapatra D. P., Trimmer J. S. (2008) Physiol. Rev. 88, 1407–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon J. E., McKinnon D. (1996) Eur. J. Neurosci. 8, 183–191 [DOI] [PubMed] [Google Scholar]
- 30.Plane F., Johnson R., Kerr P., Wiehler W., Thorneloe K., Ishii K., Chen T., Cole W. (2005) Circ. Res. 96, 216–224 [DOI] [PubMed] [Google Scholar]
- 31.Qiu M. H., Zhang R., Sun F. Y. (2003) J. Neurochem. 87, 1509–1517 [DOI] [PubMed] [Google Scholar]
- 32.Nesti E., Everill B., Morielli A. D. (2004) Mol. Biol. Cell 15, 4073–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hopkins W. F. (1998) J. Pharmacol. Exp. Ther. 285, 1051–1060 [PubMed] [Google Scholar]
- 34.Koch E. D., Olivera B. M., Terlau H., Conti F. (2004) Biophys. J. 86, 191–209 [DOI] [PMC free article] [PubMed] [Google Scholar]