Abstract
Ubiquitin is one of the most highly conserved proteins in eukaryotes and plays major biological roles as a post-translational protein modifier. Ubiquitin is also a natural constituent of plasma, and several lines of evidence suggest that extracellular ubiquitin is an immune modulator with anti-inflammatory properties. In addition, ubiquitin treatment has been shown to limit inflammation and reduce organ injury in various disease models and species in vivo. However, its mechanism of action is unknown. Here we show that extracellular ubiquitin is a natural CXC chemokine receptor 4 (CXCR4 and CD184) agonist. Extracellular ubiquitin promotes intracellular Ca2+ flux and reduces cAMP levels through a G protein-coupled receptor that signals via a Gαi/o protein in THP1 cells. Toll-like receptor 4 stimulation reduces ubiquitin-binding sites, which enabled identification of four Gαi/o PCRs as ubiquitin receptor candidates. Overexpression of candidate genes in HEK293 cells, gene silencing in THP1 cells, competition binding, and signaling studies with the CXCR4 agonist stromal cell-derived factor-1α (chemokine (CXC motif) ligand 12) and inhibitor AMD3100 identify CXCR4 as a functional ubiquitin receptor. Our finding uncovers a fundamentally new aspect of the role of ubiquitin in biology, has implications for the understanding of CXCR4-mediated events, and is expected to facilitate development of new therapeutic avenues for a variety of diseases.
Keywords: Cell Surface Receptor, G Protein-coupled Receptors (GPCR), Immunology, Inflammation, Ubiquitin, CXC Chemokine Receptor 4, Stromal Cell-derived Factor-1α, Extracellular Ubiquitin
Introduction
Ubiquitin is one of the most highly conserved proteins in all eukaryotes and plays major biological roles as a post-translational protein modifier (1). Although the discovery of ubiquitin introduced this protein as a molecule with extracellular actions (2), little attention has since been paid to its role outside the cell.
Ubiquitin is also a natural constituent of plasma, and significantly elevated systemic ubiquitin concentrations have been described in various diseases (3–6). Only a few previous studies reported on possible functions of extracellular ubiquitin and suggested that it could be involved in the regulation of cell growth, cell differentiation, and host defense (2, 7–11). Recently, we demonstrated that extracellular ubiquitin has anti-inflammatory properties and regulates leukocyte function in patients with severe injuries and sepsis (12). Along with the observation that lower systemic ubiquitin levels were accompanied by a higher degree of organ dysfunction and failure (13), these data suggested that ubiquitin release after trauma is beneficial and may function to limit exuberant inflammation. Accordingly, we showed in a series of in vivo studies that treatment with exogenous ubiquitin is anti-inflammatory and immunosuppressive and that ubiquitin administration is neuroprotective and reduces organ injury after endotoxic shock, trauma, and ischemia-reperfusion (14–19). Meanwhile the neuroprotective and immunosuppressive effects of ubiquitin have been confirmed independently (20, 21). However, the mechanism of action of extracellular ubiquitin remains unknown.
It has been shown that extracellular biotinylated and fluorescein (FITC)-labeled2 ubiquitin can be taken up into the cell (9, 11, 22), and quantification of ubiquitin uptake kinetics in the human monocytic cell line MonoMac 6 suggested a saturable process (22). Therefore, we studied whether extracellular ubiquitin interacts with a cell surface receptor. We detected that extracellular ubiquitin binds to a G protein-coupled receptor (GPCR) and signals through a Gαi/o protein. The finding that TLR4 stimulation reduced ubiquitin receptor-binding sites enabled identification of four receptor candidates. Ubiquitin binding studies after candidate receptor overexpression and gene silencing as well as pharmacological characterization of the binding and signaling properties of ubiquitin provided evidence that extracellular ubiquitin is a natural agonist of the chemokine and HIV co-receptor CXC chemokine receptor (CXCR) 4 (fusin, CD184).
EXPERIMENTAL PROCEDURES
Proteins, TLR Agonists, and Inhibitors
Ubiquitin, ovalbumin, and bovine serum albumin (BSA) were obtained from Sigma. N-terminal fluorescein-labeled ubiquitin (FITC-ubiquitin), FITC-SUMO1, FITC-SUMO2, and biotin-ubiquitin were purchased from Boston Biochem. FITC-ovalbumin was from Molecular Probes. Recombinant human stromal cell-derived factor-1α (SDF-1α; chemokine (CXC motif) ligand 12) was obtained from Peprotech. Purified Staphylococcus aureus, TLR2 ligand lipoteichoic acid; purified Escherichia coli K12 lipopolysaccharide (LPS), TLR4 ligand; ODN2216, TLR9 ligand; and ODN2216 Control, TLR9 ligand were purchased from Invivogen. Nocodazole, AMD3100, and pertussis toxin were purchased from Sigma. U73122 and U73343 were purchased from EMD Biosciences.
Cells and Cell Culture
The cell lines were cultured and maintained in RPMI1640 (Sigma) supplemented with 10% fetal bovine serum (Hyclone), 100 units/ml penicillin (Invitrogen), and 100 μg/ml Streptomycin (Invitrogen) in 75-cm2 surface area tissue culture flasks with ventilation (Nunc). Human (THP1, U937, and HL60) and murine (J774, Wehi3, and P388.D1) cell lines (all from American Type Culture Collection) were a kind gift from Ravi Shankar (Loyola University Chicago). HEK293 and HEK293 stably expressing hemagglutinin-tagged CXCR4 (HEK293-CXCR4stable) were as described (23). Human monocytes were isolated from buffy coats by density gradient centrifugation, followed by plastic adherence. Buffy coats were obtained from healthy blood donors through LifeSource, Chicagoland's blood center. Cell pretreatment with nocodazole (10 μm) was performed for 60 min at 37 °C in cell culture media.
Fluorescence and Confocal Fluorescence Microscopy
THP1 cells were incubated with FITC-ubiquitin or FITC-ovalbumin in phosphate-buffered saline (PBS), 1% BSA, and 0.01% sodium azide (Sigma) at the reported temperature for 0–60 min. After incubation, the cells were washed twice with PBS and fixed with 2% paraformaldehyde. The glass slides were prepared using Cytospin (Thermo Scientific) and imaged with a Zeiss AxioVert 200M fluorescence microscope and AxioVision 4.1 image analysis software. For confocal fluorescence microscopy, the slides were imaged with a Zeiss LSM-510 laser scanning confocal microscope and Zen software. The images were also captured with Z sectioning for three-dimensional reconstruction. Nuclear counterstaining was performed with 4′-6-diamidino-2-phenylindole (DAPI; Thermo Scientific).
Fluorescence-activated Cell Sorting (FACS) Analyses
FACS was used to analyze FITC-ubiquitin binding, FITC-ovalbumin binding and cell surface expression of the GPCRs Cnr2, CXCR4, Ebi2, and GPR35. For assessment of FITC-ubiquitin/ovalbumin binding, the cells were washed with PBS and suspended at 106 ml−1 in PBS, 1% BSA, and 0.01% sodium azide. The cells were incubated with FITC-ubiquitin or FITC-ovalbumin at the given temperature for 60 min in plastic FACS tubes (BD Biosciences). The cells were washed twice with cold (4 °C) PBS, fixed in 2% paraformaldehyde (Sigma) at 4 °C for 60 min, washed twice with cold PBS, resuspended in 100 μl of PBS with 0.1% BSA, and kept at 4 °C until analyzed with a FACSAria flow cytometer (BD Biosciences). The fluorescence intensities of at least 105 cells were recorded and analyzed using the FloJo software (Tree Star). For the quantification of CXCR4 cell surface expression, the cells were labeled with FITC-conjugated anti-human CXCR4 (R & D Systems). For quantification of Ebi2, GPR35, and Cnr2 cell surface expression, the cells were labeled with anti-human Ebi2, anti-human GPR35 (both from Genway), and anti-human Cnr2 (Thermo Scientific) in combination with FITC-labeled anti IgG/IgM (Genway). In all experiments the fluorescence signals of at least 105 cells were measured as described before. FITC-conjugated IgG2A (R & D Systems) was used as negative control under identical conditions.
Ubiquitin Binding Assays
The cells were washed with ice-cold PBS, and 105 cells were suspended in 100 μl of cold (4 °C) PBS, 1% BSA, 0.01% sodium azide in microcentrifuge tubes (VWR Scientific). FITC-ubiquitin, biotin-ubiquitin, or other FITC-labeled proteins were added and incubated for 0–60 min at 4 °C. The cells were washed twice with 1 ml of cold PBS and resuspended in 100 μl of PBS. For the detection of FITC-labeled proteins, cell suspensions were transferred into black 96-well microplates (VWR Scientific), and the fluorescence intensities were measured with a Synergy 2 microplate reader (λexcitation/emission, 485/528 nm; BioTek Instruments). Nonspecific binding was assessed as binding of FITC-ubiquitin in the presence of 300 μm native ubiquitin.
For the detection of biotin-ubiquitin binding, the cells were washed twice with 1 ml of cold PBS and fixed in 1 ml of 2% paraformaldehyde at 4 °C for 60 min. The cells were then incubated with horseradish peroxidase-conjugated anti-biotin (Sigma) for 60 min at room temperature. The cells were washed twice with 1 ml of cold PBS, and 100 μl of 3,3′,5,5′-tetramethyl-benzidine (Sigma) was added. The reaction was stopped with 100 μl of 2 n hydrochloric acid (Sigma) after 5 min. The cell suspensions were transferred to clear 96-well microplates (VWR Scientific), and the absorption intensities at 450 nm were measured using a Synergy 2 microplate reader.
Calcium Assay
Intracellular calcium was measured using the Fluo-4 NW calcium assay kit (Molecular Probes). The cells were washed twice with PBS and resuspended in assay buffer, a component of the assay kit. Fifty μl of cell suspension were pipetted into the wells of a black 96-well microplate and incubated at 37 °C and 5% CO2. After 60 min, 50 μl of 2× dye loading solution with probenecid was added to each well. The cells were then incubated at 37 °C, 5% CO2 for 30 min and an additional 30 min at room temperature. Fluorescence was then measured before and after spiking cells with ubiquitin using a Synergy 2 microplate reader (λexcitation/emission, 494/516 nm). Where applicable, AMD3100 (10 μm) was added to the wells 3 min before cells were spiked with ubiquitin; U73122 (10 μm), U73343 (10 μm), and pertussis toxin (100 ng/ml) were added at least 30 min prior to the addition of ubiquitin.
cAMP Assay
Quantitative determination of cAMP levels in THP1 cells was performed using the cAMP Complete enzyme immunoassay kit (Assay Designs), acetylated format. 5 × 105 cells were grown per well in a 12-well culture plate and treated with ubiquitin, SDF-1α, or AMD3100 at 37 °C for 0–60 min. At the end of treatment, the cells were lysed by incubating with 0.1 m HCl at room temperature for 15 min and centrifuged at 500 × g. The supernatant was used to assay for cAMP according to the manufacturer's assay protocol. All of the incubations were performed at room temperature, and optical densities were measured at a wavelength of 405 nm using a Synergy 2 microplate reader.
GPCR Candidate Gene Transfections
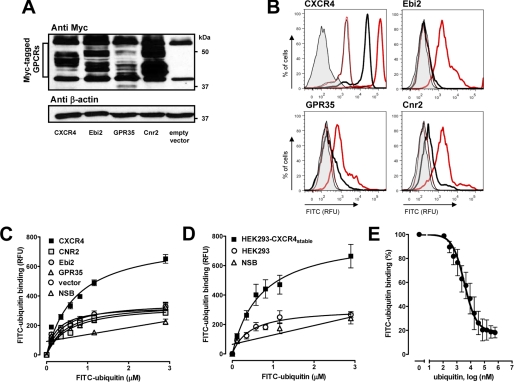
DNA encoding C-terminally MYC/DDK-tagged CXCR4 (catalog number RC202069), Ebi2 (catalog number RC204736), GPR35 (catalog number RC215174), and Cnr2 (catalog number RC210224) were purchased from OriGene Technologies. DNA (5–10 μg) encoding each respective GPCR or empty vector (pcDNA3) was transiently transfected into HEK293 cells grown on 10-cm tissue culture dishes using TansIT-LT1 (Mirus Bio) transfection reagent, according to the manufacturer's recommendation. Twenty-four hours later, the cells were divided equally onto 10-cm dishes and allowed to grow for another 48 h before performing binding experiments.
CXCR4 Gene Silencing by RNA Interference
3 × 105 THP1 cells in 1 ml of Accell siRNA delivery medium (Thermo Scientific Dharmacon) were cultured per well in a 12-well tissue culture plate (Nunc). Commercially available Accell CXCR4 siRNA (Thermo Scientific Dharmacon) was reconstituted with 1× siRNA buffer (Thermo Scientific Dharmacon) to a stock concentration of 100 μm. From this stock solution 10 μl of CXCR4 siRNA was added to each well and mixed gently. The cells were incubated at 37 °C, 5% CO2 for 72 h. Accell nontargeting siRNA pool and Accell GAPDH siRNA control were used as negative controls. After 72 h, the cells were assayed for CXCR4 cell surface expression by FACS analyses, for FITC-ubiquitin binding, and for ubiquitin-induced cell signals (cellular Ca2+ and cAMP), as described above.
Western Blots
Western blotting with anti-Myc and anti-β-actin was performed as described (23). Western blotting with anti-CXCR4 (Abcam) was performed accordingly.
Statistics
The data are expressed as the means ± S.E. from duplicate to quadruplicate measurements of n independent experiments that were performed on different days. The data were analyzed using GraphPad Prism 5 software; r2 was 0.8–0.99 for all regression curves. For the calculation of the Ki, the following parameters and constraints were used: concentration of labeled ligand = 1163 nm (10 μg/ml); Kd of labeled ligand less than 313 nm (which is the upper limit of the 95% confidence interval of the Kd for FITC-ubiquitin), upper plateau constant equal to 100%. Comparison of Bmax values from FITC-binding curves after TLR stimulation was performed by analysis of variance with Tukey's post-hoc test for multiple comparisons. A two-tailed p < 0.05 was considered significant.
RESULTS
Extracellular Ubiquitin Binds to a Cell Surface Receptor
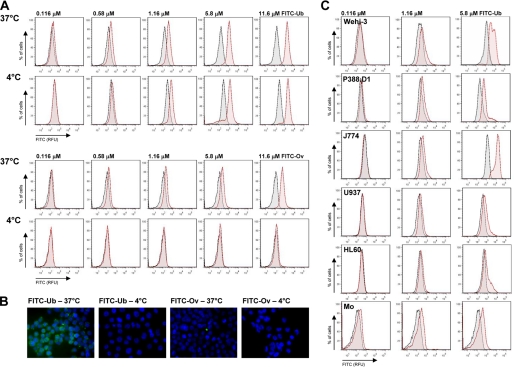
We analyzed binding of FITC-ubiquitin to the human monocytic cell line THP1 at 37 °C and at 4 °C to prevent endocytosis. All of the experiments were performed in the presence of 10 mg/ml BSA to reduce nonspecific binding and uptake. Consistent with the possible interaction of ubiquitin with a cell surface receptor and its subsequent receptor-mediated internalization, we detected FITC-ubiquitin binding to THP1 cells by flow cytometry at both temperatures (Fig. 1A, top panels) and observed its uptake into THP1 cells at 37 °C (Fig. 1B). When FITC-ovalbumin was used as a control protein under identical conditions, it did not produce a binding signal in flow cytometry at 4 °C (Fig. 1A, bottom panels), and its uptake in the presence of a large excess of BSA could not be detected by fluorescence microscopy (Fig. 1B). This suggests that the positive FITC-ovalbumin binding signal at 37 °C in flow cytometry experiments reflects nonspecific binding to the cell surface and further points toward specific binding and uptake of FITC-ubiquitin.
FIGURE 1.
Extracellular ubiquitin binds to monocytes/macrophages at 4 °C. A, FACS analyses of THP1 cells after incubation with FITC-ubiquitin (top panels) or FITC-ovalbumin (bottom panels) for 1 h at 37 °C and 4 °C. Unstained cells are shown in gray, and cells after incubation with FITC-proteins are shown in red. FITC-Ub, FITC-labeled ubiquitin. FITC-Ov, FITC-labeled ovalbumin. Both FITC-labeled proteins showed comparable fluorescence signals/mol of protein. B, fluorescence microscopy of THP1 cells after incubation with 2.9 μm FITC-ubiquitin (FITC-Ub) or FITC-ovalbumin (FITC-Ov) for 1 h at 37 and 4 °C. Green, FITC signal; blue, DAPI nuclear counterstaining. Both FITC-labeled proteins showed comparable fluorescence signals per mol of protein. C, FACS analyses of multiple monocyte/macrophage cell lines and freshly isolated human monocytes (Mo) after incubation with FITC-ubiquitin (red) for 1h at 4 °C. Unstained cells are shown in gray.
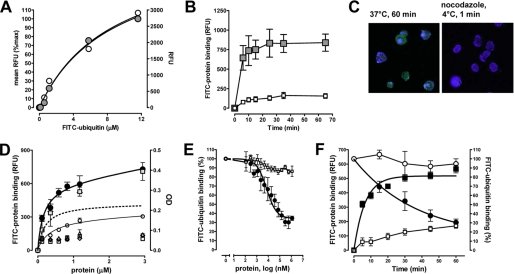
Ubiquitin binding at 4 °C was also detectable in several monocyte/macrophage cell lines of murine (Wehi-3, P388.D1, J774) and human (U937, HL60) origin and in freshly isolated human monocytes (Fig. 1B). Comparison of the fluorescence signals in THP1 cell cultures after incubation with FITC-ubiquitin with mean cell fluorescence measured by flow cytometry showed equal FITC-ubiquitin binding signals (Fig. 2A). This provided us with a simple and fast ubiquitin binding assay. Quantification of the time dependence of FITC-ubiquitin binding to THP1 cells at 4 °C suggested that binding occurs within minutes (Fig. 2B, gray squares). To be able to define ubiquitin binding to the cell surface without interference by fluorescence signals that result from FITC-ubiquitin after its cellular uptake, we incubated THP1 cells at 37 °C with the microtubule polymerization inhibitor nocodazole prior to their exposure to FITC-ubiquitin at 4 °C. Under these conditions, cellular uptake of FITC-ubiquitin could be prevented (Fig. 2C) and FITC-ubiquitin binding to THP1 cells showed typical receptor binding characteristics with a Kd of 155 ± 62 nm in saturation binding experiments (Fig. 2D). Biotin-labeled ubiquitin binding parameters were similar to FITC-ubiquitin when quantified using a secondary anti-biotin horseradish peroxidase-conjugated antibody followed by calorimetric detection (Fig. 2D, gray squares, right ordinate).
FIGURE 2.
Extracellular ubiquitin binds to a cell surface receptor. RFU, relative fluorescence units. A, THP1 cells were incubated with 1.16 μm (10 μg/ml) FITC-ubiquitin for 60 min at 37 °C and washed twice. Fluorescence was measured by flow cytometry (left ordinate, gray circles) and with a fluorescence reader (right ordinate, open circles). Both measurements correlated linear with r2 = 0.977. B, THP1 cells were incubated with 1.16 μm FITC-ubiquitin (■) or FITC-ovalbumin (□) for 0–60 min at 4 °C and washed twice, and the fluorescence signals were measured. Both FITC-labeled proteins showed comparable fluorescence signals/mol of protein. Note that cells were centrifuged for 5 min to remove free FITC-ubiquitin in the cell culture supernatant. Thus, the shortest incubation period equals 5 min exposure to FITC-ubiquitin. n = 3–4. C, confocal fluorescence microscopy of THP1 cells after incubation with FITC-ubiquitin (1.16 μm). Green, FITC-ubiquitin; blue, DAPI nuclear counterstaining. Left panel, incubation with FITC-ubiquitin for 60 min at 37 °C. Right panel, THP1 cells were preincubated with nocodazole and then incubated with FITC-ubiquitin for 1 min at 4 °C. D, FITC-ubiquitin binding to THP1 cells (1 min, 4 °C). The cells were preincubated with nocodazole. ●, FITC-ubiquitin (n = 6).  , biotin-ubiquitin (right ordinate; n = 3). ▵, FITC-SUMO1 (n = 5). ◇, FITC-SUMO2 (n = 5). □, FITC-ovalbumin (n = 3). ○, nonspecific binding (n = 6). Dashed line, specific binding curve. E, competition binding (1 min, 4 °C) curve for unlabeled ubiquitin (n = 6, ●) and ovalbumin (n = 3, □) with 1.16 μm FITC-ubiquitin. FITC-ubiquitin binding is expressed as the percentage of the fluorescence signal measured in the absence of unlabeled ubiquitin (100%). F, association and dissociation binding kinetics. For association binding studies THP1 cells were preincubated with nocodazole and then exposed to 1.16 μm FITC-ubiquitin (■, n = 5) or FITC-ovalbumin (□, n = 3, control) for 0–60 min at 4 °C. For dissociation binding studies, THP1 cells were incubated with 1.16 μm FITC-ubiquitin for 1 min at 4 °C. The cells were then washed and incubated for 0–60 min at 37 °C (○, n = 3) or 4 °C (●, nocodazole pretreatment, n = 3). FITC-ubiquitin binding is expressed as percentage of RFU measured at t = 0 min (100%) in dissociation binding studies (right ordinate).
, biotin-ubiquitin (right ordinate; n = 3). ▵, FITC-SUMO1 (n = 5). ◇, FITC-SUMO2 (n = 5). □, FITC-ovalbumin (n = 3). ○, nonspecific binding (n = 6). Dashed line, specific binding curve. E, competition binding (1 min, 4 °C) curve for unlabeled ubiquitin (n = 6, ●) and ovalbumin (n = 3, □) with 1.16 μm FITC-ubiquitin. FITC-ubiquitin binding is expressed as the percentage of the fluorescence signal measured in the absence of unlabeled ubiquitin (100%). F, association and dissociation binding kinetics. For association binding studies THP1 cells were preincubated with nocodazole and then exposed to 1.16 μm FITC-ubiquitin (■, n = 5) or FITC-ovalbumin (□, n = 3, control) for 0–60 min at 4 °C. For dissociation binding studies, THP1 cells were incubated with 1.16 μm FITC-ubiquitin for 1 min at 4 °C. The cells were then washed and incubated for 0–60 min at 37 °C (○, n = 3) or 4 °C (●, nocodazole pretreatment, n = 3). FITC-ubiquitin binding is expressed as percentage of RFU measured at t = 0 min (100%) in dissociation binding studies (right ordinate).
To assess whether these binding characteristics are specific for ubiquitin, we tested FITC-labeled ovalbumin, SUMO1, and SUMO2 as control proteins under identical conditions in parallel experiments. SUMO1 and SUMO2 were selected because they have a folded structure that is virtually superimposable on that of ubiquitin (24). All FITC-labeled control proteins showed fluorescence signals/mol of protein comparable with FITC-ubiquitin. In cell binding assays none of them produced signals above the nonspecific binding proportion of the fluorescence signal for FITC-ubiquitin (Fig. 2D).
To confirm the Kd from saturation binding experiments, we then performed homologous competition binding and kinetic binding experiments with nocodazole-pretreated cells. As shown in Fig. 2E, native ubiquitin displaced FITC-ubiquitin from binding sites on THP1 cells with a Ki of 135 nm, whereas native ovalbumin did not interfere with FITC-ubiquitin binding. In kinetic binding experiments (Fig. 2F), the observed association rate constant (kobs) for FITC-ubiquitin was 0.166 min−1, and FITC-ubiquitin dissociated from its binding site with a half-life of 26 min (dissociation rate constant (koff) = 0.027 min−1) at 4 °C, whereas the fluorescence signal remained almost constant at 37 °C. The latter was consistent with receptor-mediated uptake of ubiquitin under physiological conditions (22). Based on the kobs and the koff, we calculated a kon of 3.2 × 10−5 m−1 min−1 and a Kd of 84 nm for ubiquitin binding to its cell surface receptor.
Extracellular Ubiquitin Binds to and Signals through a Gαi/o Protein-coupled Receptor
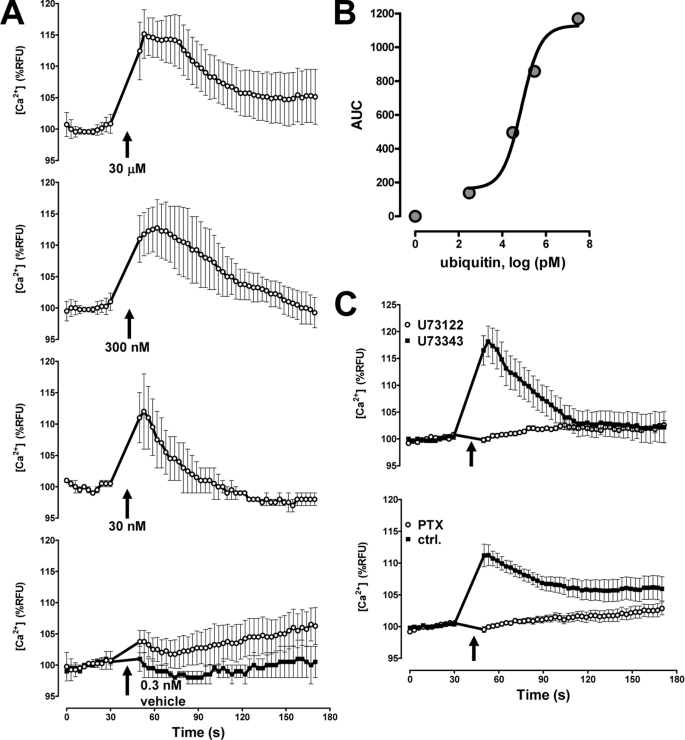
Next, we investigated whether ubiquitin binding to a cell surface receptor transduces a physiologically relevant intracellular signal and tested whether ubiquitin induces intracellular Ca2+ flux. Within seconds and at physiologically relevant concentrations, ubiquitin dose-dependently induced an increase in intracellular Ca2+ (Fig. 3A). To determine the magnitude of the Ca2+ signal, we calculated the area under the fluorescence curves and determined an EC50 for the effect of ubiquitin on intracellular Ca2+ release of 75 nm (Fig. 3B).
FIGURE 3.
Ubiquitin signals through a Gαi/o heterotrimeric G protein. RFU, relative fluorescence units. A, ubiquitin (0–30 μm)-induced Ca2+ flux in THP1 cells. n = 3. The arrows indicate the time point when ubiquitin (○) or vehicle (■) was added. B, quantification of the ubiquitin-induced Ca2+ signal from A. AUC, area under the fluorescence curves. C, inhibition of the ubiquitin (1–10 μm)-induced Ca2+ signal by U73122 (10 μm) and pertussis toxin (100 ng/ml). The weak PLC inhibitor U73343 (10 μm) was used as a negative control for U73122. n = 3. The arrows indicate the time point when ubiquitin was added.
The largest class of cell surface receptors that are able to induce an intracellular Ca2+ flux are GPCRs. Because GPCR promoted increases in intracellular Ca2+ are often mediated by phospholipase Cβ (PLCβ) activation (25), we tested the effect of the PLC inhibitor U73122 on the ability of ubiquitin to promote Ca2+ flux. In these experiments, the weak PLC inhibitor U73343 was used as a negative control for U73122. As shown in Fig. 3C (top), U73122 abolished the ubiquitin-induced Ca2+ flux, suggesting the involvement of PLCβ. Because PLCβ is typically downstream of Gαq- and Gαi/o-coupled receptors, the finding that pertussis toxin also abolished ubiquitin-induced Ca2+ release (Fig. 3C, bottom) suggested that ubiquitin signals through a GPCR that couples through the heterotrimeric Gαi/o protein (26).
Extracellular Ubiquitin Binds to CXCR4
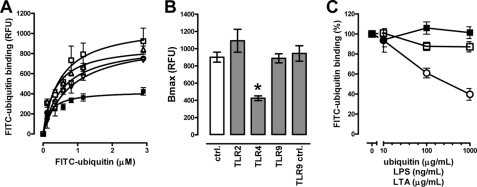
Recently, a GPCR expression profile in mouse macrophages has been reported (27). Sixty-seven GPCRs appeared to be expressed in murine macrophages. In both bone marrow-derived and peritoneal macrophages, 10 GPCRs were reported to be repressed by LPS stimulation (27). Interestingly, extracellular ubiquitin has been shown to attenuate LPS induced inflammation in vitro and in vivo (12, 15), and ubiquitin uptake into MonoMac 6 cells could be altered by LPS and lipoteichoic acid treatment (22). Thus, we further tested whether TLR 2, 4, or 9 stimulation affects ubiquitin binding to the cell surface receptor. We detected that TLR4 stimulation significantly decreased the Bmax of the FITC-ubiquitin binding characteristics, whereas TLR2 and TLR9 stimulation did not (Fig. 4, A and B). Because TLR2 and TLR4 agonists did not compete with FITC-ubiquitin for receptor binding (Fig. 4C), these data implied that TLR4 stimulation reduced the number of available ubiquitin receptor-binding sites and thus were consistent with repression of the cell surface receptor for ubiquitin by LPS.
FIGURE 4.
TLR4 stimulation reduces ubiquitin receptor-binding sites. A, FITC-ubiquitin binding (1 min, 4 °C, nocodazole pretreatment) after incubation of THP1 cells with TLR agonists (2 h, 37 °C). □, TLR2; ■, TLR4; ▵, TLR9; ▿, TLR9 control (ctrl); ○, no TLR stimulation (n = 3–5). B, Bmax values calculated from binding curves in A. *, p < 0.05 versus all other conditions. C, competition binding curve for unlabeled ubiquitin (○), TLR2 (LTA, □), and TLR4 (LPS, ■) agonists with 1.16 μm FITC-ubiquitin. n = 3. FITC-ubiquitin binding is expressed as a percentage of the fluorescence signal measured in the absence of unlabeled ubiquitin or TLR agonists (100%).
Of the 10 GPCRs that were reported to be repressed by LPS in macrophages (27), four were identified as Gαi/o-coupled seven-transmembrane receptors: cannabinoid receptor 2 (Cnr2/CB2) (28), CXCR4 (29), Ebi2 (Epstein-Barr virus induced gene 2/lymphocyte-specific G protein-coupled receptor) (30), and GPR35 (G protein-coupled receptor) (31). Because these four GPCRs appeared to be promising candidates as receptors for ubiquitin, we transfected HEK293 cells with the Myc-tagged open reading frame cDNA clones of the candidate GPCRs. To confirm expression of the candidate genes after transfection, whole cell extracts were analyzed by Western blotting with anti-Myc, and GPCR cell surface expression was analyzed by FACS after incubation of the transfected cells with GPCR-specific antibodies. We detected the expected pattern of bands (40–50 kDa) after transfection of the cells with the GPCR cDNA clones, as compared with cells that were transfected with the empty vector (Fig. 5A). FACS analyses demonstrated that the transfected cells expressed 4–11-fold more of the GPCRs on the cell surface (Fig. 5B). FITC-ubiquitin binding studies with the transfected cells showed increased specific FITC-ubiquitin binding in transiently CXCR4 overexpressing cells (Fig. 5C). The number of ubiquitin-binding sites, as estimated from the calculated Bmax value of the binding curves, increased 4.8 ± 0.9-fold in HEK293 cells transfected with CXCR4 cDNA (Fig. 5A), and these cells expressed 6 ± 2-fold more CXCR4 on the cell surface (Fig. 5B), as compared with naïve HEK293 cells and cells transfected with the other receptor candidate genes. Increased specific FITC-ubiquitin binding was also detectable in a HEK293 cell line stably expressing hemagglutinin-tagged CXCR4 (HEK293-CXCR4stable) (23). In these cells, 5.4-fold more CXCR4 was detectable on the cell surface by flow cytometry (not shown), and the number of ubiquitin-binding sites increased 5.9-fold, as compared with naïve HEK293 cells (Fig. 5D). Native ubiquitin was also able to compete with FITC-ubiquitin for receptor binding in HEK293-CXCR4stable cells (Ki: 65 nm; Fig. 5E).
FIGURE 5.
Ubiquitin receptor binding is increased in CXCR4 overexpressing cells. RFU, relative fluorescence units. FITC-ubiquitin binding (1 min, 4 °C) was assessed in nocodazole pretreated cells. A, Myc-tagged candidate receptors were transfected into HEK293 cells followed by immunoblotting of whole cell lysates with anti-Myc and anti-β-actin. B, quantification of candidate receptor expression by flow cytometry after transfection as in A. Thick lines, anti-GPCRs. Thin lines, control. Gray, unstained cells. Red, transfected with the GPCRs. Black, transfected with empty plasmid. C, FITC-ubiquitin binding after transfection as in A and B. NSB, nonspecific binding. n = 5. D, FITC-ubiquitin binding to HEK293-CXCR4stable. NSB, nonspecific binding. n = 4. E, competition binding curve for unlabeled ubiquitin in HEK293-CXCR4stable; 1.16 μm FITC-ubiquitin. n = 3. FITC-ubiquitin binding is expressed as a percentage of the fluorescence signal measured in the absence of unlabeled ubiquitin (100%).
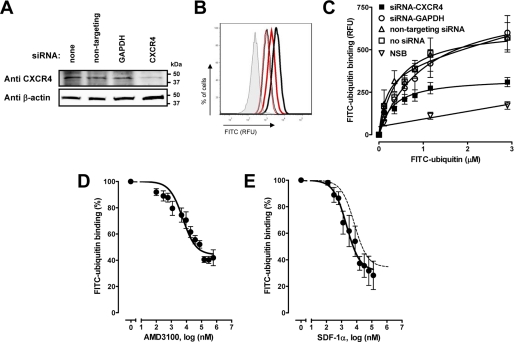
Furthermore, we assessed ubiquitin binding to THP1 cells in which CXCR4 was silenced by RNA interference. Accordingly, this resulted in 50–60% reduction of CXCR4 surface expression (Fig. 6, A and B) and ubiquitin-binding sites (Fig. 6C).
FIGURE 6.
Ubiquitin receptor binding is reduced after CXCR4 gene silencing and CXCR4 ligands compete with ubiquitin for receptor-binding sites. A, CXCR4 was silenced with siRNA in THP1 cells followed by immunoblotting of whole cell lysates with anti-CXCR4 and anti-β-actin. B, CXCR4 silencing in THP1 cells and CXCR4 quantification as in Fig. 5B. Red, CXCR4 siRNA. Black, nontargeting siRNA. n = 4. C, FITC-ubiquitin binding curves after CXCR4 silencing, as in A and B. n = 5. FITC-ubiquitin binding (1 min, 4 °C) was measured in nocodazole pretreated cells. RFU, relative fluorescence units. NSB, nonspecific binding. D and E, competition binding curves with AMD3100 (D, n = 4) and SDF-1α (E, n = 4) in THP1 cells; 1.16 μm FITC-ubiquitin. The dashed line in E shows the competition curve for native ubiquitin (from Fig. 2E). FITC-ubiquitin binding is expressed as a percentage of the fluorescence signal measured in the absence of the CXCR4 ligands (100%).
Consistent with ubiquitin binding to CXCR4, we detected that the CXCR4 antagonist AMD3100 competed with FITC-ubiquitin for receptor binding (Ki, 250 nm; Fig. 6D). Moreover, the specific CXCR4 ligand SDF-1α also competed with FITC-ubiquitin for the receptor with a Ki that was 50% lower than the Ki for native ubiquitin (Ki: 68 nm; Fig. 6E). The bottom plateaus of the competition curves for native ubiquitin (32.2 ± 3.7%; Fig. 2E) and SDF-1α (31.45 ± 6%; Fig. 6E) were comparable and reached the nonspecific binding proportion of the fluorescence signal of ∼30% (Fig. 2D, open circles).
Extracellular Ubiquitin Is a CXCR4 Agonist
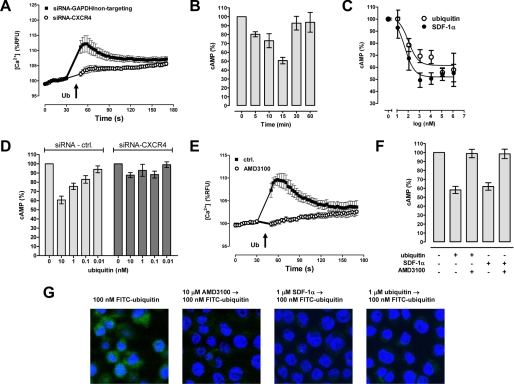
Because our data suggested that ubiquitin binds to CXCR4, we studied whether ubiquitin also signals via CXCR4. Consistent with ubiquitin signaling through CXCR4, we detected that reduction of CXCR4 cell surface expression by gene silencing in THP1 cells (as in Fig. 6, A and B) attenuated the ubiquitin-induced Ca2+ flux to a comparable proportion (Fig. 7A).
FIGURE 7.
Ubiquitin is a CXCR4 agonist. A, ubiquitin (30 μm)-induced Ca2+ flux after CXCR4 silencing in THP1 cells, as in Fig. 6 (A and B). n = 4. B, cAMP levels in THP1 cells 0–60 min after ubiquitin (1.16 μm) treatment. n = 4. The data are expressed as percentages of untreated cells (100%). C, dose-dependent reduction of cAMP levels in THP1 cells by ubiquitin (○) and SDF-1α (●). n = 4–8. The data are expressed as percentages of untreated cells (100%). D, effects of ubiquitin on cAMP levels after CXCR4 silencing in THP1 cells, as in Fig. 6 (A and B). n = 4. Light gray bars, cells transfected with nontargeting siRNA (n = 2) or siRNA-GAPDH (n = 2). Dark gray bars, cells transfected with siRNA-CXCR4 (n = 4). The data are expressed as percentages of untreated cells (100%). E, AMD3100 (10 μm) inhibits ubiquitin (30 μm)-induced Ca2+ flux in THP1 cells. n = 3. F, AMD3100 (10 μm) abolishes ubiquitin (100 nm)- and SDF-1α (100 nm)-induced reduction of cAMP levels in THP1 cells (n = 4). The data are expressed as percentages of untreated cells (100%). G, FITC-ubiquitin uptake into THP1 cells is reduced by preincubation of cells with CXCR4 ligands. The cells were incubated (30 min, 37 °C) with 100 nm FITC-ubiquitin and used for confocal fluorescence microscopy, as in Fig. 1A. The cells were not pretreated or pretreated (30 min, 37 °C) with 10 μm AMD3100, 1 μm SDF-1α, or 1 μm native ubiquitin (from left to right). Green, FITC-ubiquitin. Blue, DAPI nuclear counterstaining.
Because CXCR4 is coupled to Gαi/o heterotrimeric G protein, CXCR4 activation inhibits adenylyl cyclase activity and decreases intracellular concentrations of the second messenger cAMP (25). Therefore, we assessed whether ubiquitin also reduces cellular cAMP levels. We found that ubiquitin reduced cAMP levels time and dose-dependently (Fig. 7, B and C). As compared with SDF-1α, the IC50 for the effects of ubiquitin on cAMP levels was 2-fold higher (SDF-1α, 49 nm; ubiquitin, 104 nm; Fig. 7C). CXCR4 gene silencing also abolished the capacity of ubiquitin to reduce cAMP levels (Fig. 7D). In addition, the CXCR4 antagonist AMD3100 prevented ubiquitin-promoted Ca2+ flux (Fig. 7E) and abolished ubiquitin and SDF-1α-induced reduction of cAMP levels (Fig. 7F).
Because ubiquitin undergoes rapid endocytosis (9, 11, 22), we then tested whether cellular uptake of FITC-ubiquitin is blocked by pretreatment of cells with CXCR4 ligands. We observed that preincubation of THP1 cells with either AMD3100, SDF-1α, or unlabeled ubiquitin reduced subsequent cellular uptake of FITC-ubiquitin (Fig. 7G), further indicating that CXCR4 is a receptor for extracellular ubiquitin and that ubiquitin is internalized into cells via CXCR4.
DISCUSSION
In the present study we demonstrate that the Gαi/o-coupled GPCR CXCR4 is a cell surface receptor for extracellular ubiquitin. Ubiquitin binding studies after CXCR4 overexpression and gene silencing, ubiquitin receptor displacement with specific CXCR4 ligands, attenuation of the biological actions of ubiquitin after CXCR4 knockdown, and inhibition of its biological effects by pertussis toxin, U73122, and AMD3100 provide multiple layers of evidence for CXCR4 as a functional ubiquitin cell surface receptor.
CXCR4 is abundantly expressed in leukocytes and in most human tissues (32). The SDF-1α/CXCR4 axis is important for normal development and hematopoeisis, has pleiotropic roles in the immune system, and is involved in cancer metastasis, leukemia, and HIV infection (26, 33). In agreement with our findings, previous reports on the biological actions of extracellular ubiquitin and SDF-1α are equally consistent with CXCR4 as their common receptor. For example, under inflammatory conditions, both molecules enhance the production of the anti-inflammatory cytokine interleukin-10 and inhibit secretion of the pro-inflammatory cytokine tumor necrosis factor α (12, 18, 34). Furthermore, ubiquitin and SDF-1α decrease apoptosis via a phosphoinositide 3-kinase-dependent mechanism in vitro (11, 35), and administration of exogenous ubiquitin and SDF-1α limits inflammation and prevents cell damage in various in vivo models (14–20, 34, 36, 37).
Determination of the affinity of ubiquitin for CXCR4 from saturation binding curves, homologous competition binding curves, and kinetic binding experiments show that ubiquitin binds to its receptor with a Kd of ∼100 nm. As expected for signaling through a Gαi/o-coupled GPCR, ubiquitin reduces cellular cAMP levels and promotes intracellular Ca2+ flux likely through activation of PLCβ via G protein βγ subunits (25, 26). The biological effects of ubiquitin are dose-dependent, and the half-maximal concentrations required for these effects are in agreement with its affinity for CXCR4.
AMD3100 is a specific CXCR4 antagonist, and its CXCR4-binding site has been characterized (38). The Ki of 250 nm that we determined for the displacement of FITC-ubiquitin from the CXCR4-binding site by AMD3100 is consistent with the Ki of 100–650 nm that has been reported previously for its inhibition of [125I]SDF-1α binding to CXCR4 (39, 40). To our knowledge, in contrast to ubiquitin, the affinity of SDF-1α for CXCR4 has as yet not been determined from saturation or kinetic binding experiments; previously reported affinities are derived from homologous competition binding experiments using [125I]SDF-1α (39, 41–45). The Ki of 65 nm for SDF-1α that we determined in heterologous competition binding experiments with FITC-ubiquitin in THP1 cells is higher than its reported Kd for displacement of [125I]SDF-1α binding to CXCR4 in human peripheral blood monocytes, T-cells, and T-cell lines (1.5–24 nm) (39, 41–44) and similar to its Kd in human hNT neurons (54 nm) (45). These data demonstrate that the affinity of SDF-1α for CXCR4 shows considerable differences among various cell types; however, the reasons remain unknown. Because we did not determine the affinity of SDF-1α for CXCR4 in THP1 cells, it is possible that the Ki of SDF-1α for the displacement of ubiquitin from CXCR4 in THP1 cells is different from its Kd for CXCR4, which could point toward distinct CXCR4-binding sites for ubiquitin and SDF-1α. On the other hand, the slopes of the competition binding curves for SDF-1α and ubiquitin are indistinguishable, and both molecules reduce the FITC-ubiquitin signal to the nonspecific binding proportion of the fluorescence signal, suggesting complete displacement of the labeled ligand. Furthermore, the IC50 for the reduction of cellular cAMP levels by ubiquitin and SDF-1α are consistent with their determined Kd and Ki values from competition binding experiments with FITC-ubiquitin. Thus, these data suggest that both molecules bind to the same binding site on CXCR4 and that the affinity of ubiquitin for CXCR4 is ∼2-fold lower than the affinity of SDF-1α.
The determined affinity of the ubiquitin CXCR4 interaction implies physiological relevance in inflammatory conditions and explains why treatment with exogenous ubiquitin was effective in previous in vivo studies. Under physiological base-line conditions, systemic concentrations of extracellular ubiquitin are ∼5–10 nm but increase to 40–70 nm during infectious and noninfectious inflammation in patients (3, 9, 12, 13). Remarkably, this ubiquitin concentration approaches its Kd for CXCR4, its EC50 for the reduction of cAMP levels and induction of Ca2+ fluxes, and its effective concentration to reduce LPS stimulated tumor necrosis factor α production that we have described previously (12). Treatment of animals with 1.3–1.5 mg of ubiquitin/kg intravenously produces peak concentrations of more than 1 μm (16, 17). This is more than an order of magnitude higher than its Kd for CXCR4, suggesting complete receptor engagement and is consistent with the notion that ubiquitin exerts its anti-inflammatory effects via activation of CXCR4.
Systemic ubiquitin concentrations in physiologic base-line conditions are 20–50-fold higher than SDF-1α concentrations and increase severalfold in various diseases (9, 12, 13, 46). Therefore, despite the lower affinity of ubiquitin for CXCR4, it is conceivable that extracellular ubiquitin is involved many CXCR4-related functions in health and disease states. During inflammation activated neutrophils release cathepsin G and neutrophil elastase, both of which are able to cleave the N terminus of SDF-1α, leading to a truncated form that is unable to interact with CXCR4 (26). Although the precise concentrations of SDF-1α that are degraded under these conditions remain unknown, it is tempting to speculate that this could shift the equilibrium between biologically active SDF-1α, ubiquitin, and CXCR4 even further toward ubiquitin-mediated CXCR4 signaling in inflammation.
CXCR4 undergoes rapid SDF-1α-mediated internalization onto the early endosomal compartment (23). Our findings that blocking of CXCR4 with AMD3100 and reduction of available CXCR4-binding sites by SDF-1α pretreatment prevent FITC-ubiquitin uptake suggests that extracellular ubiquitin also undergoes agonist-promoted endocytosis via CXCR4. Interestingly, it has been shown recently that cellular uptake of extracellular ubiquitin is followed by its covalent conjugation to intracellular proteins of the target cell (9, 22). This implies that extracellular ubiquitin escapes from the endosomal compartment and is utilized by the endogenous ubiquitin protein ligase system of the target cell. SDF-1α has been shown to activate STAT3 within a few minutes (47). Furthermore, previous observations suggest that extracellular ubiquitin can be conjugated to STAT3, leading to its proteasomal degradation at later time points (>30 min) (9). Therefore, it is an attractive hypothesis that post-translational modification of intracellular proteins with extracellular ubiquitin produces a secondary cell signal, which could function as an autoregulatory mechanism to terminate its receptor-mediated biological signals on the level of secondary effector molecules.
Previous studies suggested that the majority of extracellular ubiquitin originates from damaged cells and tissues, and we identified erythrocytes as a major source (12, 13, 48). Although mature erythrocytes contain high amounts of ubiquitin, they have lost substantial amounts of enzymes of the ubiquitin proteasome pathway (4, 49). As a consequence, it is generally believed that erythrocyte ubiquitin is a remnant of the cellular remodeling that occurs during maturation of reticulocytes to erythrocytes. Alternatively, ubiquitin release from clotted erythrocytes could constitute a strikingly simple mechanism to deliver it specifically and at high local concentrations at the site of cellular injury and inflammation.
Conclusively, our findings reveal that ubiquitin, one of the most highly conserved proteins with essential intracellular functions in all eukaryotes, is also a natural CXCR4 agonist when it is present outside the cell. Besides the HIV envelope protein gp120, the cognate CXCR4 ligand SDF-1α, and ligand macrophage migration inhibitory factor, ubiquitin is now the third endogenous protein that has been shown to bind to CXCR4 (26, 44, 45). Whether ubiquitin also binds to CXCR7/RDC1, a recently identified high affinity binding partner for SDF-1α (50), remains to be determined.
The identification of extracellular ubiquitin as a natural CXCR4 agonist has implications for our understanding of CXCR4-mediated events and also suggests that CXCR4 signaling plays an important role during the innate immune response to trauma and infection. Ubiquitin treatment has been shown to have profound therapeutic potential to reduce exuberant inflammation and organ injury in various models and species (14–20). Thus, it is expected that the identification of CXCR4 as a cell surface receptor for ubiquitin opens up new therapeutic avenues for diseases as diverse as trauma, ischemia-reperfusion injury, sepsis, HIV, or cancer.
Acknowledgments
We thank Jacqueline Romero for technical assistance, Adriana Caballero for help with initial calcium mobilization experiments, Kuzhali Muthu for help with initial FACS analyses, and Ravi Shankar for providing cell lines.
This work was supported by Deutsche Forschungsgemeinschaft Grant MA 2474/2-2 (to M. M.). This work was also supported in part by National Institutes of Health Grant GM075159 (to A. M.). The therapeutic use of ubiquitin has been patented (United States Patent 7,262,162), and M. M. is an inventor. Based on the results of this work, Loyola University Chicago has filed a provisional patent application (serial number 61/222,267). The authors are the inventors. None of the authors has received any income related to the patent or patent application.
- FITC
- fluorescein isothiocyanate
- FACS
- fluorescence-activated cell sorting
- GPCR
- G protein-coupled receptor
- HIV
- human immunodeficiency virus
- CXCR
- CXC chemokine receptor
- TLR
- Toll-like receptor
- BSA
- bovine serum albumin
- SDF
- stromal cell-derived factor
- LPS
- lipopolysaccharide
- PBS
- phosphate-buffered saline
- DAPI
- 4′-6-diamidino-2-phenylindole
- SUMO
- small ubiquitin-related modifier
- PLC
- phospholipase C
- STAT
- signal transducer and activator of transcription.
REFERENCES
- 1.Hershko A., Ciechanover A. (1998) Annu. Rev. Biochem. 67, 425–479 [DOI] [PubMed] [Google Scholar]
- 2.Goldstein G., Scheid M., Hammerling U., Schlesinger D. H., Niall H. D., Boyse E. A. (1975) Proc. Natl. Acad. Sci. U.S.A. 72, 11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asseman C., Pancré V., Delanoye A., Capron A., Auriault C. (1994) J. Immunol. Methods 173, 93–101 [DOI] [PubMed] [Google Scholar]
- 4.Takada K., Nasu H., Hibi N., Tsukada Y., Shibasaki T., Fujise K., Fujimuro M., Sawada H., Yokosawa H., Ohkawa K. (1997) Clin. Chem. 43, 1188–1195 [PubMed] [Google Scholar]
- 5.Takagi M., Yamauchi M., Toda G., Takada K., Hirakawa T., Ohkawa K. (1999) Alcohol. Clin. Exp. Res. 23, (Suppl. 4) 76S–80S [DOI] [PubMed] [Google Scholar]
- 6.Akarsu E., Pirim I., Selçuk N. Y., Tombul H. Z., Cetinkaya R. (2001) Nephron 88, 280–282 [DOI] [PubMed] [Google Scholar]
- 7.Kagan W. A., O'Neill G. J., Incefy G. S., Goldstein G., Good R. A. (1977) Blood 50, 275–288 [PubMed] [Google Scholar]
- 8.Pancré V., Pierce R. J., Fournier F., Mehtali M., Delanoye A., Capron A., Auriault C. (1991) Eur. J. Immunol. 21, 2735–2741 [DOI] [PubMed] [Google Scholar]
- 9.Daino H., Matsumura I., Takada K., Odajima J., Tanaka H., Ueda S., Shibayama H., Ikeda H., Hibi M., Machii T., Hirano T., Kanakura Y. (2000) Blood 95, 2577–2585 [PubMed] [Google Scholar]
- 10.Kieffer A. E., Goumon Y., Ruh O., Chasserot-Golaz S., Nullans G., Gasnier C., Aunis D., Metz-Boutigue M. H. (2003) FASEB J. 17, 776–778 [DOI] [PubMed] [Google Scholar]
- 11.Singh M., Roginskaya M., Dalal S., Menon B., Kaverina E., Boluyt M. O., Singh K. (2010) Cardiovasc. Res. 86, 20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majetschak M., Krehmeier U., Bardenheuer M., Denz C., Quintel M., Voggenreiter G., Obertacke U. (2003) Blood 101, 1882–1890 [DOI] [PubMed] [Google Scholar]
- 13.Majetschak M., Zedler S., Hostmann A., Sorell L. T., Patel M. B., Novar L. T., Kraft R., Habib F., de Moya M. A., Ertel W., Faist E., Schade U. (2008) J. Trauma 64, 586–598 [DOI] [PubMed] [Google Scholar]
- 14.Earle S. A., El-Haddad A., Patel M. B., Ruiz P., Pham S. M., Majetschak M. (2006) Transplantation 82, 1544–1546 [DOI] [PubMed] [Google Scholar]
- 15.Majetschak M., Cohn S. M., Nelson J. A., Burton E. H., Obertacke U., Proctor K. G. (2004) Surgery 135, 536–543 [DOI] [PubMed] [Google Scholar]
- 16.Majetschak M., Cohn S. M., Obertacke U., Proctor K. G. (2004) J. Trauma 56, 991–999 [DOI] [PubMed] [Google Scholar]
- 17.Earle S. A., Proctor K. G., Patel M. B., Majetschak M. (2005) Surgery 138, 431–438 [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Covarrubias L., Manning E. W., 3rd, Sorell L. T., Pham S. M., Majetschak M. (2008) Crit. Care Med. 36, 979–982 [DOI] [PubMed] [Google Scholar]
- 19.Griebenow M., Casalis P., Woiciechowsky C., Majetschak M., Thomale U. W. (2007) J. Neurotrauma 24, 1529–1535 [DOI] [PubMed] [Google Scholar]
- 20.Ahn H. C., Yoo K. Y., Hwang I. K., Cho J. H., Lee C. H., Choi J. H., Li H., Cho B. R., Kim Y. M., Won M. H. (2009) Exp. Neurol. 220, 120–132 [DOI] [PubMed] [Google Scholar]
- 21.Jaremko L., Jaremko M., Pasikowski P., Cebrat M., Stefanowicz P., Lisowski M., Artym J., Zimecki M., Zhukov I., Szewczuk Z. (2009) Biopolymers 91, 423–431 [DOI] [PubMed] [Google Scholar]
- 22.Majetschak M., Ponelies N., Hirsch T. (2006) Immunol. Cell Biol. 84, 59–65 [DOI] [PubMed] [Google Scholar]
- 23.Bhandari D., Trejo J., Benovic J. L., Marchese A. (2007) J. Biol. Chem. 282, 36971–36979 [DOI] [PubMed] [Google Scholar]
- 24.Bayer P., Arndt A., Metzger S., Mahajan R., Melchior F., Jaenicke R., Becker J. (1998) J. Mol. Biol. 280, 275–286 [DOI] [PubMed] [Google Scholar]
- 25.McCudden C. R., Hains M. D., Kimple R. J., Siderovski D. P., Willard F. S. (2005) Cell Mol. Life Sci. 62, 551–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busillo J. M., Benovic J. L. (2007) Biochim. Biophys. Acta 1768, 952–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lattin J. E., Schroder K., Su A. I., Walker J. R., Zhang J., Wiltshire T., Saijo K., Glass C. K., Hume D. A., Kellie S., Sweet M. J. (2008) Immunome Res. 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayewitch M., Avidor-Reiss T., Levy R., Barg J., Mechoulam R., Vogel Z. (1995) FEBS Lett. 375, 143–147 [DOI] [PubMed] [Google Scholar]
- 29.Chen W. J., Jayawickreme C., Watson C., Wolfe L., Holmes W., Ferris R., Armour S., Dallas W., Chen G., Boone L., Luther M., Kenakin T. (1998) Mol. Pharmacol. 53, 177–181 [DOI] [PubMed] [Google Scholar]
- 30.Rosenkilde M. M., Benned-Jensen T., Andersen H., Holst P. J., Kledal T. N., Lüttichau H. R., Larsen J. K., Christensen J. P., Schwartz T. W. (2006) J. Biol. Chem. 281, 13199–13208 [DOI] [PubMed] [Google Scholar]
- 31.Taniguchi Y., Tonai-Kachi H., Shinjo K. (2006) FEBS Lett. 580, 5003–5008 [DOI] [PubMed] [Google Scholar]
- 32.Gupta S. K., Pillarisetti K. (1999) J. Immunol. 163, 2368–2372 [PubMed] [Google Scholar]
- 33.Burger J. A., Kipps T. J. (2006) Blood 107, 1761–1767 [DOI] [PubMed] [Google Scholar]
- 34.Meiron M., Zohar Y., Anunu R., Wildbaum G., Karin N. (2008) J. Exp. Med. 205, 2643–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng H., Dai T., Zhou B., Zhu J., Huang H., Wang M., Fu G. (2008) Atherosclerosis 201, 36–42 [DOI] [PubMed] [Google Scholar]
- 36.Shyu W. C., Lin S. Z., Yen P. S., Su C. Y., Chen D. C., Wang H. J., Li H. (2008) J. Pharmacol. Exp. Ther. 324, 834–849 [DOI] [PubMed] [Google Scholar]
- 37.Hu X., Dai S., Wu W. J., Tan W., Zhu X., Mu J., Guo Y., Bolli R., Rokosh G. (2007) Circulation 116, 654–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenkilde M. M., Gerlach L. O., Jakobsen J. S., Skerlj R. T., Bridger G. J., Schwartz T. W. (2004) J. Biol. Chem. 279, 3033–3041 [DOI] [PubMed] [Google Scholar]
- 39.Fricker S. P., Anastassov V., Cox J., Darkes M. C., Grujic O., Idzan S. R., Labrecque J., Lau G., Mosi R. M., Nelson K. L., Qin L., Santucci Z., Wong R. S. (2006) Biochem. Pharmacol. 72, 588–596 [DOI] [PubMed] [Google Scholar]
- 40.Zhang W. B., Navenot J. M., Haribabu B., Tamamura H., Hiramatu K., Omagari A., Pei G., Manfredi J. P., Fujii N., Broach J. R., Peiper S. C. (2002) J. Biol. Chem. 277, 24515–24521 [DOI] [PubMed] [Google Scholar]
- 41.Di Salvo J., Koch G. E., Johnson K. E., Blake A. D., Daugherty B. L., DeMartino J. A., Sirotina-Meisher A., Liu Y., Springer M. S., Cascieri M. A., Sullivan K. A. (2000) Eur. J. Pharmacol. 409, 143–154 [DOI] [PubMed] [Google Scholar]
- 42.Hesselgesser J., Liang M., Hoxie J., Greenberg M., Brass L. F., Orsini M. J., Taub D., Horuk R. (1998) J. Immunol. 160, 877–883 [PubMed] [Google Scholar]
- 43.Loetscher P., Gong J. H., Dewald B., Baggiolini M., Clark-Lewis I. (1998) J. Biol. Chem. 273, 22279–22283 [DOI] [PubMed] [Google Scholar]
- 44.Bernhagen J., Krohn R., Lue H., Gregory J. L., Zernecke A., Koenen R. R., Dewor M., Georgiev I., Schober A., Leng L., Kooistra T., Fingerle-Rowson G., Ghezzi P., Kleemann R., McColl S. R., Bucala R., Hickey M. J., Weber C. (2007) Nat. Med. 13, 587–596 [DOI] [PubMed] [Google Scholar]
- 45.Hesselgesser J., Halks-Miller M., DelVecchio V., Peiper S. C., Hoxie J., Kolson D. L., Taub D., Horuk R. (1997) Curr. Biol. 7, 112–121 [DOI] [PubMed] [Google Scholar]
- 46.Xiao Q., Ye S., Oberhollenzer F., Mayr A., Jahangiri M., Willeit J., Kiechl S., Xu Q. (2008) PLoS ONE 3, e4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao H., Priebe W., Glod J., Banerjee D. (2009) Stem Cells 27, 857–865 [DOI] [PubMed] [Google Scholar]
- 48.Majetschak M., King D. R., Krehmeier U., Busby L. T., Thome C., Vajkoczy S., Proctor K. G. (2005) Crit. Care Med. 33, 1589–1594 [DOI] [PubMed] [Google Scholar]
- 49.Haas A. L. (1991) Adv. Exp. Med. Biol. 307, 191–205 [DOI] [PubMed] [Google Scholar]
- 50.Balabanian K., Lagane B., Infantino S., Chow K. Y., Harriague J., Moepps B., Arenzana-Seisdedos F., Thelen M., Bachelerie F. (2005) J. Biol. Chem. 280, 35760–35766 [DOI] [PubMed] [Google Scholar]