Abstract
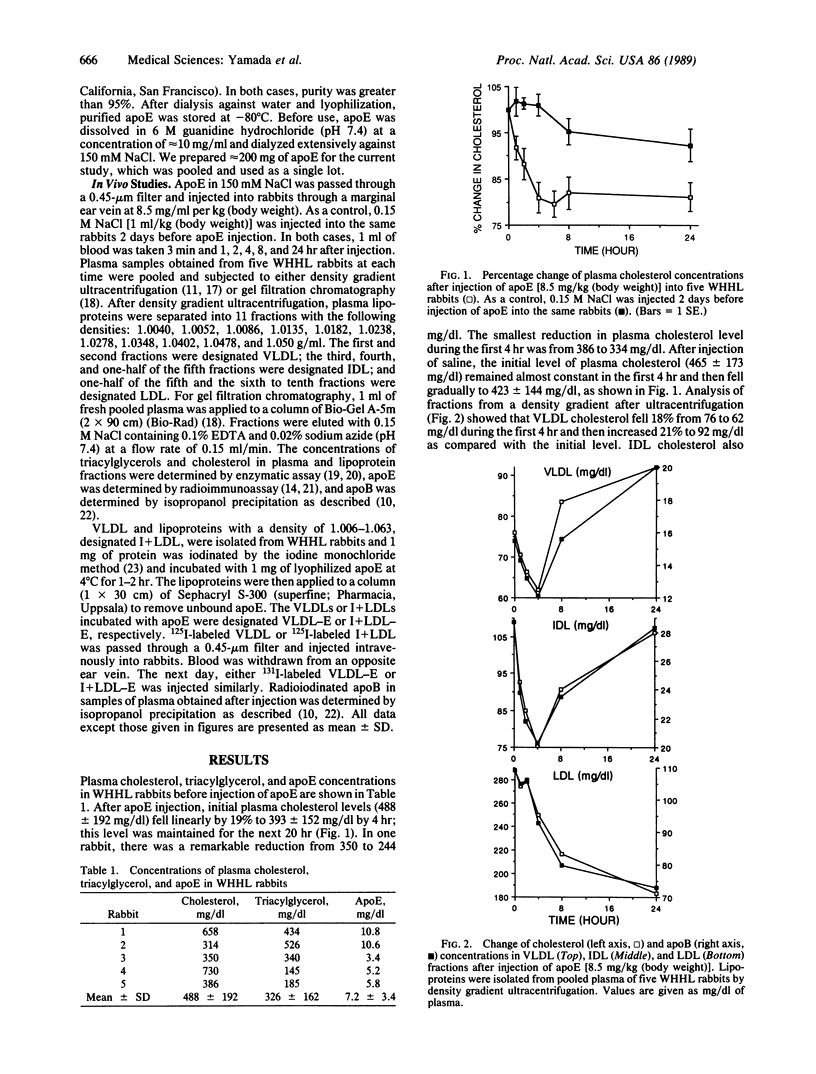
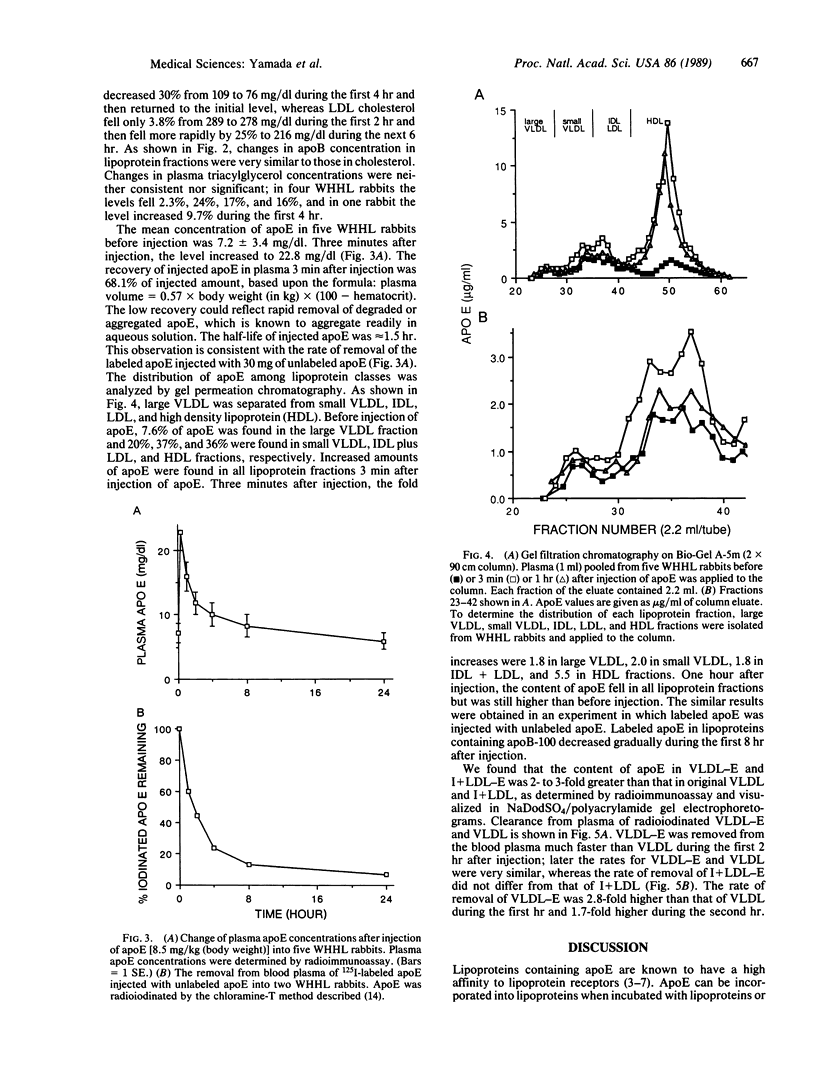
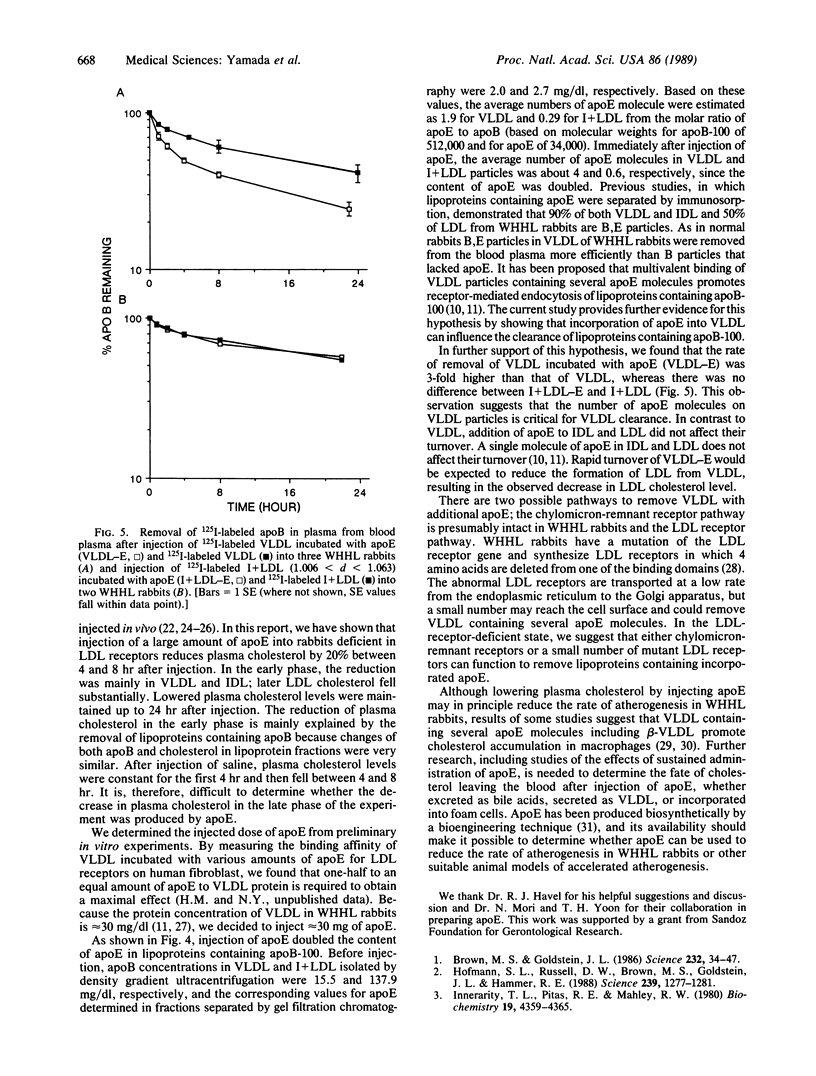
Apolipoprotein E (apoE) is known to play an important role in lipoprotein metabolism. We have studied the effect of apoE on the metabolism of plasma cholesterol by injecting apoE intravenously into rabbits deficient in low density lipoprotein receptors [Watanabe heritable hyperlipidemic (WHHL) rabbits]. Approximately 30 mg of apoE was injected per rabbit; a total of five WHHL rabbits were used. One hour later, plasma cholesterol levels fell 8.3% (from 488 +/- 192 to 446 +/- 174 mg/dl). After 3 hr, cholesterol levels had fallen by 19% (to 392 +/- 152 mg/dl). The reduced levels were maintained for at least 8 hr after injection of apoE. Cholesterol in very low density lipoproteins (VLDLs) and intermediate density lipoproteins fell rapidly during the first 2 hr after injection, followed by a reduction in the low density lipoprotein cholesterol level. Changes in apolipoprotein B levels in each lipoprotein fraction were very similar to those of cholesterol. Plasma apoE levels 3 min after injection were elevated 3-fold to 22.8 +/- 6.3 mg/dl and returned to initial levels 8 hr after injection. The rate of removal of intravenously injected 125I-labeled VLDL that had been incubated with apoE was 3-fold higher than that of unmodified VLDL. From these results, we conclude that the injected apoE is incorporated into VLDLs and that VLDL particles carrying more apoE are removed from the blood more rapidly, resulting in reduced formation of low density lipoprotein and lowered cholesterol levels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- Bates S. R., Coughlin B. A., Mazzone T., Borensztajn J., Getz G. S. Apoprotein E mediates the interaction of beta-VLDL with macrophages. J Lipid Res. 1987 Jul;28(7):787–797. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Bucolo G., David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973 May;19(5):476–482. [PubMed] [Google Scholar]
- Fainaru M., Havel R. J., Imaizumi K. Radioimmunoassay of arginine-rich apolipoprotein of rat serum. Biochim Biophys Acta. 1977 Jan 25;490(1):144–155. doi: 10.1016/0005-2795(77)90114-3. [DOI] [PubMed] [Google Scholar]
- Fielding P. E., Fielding C. J. An apo-E-free very low density lipoprotein enriched in phosphatidylethanolamine in human plasma. J Biol Chem. 1986 Apr 25;261(12):5233–5236. [PubMed] [Google Scholar]
- Gianturco S. H., Bradley W. A., Gotto A. M., Jr, Morrisett J. D., Peavy D. L. Hypertriglyceridemic very low density lipoproteins induce triglyceride synthesis and accumulation in mouse peritoneal macrophages. J Clin Invest. 1982 Jul;70(1):168–178. doi: 10.1172/JCI110590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Kita T., Kotite L., Kane J. P., Hamilton R. L., Goldstein J. L., Brown M. S. Concentration and composition of lipoproteins in blood plasma of the WHHL rabbit. An animal model of human familial hypercholesterolemia. Arteriosclerosis. 1982 Nov-Dec;2(6):467–474. doi: 10.1161/01.atv.2.6.467. [DOI] [PubMed] [Google Scholar]
- Hofmann S. L., Russell D. W., Brown M. S., Goldstein J. L., Hammer R. E. Overexpression of low density lipoprotein (LDL) receptor eliminates LDL from plasma in transgenic mice. Science. 1988 Mar 11;239(4845):1277–1281. doi: 10.1126/science.3344433. [DOI] [PubMed] [Google Scholar]
- Hui D. Y., Innerarity T. L., Mahley R. W. Lipoprotein binding to canine hepatic membranes. Metabolically distinct apo-E and apo-B,E receptors. J Biol Chem. 1981 Jun 10;256(11):5646–5655. [PubMed] [Google Scholar]
- Imaizumi K., Fainaru M., Havel R. J. Composition of proteins of mesenteric lymph chylomicrons in the rat and alterations produced upon exposure of chylomicrons to blood serum and serum proteins. J Lipid Res. 1978 Aug;19(6):712–722. [PubMed] [Google Scholar]
- Innerarity T. L., Arnold K. S., Weisgraber K. H., Mahley R. W. Apolipoprotein E is the determinant that mediates the receptor uptake of beta-very low density lipoproteins by mouse macrophages. Arteriosclerosis. 1986 Jan-Feb;6(1):114–122. doi: 10.1161/01.atv.6.1.114. [DOI] [PubMed] [Google Scholar]
- Innerarity T. L., Pitas R. E., Mahley R. W. Receptor binding of cholesterol-induced high-density lipoproteins containing predominantly apoprotein E to cultured fibroblasts with mutations at the low-density lipoprotein receptor locus. Biochemistry. 1980 Sep 2;19(18):4359–4365. doi: 10.1021/bi00559a032. [DOI] [PubMed] [Google Scholar]
- Kita T., Goldstein J. L., Brown M. S., Watanabe Y., Hornick C. A., Havel R. J. Hepatic uptake of chylomicron remnants in WHHL rabbits: a mechanism genetically distinct from the low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3623–3627. doi: 10.1073/pnas.79.11.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Hui D. Y., Innerarity T. L., Weisgraber K. H. Two independent lipoprotein receptors on hepatic membranes of dog, swine, and man. Apo-B,E and apo-E receptors. J Clin Invest. 1981 Nov;68(5):1197–1206. doi: 10.1172/JCI110365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Shelburne F. A., Quarfordt S. H. The interaction of heparin with an apoprotein of human very low density lipoprotein. J Clin Invest. 1977 Oct;60(4):944–950. doi: 10.1172/JCI108849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M. M., Krauss R. M., Lindgren F. T., Forte T. M. Heterogeneity of serum low density lipoproteins in normal human subjects. J Lipid Res. 1981 Feb;22(2):236–244. [PubMed] [Google Scholar]
- Soltys P. A., Gump H., Hennessy L., Mazzone T., Carey K. D., McGill H. C., Jr, Getz G. S., Bates S. R. Hepatic perfusate very low density lipoproteins obtained from fat-fed nonhuman primates stimulate cholesterol esterification in macrophages. J Lipid Res. 1988 Feb;29(2):191–201. [PubMed] [Google Scholar]
- Vogel T., Weisgraber K. H., Zeevi M. I., Ben-Artzi H., Levanon A. Z., Rall S. C., Jr, Innerarity T. L., Hui D. Y., Taylor J. M., Kanner D. Human apolipoprotein E expression in Escherichia coli: structural and functional identity of the bacterially produced protein with plasma apolipoprotein E. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8696–8700. doi: 10.1073/pnas.82.24.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Windler E. E., Kovanen P. T., Chao Y. S., Brown M. S., Havel R. J., Goldstein J. L. The estradiol-stimulated lipoprotein receptor of rat liver. A binding site that membrane mediates the uptake of rat lipoproteins containing apoproteins B and E. J Biol Chem. 1980 Nov 10;255(21):10464–10471. [PubMed] [Google Scholar]
- Windler E., Chao Y., Havel R. J. Regulation of the hepatic uptake of triglyceride-rich lipoproteins in the rat. Opposing effects of homologous apolipoprotein E and individual C apoproteins. J Biol Chem. 1980 Sep 10;255(17):8303–8307. [PubMed] [Google Scholar]
- Yamada N., Havel R. J. Measurement of apolipoprotein B radioactivity in whole blood plasma by precipitation with isopropanol. J Lipid Res. 1986 Aug;27(8):910–912. [PubMed] [Google Scholar]
- Yamada N., Murase T., Akanuma Y., Itakura H., Kosaka K. Plasma apolipoprotein E levels in hypertriglyceridemia. Horm Metab Res. 1982 Jun;14(6):303–306. doi: 10.1055/s-2007-1019000. [DOI] [PubMed] [Google Scholar]
- Yamada N., Shames D. M., Havel R. J. Effect of low density lipoprotein receptor deficiency on the metabolism of apolipoprotein B-100 in blood plasma. Kinetic studies in normal and Watanabe heritable hyperlipidemic rabbits. J Clin Invest. 1987 Aug;80(2):507–515. doi: 10.1172/JCI113099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N., Shames D. M., Stoudemire J. B., Havel R. J. Metabolism of lipoproteins containing apolipoprotein B-100 in blood plasma of rabbits: heterogeneity related to the presence of apolipoprotein E. Proc Natl Acad Sci U S A. 1986 May;83(10):3479–3483. doi: 10.1073/pnas.83.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Bishop R. W., Brown M. S., Goldstein J. L., Russell D. W. Deletion in cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science. 1986 Jun 6;232(4755):1230–1237. doi: 10.1126/science.3010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van't Hooft F., Havel R. J. Metabolism of chromatographically separated rat serum lipoproteins specifically labeled with 125I-apolipoprotein E. J Biol Chem. 1981 Apr 25;256(8):3963–3968. [PubMed] [Google Scholar]



