Abstract
Activation of the cyclic GMP phosphodiesterase (PDE6) by transducin is the central event of visual signal transduction. How the PDE6 inhibitory γ-subunit (Pγ) interacts with the catalytic subunits (Pαβ) and the transducin α-subunit (αt) in this process is not entirely clear. Here we have investigated this issue, taking advantage of site-specific label transfer from throughout the full-length Pγ molecule to both αt and Pαβ. The interaction profiling and pull-down experiments revealed that the Pγ C- terminal domain accounted for the major interaction with αt bound with guanosine 5′-3-O-(thio)triphosphate (αtGTPγS) in comparison with the central region, whereas an opposite pattern was observed for the Pγ-Pαβ interaction. This complementary feature was further exhibited when both αtGTPγS and Pαβ were present and competing for Pγ interaction, with the Pγ C-terminal domain favoring αt, whereas the central region demonstrated a preference for Pαβ. Furthermore, αtGTPγS co-immunoprecipitated with PDE6 and vice versa in a Pγ-dependent manner. Either Pαβ or αtGTPγS could be pulled down by the Btn-Pγ molecules on streptavidin beads that were saturated by the other partner, indicating simultaneous binding of these two partners to Pγ. These data together indicate that complementary Pγ interactions with its two targets facilitate the αt·PDE6 “transducisome” formation. Thus, our study provides new insights into the molecular mechanisms of PDE6 activation.
Keywords: Cyclic GMP (cGMP), G Proteins, Phosphodiesterases, Phototransduction, Protein-protein Interactions, Transducin, Transducisome
Introduction
The intricate visual transduction in rod photoreceptor cells provides a paradigm for G protein-coupled signaling. The outstanding visual sensitivity of the rod is largely due to the great signal amplification achieved by the cGMP2 phosphodiesterase PDE6 (rod photoreceptor cGMP phosphodiesterase), the central effector enzyme (1). Upon absorption of a single photon, light-excited rhodopsin stimulates an exchange of GTP for GDP bound in the transducin α subunit (αt) (2), which in turn relieves PDE6 from the inhibitory constraint exerted by its γ-subunit (Pγ). PDE6 activation causes rapid cGMP breakdown, which closes the cGMP-coupled ion channels, thus relaying visual signals to the brain in a form of electrical pulses (3). PDE6 in the rod is uniquely composed of a large catalytic heterodimer (Pαβ, ∼100 kDa each subunit) to which bind two small identical Pγ subunits (∼10 kDa) keeping the enzyme inactive in the dark (1, 4). The PDE6 structure is less well understood compared with the other key players in phototransduction. This is primarily due to the fact that solving the atomic structure of PDE6 has been hindered by the lack of an expression system to produce active Pαβ heterodimers in large amounts (5). A low resolution electron microscopy image of Pαβ has revealed a linear alignment of three distinct domains of each subunit: the tandem GAFa and GAFb domains on the N-terminal side that host non-catalytic cGMP binding and the C-terminal catalytic domain that performs cGMP hydrolysis (6). Direct allosteric communication between GAF domains and the catalytic domain has been recently reported (7).
The inhibitory Pγ subunit is an intrinsically disordered protein, yet structural elements important for its function are encoded in the free Pγ molecule (8). The Pγ sequence of 87 amino acids features a polycationic central domain (Gly19–Gly49) and a negatively charged C-terminal half that contains a linker region (Phe50–Gly61) and a hydrophobic C-terminal domain (Thr62–Ile87) (1, 9). The last C-terminal dozen or so residues (herein termed the inhibitory region) are involved in the interaction with the Pαβ catalytic domain (8, 10, 11). The very recently reported crystal structure of the chimeric PDE5/6 catalytic domain complexed with the Pγ(70–87) inhibitory peptide (5) has confirmed the previous suggestion that the highly hydrophobic C terminus (Y84GII87) directly blocks the cGMP entry into the catalytic pocket (12, 13). The other important Pαβ-interacting site on Pγ is the central domain, which has been shown to provide most of the binding strength for Pαβ (14). The central domain of Pγ binds to the Pαβ GAF domain (15, 16) and couples non-catalytic cGMP binding in a positively cooperative manner, thus regulating the PDE-inhibiting function of Pγ (14). Remarkably, the C-terminal domain and the central domain also constitute αt-interacting sites (17–19).
An overlap of the Pγ C-terminal αt-binding region (Thr62–Ile87) and the inhibitory region (Asn74–Ile87) forms the structural basis for transducin-mediated PDE6 activation (5, 8, 20). Various lines of evidence suggest that GTP-bound αt activates PDE6 by physically displacing the inhibitory region of Pγ from the Pαβ catalytic pocket, thus initiating the signaling state of phototransduction (5, 8, 10–12, 20). In the ensuing transition state, αtGTP is converted back to the GDP-bound inactive structure, which has lowered affinity with Pγ, thus releasing it to reinhibit PDE6 and terminate signaling (3). Fast visual recovery is ensured by great acceleration of the αt GTPase activity, which is achieved by the GTPase-activating protein (GAP) complex composed of αt, Pγ, RGS9-1 (the ninth member of the regulators of G-protein signaling in photoreceptors), and its constitutive partner Gβ5 as well as the membrane anchoring protein R9AP (3, 21). Much of the molecular details of the Pγ-αtGTP interaction in the signaling state have been learned from the crystal structure of the partial transition state complex, which includes the GDP-AlF4−-bound αt/i1 chimera, the half-Pγ (Gly46–Ile87), and the catalytic core of RGS9-1 (20). As visualized by this structure, a stretch of Pγ residues around Trp70 forms a tight interaction with αt that is further reinforced by additional contacts provided by some residues in the Pγ inhibitory region. Recent NMR (8) and crystallography (5) studies indicated that when the Pγ inhibitory region was associated with the chimeric PDE5/6 catalytic domain, the critical αt-binding residues Trp70 and Leu76, however, were not involved. These studies lend further support to a model of PDE6 activation (5, 11); i.e. an engagement of αtGTP with the Pγ residues Trp70 and Leu76 triggers a conformational change involving a hingelike rigid body movement of Pγ(78–87) away from the PDE6 catalytic pocket.
Thus, Pγ plays a pivotal role, not only for turning on but also for turning off phototransduction and keeping the signaling system inactive in the dark (9). Despite a wealth of information regarding phototransduction mechanisms, dynamic interactions of Pγ with αt and Pαβ, as well as RGS9-1, are not well understood. There has been controversy as to whether Pγ completely dissociates from Pαβ in the process of PDE6 activation. It is possible that whereas αt sequesters the Pγ C-terminal region from the Pαβ catalytic domain, the central domain of Pγ stays bound to the Pαβ GAF domain until the binding is allosterically reduced by the dissociation of cGMP from the GAF domain (1). This scenario of simultaneous Pγ interactions with both αt and Pαβ is consistent with the proposition of an intermediate αt·PDE6 complex during PDE6 activation (17, 22–26). Earlier studies suggested that direct αt-Pαβ contacts may be a driving force in forming the intermediate complex in the presence of disc membranes (24, 27). However, it has not been determined whether the Pγ interactions with αt and Pαβ contribute important elements to the intermediate PDE6 activation complex.
As presented in this study, the label transfer approach, which has proven to be powerful for systematically detecting interactions of full-length molecules (16, 28, 29), offered us an opportunity to investigate this issue from a unique perspective. The data obtained through label transfer, immunoprecipitation, and pull-down suggest that complementary interactions, in which the Pγ C-terminal domain forms a strong interaction with αt while the central region binds tightly with Pαβ, assist the transducin·PDE6 complex formation, which elicits PDE6 activation.
EXPERIMENTAL PROCEDURES
The chemicals and reagents used in this study were from the sources described previously (16, 28) unless otherwise stated. The C-terminal Pγ peptide (Pγ(62–87)) was custom-synthesized at the Peptide Synthesis Facility of the Biotechnology Center, University of Wisconsin (Madison, WI).
Transducin Preparation
Using frozen dark-adapted bovine retinas (J. A. & W. L. Lawson Co.), rod outer segment (ROS) membranes were isolated, from which holotransducin was prepared as described previously (29, 30). αtGDP and βγt were then purified from holotransducin using a blue Sepharose CL-6B column. To prepare αtGTPγS, GTPγS was added to ROS membranes, and αtGTPγS was thus released and purified on the blue Sepharose CL-6B column. The purity of αt was determined to be >95% by SDS-PAGE and Coomassie staining. The purified proteins were stored at −80 °C.
Preparation of PDE6
The samples of bovine PDE6 were kindly provided by Dr. Nikolai O. Artemyev at the University of Iowa and prepared according to established methods (4). Briefly, holo-PDE6 was extracted from bleached ROS membranes, and Pαβ was then obtained by removing Pγ through mild tryptic proteolysis of holo-PDE6. More vigorous tryptic treatment generated the Pαβ heterodimer with a nick at Lys146/Lys147 on Pβ. It has been reported that nicked Pαβ has unaltered functional properties (12, 16). Unless otherwise stated, “Pαβ” refers to nicked Pαβ throughout this paper. The Pαβ preparations were purified to >95% by a Mono-Q column (Amersham Biosciences), as judged from Coomassie-stained SDS gels.
Preparation of Pγ Photoprobes
The constructs for expressing the full-length wild type Pγ with the single cysteine at position 68 (29), and the single cysteine mutants were generated as described previously (28). They were expressed in E. coli and purified by chitin beads, followed by reversed-phase HPLC using the POROS 20 R2 resin (31). The truncated Pγ variants (29) with and without a His6 tag at the N terminus (HisPγ(1–61) and Pγ(1–61), respectively) were prepared using the same protocol. Full-length Pγ (>95% pure) was used for preparation of Pγ photoprobes. The radioactive [125I]ACTP-Pγ and non-radioactive [127I]ACTP-Pγ photoprobes were prepared as described earlier (28).
The maleimido benzophenone (mBP)-Pγ photoprobes were prepared as described previously (16). Briefly, Pγ was derivatized with mBP in 10–20-fold molar excess, and mBP-Pγ was then separated from unreacted Pγ and free mBP through reversed phase HPLC. Correct molecular masses of the [127I]ACTP-Pγ and mBP-Pγ photoprobes have been confirmed by electrospray ionization mass spectrometry conducted at the Chemistry Department Mass Spectrometry Facility of the University of Wisconsin (Madison, WI).
Functional Assay of the Pγ Photoprobes
The transducin GTPase activity assay was kindly conducted by Dr. Kirill A. Martemyanov (now at the University of Minnesota) and Dr. Vadim Y. Arshavsky (now at Duke University), using a single turnover technique as described previously (32). The assay was conducted at room temperature (22–24 °C) in a buffer containing 25 mm Tris-HCl (pH 8.0), 140 mm NaCl, and 8 mm MgCl2. The urea-treated ROS membranes, lacking endogenous activity of RGS9-1, were used as a source for the photoexcited rhodopsin required for transducin activation. The reactions were initiated by the addition of 10 μl of 0.6 μm [32P]GTP (∼105 dpm/sample) to 20 μl of urea-treated ROS membranes (20 μm final rhodopsin concentration) reconstituted with transducin heterotrimer (1 μm) and recombinant RGS9-1·Gβ5 complex (0.5 μm). The reactions were performed in either the absence or presence of Pγ derivatives (1 μm). The reaction was stopped by the addition of 100 μl of 6% perchloric acid. The 32P formation was measured with activated charcoal. All assays were conducted in the absence of reducing agent due to the presence of the disulfide linkage between the photoreactive group and Pγ.
Photocross-linking/Label Transfer Using Pγ Photoprobes
A scheme is presented in supplemental Fig. S1A to explain the label transfer strategy. Unless otherwise described, photocross-linking reactions were performed in the HEPES buffer (10 mm HEPES, pH 7.5, 120 mm NaCl, 5 mm MgCl2). Samples were contained in ultraclear polypropylene microcentrifuge tubes (Axygen). The reactions using [125I]ACTP-Pγ photoprobes were exposed to the UV light generated by an AH-6 water-jacketed 1000-watt high pressure mercury lamp for 5 s at a distance of 10 cm (28). The reactions with mBP-Pγ were photolyzed at 5–10 °C for 2 × 15 min with a 5-min dark interval on ice in an RPR-100 Rayonet photochemical reactor equipped with 18 bulbs of 350 nm (Southern New England Ultraviolet Company). Immediately after photolysis, sample buffer was added to the reactions to final concentrations of 1% SDS and 50 mm DTT. The proteins were separated by SDS-PAGE and then subjected to Coomassie Blue staining and autoradiography. Autoradiography and protein quantitation were performed as described previously (28).
Pull-down Assays of Pγ Interactions with αtGTPγS and Pαβ Using Affinity Beads
To immobilize the full-length Pγ to the streptavidin beads, biotinylated Pγ was prepared by covalently attaching maleimide-PEO2-biotin (Pierce Biotechnology) to the single cysteine at position 3 of the Pγ mutant, L3C (28). The derivatization reaction and purification of the Btn-L3C derivative were performed following the protocol of mBP-Pγ preparation (16). To prepare Btn-Pγ(46–87), the Pγ 87C mutant was first derivatized with maleimide-PEO2-biotin and then trypsinized, and Btn-Pγ(46–87) was purified by reversed phase HPLC using a C4 column (31).
For each pull-down reaction, 0.4 μl of Ultra-Link Plus immobilized streptavidin gel (Pierce) was first equilibrated with the pull-down buffer, which contains 20 mm HEPES, pH 7.5, 120 mm NaCl, 5 mm MgCl2, 1 mm DTT, 0.1% n-dodecanoylsucrose (Calbiochem), and 50 μg/ml BSA, and then incubated with 4 μm Btn-Pγ by rotating the microcentrifuge tube for 10 min at room temperature. A high concentration (1 μg/μl) of BSA or soybean trypsin inhibitor was added at this step to block possible nonspecific protein-bead interactions. After this incubation, Btn-Pγ was found completely bound to the streptavidin beads (data not shown). To test if the Pγ peptides disrupt the Pγ-αtGTPγS or Pγ-Pαβ interaction, Pγ(62–87) or Pγ(1–61) in excess over Btn-Pγ was first incubated with αtGTPγS or Pαβ in the pull-down buffer for 1 h on ice and then added to the Btn-Pγ-streptavidin beads. After rotating the reactions at 4 °C for 1–2 h, the beads were washed twice with 400 μl of ice-cold pull-down buffer. Proteins were then eluted from the beads with SDS/DTT-containing sample buffer, run on a low cross-link 15% acrylamide gel (33), and visualized by staining with Coomassie Blue R-250, or SilverSNAP Stain Kit II (Pierce) when lower amounts of proteins were used.
To study the interactions of the Pγ central region with Pαβ and αt, the Pγ construct HisPγ(1–61) was used. For each reaction, 0.5 μl of His-Select High-Flow nickel beads (Sigma) were first washed with 500 μl of H2O and then with 300 μl of pull-down buffer. HisPγ(1–61) of 5 μm was immobilized to the beads by incubation in the pull-down buffer (supplemented with 1 μg/μl trypsin inhibitor at this step) at room temperature for 10 min on rotating. Twenty mm imidazole was included in the pull-down buffer throughout the experimental procedures to prevent possible nonspecific binding of proteins to nickel beads. After the beads were washed with 2 × 500 μl of pull-down buffer to remove unbound HisPγ(1–61), Pαβ or/and αtGTPγS were added and incubated with the beads for 1–2 h at 4 °C. The beads were then washed twice with 200 μl of pull-down buffer. The proteins on the beads were eluted with the sample buffer and resolved by SDS-PAGE using a low cross-link 15% gel (33), which was then silver-stained using SilverSNAP Stain Kit II (Pierce).
Immunoprecipitation Assay of the αtGTPγS-PDE6 Interaction
Co-immunoprecipitation of αtGTPγS with holo-PDE6 was carried out using nProtein A Sepharose Fast-Flow beads (Amersham Biosciences) and the antibody against bovine rod Pα (Affinity Bioreagents). For each reaction, 0.5 μl of Protein A beads were first equilibrated with the HEPES buffer (10 mm HEPES, pH 7.5, 120 mm NaCl, 5 mm MgCl2) and then incubated with 0.5 μg of the anti-Pα antibody by rotating for 1 h at 4 °C. One μg/μl soybean trypsin inhibitor was included to block possible nonspecific protein binding sites on Protein A beads. The beads were washed three times with 300 μl of HEPES buffer prior to the immunoprecipitation reaction. Meanwhile, 0.5 μg of holo-PDE6 was incubated for 1–2 h on ice with 0.5 μg of αtGTPγS in the HEPES buffer containing 50 μg/ml trypsin inhibitor and 1 mm DTT. The reaction was then added to the washed Protein A beads with anti-Pα bound and incubated for 1 h by rotating at 4 °C. Pγ peptide Pγ(1–61) or Pγ(62–87) in a 200-fold molar excess over holo-PDE6 was added as a competitor to disrupt Pγ interactions. After washing the beads three times with 300 μl of HEPES buffer (containing trypsin inhibitor and DTT), the immunoprecipitate was eluted with the sample buffer and subjected to SDS-PAGE and then detected by Western blotting using the anti-αt antibody (K-20, Santa Cruz Biotechnology, Inc. (Santa Cruz, CA)).
Co-immunoprecipitation of Pαβ with αtGTPγS was performed similarly but in a reverse manner. Briefly, 1.5 μg of anti-αt was immobilized onto 0.75 μl of Protein-A beads. In separate tubes, 0.95 μg of Pαβ was incubated with 0.1 μg of Pγ on ice for 0.5 h with or without a competitor (Pγ(1–61) or Pγ(62–87) in a 200-fold molar excess over Pαβ), and 0.2 μg of αtGTPγS was then added. Following incubation on ice for 1 h, the reaction was mixed with washed anti-αt/Protein A beads and rotated for 1 h at 4 °C. The Pαβ immunoprecipitate was then eluted off of the washed beads and detected by Western blotting using the anti-Pα antibody.
Western Blot
Western blotting was performed as described previously (16). Low cross-link 15% acrylamide gels (33) were used for SDS-PAGE. Proteins were electrotransferred from the gel to the polyvinylidene difluoride membrane for 2 h at 45 V. Antibody dilutions were as follows: anti-Pα (Affinity Bioreagents), 1 μg/ml; anti-αt (Affinity Bioreagents), 0.5 μg/ml; horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Sigma), 100,000–300,000-fold. The Millipore Immobilon Western horseradish peroxidase substrate was used for chemiluminescence detection of the horseradish peroxidase-labeled bands.
RESULTS
Profiling of the αtGTPγS Interaction with the Full-length Pγ
A full spectrum profiling of Pγ-αt interaction has not been reported. Previous peptide mapping studies showed that C-terminal Pγ peptides had slightly lower affinity for αtGTPγS than peptides from the central region (17, 18). The affinity of these peptides with activated αt (∼1 μm), however, is nearly 100-fold lower than the full-length Pγ (10–12 nm (18, 34)). This conspicuous affinity difference indicates that the full-length Pγ molecule is required to assume an optimal conformation for binding with αt. Obviously, peptide mapping is not an optimal approach to measure relative Pγ domain contributions in the full-length Pγ-αtGTPγS interaction. Our label transfer experiments, in which the full-length Pγ could be used, however, offered a better means to address this issue.
Eleven photoprobes were prepared, with [125I]ACTP site-specifically attached through mixed disulfide to the single cysteines placed at various positions throughout the Pγ molecule. Functional properties of these ACTP-Pγ probes have been carefully characterized when previously used to map the Pγ-Pαβ interaction interface, and no major change due to the ACTP modification was observed for the PDE6 inhibition potency of Pγ (28). A possible impact of ACTP on the Pγ function of αt GTPase stimulation was further assessed in this study, which showed that the functional activities of these Pγ photoprobes were similar to that of the unmodified native Pγ (supplemental Fig. S1B).
We therefore used these probes in the photocross-linking/label transfer experiments to profile the Pγ-αtGTPγS interaction. Upon UV illumination, the azide group of ACTP is photoactivated into a nitrene (35), which then inserts into the nearby Pγ-interacting site(s) on αt, forming a covalent bond with the αt backbone. After DTT reversal of the S–S link between ACTP and the cysteine on Pγ, the 125I radiolabel is transferred from Pγ to αt (see the diagram in supplemental Fig. S1A), which can be detected by autoradiography (Fig. 1A). Thus, the label transfer efficiency reflects the interaction intensity between αt and a given Pγ position where [125I]ACTP is attached.
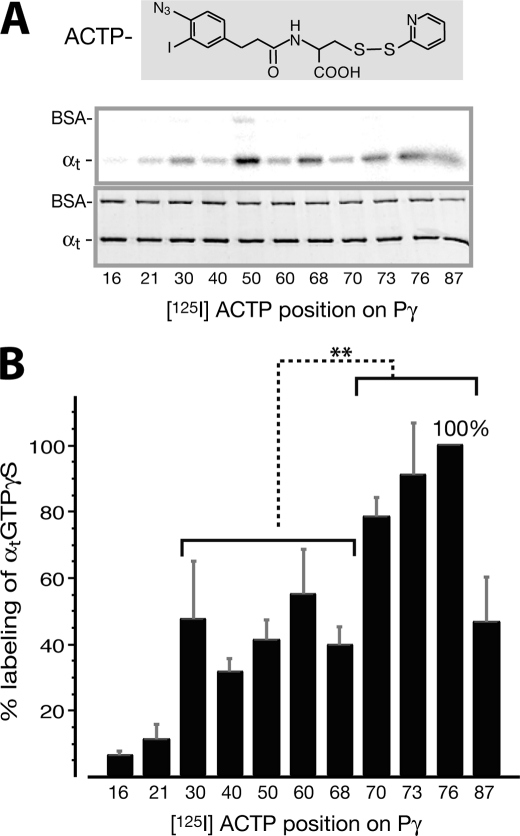
FIGURE 1.
Profiling of [125I]ACTP label transfer to αtGTPγS from various positions throughout the Pγ molecule. A, radiolabel transfer from [125I]ACTP-Pγ to αtGTPγS was detected by autoradiography (top panel). Shown in the bottom panel is the Coomassie-stained gel, below which the corresponding [125I]ACTP derivatization positions on Pγ are listed. Photocross-linking reactions were performed with 1 μm αtGTPγS and 0.8 μm [125I]ACTP-Pγ (see “Experimental Procedures”). BSA was included in the reactions as an internal control. B, profile of quantified label transfer to αtGTPγS. Each data value is expressed as a percentage relative to the maximum labeling (position 76, ±15.7%) and presented as an average ±S.D. (error bars) of four separate experiments. The amount of label transfer to αtGTPγS from each Pγ position was normalized for the αt protein amount and the specific activity of the corresponding [125I]ACTP-Pγ probe (supplemental Table S1 or Ref 28). Statistical analyses were performed by t test (Microsoft Excel). *, p = 0.01–0.05 (significant); **, p = 0.001–0.01 (very significant); ***, p < 0.001 (extremely significant); ns, p > 0.05 (not significant). As shown in the figure, there is a very significant difference between label transfer from the Pγ Phe30–Cys68 region and that from the Trp70–Leu76 region. Position 87 is not included in the latter group for statistical analysis because label transfer from the hydrophilic ACTP probe substituting the hydrophobic Ile87 residue could not accurately reflect the interaction between this position and αtGTPγS (see the discussion of Figs. 1 and 2 under “Results”). The difference between each position and position 76 is also analyzed: positions 16 and 21 (***); 40, 50, and 68 (**); 30, 60, and 87 (*); 70 and 73 (not significant).
The [125I]ACTP-Pγ photoprobes, which were previously proven to transfer radiolabel to Pαβ specifically (28), were shown here to also specifically transfer radiolabel to αtGTPγS. Specificity of the observed label transfer to αt was manifested not only by the absence of radiolabel on BSA (Fig. 1A), which was included as an internal control, but also by the Pγ position dependence of label transfer (Fig. 1B).
The label transfer yield from each Pγ position was quantified by normalizing the intensity of radiolabel on αt with the specific radioactivity of the corresponding [125I]ACTP-Pγ photoprobe (supplemental Table S1) (28). Interestingly, the resultant profile of label transfer to αt showed a pattern in which αtGTPγS was highly labeled by [125I]ACTP from the Pγ C-terminal positions around Trp70 (positions 70, 73, and 76) (Fig. 1B). This region is known to have intimate contacts with αt (20). [125I]ACTP from the Pγ central positions Phe30-Leu60 (and the C-terminal position Ile87), however, only moderately labeled αtGTPγS. A low level of label transfer from the Pγ N-terminal positions 16 and 21 most likely represents a background level. Consistently, experiments performed under the same conditions but using αtGDP-AlF4−, which shares a high similarity with αtGTPγS in their three-dimensional structures and functional properties (20, 36), showed a similar profiling pattern of label transfer (data not shown).
To further confirm the observed Pγ-αtGTPγS interaction pattern (Fig. 1B), label transfer profiling was also carried out using Pγ photoprobes containing mBP, a photoreactive group different from ACTP (Fig. 2A). Under UV light (350–365 nm), the ketone in benzophenone is activated into a diradical by disproportionation and reacts with neighboring C–H bonds (35) in αt to form a C–C link. Because the maleimide group forms a C–S bond with the cysteine on Pγ, which cannot be cleaved by DTT, the cross-linked Pγ-αt complex stays covalently linked and migrates as a band higher than the αt band on the gel after SDS/DTT treatment (Fig. 2A). The mBP-Pγ photoprobes, which have been previously characterized, showed no significant functional changes in PDE6 inhibition due to mBP modifications on Pγ (16). Moreover, in a previous study from our laboratory (29), another benzophenone probe, benzoyl-l-phenylalanine, which is very similar to mBP, caused only minor changes in the Pγ stimulation of αt GTPase when incorporated into a Pγ C-terminal position at 66, 73, 76 or 86.
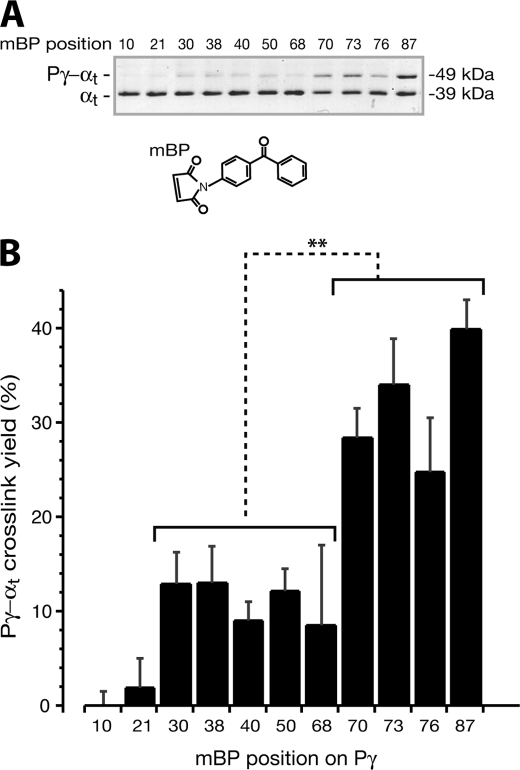
FIGURE 2.
Profiling of the Pγ-αtGTPγS interaction using mBP photoprobes. A, photocross-linked mBP-Pγ-αtGTPγS is shown as a higher band above the αt band on the Coomassie-stained SDS gel. The photocross-linking reactions were performed as described under “Experimental Procedures.” Each reaction included 1.6 μg of αtGTPγS and an equal molar amount of mBP-Pγ in the HEPES buffer (10 mm HEPES, pH 7.5, 120 mm NaCl, 5 mm MgCl2). DTT was added at a concentration of 2 mm to prevent possible nonspecific cross-link. B, the photocross-link efficiency of mBP-Pγ and αtGTPγS at each Pγ position was quantified as a percentage ratio of the protein amount in the cross-link band versus the sum in both the αt band and the cross-link band. Each bar represents an average ± S.D. (error bars) of six separate experiments. The mBP derivatization positions on Pγ are listed at the bottom. The difference in cross-link efficiency of αtGTPγS with two groups of Pγ positions, Phe30–Cys68 and Trp70–Ile87, is very significant. t test of each position against position 87 is shown: positions 10, 21, 30, 38, 40, 50, and 68 (***); positions 70 and 76 (**); and position 73 (not significant (ns)).
In a good agreement with the above results of [125I]ACTP label transfer, the cross-linking experiments using mBP-Pγ resulted in a similar profiling pattern (Fig. 2B). Thus, the fact that profiling with two different photophores (ACTP and mBP) led to similar patterns eliminates concerns regarding possible chemical selectivities of the photoprobes.
Compared with [125I]ACTP-Pγ, the mBP-Pγ probes showed a more profound preference for the Pγ C-terminal positions in cross-linking with αtGTPγS. This difference very likely stemmed from the fact that mBP is more hydrophobic (than ACTP) and thus more similar to the C-terminal hydrophobic residues that were replaced by the photoprobe. An interesting example is Ile87, the prominent hydrophobicity of which is known to play an important role in the function of Pγ (12, 13). Accordingly, the hydrophobic mBP at position 87 yielded the highest Pγ-αtGTPγS cross-link efficiency (Fig. 2B), whereas [125I]ACTP, a relatively hydrophilic probe due to the presence of a carboxyl group (Fig. 1A), resulted in less cross-link efficiency at position 87 (Fig. 1B). In this regard, the mBP cross-linking profile (Fig. 2B) may better represent Pγ domain contributions to the Pγ-αtGTPγS interaction.
Notably, both [125I]ACTP and mBP yielded a substantial cross-link at Pγ position 70 (Figs. 1B and 2B), which is critical for the Pγ-αt interaction (20). This observation agrees with our previous study in which benzoyl-l-phenylalanine replacement of Trp70 also yielded a relatively high cross-link efficiency (29). The simplest explanation is that, due to a similarity to tryptophan, the photoprobe placed at position 70 could remain in close proximity to the Trp70-interacting residues of αtGTPγS. This proposition is also supported by the native gel assay in which the ACTP-Pγ·αt complexes still formed substantially, although the ACTP derivatization at position 70, 76, or 87 affected the Pγ·αt interaction to some extent (supplemental Fig. S1C). It is thus reasonable to conclude that the αtGTPγS interaction with Trp70 would be actually stronger than observed here with a photoprobe substitution at this position. Nevertheless, our profiling experiments using full-length purified proteins revealed a clear pattern of the Pγ-αtGTPγS interaction; the C-terminal domain of Pγ contributed the major strength for the interaction with αtGTPγS compared with the remainder of the Pγ molecule (Figs. 1B and 2B).
The C-terminal Domain of Pγ Provides Major Binding Strength for the Pγ Interaction with αtGTPγS, as Does the Central Domain for the Interaction with Pαβ
In order to further assess the role of the Pγ C-terminal domain in the interaction with αtGTPγS, a different approach (pull-down) was applied, using Btn-Pγ bound to streptavidin beads. As shown in Fig. 3A, αtGTPγS was specifically pulled down by Btn-Pγ (lane 2), because no αt was detected in the control with no Btn-Pγ (lane 1). Interestingly, the Pγ C-terminal peptide Pγ(62–87) effectively abolished αtGTPγS pull-down (lane 3), but the N-terminal peptide Pγ(1–61) of the same concentration did not (lane 4). This result demonstrates a dominant role of the Pγ C-terminal domain in the Pγ-αtGTPγS interaction. This conclusion is also supported by the observation that the C-terminal half of Pγ (Btn-Pγ(46–87)) efficiently pulled down αtGTPγS (Fig. 3D, lane 3), but the N-terminal HisPγ(1–61) peptide did not (Fig. 3C, lane 4). Importantly, the observation that the Pγ C-terminal domain provided the major Pγ-αtGTPγS interaction strength (Figs. 1–3) is in accord with the crystal structure of the partial GAP complex (20), in which the Pγ C-terminal domain is engaged in a hydrophobic interlock between the αt/i1 switch II and α3 helix.
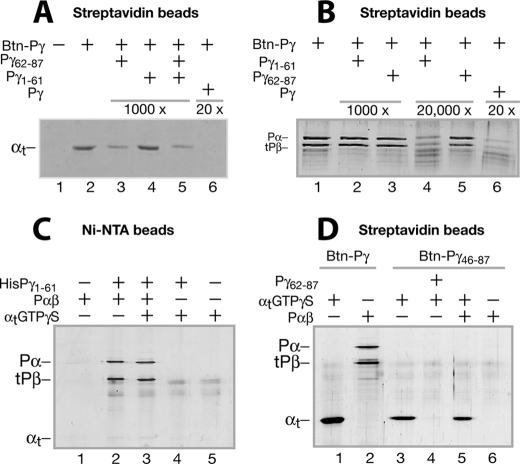
FIGURE 3.
Differential Pγ domain interactions with αtGTPγS and Pαβ evidenced by pull-down experiments. Pull-down experiments were performed with Btn-Pγ or Btn-Pγ(46–87) immobilized on strepavidin beads or HisPγ(1–61) immobilized on Ni2+-nitrilotriacetic acid beads. Conditions are described under “Experimental Procedures” unless otherwise stated. Trypsin inhibitor instead of BSA was included to block nonspecific protein-bead interactions because BSA (66 kDa) migrates too close to tPβ (∼70 kDa) on the gel. The gels in A–D each represent at least three similar experiments. A, pull-down of αtGTPγS by Btn-Pγ. The control without Btn-Pγ is shown in lane 1. The Pγ peptide Pγ(62–87) (lane 3), Pγ(1–61) (lane 4), or both (lane 5) in a 1000-fold molar excess over Btn-Pγ or the full-length Pγ in a 20-fold excess (lane 6) was added to compete with the Btn-Pγ-αtGTPγS interaction. B, pull-down of Pαβ by Btn-Pγ. Pγ(1–61) (lanes 2 and 4) or Pγ(62–87) (lanes 3 and 5), in a 1000- or 20,000-fold molar excess over Btn-Pγ, was present, competing with the Btn-Pγ-Pαβ interaction. C, pull-down of Pαβ or αtGTPγS using the HisPγ(1–61) peptide immobilized to nickel beads. Lanes 1 and 5, controls without HisPγ(1–61) for pulling down Pαβ (lane 2) and αtGTPγS (lane 4), respectively. Both Pαβ and αtGTPγS were added in the reaction of lane 3. D, pull-down of αtGTPγS (lanes 3 and 4) or Pαβ (lane 6) by Btn-Pγ(46–87). Pγ(62–87) in a 200-fold molar excess over Btn-Pγ(46–87) was used to compete with the Btn-Pγ(46–87)-αtGTPγS interaction. Pull-down of αtGTPγS (lane 1) or Pαβ (lane 2) by the full-length Btn-Pγ was also performed to compare with the Btn-Pγ(46–87) pull-down conditions.
It is noteworthy that the full-length Pγ in a 20-fold excess completely abolished the αtGTPγS pull-down (Fig. 3A, lane 6), and the full-length Pγ pulled down αtGTPγS much more efficiently than the C-terminal half-peptide (compare lane 1 with lane 3 in Fig. 3D). This is consistent with the previous assessment that although either the central or the C-terminal peptides could interact with αtGTPγS separately, the full-length Pγ interacted with αt with a much higher affinity (17, 18). Here we further assert that in the full-length Pγ-αt interaction, although the C-terminal domain contributes more than the central domain, both domains are required to forge a strong Pγ-αtGTPγS interaction.
Interestingly, in contrast to the major role of the Pγ C-terminal domain in the Pγ-αt interaction, it is Pγ(1–61) (Fig. 3C, lane 2) rather than the C-terminal half (Fig. 3D, lane 6) that efficiently pulled down Pαβ. The fact that Pγ(1–61) (lane 4 in B) but not Pγ(62–87) (lane 5 in B) abrogated the Pγ-Pαβ interaction also indicates a dominant role of the Pγ N-terminal side for interacting with Pαβ. This conclusion is consistent with the previous observations that the Pγ central domain binds Pαβ much more strongly than the C-terminal domain (14). It is noteworthy that Pγ(1–61) that was 20,000-fold (lane 4 in B) but not 1000-fold (lane 2 in B) in molar excess could disrupt the Pγ-Pαβ interaction. This result reflects an exceptionally tight full-length Pγ-Pαβ interaction, the optimum Kd of which is in the subpicomolar range (1, 14). Therefore, the stark contrast of the binding of Pαβ and αtGTPγS to the same Pγ domain raised an important question as to whether Pγ interacts with αtGTPγS and Pαβ differentially.
Pγ Interacts with αtGTPγS and Pαβ in a Complementary Manner
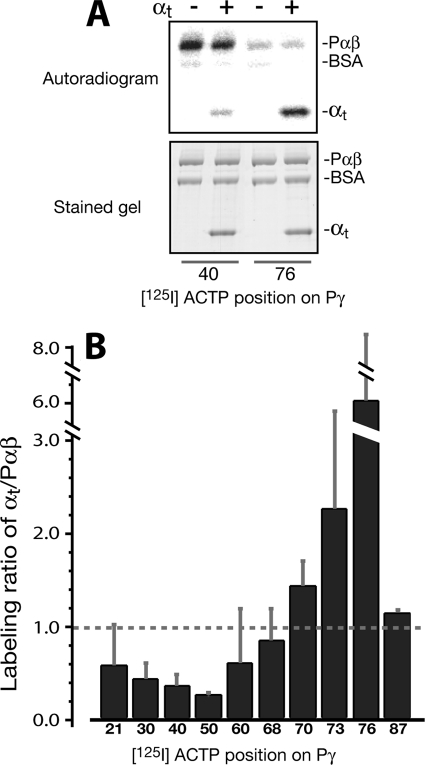
Label transfer profiling with the full-length, photoprobe-derivatized Pγ constructs allowed us to compare the pattern of Pγ-αtGTPγS interaction (Figs. 1B and 2B) with the previously observed Pγ-Pαβ interaction profile (28). A “complementary” feature of the two separately determined profiles was thus revealed, in which the Pγ central region provides most of the strength for binding with Pαβ, whereas the C-terminal region accounts for the major interaction with αtGTPγS. These data prompted us to investigate the competition between αtGTPγS and Pαβ for interacting with Pγ, by comparing the [125I]ACTP label transfer to αtGTPγS and to Pαβ from various Pγ positions in a systematic manner. For this purpose, label transfer to αtGTPγS and Pαβ from a certain Pγ position in a photocross-linking reaction with these two partners present could be directly compared in the same lane on the SDS gel (Fig. 4A). These experiments were intended to mimic the signaling state when αtGTP interacts with and displaces Pγ from PDE6 to activate the enzyme. Pγ was utilized substoichiometrically in comparison with αtGTPγS and Pαβ (0.7 Pγ, 1 αt, 1 subunit of Pαβ) so that αtGTPγS and Pαβ could effectively “compete” in their interactions with Pγ and therefore provide a greater opportunity for revealing a preferential labeling on αtGTPγS or Pαβ from a given Pγ position. As shown in the autoradiogram (Fig. 4A) and the data that are summarized as the labeling ratios for the two targets (Fig. 4B), a distinct preference of photolabel transfer for αtGTPγS over Pαβ occurred from the Pγ C-terminal positions, in particular from position 76. Accordingly, the recent crystal structure (5) showed that whereas the Pγ C terminus bound to the chimeric PDE5/6 catalytic domain, Leu76 pointed away, positioning itself in a direction ready for interaction with αt. In clear contrast to the C-terminal positions, however, more labeling from the Pγ central positions (Val21–Leu60) was observed on Pαβ than on αtGTPγS (Fig. 4B). These experiments of label transfer competition further confirm the complementary nature of the Pγ interactions with αtGTPγS and Pαβ, which was first exhibited by the different interaction profiles.
FIGURE 4.
Distinct Pγ domain preference of label transfer to αtGTPγS and Pαβ competing for interaction with Pγ. A, label transfer from the [125I]ACTP-Pγ photoprobes to αtGTPγS in competition with Pαβ. [125I]ACTP-Pγ photoprobes of the same batch as in Fig. 1 were used. Photocross-linking reactions were conducted with the [125I]ACTP-Pγ photoprobes in the presence of both αtGTPγS (4 μm) and Pαβ (without a nick) at a molar ratio of 0.7 Pγ, 1.0 αt, 1.0 Pαβ subunit. The reactions were then subjected to SDS-PAGE (bottom) and autoradiography (top). BSA was used as an internal control to demonstrate the specificity of label transfer. B, labeling ratio of αtGTPγS versus Pαβ. Each bar value represents a mean ± S.D. (error bars) (of 3–5 experiments). The amount of label transfer to αtGTPγS or Pαβ from each Pγ position was normalized for the αt (or Pαβ) protein amount and the specific activity of the corresponding [125I]ACTP-Pγ probe (supplemental Table S1 and Ref. 28). A t test was performed for each position against the value 1.0 (dashed line): positions 40, 50, and 76 (***); positions 30 and 87 (**); positions 70 and 73 (*); and positions 21, 60, and 68 (not significant (ns)). A bar value less than 1 indicates a labeling preference for Pαβ; accordingly, a value greater than 1 indicates a preference for αt.
Complementary Pγ Interactions with αt and Pαβ Constitute the αt·PDE6 Complex
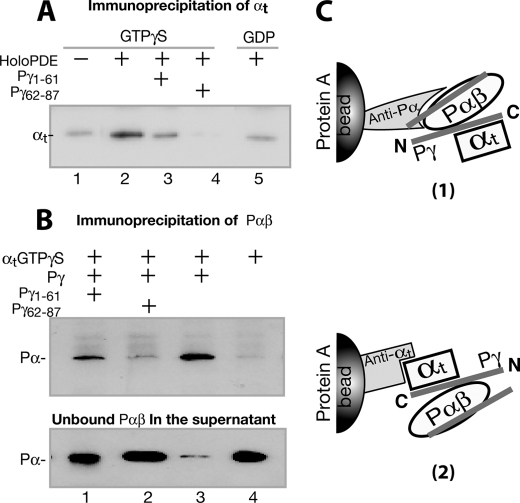
Given the above evidence that Pγ differentially interacted with its two targets, with the C-terminal domain favoring αtGTPγS and the central region preferring Pαβ, we sought to test whether the complementary interactions play an important role in the intermediate transducin·PDE6 complex, using co-immunoprecipitation approaches (Fig. 5, A and B). If the αtGTPγS·PDE6 complex is indeed primarily held together by the Pγ C-terminal interaction with αtGTPγS and the central region interaction with Pαβ (diagramed in Fig. 5C), disrupting either of these interactions using a Pγ peptide should dissociate the αt·PDE6 complex.
FIGURE 5.
Pγ-dependent αt·PDE6 interaction detected through immunoprecipitation. The immunoprecipitation experiments were conducted using purified proteins, antibodies, and Protein A beads, as described under “Experimental Procedures.” The blots shown in the figure each represent at least three similar experiments. A, αtGTPγS was immunoprecipitated by the holo-PDE6·anti-Pα·Protein A beads (lane 2). Lane 1, the control with no holo-PDE6. Pγ peptide Pγ(1–61) (lane 3) or Pγ(62–87) (lane 4) in a 200-fold molar excess was added to compete with the Pγ interactions. In lane 5, αtGDP instead of αtGTPγS was used. B, Pαβ was immunoprecipitated by the αtGTPγS·anti-αt·Protein A beads (lane 3). Lane 4 is the control in the absence of Pγ. In lanes 1 and 2, Pγ(1–61) and Pγ(62–87) of 200-fold molar excess were added, respectively, to compete with the Pγ interactions. Immunoblotting of supernatants containing unbound Pαβ is shown in the bottom panel. C, diagrams depicting the experimental strategies in A and B. For simplicity, the second αt molecule that could also bind to Pγ·Pαβ (46) is not shown.
We first observed that αtGTPγS was co-immunoprecipitated specifically with the holo-PDE6 that was immobilized to Protein A beads via the anti-Pα antibody (Fig. 5A, lane 2), as compared with the control with no holo-PDE6 added (lane 1). The immunoprecipitation of αt proved to be GTP-dependent because only a background level of αtGDP was co-immunoprecipitated with holo-PDE6 (lane 5). Moreover, the αt precipitation diminished in the presence of either Pγ(1–61) (lane 3) or Pγ(62–87) (lane 4), indicative of complementary Pγ interactions with αt and Pαβ.
To further explore the Pγ-dependent nature of the αt·PDE6 co-immunoprecipitation, experiments were performed in a reverse manner, detecting co-immunoprecipitation of Pαβ with αtGTPγS, which was immobilized on Protein A beads through the anti-αt antibody (Fig. 5B). Similar to the results shown in Fig. 5A, Pαβ was co-immunoprecipitated with αtGTPγS in the presence of Pγ (lane 3). Obviously, Pαβ was precipitated by the Pγ·αtGTPγS complex, because no Pαβ precipitation was observed when Pγ was absent (lane 4). Pαβ precipitation was diminished in the presence of Pγ(1–61) (lane 1) or Pγ(62–87) (lane 2). A significant portion of Pαβ was still precipitated with excess Pγ(1–61) present (lane 1), reflecting tight binding of the Pγ N-terminal half with Pαβ (see Fig. 3, B and C). These data further confirm the Pγ-mediated complementary interactions.
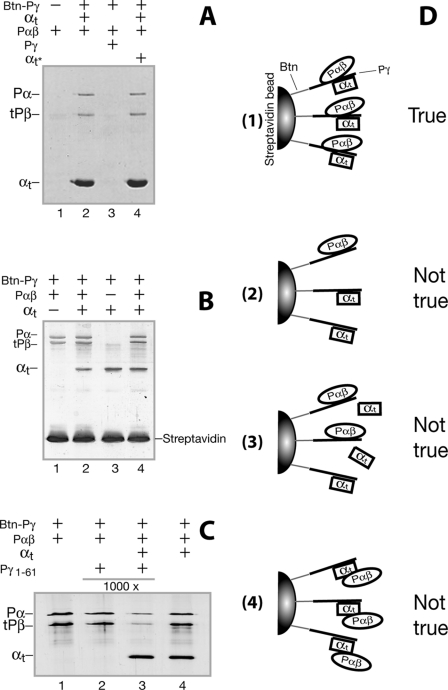
With the data in Fig. 5 showing Pγ-dependent co-immunoprecipitation of αtGTPγS with PDE6 as well as co-immunoprecipitation of PDE6 with αtGTPγS, we obtained additional evidence (Fig. 6, A–C) supporting the conclusion that complementary and simultaneous binding of Pγ to its two partners constituted the αt·PDE6 complex.
FIGURE 6.
Simultaneous Pγ binding with αtGTPγS and Pαβ evidenced by pull-down experiments. Pull-down was performed as described under “Experimental Procedures.” Trypsin inhibitor was used to block nonspecific protein-bead interactions. 4 μm Btn-Pγ was used in A, and the gel was Coomassie-stained; 0.5 μm Btn-Pγ was used in B and C, and the gels were silver-stained. Each gel shown in the figure is a representative of at least three similar experiments. A, αtGTPγS in a 5-fold molar excess versus Btn-Pγ (4 μm) was added to the Btn-Pγ streptavidin beads to saturate the αt-binding sites on Btn-Pγ by incubation for 1 h at 4 °C. After washing the beads, Pαβ (0.2 μm dimer) was added and incubated for another 1 h. In the reaction of lane 3, Pγ in 5-fold molar excess was added to compete with the Btn-Pγ-Pαβ interaction. In lane 4, αtGTPγS in a 5-fold molar excess was added again (marked as αt*) after washing the αt-saturated Btn-Pγ beads to resaturate the Pγ molecules that could have become free due to extensive washing. B, in order to determine whether αt could replace Pαβ that is prebound with Btn-Pγ, the Pαβ-binding sites of Btn-Pγ were first saturated by incubation with 1 μm Pαβ, the beads were washed, and 1 μm αtGTPγS (lane 2) or equal volume of pull-down buffer (lane 1) was then added. Similarly, to address whether Pαβ could replace αt that is bound on the Btn-Pγ beads, the αt-binding sites were first saturated with 2 μm αtGTPγS, 1 μm Pαβ (lane 4), or an equal volume of pull-down buffer (lane 3) was then added after washing the beads. C, the Pαβ-binding sites of Btn-Pγ were first saturated by incubation with 1 μm Pαβ, and the effect of Pγ(1–61) on Pαβ pull-down was then compared in the absence (lane 2) or presence (lane 3) of 2 μm αtGTPγS. D, schematic diagrams of alternative explanations for the co-pull-down of αtGTPγS and Pαβ by Btn-Pγ. For ease in distinguishing the proteins, αtGTPγS, Pαβ, and Pγ are shown as squares, ovals, and lines, respectively.
The experiments were designed based on the following idea. If Pαβ binds Pγ simultaneously along with αtGTPγS (diagramed in Fig. 6D, 1), Pαβ should be pulled down by the Btn-Pγ molecules on streptavidin beads that are saturated by excess αtGTPγS, and vice versa. As shown in Fig. 6A, Pαβ was readily pulled down by Btn-Pγ, which was bound to the beads and preincubated with αtGTPγS at a 5-fold molar excess (lanes 2 and 4), supporting the notion that co-binding of αtGTPγS and Pαβ to Btn-Pγ occurred as depicted in Fig. 6D, 1. We cautioned that the observed co-pull-down of Pαβ and αt might be subject to such alternate explanations as depicted in Fig. 6D, 2–4. However, these concerns can be ruled out by detailed analysis of our data. First, Pαβ was not pulled down by a population of Btn-Pγ that could have become free due to extensive washing of the αt-saturated beads (see Fig. 6D, 2), because the same amount of Pαβ was pulled down in the presence of excess αt that was added back to the αt-saturated beads (Fig. 6A, lane 4). Second, the addition of Pαβ to αt-saturated beads did not reduce αt pull-down (Fig. 6B, compare lane 4 with lane 3), indicating that the Pαβ pull-down did not result from replacement of a portion of αt by the Pαβ binding to Btn-Pγ (see D3). In this case, replaced αt should have been washed off and would have led to a lowered intensity of the αt band. This was also true for the αt pull-down on Pαβ-saturated beads (compare lane 2 with lane 1 in B). Third, Pαβ was not pulled down by directly interacting with αt (see D4), because αt was readily pulled down by Btn-Pγ(46–87) but Pαβ was not pulled down together with αt (Fig. 3D, lane 5). Similarly, αt was not pulled down together with Pαβ, which was pelleted with His-Pγ(1–61) on nickel beads (Fig. 3C, lane 3).
Finally, Pγ(1–61) in a 1000-fold excess could not compete with the strong full-length Pγ-Pαβ interaction (Fig. 3B, lane 2, and Fig. 6C, lane 2) but could do so in the presence of αtGTPγS (Fig. 6C, lane 3). The simplest explanation is that simultaneous binding of αtGTPγ to Btn-Pγ along with Pαβ weakened the Pγ-Pαβ interaction by sequestering the Pγ C-terminal domain and thus kept Pαβ from a high affinity binding with the full-length Pγ. Taken together, these lines of evidence suggest that Pγ facilitated the formation of the αtGTPγS·PDE6 complex by binding to both Pαβ and αtGTPγS simultaneously.
DISCUSSION
PDE6 activation mediated by αt is the central step in the visual transduction cascade. How Pγ interacts with its two targets (αt and Pαβ) is the key to understanding the molecular mechanism of PDE6 activation, yet it has been difficult to capture a snapshot of this dynamic process through structural biology. We have obtained evidence here supporting the conclusion that complementary interactions (a strong Pγ C-terminal interaction with αtGTP and a tight binding of the central region to Pαβ) occur in favor of PDE6 activation and at least partly account for the binding force in the intermediate αt·PDE6 complex.
It is known that the Pγ central region and the C-terminal domain are the two primary binding sites not only for αtGTPγS but also for Pαβ (8, 10, 16–18, 29, 37, 38), but it is not yet clear how the two Pγ domains differentiate their interactions with αt and Pαβ (9) when both targets are involved during PDE6 activation.
The observation of complementary Pγ domain interactions with αtGTPγS and Pαβ was based on comparison of the interaction profiles with the two targets, both obtained from systematic mapping of the entire interaction interfaces. A stronger αtGTPγS interaction with the Pγ C-terminal domain than with the Pγ central region (Figs. 1B and 2B) was in interesting contrast to a stronger Pαβ interaction with the Pγ central region than with the Pγ C-terminal domain (28), as also indicated by the pull-down experiments (Fig. 3). More significantly, this complementary feature was also observed when both of the targets were present competing for Pγ interaction (Fig. 4).
The complementary Pγ interactions with its two targets may have important implications for the molecular mechanism of PDE6 activation. The significance is 2-fold. First, because the αt-interacting C-terminal domain (Thr62–Ile87) includes the PDE6-inhibiting region, a strong Pγ C-terminal interaction with αtGTP is essential to compete with the Pγ-Pαβ interaction in order to displace the Pγ C terminus from the Pαβ catalytic site (5, 8, 20). Accordingly, a weak interaction between the Pαβ catalytic domain and the Pγ C-terminal domain should therefore provide αtGTP a stronger competitive edge over Pαβ, ensuring an efficient PDE6 activation. Moreover, tight binding of the Pγ C-terminal domain to αtGTP helps keep Pγ from reinhibiting PDE6 before the visual signal is adequately amplified (20).
Second, a greater binding strength of Pαβ for the Pγ central region keeps it sequestered by Pαβ. Because it is the complex formed by αtGTP and the full-length Pγ that can be readily recognized by RGS9-1·Gβ5 to maximally fulfill the GAP function (32), sequestration of the Pγ N-terminal half by Pαβ may prevent visual signaling from being terminated too early. An extremely high rod visual sensitivity is thus achieved not only because of an exceptionally high efficiency of the activated PDE6 but also for its extended lifetime (1).
Furthermore, the complementarity of the Pγ-αt interaction and the Pγ-Pαβ interaction also reveals some insights with regard to the molecular topology of the αt·PDE6 complex. αtGTPγS was co-immunoprecipitated by holo-PDE6, and Pαβ was co-immunoprecipitated by αtGTPγS, all in a Pγ-dependent fashion (Fig. 5). This indicates a molecular organization such that the C-terminal domain of Pγ binds tightly with αtGTPγS while the central region forms a strong interaction with Pαβ, thus “gluing” αtGTPγS and Pαβ into the PDE6 activation complex, or “transducisome.” This proposed organization is also supported by the data from co-pull-down of αtGTPγS and Pαβ by Btn-Pγ (Fig. 6). Thus, the complementary Pγ interactions may explain a long held puzzle; although the Pαβ-binding regions and αt-binding regions overlap on Pγ (1, 9), an intermediate transducin·PDE6 complex could still occur during visual transduction (22–24). Our data do not exclude the possibility that in the PDE6 activation complex, the Pγ central region may also be involved in binding with αtGTP, albeit probably through weak interactions. A mutagenesis study showed that Lys41, Lys44, and Lys45 on the C-terminal side of the Pγ polycationic region were involved in the interaction with αt but not with Pαβ (39), raising the possibility of simultaneous non-competitive αtGTPγS and Pαβ binding to the Pγ central region.
Early studies using rod disc membranes suggested that direct αt-Pαβ interaction accounted for an important binding force in the αt·PDE6 complex (24, 27). In the current study, however, αtGTPγS and Pαβ were not co-immunoprecipitated with each other in the absence of Pγ (Fig. 5). Moreover, when either αtGTPγS or Pαβ was bound to Btn-Pγ(46–87) or HisPγ(1–61) on affinity beads, the other was not co-pulled down (Fig. 3). These results indicate a lack of direct αt-Pαβ interaction in the αt·PDE6 complex under our experimental conditions. Because no disc membranes were involved in our experiments, we suggest that the disc membranes used in the early studies may have played a role in organizing the proteins in such a way that αt and Pαβ make direct contacts that further tighten the αt·PDE6 complex. In support of this proposition, increasing evidence indicates that disc membranes enhance protein functions in phototransduction (40–42). Nevertheless, our data indicate that the complementary Pγ interactions with its two targets, at least in part, account for the binding force in the αt·PDE6 complex.
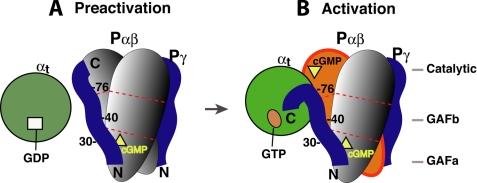
Based on the data presented herein and evidence from previous studies, a possible scenario of protein-protein interactions during PDE6 activation is depicted in Fig. 7. GTP-bound αt may initially engage PDE6 by making contacts with the Pγ residues Trp70 and Leu76, which are not involved in intimate interactions with the Pαβ catalytic site (5, 8, 11), and may also interact via part of the polycationic region (19, 39) (Figs. 1B and 2B). These initial αt contacts with Pγ may trigger a conformational change that results in a rigid body movement of the Pγ C terminus away from the Pαβ catalytic site with Leu76 serving as a “hinge.” The Pγ C-terminal domain can now bind tightly with αt, and PDE6 is deinhibited (5). At this stage, an intermediate complex containing αtGTP·Pγ·Pαβ, or transducisome, probably exists due to the complementary binding of Pγ to αtGTP and Pαβ (Figs. 4–6), with the Pγ C-terminal domain tightly bound to αtGTP and the central region bound to the Pαβ GAF domain with high affinity. The Pγ central region could stay bound to the Pαβ GAF domain until the binding is weakened by lowered cGMP levels, through a mechanism of positive cooperativity of Pγ and non-catalytic cGMP in binding to the GAF domain (1, 14, 26, 43, 44).
FIGURE 7.
Schematic diagram of the complementary Pγ interactions with its two targets during PDE6 activation. The molecules involved in PDE6 activation are represented by different shapes. Egg, Pα and Pβ; circle, αt; ribbon, Pγ; square, GDP; oval, GTP; triangle, cGMP. For simplicity, the three domains of Pα and Pβ are shown as segments separated by the dotted red lines. Our previous studies revealed that the Pγ Phe30 region preferred binding to Pα, whereas the Ser40 region favored binding to Pβ, suggesting simultaneous Pγ interactions with Pα and Pβ (16, 28). Because PDE6 can only be efficiently activated 50% by transducin (26, 46), it is highly likely that only one Pγ is displaced by αtGTP during PDE6 activation in the mammalian retina, whereas the other Pγ (on the opposite side) stays tightly bound to Pαβ (1). A movement of the Pγ(78–87) segment from Pαβ to αtGTP was suggested by Barren et al. (5), based on the crystal structure of the Pγ(70–87)·PDE5/6 catalytic domain complex.
Thus, the duration of the αtGTP·Pγ·Pαβ transducisome is probably subject to regulation by the cGMP occupancy in the Pαβ GAF domain. However, the activity of the GAP complex may have a more significant effect on the lifetime of the transducisome, because GTP hydrolysis in αt accelerated by RGS9-1 is the rate-limiting step of the rod photoresponse (45). Interesting questions hereby arise as to how Pγ dynamically and differentially interacts with RGS9-1/Gβ5, αt, and Pαβ upon a transition from its role in the PDE6 activation complex to that in the GAP complex and how the cGMP binding in the GAF domain regulates this process. Because disruption of Pγ interactions with its partners in phototransduction causes impaired visual functions (9), systematic investigations of these interactions will advance our understanding of the molecular mechanisms of the related retinal diseases.
Acknowledgments
We thank K. A. Martemyanov and V. Y. Arshavsky for functional assays of the Pγ photoprobes and H. Muradov and N. O. Artemyev for the PDE6 preparations. We also thank M. Arbabian for help in radiosynthesis and transducin preparations and M. M. Vestling for assistance in mass spectrometry.
This work was supported, in whole or in part, by National Institutes of Health Grant GM33138 (to A. E. R. and L.-W. G.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Fig. S1.
- cGMP
- cyclic GMP
- αt
- transducin α-subunit
- Pαβ
- PDE6 catalytic heterodimer
- GAF
- a domain derived from cGMP phosphodiesterases, adenylyl cyclases, and the Escherichia coli protein Fh1A
- Pγ
- PDE6 inhibitory subunit
- GAP
- GTPase-activating protein
- ACTP
- N-[3-iodo-4-azidophenylpropioamido-S-(2-thiopyridyl)]cysteine
- mBP
- 4-(N-maleimido)benzophenone
- HPLC
- high performance liquid chromatography
- ROS
- rod outer segment
- DTT
- dithiothreitol
- BSA
- bovine serum albumin
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate.
REFERENCES
- 1.Cote R. H. (2006) Photoreceptor Phosphodiesterase (PDE6): A G-protein-activated PDE Regulating Visual Excitation in Rod and Cone Photoreceptor Cells, pp. 165–193, CRC Press, Inc., Boca Raton, FL [Google Scholar]
- 2.Oldham W. M., Hamm H. E. (2008) Nat. Rev. Mol. Cell Biol. 9, 60–71 [DOI] [PubMed] [Google Scholar]
- 3.Burns M. E., Arshavsky V. Y. (2005) Neuron 48, 387–401 [DOI] [PubMed] [Google Scholar]
- 4.Artemyev N. O., Arshavsky V. Y., Cote R. H. (1998) Methods 14, 93–104 [DOI] [PubMed] [Google Scholar]
- 5.Barren B., Gakhar L., Muradov H., Boyd K. K., Ramaswamy S., Artemyev N. O. (2009) EMBO J. 28, 3613–3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kameni Tcheudji J. F., Lebeau L., Virmaux N., Maftei C. G., Cote R. H., Lugnier C., Schultz P. (2001) J. Mol. Biol. 310, 781–791 [DOI] [PubMed] [Google Scholar]
- 7.Zhang X. J., Cahill K. B., Elfenbein A., Arshavsky V. Y., Cote R. H. (2008) J. Biol. Chem. 283, 29699–29705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song J., Guo L. W., Muradov H., Artemyev N. O., Ruoho A. E., Markley J. L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 1505–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo L. W., Ruoho A. E. (2008) Curr. Protein Pept. Sci. 9, 611–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skiba N. P., Artemyev N. O., Hamm H. E. (1995) J. Biol. Chem. 270, 13210–13215 [DOI] [PubMed] [Google Scholar]
- 11.Granovsky A. E., Artemyev N. O. (2001) Biochemistry 40, 13209–13215 [DOI] [PubMed] [Google Scholar]
- 12.Artemyev N. O., Natochin M., Busman M., Schey K. L., Hamm H. E. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 5407–5412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granovsky A. E., Natochin M., Artemyev N. O. (1997) J. Biol. Chem. 272, 11686–11689 [DOI] [PubMed] [Google Scholar]
- 14.Mou H., Cote R. H. (2001) J. Biol. Chem. 276, 27527–27534 [DOI] [PubMed] [Google Scholar]
- 15.Muradov K. G., Granovsky A. E., Schey K. L., Artemyev N. O. (2002) Biochemistry 41, 3884–3890 [DOI] [PubMed] [Google Scholar]
- 16.Guo L. W., Muradov H., Hajipour A. R., Sievert M. K., Artemyev N. O., Ruoho A. E. (2006) J. Biol. Chem. 281, 15412–15422 [DOI] [PubMed] [Google Scholar]
- 17.Artemyev N. O., Rarick H. M., Mills J. S., Skiba N. P., Hamm H. E. (1992) J. Biol. Chem. 267, 25067–25072 [PubMed] [Google Scholar]
- 18.Skiba N. P., Bae H., Hamm H. E. (1996) J. Biol. Chem. 271, 413–424 [DOI] [PubMed] [Google Scholar]
- 19.Granovsky A. E., McEntaffer R., Artemyev N. O. (1998) Cell Biochem. Biophys. 28, 115–133 [DOI] [PubMed] [Google Scholar]
- 20.Slep K. C., Kercher M. A., He W., Cowan C. W., Wensel T. G., Sigler P. B. (2001) Nature 409, 1071–1077 [DOI] [PubMed] [Google Scholar]
- 21.Cheever M. L., Snyder J. T., Gershburg S., Siderovski D. P., Harden T. K., Sondek J. (2008) Nat. Struct. Mol. Biol. 15, 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navon S. E., Fung B. K. (1988) J. Biol. Chem. 263, 489–496 [PubMed] [Google Scholar]
- 23.Catty P., Pfister C., Bruckert F., Deterre P. (1992) J. Biol. Chem. 267, 19489–19493 [PubMed] [Google Scholar]
- 24.Clerc A., Bennett N. (1992) J. Biol. Chem. 267, 6620–6627 [PubMed] [Google Scholar]
- 25.Tsang S. H., Woodruff M. L., Chen C. K., Yamashita C. Y., Cilluffo M. C., Rao A. L., Farber D. B., Fain G. L. (2006) J. Neurosci. 26, 4472–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norton A. W., D'Amours M. R., Grazio H. J., Hebert T. L., Cote R. H. (2000) J. Biol. Chem. 275, 38611–38619 [DOI] [PubMed] [Google Scholar]
- 27.Clerc A., Catty P., Bennett N. (1992) J. Biol. Chem. 267, 19948–19953 [PubMed] [Google Scholar]
- 28.Guo L. W., Grant J. E., Hajipour A. R., Muradov H., Arbabian M., Artemyev N. O., Ruoho A. E. (2005) J. Biol. Chem. 280, 12585–12592 [DOI] [PubMed] [Google Scholar]
- 29.Grant J. E., Guo L. W., Vestling M. M., Martemyanov K. A., Arshavsky V. Y., Ruoho A. E. (2006) J. Biol. Chem. 281, 6194–6202 [DOI] [PubMed] [Google Scholar]
- 30.Liu Y., Arshavsky V. Y., Ruoho A. E. (1996) J. Biol. Chem. 271, 26900–26907 [DOI] [PubMed] [Google Scholar]
- 31.Guo L. W., Assadi-Porter F. M., Grant J. E., Wu H., Markley J. L., Ruoho A. E. (2007) Protein Expr. Purif. 51, 187–197 [DOI] [PubMed] [Google Scholar]
- 32.Martemyanov K. A., Arshavsky V. Y. (2002) J. Biol. Chem. 277, 32843–32848 [DOI] [PubMed] [Google Scholar]
- 33.Baehr W., Devlin M. J., Applebury M. L. (1979) J. Biol. Chem. 254, 11669–11677 [PubMed] [Google Scholar]
- 34.Slepak V. Z., Artemyev N. O., Zhu Y., Dumke C. L., Sabacan L., Sondek J., Hamm H. E., Bownds M. D., Arshavsky V. Y. (1995) J. Biol. Chem. 270, 14319–14324 [DOI] [PubMed] [Google Scholar]
- 35.Tanaka Y., Bond M. R., Kohler J. J. (2008) Mol. Biosyst. 4, 473–480 [DOI] [PubMed] [Google Scholar]
- 36.Noel J. P., Hamm H. E., Sigler P. B. (1993) Nature 366, 654–663 [DOI] [PubMed] [Google Scholar]
- 37.Artemyev N. O., Hamm H. E. (1992) Biochem. J. 283, 273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Artemyev N. O., Mills J. S., Thornburg K. R., Knapp D. R., Schey K. L., Hamm H. E. (1993) J. Biol. Chem. 268, 23611–23615 [PubMed] [Google Scholar]
- 39.Brown R. L. (1992) Biochemistry 31, 5918–5925 [DOI] [PubMed] [Google Scholar]
- 40.Melia T. J., Malinski J. A., He F., Wensel T. G. (2000) J. Biol. Chem. 275, 3535–3542 [DOI] [PubMed] [Google Scholar]
- 41.Nair K. S., Balasubramanian N., Slepak V. Z. (2002) Curr. Biol. 12, 421–425 [DOI] [PubMed] [Google Scholar]
- 42.Kerov V., Rubin W. W., Natochin M., Melling N. A., Burns M. E., Artemyev N. O. (2007) J. Neurosci. 27, 10270–10277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arshavsky V. Y., Dumke C. L., Bownds M. D. (1992) J. Biol. Chem. 267, 24501–24507 [PubMed] [Google Scholar]
- 44.Cote R. H., Bownds M. D., Arshavsky V. Y. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 4845–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krispel C. M., Chen D., Melling N., Chen Y. J., Martemyanov K. A., Quillinan N., Arshavsky V. Y., Wensel T. G., Chen C. K., Burns M. E. (2006) Neuron 51, 409–416 [DOI] [PubMed] [Google Scholar]
- 46.Liu Y. T., Matte S. L., Corbin J. D., Francis S. H., Cote R. H. (2009) J. Biol. Chem. 284, 31541–31547 [DOI] [PMC free article] [PubMed] [Google Scholar]