Abstract
Protein kinase A (PKA) is the main receptor for the universal cAMP second messenger. PKA is a tetramer with two catalytic (C) and two regulatory (R) subunits, each including two tandem cAMP binding domains, i.e. CBD-A and -B. Structural investigations of RIα have revealed that although CBD-A plays a pivotal role in the cAMP-dependent inhibition of C, the main function of CBD-B is to regulate the access of cAMP to site A. To further understand the mechanism underlying the cross-talk between CBD-A and -B, we report here the NMR investigation of a construct of R, RIα-(119–379), which unlike previous fragments characterized by NMR, spans in full both CBDs. Our NMR studies were also extended to two mutants, R209K and the corresponding R333K, which severely reduce the affinity of cAMP for CBD-A and -B, respectively. The comparative NMR analysis of wild-type RIα-(119–379) and of the two domain silencing mutations has led to the definition at an unprecedented level of detail of both intra- and interdomain allosteric networks, revealing several striking differences between the two CBDs. First, the two domains, although homologous in sequence and structure, exhibit remarkably different responses to the R/K mutations especially at the β2-3 allosteric “hot spot.” Second, although the two CBDs are reciprocally coupled at the level of local unfolding of the hinge, the A-to-B and B-to-A pathways are dramatically asymmetrical at the level of global unfolding. Such an asymmetric interdomain cross-talk ensures efficiency and robustness in both the activation and de-activation of PKA.
Keywords: Allosteric Regulation, Cooperativity, Cyclic AMP (cAMP), NMR, Protein Kinase A (PKA), CBD, CNB
Introduction
Cyclic adenosine monophosphate (cAMP) is an essential and ubiquitous second messenger that relays extracellular signals and translates them into tightly regulated intracellular responses (1). In eukaryotes, one of the main cAMP receptors is protein kinase A (PKA),4 which catalyzes Ser/Thr phosphorylation in downstream protein substrates that in turn control a wide range of cellular processes including metabolism and cell death (2). PKA exists in two forms: an inactive tetrameric holoenzyme and an active free catalytic subunit (C). In the holoenzyme, two C molecules form a complex with a dimeric regulatory subunit (R2). Upon binding to cAMP synthesized in response to external signals, each R-subunit undergoes a conformational change, releasing the C-subunit in its active state (1). The cAMP-dependent activation of PKA is reversible, and when phosphodiesterases deplete cAMP levels, the R-subunits revert to their dormant C-bound state in which the kinase function is inhibited (3). The reversibility in the activation of PKA represents a critical aspect of the regulation of its function, as it is required for the cell to revert to resting conditions after cAMP levels have been temporarily raised by transient external stimuli (4).
The R-subunit of PKA is a multidomain and highly dynamic protein. All known R-subunit isoforms share a common organization with an N-terminal dimerization/docking domain followed by a flexible linker region that includes the autoinhibitory segment and is C-terminally connected to two tandem CBDs (CBD-A and B, Fig. 1a) (5). Whereas the dimerization/docking domain mediates the subcellular localization of PKA via protein kinase A-anchoring proteins (6), the interactions with the C-subunit and with cAMP involve the autoinhibitory region and CBDs A and B (7). Both CBDs share a typical α/β-subdomain architecture, with the β-barrels embedding the two cAMP binding sites and with the non-contiguous α-helical subdomains joined at the interface between the two domains (Fig. 1e) (8).
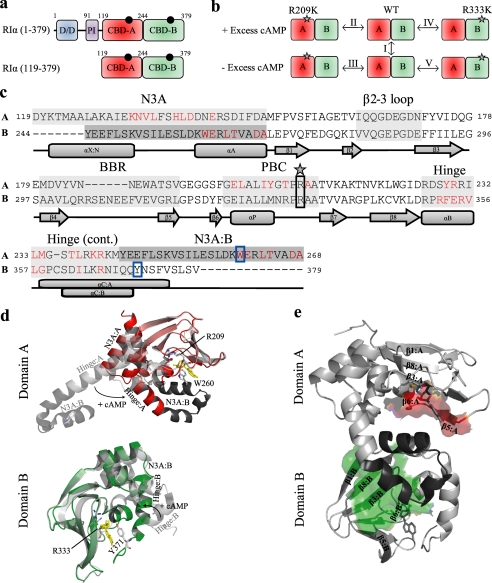
FIGURE 1.
Domain and structure overview of RIα. a, shown is a schematic representation of the domain organization of the full-length RIα-subunit of PKA as well as the RIα-(119–379) construct studied here by NMR. The black circles indicate molecules of cAMP bound to the two tandem C-terminal CBDs, which are shown in different shades of green. The dimerization/docking (D/D) domain is shown in blue, and the pseudoinhibitory (PI) site is shown in purple. Domain boundaries are indicated by residue numbers located above the respective domains. The domain boundary is defined by residue Tyr-244. b, shown is an overview of the H/D comparisons (I-V) between various states of the RIα-(119–379) construct for the wild-type (WT), R209K, and R333K mutants, which were all probed by H/D exchange in the presence and absence of 2 mm excess cAMP. The gray stars indicate the site of the mutation. c, shown is sequence alignment of the two CBDs of the RIα-(119–379) construct. The A and B labels to the left of the sequence refer to CBD-A and -B, respectively. Major allosteric hot spots are shaded with gray, which include the N3A motif, β2-3 loop, BBR, PBC, and hinge. The lid of domain A is contained within the N3A motif of B, whereas the lid of domain B is contained with the hinge of domain B. A darker shade of gray has been used to indicate that the region from Tyr-244 to Ala-268 belongs to domain B. Residues shown in red are those that contact the C-subunit, according to changes in solvent-accessible surface area for the PDB code 2QCS structure with and without the C-subunit present. The star and solid black box indicate the site of the R209K and R333K mutations. The solid blue box shows the position of the capping residues, Trp-260 and Tyr-371, for domains A and B, respectively. Secondary structure elements are shown below the sequence. d, shown is an overlay of the cAMP-bound (PDB code 1RGS) and C-subunit bound (PDB code 2QCS) crystal structures for domain A (top) and domain B (bottom). The cAMP-bound structure is shown in color, whereas the C-subunit bound structure is shown in gray. The N3A motif of domain B is depicted in the top panel as a darker shade of gray. The hinge and N3A motifs are labeled as well as are the conserved arginines (i.e. Arg-209 and Arg-333) and capping residues (i.e. Trp-260 and Tyr-371), which are shown as sticks and colored according to their atom types. Movement of the hinge upon binding of cAMP is shown with a black arrow, and molecules of cAMP are shown with yellow sticks. e, interdomain contacts between domains A and B are shown. The red- and green-colored surfaces show the contacts that the β-subdomain of CBD-A and CBD-B make with the other domain, respectively. The Tyr-244—Ala-268 region shaded in dark gray in panel c is shown in panel e with a dark gray ribbon. The domain interfaces were identified by comparing the solvent-accessible surface area values of domain deletion mutants to those generated for the wild-type protein (further details are available in supplemental Fig. S5 and its caption).
The structures of the R-subunit bound to either cAMP or the C-subunit (7–11) have revealed an ordered sequential mechanism of PKA activation whereby cAMP binds first to CBD-B, making site A accessible to a second molecule of cAMP, which in turn causes the release of the active C-subunit. In other words CBD-B functions as a gatekeeper for CBD-A, whereas the latter acts as the central controlling unit of the PKA system and provides the primary interfaces with the C-subunit (7–11). The structures of the R-subunit in its active and inhibited states have also demonstrated that although CBD-A and CBD-B play clearly distinct roles in the activation of PKA, they both share similar allosteric features (Fig. 1c). Specifically, in both domains cAMP docks into a conserved phosphate binding cassette (PBC) and a base binding region (BBR), causing a hinge rotation of the helical region C-terminal to the β-barrel (“hinge” or αB/C-helices) and a closure of the hinge region over the adenine base (Fig. 1d).
In all CBD domains there is a conserved element called the “lid” that interacts with the adenine ring, but the origin of this lid varies in the different CBD domains (1). In the CBD-B of RIα, the lid comes from the αC-helix of the same CBD (i.e.“cis-domain lid”), whereas for CBD-A of RIα the lid is provided by the B domain (i.e.“trans-domain lid”) and specifically by the N3A motif (N3A) of CBD-B (Fig. 1c). The N3A constitutes the fourth conserved motif in both CBDs, which is the helical element that lies N-terminal to the β-barrel and is composed of an N-terminal helix, a 310 loop, and the following αA helix (Fig. 1, c–e). The cAMP-dependent rearrangement of the helices C-terminal to the β-subdomain also leads to a concurrent partial re-positioning of the N3A motif that in the case of CDB-B is fused to the αC-helix of CBD-A but is still functionally a part of CBD-B (Fig. 1, c and d) (7). This disposition of two CBDs in tandem and fused directly to each other is a distinctive feature of the R-subunits of PKA. However, structural investigations of other CBDs have shown that the four elements outlined above (i.e. PBC, BBR, hinge, N3A, Fig. 1, c and d) are universally conserved among eukaryotic domains that function as cAMP sensors (12, 13).
The crystallographic studies on the structure of CBDs have recently been complemented by NMR analyses of constructs including a single CBD of either PKA or EPAC (exchange protein directly activated by cAMP), which have unveiled an additional conserved allosteric “hot spot” at the loop between strands β2 and β3 (14–18). Furthermore, the solution studies have also revealed that cAMP binding controls not only the local environment of the allosteric sites as sensed by chemical shift changes but also the hierarchical distribution of excited states that defines the free energy landscape of these domains, as probed through H/D exchange monitored at residue resolution by NMR. Specifically, it is now clear that cAMP modulates not only global unfolding of the CBDs but also local unfolding at α-subdomain sites well removed from the PBC where cAMP docks (15, 17). Whereas the definition of these long range perturbations represents a first step toward mapping intra-CBD signaling pathways and provides initial clues on how cAMP reshapes the free energy landscape of CBDs, the picture emerging from the NMR analysis of single CBD constructs is still somewhat limited by the lack of the adjacent domain. In particular, because of the absence of the “gatekeeper” CBD-B, the previous RIα construct used for NMR purposes (i.e. RIα-(119–244)) did not contain a full integral lid region (15, 18, 19) and did not provide any insight into the cross-talk between the two cAMP binding domains.
Here, we report the investigation by NMR of a two-domain construct of RIα, i.e. RIα-(119–379) (Fig. 1a). This construct not only includes the whole CBD-B, but it provides also an unprecedented representation of eukaryotic CBDs in general because, unlike previous NMR-based investigations (14, 15, 17–19), it contains the entire lid regions of both CBDs (Fig. 1, a and c). Furthermore, as a first step toward mapping the inter-CBD cross-talk, the NMR investigation of the wild-type two-domain construct RIα-(119–379) was also extended to two single mutants that target two highly conserved Arg residues in the PBCs, i.e. R209K in PBC-A and R333K in PBC-B (Fig. 1, c and d). The guanidiniums of Arg-209 and Arg-333 form salt bridges with the phosphate of cAMP and are an integral part of PBC-A and -B, respectively (8, 13) (Fig. 2, a–e). Indeed, these arginines are highly conserved not only in the PBCs of RIα (Fig. 1, b–d) but also in the majority of other known CBDs (20).
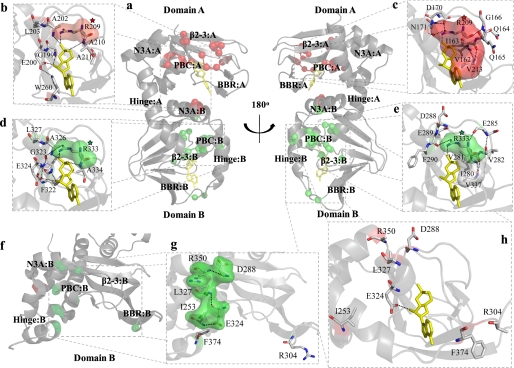
FIGURE 2.
Map of significant mutation-induced chemical shift changes onto the crystal structures of the R-subunit. a, shown are chemical shift changes (red spheres, R209K; green spheres, R333K) mapped onto the cAMP-bound state of the R-subunit. Chemical shift changes observed within the PBC of domains A (b) and B (d) as well as the β2-3 loop of domains A (c) and B (e) can largely be explained by the extensive hydrogen bond networks within the cAMP-bound structure. f, chemical shift changes (red spheres, R209K; green spheres, R333K) mapped onto domain B of the C-subunit-bound state of the R-subunit are shown. Several residues that appear unrelated in the cAMP-bound structure (g) form a series of interactions within the C-subunit-bound state (h). Surfaces are shown for selected residues to indicate hydrophobic contacts (panels b–e) or to show clustering of residues that form a hydrogen bond network in the C-subunit bound state (panel g). Surfaces for residues that show chemical shift changes in response to the R209K or R333K mutations are shown as red and green, respectively, whereas surfaces shown in white belong to residues that do not show a significant chemical shift change in response to either mutation. The red- and green- colored stars are shown to indicate the conserved Arg mutation in CBD-A or CBD-B, respectively. Molecules of cAMP are shown as yellow sticks.
The mutation of Arg-209 to Lys has been shown to reduce the affinity for cAMP at site A by about 3 orders of magnitude (i.e. KD from 30 nm to the ≥μm range) as well as at the non-mutated site B by about 1 order of magnitude (i.e. KD from 15 to 130 nm) (21). A similar reciprocal “domain silencing” effect has been reported also for R333K (21). The ability of the R209K and R333K mutations to decrease the cAMP affinity for both PBCs indicates that these point substitutions cause long range perturbations and represent useful tools to start dissecting the pathways that mediate the interdomain communication in RIα. Furthermore, despite the increased dissociation constants for cAMP, the inhibition of the C-subunit by either R209K or R333K RIα is still cAMP-dependent (21), indicating that both RIα mutants are functionally active. Overall, the three state (i.e. wild-type, R209K, and R333K RIα-(119–379)) comparative NMR analysis of chemical shifts and solvent protection factors for wild-type, R209K, and R333K RIα-(119–379) (Fig. 1b) has revealed several striking differences between domains A and B. Such differences pertain to intradomain as well as to interdomain cAMP signal propagation and provide new insight not only on the deactivation but also on the activation mechanism of PKA.
EXPERIMENTAL PROCEDURES
NMR Sample Preparation of RIα-(119–379)
The two-domain construct RIα-(119–379) of PKA was subcloned into the pRSET-B expression system (Invitrogen), and mutants were prepared by site-directed mutagenesis. The protein was transformed into Escherichia coli BL21(DE3) cells and overexpressed in M9 minimal medium. For 1H,15N HSQC and H/D exchange experiments, a single-labeled 15N sample with >98% 15N incorporation was used. For triple resonance experiments, triple-labeled 13C,2H,15N samples were prepared. Both 15N and 13C were incorporated at levels >98%, whereas 2H was present at levels of 50–98% at the Hα position of the protein after back exchange with purification buffers, depending on what percentage of 2H2O was used in the growth process. Selectively labeled samples of 15N-labeled Gly, Ala, Val, and Leu were also prepared for the wild type to assist in assignment. The Gly-, Ala-, and Val-selective samples were prepared by growing the cells in LB medium and adding 0.5 g of the 15N-labeled amino acid, predissolved in H2O, into the culture 1 h before induction by isopropyl 1-thio-β-d-galactopyranoside, as previously described (22). The [15N]Leu sample was prepared in M9 medium with the NH4Cl replaced by 100 mg each of the other unlabeled 19 amino acids and 200 mg of [15N]Leu. The cells were grown and induced under the same conditions as the other isotopically enriched M9 culture. The remaining portion of the sample preparations followed, to a large extent, previously published protocols and the details are available as supplemental material. During the last purification step, i.e. gel filtration, the protein was separated from the cAMP used during the prior elution steps, and the final product was a protein free of excess cAMP. The NMR samples were prepared by concentrating the protein to 0.5 mm, as determined by the Bradford assay (23), and adding 2H2O to a final concentration of 5% (v/v). To ensure the long term stability of the NMR samples, 0.4 mm 4-(2-aminoethyl)benzenesulfonyl fluoride and 1 mm Tris(2-carboxyethyl)phosphine were also added.
NMR Spectroscopy
All NMR spectra were recorded at a temperature of 306 K on a Bruker AV 700 spectrometer equipped with a TCI cryo-probe. In all experiments, the 1H and 15N carrier frequencies were set at the water resonance and at the center of the amide region, respectively. The 13C carrier frequency varied depending on the experiment. All one-dimensional data sets were processed and analyzed using Xwinnmr (Bruker Inc.), and all multidimensional data sets were processed using NMRPipe (24). Unless otherwise specified, a phase-shifted-squared sine bell window function was employed for both dimensions before zero filling. Processed spectra were analyzed using NMRDraw and Sparky 3.114 (25).
All of the two-dimensional HSQC spectra acquired were sensitivity- and gradient-enhanced and included water flip-back pulses. After 128 dummy scans, 8 scans were accumulated per t1 point using an inter scan delay of 1 s. Unless otherwise specified, the 15N dimension was digitized with 128 complex points for a spectral width of 31.8 ppm, and the 1H dimension was digitized with 1024 complex points for a spectral width of 15.939 ppm. All TROSY triple resonance three-dimensional experiments (i.e. HNCA, HN(CO)CA, HN(CO)CACB, HNCACB, HN(CA)CO, and HNCO) as well as the HSQC-NOESY-HSQC spectra were acquired on triple-labeled (>98% 15N, >98% 13C, and 50% 2H isotopic enrichment) protein samples with a concentration of 0.5 mm and with an inter-scan delay ranging from 1 to 2 s, depending on the sensitivity of the experiment. Chemical shift predictions were generated with ShiftX (26) using the RIα-(91–379) crystal structure (PBD code 1RGS) (8) with corrections to account for deuteration. Secondary structure profiles were calculated with the program PECAN using the NH, HN, Cα, Cβ, and C′ backbone assignments (27). The compounded changes in chemical shift when comparing the wild-type protein with the mutants were calculated with the equation Δδcompound = ((Δδ1H)2 + (Δδ15N/6.5)2)1/2, where Δδ 1H and Δδ 15N are the ppm differences in nitrogen and hydrogen amide chemical shifts, respectively, between the two states being compared.
H/D Exchange Experiments
The samples for the H/D exchange experiments were prepared by first concentrating 7.5 mg of protein to ∼2.5 mm (<100 μl final volume). The sample was prepared as previously described (28) by passing the sample through a Sephadex G10 column with a 3-ml bed volume pre-equilibrated with 98% 2H2O-based gel filtration buffer. A series of HSQC experiments was recorded with preset parameters for a sample with similar buffer and sample height to minimize the dead time (∼25 min) of the H/D exchange experiment. The fast initial decay was monitored through a series of 30 HSQC spectra acquired with four scans (∼10 min per HSQC). The remaining slower decay was monitored through a series of HSQC spectra acquired with eight scans. Data were acquired for a total of 20 h. The sample was kept at 33 °C, and HSQC spectra were acquired once per week to provide additional data points for slowly decaying peaks. For each construct (i.e. wild type and two mutants) H/D exchange data were acquired for both samples with no excess cAMP and 2 mm excess cAMP, resulting in a total of six H/D data sets (Fig. 1b).
The HSQC cross-peak heights were quantified by NMRPipe as the sum of the intensities in a 3 × 3 grid centered on the peak maximum (29). The rates of exchange were measured using the Levenberg-Marqardt nonlinear least-square exponential fitting implemented through the Curvefit software as previously described (15). The error on the intensities was based on the standard deviation of the spectral noise. The H/D decays were normalized relative to the intensities in the first HSQC spectrum acquired after exposure to 2H2O.
RESULTS
NMR Assignments for the RIα-(119–379)·cAMP2 Complex
As a first step toward a high resolution characterization of the tandem CBDs of the PKA R-subunit in solution, the backbone assignment of the cAMP-bound state of RIα-(119–379) was obtained using a combination of TROSY-based triple resonance experiments, HSQC-NOESY-HSQC spectra, selectively labeled amino acid samples (i.e. 15N-labeled Gly, Ala, Val, and Leu), and Cα and Cβ chemical shift calculations. Further details are available in the supplemental material. Overall, our integrated assignment strategy led to a total of 1268 assignments for the NH, HN, Cα, Cβ, and C′ resonances (i.e. ∼97% coverage of the backbone resonances) of the wild-type RIα-(119–379) construct in the presence of a 2 mm excess cAMP. Only two residues were not assigned, the first being Asp-119, which is the N-terminal residue and is typically broadened because of its rapid exchange with the solvent, and the second residue is His-138, which is broadened due to ms-μs internal dynamics of the N3A 310 loop to which it belongs. The NMR assignment of RIα-(119–379)·cAMP2 provides the foundation to further investigate this construct in solution at residue and atomic resolution.
High Resolution Picture of the Native Ensemble Accessible to the RIα-(119–379)·cAMP2 Complex
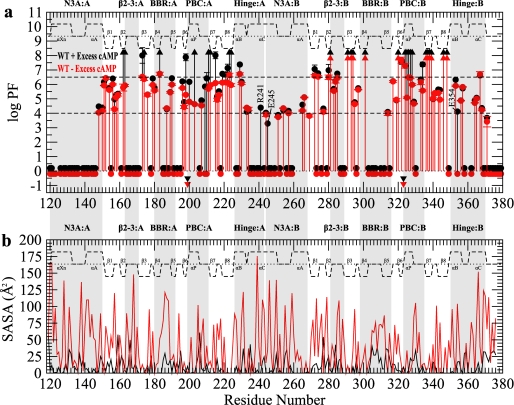
The ensemble of locally and globally unfolded states accessible to the RIα-(119–379)·cAMP2 complex was probed at high resolution by NMR-monitored H/D exchange experiments, and the resulting residue-specific protection factors (PFs) are reported in Fig. 3a (black symbols). Based on the magnitude of the PF values shown in Fig. 3a, the residues of each CBD can be subdivided into three major classes. Class I consists of residues that are highly protected (PF > 6.5) and rely mainly on transient global unfolding H/D exchange pathways, i.e. the solvent protection of class I residues is not modulated by local unfolding. Class II encompasses residues with intermediate PF values (4.0 < PF ≤ 6.5). These sites are generally protected from the solvent, but unlike class I residues, they do not strictly require global unfolding to be exchanged with deuterium. Class III includes those amides that exchange rapidly (PF ≤ 4.0), i.e. they are either unprotected from the solvent in the ground state structure or are protected but promptly exposed to the solvent through transient local unfolding events. The partitioning across these three broad H/D exchange classes of the key structural and functional elements of RIα-(119–379) provides an initial basic framework to start comparing the two CBDs of the regulatory subunit of PKA Iα.
FIGURE 3.
H/D exchange profiles for wild-type RIα-(119–379). a, shown is a PF plot for wild-type RIα-(119–379) in the presence (black) or absence (red) of 2 mm excess cAMP. Circles appearing at PF values >1 indicate residues with a quantifiable PF, whereas upwards triangles at PF ∼8 indicate lower limit PF values for residues that did not exchange for the duration of the experiment. Circles located around a PF of 0 indicate residues that completely exchanged within the dead-time of the experiments. Inverted triangles indicate residues that were too broad in the HSQC of the 15N-labeled sample to be used as a probe of H/D exchange. The secondary structure is shown as dashed lines above the protection factors with the α-helices and β-sheets labeled. Major allosteric hot-spots are shaded in gray. WT, wild type. b, shown are calculated solvent-accessible surface area (SASA) values for the side-chain (red) and backbone (black) atoms using the cAMP-bound form of the R-subunit.
For both CBD-A and CBD-B of the RIα-(119–379)·cAMP2 complex, class I residues are largely confined to the β-subdomain (Fig. 3a), with the main exception of several residues in the αP helices of both PBCs that are slowly exchanging and display class I protection factors as well. Unlike the PBC sites, almost all residues in the other key functional sites (e.g. the BBR, the N3A motif, and the hinge regions) belong to the intermediate or fast exchange classes (i.e. classes II or III) (Fig. 3a). Whereas this observation applies to both domains, the partitioning of specific loci to class II versus class III is highly domain-specific. For instance, a striking difference between the two domains is the degree of protection observed for the N3A motif and the BBR in each CBD. In domain A, the BBR has several class II residues, and the N3A motif is mostly composed of class III residues (Fig. 3a). On the other hand, a reversed H/D exchange pattern is observed for domain B, with its BBR being highly solvent-exposed (i.e. most BBR-B residues belong to class III) and its N3A motif exhibiting some degree of protection (i.e. several N3A-B residues fall in class II) (Fig. 3a). The variability in the N3A and BBR H/D exchange patterns, therefore, defines a marked difference between CBD-A and -B (Fig. 3a). Further differences between the two domains are revealed by investigating how the H/D protection factors depend on cAMP.
cAMP-dependent Variations in Solvent Protection for RIα-(119–379)
Because of the instability of the aggregation-prone apo state of RIα-(119–379) (28), we investigated the cAMP dependence of its H/D PF values through a previously described equilibrium perturbation approach (28). This method effectively circumvents experimental challenges caused by aggregation and/or precipitation of the apo RIα-subunit by requiring only the creation of minor populations of apo state in dynamic equilibrium with the more stable cAMP-bound form (28). For this purpose it is sufficient to measure H/D exchange rates of RIα-(119–379) in the absence of excess cAMP and compare them to those observed in the presence of excess cAMP (i.e. comparison I in Fig. 1b). The comparative analysis of the H/D exchange profiles in the presence and absence of excess cAMP (Fig. 3a, black and red symbols) reveals that the major decreases in PF values occur for the two PBCs, confirming the creation of the desired minor apo states for both cAMP binding sites. For either CBD, the populations of these apo states are too low to cause detectable chemical shift changes between the excess and no excess samples (supplemental Fig. S2). However, Fig. 3a shows that the loss of protection observed for PBC-A upon removal of excess ligand is on average larger than that detected for PBC-B, indicating a higher proportion of apo state present in domain A than in domain B, i.e. a lower occupancy of domain A by cAMP relative to domain B in the absence of excess cAMP. This result is consistent with earlier reports of a larger dissociation constant of cAMP from site A relative to site B, reflecting an off-rate 1–2 orders of magnitude faster for the former binding pocket (30). The different cAMP occupancies of the A and B sites also explain why a dramatic drop of class I protection factors to class II is observed for domain A but not for domain B in the absence of excess cAMP (Fig. 3a).
Despite the difference in the A versus B cAMP occupancies for the sample without excess cAMP, it is notable that the hinge-helices of both domains display dramatic losses in protection for selected residues (Fig. 3a). For example, Arg-241 in the hinge of CBD-A and Glu-354 in the hinge of CBD-B both show a remarkable class II to III decrease in protection as the concentration of free cAMP decreases (Fig. 3a). These observations are consistent with the release of cAMP from each binding site causing the expected (31) hinge-based rotation of the lid moiety away from the rest of the CBD (Fig. 1d). However, the data in Fig. 3a are not sufficient on their own to dissect the domain-specific contributions to the cAMP-dependent changes or to map the interdomain communication pathways because the comparison of the wild-type RIα-(119–379) with and without excess cAMP (comparison I in Fig. 1b) affects both domains. For this purpose it is necessary to further extend our investigation to domain-specific perturbations as outlined in the experimental design scheme of Fig. 1b, which is largely based on the R209K mutation in CBD-A and on the corresponding R333K substitution in CBD-B (Fig. 1b).
Mapping the Effects of the R209K Mutation on RIα-(119–379) by Chemical Shifts
As a first step toward mapping the inter-CBD cross-talk in RIα-(119–379), domain A was selectively perturbed by mutating Arg-209 to lysine. Details about the NMR assignment of the R209K mutants are available as supplemental material. Whereas the analysis of the secondary chemical shifts measured in the presence of 2 mm excess cAMP (supplemental Fig. S1, a and b) did not reveal any significant differences between the overall structures of the wild-type and of the R209K RIα-(119–379)·cAMP2 complexes, the map of the mutation-induced NH chemical shift changes (Fig. 4, a and c, and supplemental Fig. S3a) indicates that several local perturbations still occur as a result of the mutation.
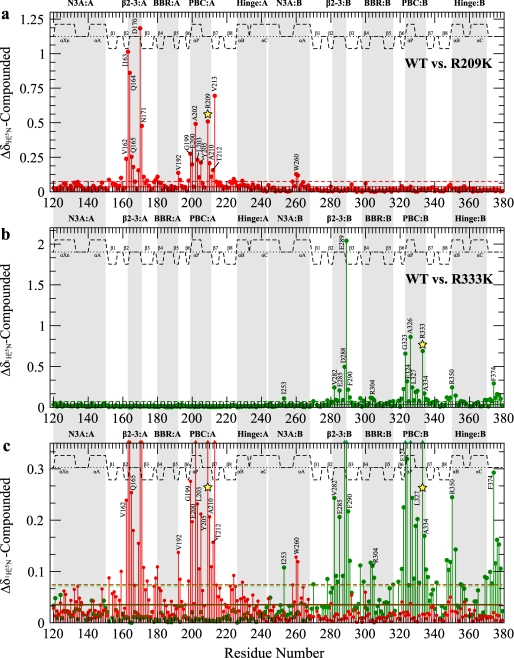
FIGURE 4.
Compounded amide chemical shift differences between wild-type (WT) RIα-(119–379)·cAMP2 and the R209K (a) and R333K (b) mutants, all in the presence of 2 mm excess cAMP, are shown. The average chemical shift (solid line) as well as 1 S.D. above the average (dashed line), are shown with red (R209K) or green (R333K) horizontal lines. c, overlap of plots in panels a and b vertically re-scaled to facilitate the comparison of smaller chemical shift changes induced by R209K (red) and R333K (green). Colored triangles at the top of the panel indicate data that is off-scale. Major allosteric hot spots are shaded in gray, and the site of the mutation is indicated by a yellow star. The secondary structure is shown as dashed lines above the data with the α-helices and β-sheets labeled.
The largest overall chemical shift changes are not observed for the mutated residue, but rather, they occur for the residues that directly interact with the Arg-209 side-chain, i.e. Asp-170, which forms a salt bridge with the guanidinium of Arg-209, and Ile-163, which contacts the methylenes of Arg-209 (Figs. 4a and 2, a–c). Both Ile-163 and Asp-170 are part of the β2-3 loop, and their dramatic chemical shift variations (Fig. 4a) demonstrate the tight coupling of this allosteric site to the PBC. Interestingly, the mutant-induced perturbation of Ile-163 propagates even beyond the β2-3 loop to Val-213 in β7, which is connected to Ile-163 by a double backbone-to-backbone hydrogen bond (Figs. 4a and 2c).
The R209K mutation-dependent chemical shifts also reveal a second group of residues with reduced but still highly significant ppm variations (Fig. 4, a and c). These include not only the site of the mutation but also several other residues involved in direct interactions with cAMP bound to domain A. For instance, some of the largest chemical shift changes occurring within PBC-A are for Gly-199, Ala-202, and Ala-210, which donate a hydrogen bond to cAMP through their amides (Figs. 4, a and c, and 2, a and b). Overall, several of these PBC-A ppm variations linked to the R209K substitution as well as those reported for the β2-3 loop region of domain A (Fig. 4a) are similar to the cAMP-dependent chemical shift changes previously reported for the RIα-(119–244) construct (15), suggesting that the R209K mutation at least partially mimics the release of cAMP from domain A. Furthermore, some additional minor ppm changes are also found for other regions known to be involved in cAMP binding, such as the BBR (e.g. Val-192 in Fig. 4, a and c) and N3A-B, which contains the lid of CBD-A (e.g. W260 in Figs. 4, a and c, and 2b), confirming that R209K perturbs the mode of cAMP binding to domain A. Apart from changes in the N3A motif, no other significant chemical shift changes are observed for domain B in Fig. 4a, indicating that the previously reported long range effects of the R209K mutation (21) across the interdomain boundary pertain mostly to minimally populated excited states that are not sensed by chemical shift changes (Fig. 4a) but are better probed by variations in H/D exchange protection factors.
Mapping the Effects of the R209K Mutation on RIα-(119–379) by H/D Exchange
The PFs measured for R209K RIα-(119–379) in the presence of a 2 mm excess cAMP are reported in Fig. 5a (red symbols). For comparative purposes, Fig. 5a also includes the PF values of the wild-type construct measured under the same experimental conditions (Fig. 5a, black symbols). This comparison (II, Fig. 1b) reveals that the most dramatic R209K-induced changes are for the PBC of domain A and the adjacent β6,7 strands with several residues displaying a remarkable loss in protection from the slowly exchanging class I to the fast exchanging class III (Fig. 5a). Decreased protection factors are also observed for the domain A region that interacts with the adenine base (BBR-A, Fig. 5a). The increased solvent exposure observed for the PBC-A and BBR-A of the mutant relative to the wild type confirms that the R209K mutation significantly perturbs the mode of cAMP binding to domain A.
FIGURE 5.
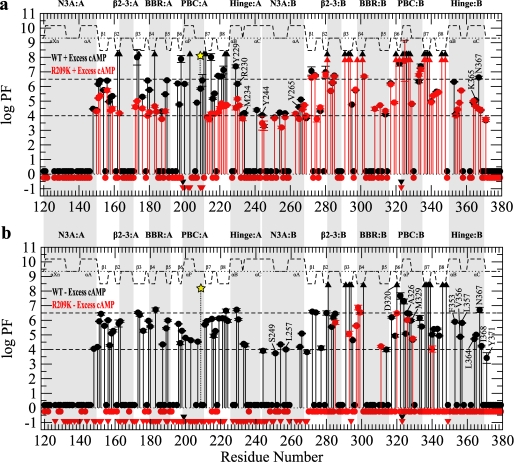
H/D exchange profiles for RIα-(119–379) with domain A silenced by the R209K mutation. a, shown is a PF plot for the wild-type RIα-(119–379) (black) and R209K (red) in the presence of 2 mm excess cAMP. b, shown is a PF plot for the wild-type (black) and R209K (red) in the absence of excess cAMP. All symbols and labels are the same as in Fig. 3.
The other PF variations caused by R209K in domain A (Fig. 5a) are somewhat comparable with those caused by the partial removal of cAMP from site A (Fig. 3a). Specifically, most of the highly protected residues of the wild-type domain A that belong to class I drop to class II in the R209K mutant, resulting in a significantly more homogeneous distribution of PF values across CBD-A that is now devoid of any clear hierarchical pattern (Fig. 5a). In addition, a mutation-induced increase in exposure was observed also for the hinge helices of domain A (e.g. Tyr-229, Arg-230, and Met-234 in Fig. 5a). These changes are consistent with the R209K mutant promoting the stabilization of a C-subunit-bound-like state of domain A (Fig. 1d), and a similar equilibrium shift toward a C-subunit-bound-like state was observed for the wild-type construct upon reducing the occupancy of cAMP in site A (Fig. 3a), thus suggesting that R209K mimics at least partially the selective release of cAMP from domain A.
Unlike CBD-A, the detectable R209K-induced changes in solvent protection for CBD-B are confined to its hinge region, which becomes more exposed in the mutant (Fig. 5a). For instance, the PF of Asn-367 drops by about 2 orders of magnitude when the distal Arg-209 is mutated to Lys (Fig. 5a). Interestingly, no significant PF variations were observed for PBC-B (Fig. 5a), indicating that the decreased solvent protection observed for the hinge-B reflects a genuine long range effect caused by the R209K mutation in PBC-A. This interdomain coupling does not result in detectable PF changes for the class I residues of domain B based on the data in Fig. 5a, which were acquired in the presence of 2 mm excess cAMP. However, it should be noted that, under these conditions of high cAMP concentrations, only PF lower limits, as opposed to exact PF values, can be measured for the domain B class I residues (Fig. 5a). As a result, it cannot be ruled out that the R209K mutation affects the class I PF values of domain B, but these changes escape detection due to their slow H/D exchange timescale. It is, therefore, critical to reassess the effect of the R209K mutation on the H/D exchange profiles using a sample without excess cAMP, for which the highest PF values are likely to be reduced.
The H/D protection factors measured for R209K RIα-(119–379) in the absence of excess cAMP are shown in Fig. 5b (red symbols). Fig. 5b also includes, as a term of comparison, the PF values measured for wild-type RIα-(119–379) under the same experimental conditions (black symbols). The wild-type versus R209K comparison in the absence of excess cAMP (comparison III, Fig. 1b) reveals a dramatic mutation-induced global loss of protection not only for domain A but also for domain B (Fig. 5b). Specifically, the R209K mutant is devoid of any class I residues (Fig. 5b, red symbols) with the majority of detectable residues in both domains falling within the fast exchanging class III. For CBD-B of the R209K mutant, a limited number of residues display an intermediate exchange pattern (class II), such as Ala-326 and Met-329 in PBC-B (Fig. 5b, red symbols). Although this residual degree of PBC-B protection in R209K is comparable with the protection of PBC-B measured for R333K under the same experimental conditions (see below and supplemental Fig. S4, red symbols), the overall protection for the rest of the CBD-B is lower in R209K than in R333K (supplemental Fig. S4, red symbols). This observation is most apparent for the region C-terminal to PBC-B, including the β7, β8, and the hinge (Fig. 5b and supplemental Fig. S4, red symbols) that are almost completely in the fast H/D exchange regime (class III) in R209K (Fig. 5b). These observations indicate that the remarkable increase in solvent exposure of CBD-B observed for R209K relative to the wild-type construct (Fig. 5b) is to a large extent due to the mutation-induced perturbations in PBC-A rather than to the disruption of cAMP binding at PBC-B. In other words, the data of Fig. 5b support the notion that domain A controls domain B both at the level of local and global unfolding, even in the absence of the C-subunit. The Arg-209 site in domain A, therefore, emerges as a critical nucleation point for the network of interactions of both CBD-A and -B. To evaluate whether the corresponding residue in CBD-B (i.e. Arg-333) plays a similar role, we have extended our comparative chemical shift and H/D analyses to the R333K RIα-(119–379) mutant (Fig. 4, b and c, and Fig. 6). The R333K mutation also serves as a control for R209K in the dissection of the domain-specific responses to cAMP binding.
FIGURE 6.
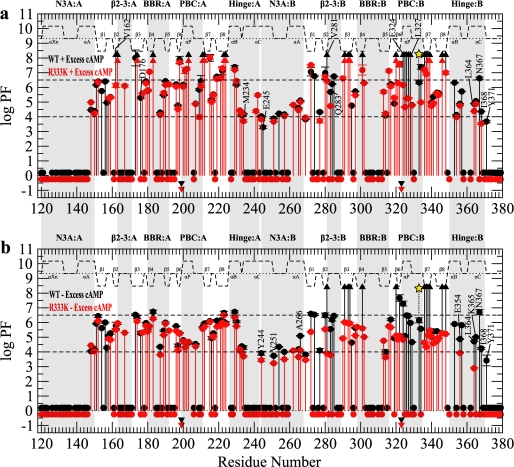
H/D Exchange profiles for RIα-(119–379) with domain B silenced by the R333K mutation. a, shown is a PF plot for the wild-type RIα-(119–379) (black) and R333K (red) in the presence of 2 mm excess cAMP. b, shown is a PF plot for the wild-type (black) and R333K (red) in the absence of excess cAMP. All symbols and labels are the same as in Fig. 3.
Mapping the Effects of the R333K Mutation on RIα-(119–379) by Chemical Shifts
Similarly to the R209K mutant, the secondary chemical shifts measured for R333K in the presence of 2 mm excess cAMP do not exhibit any appreciable differences from those of the wild-type construct (supplemental Fig. S1, a and c), indicating that the overall structure is not significantly perturbed by the R333K mutation. However, the comparative analysis of the amide chemical shifts (Fig. 4, b and c, and supplemental Fig. S3b) reveals multiple R333K-induced local perturbations. As in the case of R209K, the largest overall chemical shift change caused by R333K is not observed for the mutation site but for the negatively charged residue that interacts directly with the mutated guanidinium, i.e. Glu-289 at the C terminus of the β2-3 loop of CBD-B (Figs. 4b and 2e). However, other residues in the β2-3 loop (i.e. Val-281 in CBD-B and the corresponding Ile-163 in CBD-A; Figs. 1c and 2, c and e) respond differently to the R/K mutation in the two domains. Whereas in CBD-A the R209K mutation causes a chemical shift variation for Ile-163 comparable with that observed for Asp-170 (Fig. 4a), in CBD-B the R333K mutation does not result in any major chemical shift change for Val-281 (Fig. 4b) despite the fact that the side chains of both Ile-163 and Val-281 are packed against the methylenes of the neighboring conserved arginines, i.e. Arg-209 and Arg-333, respectively (Fig. 2, c and e). The N terminus of the β2-3 loop, therefore, defines the first striking difference between the responses of the two CBDs to the homologous R/K mutations in their respective PBCs. This domain-specific response to the R333K mutation is further corroborated by the absence of any significant R333K-induced chemical shift change for Val-337 in CBD-B (Fig. 4b), which aligns with Val-213 in CBD-A (Fig. 1c), i.e. the residue doubly hydrogen-bonded to the CBD-A Ile-163 and significantly affected by the R209K mutation (Figs. 4a and 2, c and e). It is remarkable that such structurally homologous binding domains within the same protein can elicit such different responses as a result of a homologous mutation.
The majority of the other R333K-induced chemical shift changes observed for CBD-B (Fig. 4, b and c) are quite similar to those detected for the R209K mutation in CBD-A (Fig. 4, a and c), with significant variations observed for most of the PBC-B amides donating a hydrogen bond to cAMP (i.e. Gly-323 and Ala-326) and minor perturbations detected in the vicinity of other regions known to be affected by cAMP, such as the BBR and the hinge of CBD-B (Fig. 4b). These chemical shift patterns suggest that in R333K the positioning of cAMP within the PBC-B is at least partially perturbed. The chemical shift map of the perturbations caused by the R333K mutation (Fig. 4, b and c) also reveals a unique feature of the N3A motif of domain B. Specifically, Ile-253 displays a chemical shift change of ∼0.1 ppm (Fig. 4c), which is larger than any other ppm variations observed for the N3A motif of domain A as a result of the R209K mutation (Fig. 4c). Therefore, the N3A-B appears to be more sensitive than N3A-A to perturbations in the respective PBC. Furthermore, Ile-253 is part of a group of residues located throughout CBD-B (i.e. Ile-253, Asp-288, Leu-327, Arg-350, and Phe-374) that display significant chemical shift changes (Fig. 4, b and c) and cluster together better when mapped onto the C-subunit-bound R-subunit than onto the cAMP-bound R-subunit structures (Fig. 2, g and h). This observation is consistent with a proportion of a C-subunit-bound-like state being present even in the absence of the C-subunit. Last, Fig. 4, b and c, also shows that all R333K-induced local perturbations are confined to domain B. However, a more conclusive assessment of possible long range effects of R333K requires that the wild-type versus R333K comparison (Fig. 1b) be examined at the level of H/D exchange profiles (Fig. 6).
Mapping the Effects of the R333K Mutation on RIα-(119–379) by H/D Exchange
The H/D exchange profile measured for R333K RIα-(119–379) in the presence of 2 mm excess cAMP is reported in Fig. 6a (red symbols). For the purpose of facilitating comparison IV (Fig. 1b), Fig. 6a also includes the PF values observed for the wild-type construct under the same experimental conditions (Fig. 6a, black symbols). It is clear in Fig. 6a that the largest R333K-induced protection losses are detected for PBC-B. A similar pattern was observed also for PBC-A of R209K (Fig. 5a); however, the overall magnitude of the PBC-B decrease in solvent protection for R333K is somewhat reduced as it is limited to a class I to II shift (e.g. Glu-324 and Leu-327 in Fig. 6a) as opposed to the I to III changes seen for PBC-A in R209K (Fig. 5a). Furthermore, for R209K, the class I to III variations extended to the adjacent β6 and 7 strands (Fig. 5a), whereas in R333K these β-strands remain highly protected (Fig. 6a). Overall, these observations point to PBC-B as being less disrupted than PBC-A by the respective R/K mutation. Consistent with this result, no major R333K-induced changes are reported for BBR-B (Fig. 6a), although it was already quite exposed in the wild type (Fig. 3, a and b).
The other PF changes caused by R333K (Fig. 6a) suggest that the R333K RIα mutant mimics at least partially a C-subunit-bound-like state of the R-subunit (Fig. 1d). Specifically, R333K-induced decreases in protection are observed for the hinge region of domain B (e.g. Leu-364, Asn-367, Ile-368, and Tyr-371, Fig. 6a) as well as the N3A of domain B (e.g. Glu-245, Fig. 6a) and the hinge of domain A (e.g. Met-234, Fig. 6a). These PF losses also extend to the β2-3 segments of both CBD-B (e.g. Val-281 and Gln-283, Fig. 6a) and CBD-A (e.g. Val-162 and Asp-176, Fig. 6a). However, PBC-A appears to be only minimally affected by the R333K mutation (Fig. 6a), indicating that the R333K-induced PF changes observed in the β2-3 and hinge of domain A (Fig. 6a) mainly reflect long range interdomain perturbations initiated at the level of PBC-B. Overall, the class II (intermediate H/D exchange) residues appear to be the most perturbed group of residues as a result of the R333K mutation (Fig. 6a), and most of the class I (slow H/D exchange) sites are not significantly affected by this perturbation (Fig. 6a). A possible explanation for the lack of appreciable changes for class I residues in the R333K mutant in the presence of 2 mm excess cAMP is that these PF values still fall above the upper limit of detection. It is, therefore, essential to re-assess the comparative R333K versus wild-type H/D analysis in the absence of excess cAMP (comparison V, Fig. 1b).
The H/D protection factors for R333K RIα-(119–379) measured in the absence of excess cAMP are reported in Fig. 6b (red symbols), which also includes for comparative purposes the PF values measured for wild-type RIα-(119–379) under the same experimental conditions (black symbols). The wild-type versus R333K H/D comparison in the absence of excess cAMP (Fig. 6b) not only further confirms that the PBC-B perturbation propagates to the hinge regions of both domains, as seen in the presence of excess cAMP, but also clearly shows that the buried CBD-B residues shift from class I in wild-type to class II in the mutant (Fig. 6b), indicating a significant degree of global unfolding in domain B as a result of the R333K substitution. Interestingly, the global unfolding of CBD-B does not appear to appreciably propagate to CBD-A, where the highest PF values remain largely unaffected by the R333K substitution either in the presence or absence of excess cAMP (Fig. 6, a and b). It is notable, however, that CBD-B still exerts a significant control over CBD-A at the level of local unfolding as sensed by the variations in the intermediate and fast exchange (classes II and III) protection factors (Fig. 6).
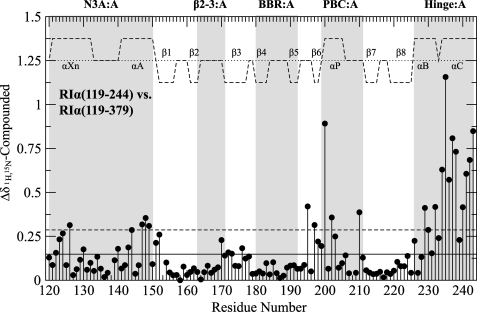
The control of local unfolding events in CBD-A by CBD-B is especially evident for the hinge-A and lid-A regions (Figs. 1c and 6). The influence of PBC-B on these sites represents a crucial component of the B-to-A interdomain communication pathways because perturbations at the C-terminal region of CBD-A propagate to other distal allosteric hot spots within domain A. This long range cross-talk is best appreciated by comparing the chemical shifts of the previously characterized domain A fragment, i.e. wild-type RIα-(119–244) (15, 32), with those assigned here for the wild-type two-domain construct RIα-(119–379), as illustrated in Fig. 7. Fig. 7 shows that the largest chemical shift variations are observed in the vicinity of the truncated C terminus, as expected, but significant ppm changes are also detected for the short helix of the PBC as well as for the N3A motif, indicating that perturbations in the hinge of domain A are likely to propagate to most of the other residues of the α-helical subdomain.
FIGURE 7.
Compounded chemical shift differences between the wild-type RIα(119–244) CBD-A construct and the wild-type RIα-(119–379) CBD-A,B construct both in the presence of excess cAMP. The average chemical shift difference (solid line) as well as 1 S.D. above the average (dashed line) are shown with black horizontal lines. Major allosteric hot spots are shaded in gray, and the secondary structure is shown as dashed lines above the data with the α-helices and β-sheets labeled.
DISCUSSION
A-to-B Interdomain Communication Pathways
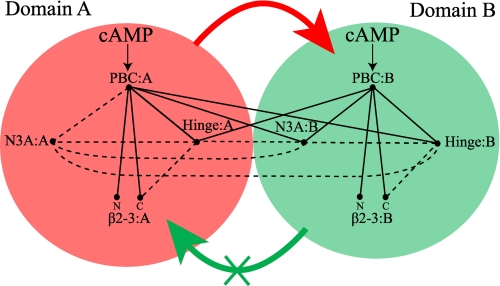
The combined comparative analysis of chemical shift maps and H/D exchange profiles presented here for the wild-type RIα-(119–379)·cAMP2 complex as well as the R209K and R333K mutants reveals that domain A controls domain B at multiple levels. First, CBD-B is coupled to CBD-A at the level of global unfolding as sensed primarily by the maximal protection factors (class I) measured for the highly buried residues, showing that a collective class I PF decrease in domain A results in a dramatic loss of class I protection in domain B as well, as observed most clearly in Fig. 5b. Second, CBD-B is also coupled to CBD-A at the level of local unfolding as probed mainly by the intermediate protection factors (class II), which indicate that local unfolding of PBC-A leads to increased exposure for the hinge helices not only of domain A but of domain B as well (Fig. 5, a and b). A schematic diagram summarizing these findings is shown in Fig. 8.
FIGURE 8.
Schematic diagram of the intra- and interdomain couplings among the critical allosteric sites of RIα-(119–379) as revealed by the present investigation. The red- and green-shaded circles refer to CBD-A and -B, respectively. The N3A motif, β2-3 loop, PBC, and hinge regions are labeled, whereas the BBR was omitted to simplify the figure. The N and C labels denote the N and C terminus of the β2-3 loop, respectively. The binding of cAMP to the PBC of both domains is indicated by arrows. The PBC sites of each CBD are connected by solid lines to other allosteric hot spots if they fulfill the following conditions: 1) they include residues in direct contact with the PBC either in the presence or absence of cAMP (7, 8), and 2) the region connected to the PBC is perturbed through local unfolding pathways as probed by class II or III protection factors and/or through changes in the local environment as probed by amide chemical shift variations. The dashed lines indicate regions with contacting residues but for which there is no experimental evidence in the present study to indicate whether the two regions are indeed allosterically linked. The absence of a given line between a pair of allosteric sites implies that there are no contacts between those two allosteric sites either in the presence or absence of cAMP. The thick red arrow means that the global unfolding of CBD-A promotes the global unfolding of CBD-B, whereas the thick green arrow with an X indicates that CBD-B is not able to appreciably affect the global unfolding of domain A.
The A-to-B coupling at the level of global unfolding can be rationalized in terms of a network of hydrophobic residues that spans mostly the helical bundle at the interface between the two domains and strands β3 and β8 of domain B as shown in Fig. 1e (8, 10). The β3:B and β8:B strands are two of the inner strands of the β-barrel:B and constitute an integral part of the buried core of CBD-B (Figs. 1e and 3b). Perturbations of the β3:B and β8:B sites, such as those caused by unfolding domain A, are therefore likely to promote unfolding of domain B as well. However, this mechanism does not explain the interdomain cross-talk at the level of local unfolding as strands β3:B and β8:B in RIα-(119–379)·cAMP2 do not include any class II PF values (Fig. 3a). The A-to-B coupling at the level of local unfolding can instead be explained by a simple “tandem transdomain lid” model.
The tandem transdomain lid model is based on the observation that not only is the hinge region of domain A covalently linked to the N3A motif of domain B (i.e. tandem CBD arrangement), but also the lid for cAMP:A is embedded in N3A:B (Fig. 1c). The tandem domain disposition in combination with the transdomain lid serves as an effective signal relay mechanism between the two CBDs (7) (Fig. 8). Specifically, the combined analysis of the structures of eukaryotic CBDs (7, 31) has revealed that when cAMP docks into the PBC, it causes a rotation of the hinge helices toward the PBC, which in turn causes a repositioning of the adjacent N3A motif belonging to the same CBD (Fig. 1d). Furthermore, it has been shown that the cAMP-dependent hinge rotation and re-positioning of the N3A motif involves several modulations in the dynamic profiles of both sites (18). In other words, within each CBD, not only is the PBC coupled to the hinge region, but the latter is also coupled to the N3A motif, and these intradomain couplings include cAMP-dependent modulations in both conformation and internal dynamics (14, 18). A possible mechanism for the control by PBC-A of the hinge region of both domains can, therefore, be outlined as the release of cAMP from PBC-A causing an outward rotation of the hinge of domain A away from PBC-A and promoting local unfolding in this region as well as in the adjacent N3A-B, which includes the lid-A and in turn causes increased exposure in the hinge of domain B to which it is coupled (Fig. 8).
Proposed Physiological Significance of the A-to-B Interdomain Communication Pathways
The control elicited by domain A on domain B at the level of global and local unfolding provides new insight into the deactivation mechanism of PKA in general and specifically on how the two cAMP molecules bound to each R-subunit are released from their respective PBC sites. Considering that the residence time of cAMP in PBC-A is significantly shorter than that of cAMP in PBC-B (i.e. ∼1 min versus ∼45 min (21)), it is likely that phosphodiesterases cause the depletion of cAMP first from site A, resulting in the transient formation of a mixed intermediate with an apo site A and a cAMP-bound site B. If a new external signal reaches the cell when the R-subunit is in the mixed intermediate state, the cAMP-dependent response of PKA will be suboptimal as the evolutionary selected gating function of CBD-B is impaired by the cAMP still bound to site B due to previous signals. To avoid such a “memory” effect between subsequent signals, it is necessary that phosphodiesterases be able to promptly release cAMP from both sites. An effective mechanism to achieve this double cAMP release relies on the global unfolding A-to-B pathway outlined above. As phosphodiesterases start to catalyze the removal of cAMP from site A, CBD-A begins to undergo partial but global unfolding, which in turn promotes global unfolding of CBD-B, thus, accelerating the release of cAMP from site B as well. This conclusion is also corroborated by the observation that the residence time of cAMP in site B decreases by 60% in the R209K mutant relative to wild-type RIα (21).
The acceleration of the off rate of cAMP at site B is not the only effect of the A-to-B global unfolding pathway. Another critical consequence of this A-to-B coupling pertains to the interface between the α- and β-subdomains of domain B. Specifically, it has been shown that several residues displaying class I PF values, i.e. relying on global unfolding exchange pathways, typically lie at the α/β-subdomain interface, suggesting that as a CBD is progressively unfolded, its α- and β-subdomains become increasingly decoupled (15, 16). Consequently, it is anticipated that as phosphodiesterases begin to decrease the cAMP occupancy at site A, causing global unfolding of both domains, the coupling between the α- and β-subdomains within each CBD is weakened, priming the R-subunit for a C-subunit-driven conformational change even before cAMP is fully removed from the “slow” site B. This process is further facilitated by the A-to-B local unfolding pathway discussed above, whereby release of cAMP from domain A disengages the hinge regions not only of domain A but also of domain B.
B-to-A Interdomain Communication Pathways
Our data reveal a striking difference between the A-to-B and B-to-A pathways at the level of global unfolding. Whereas global unfolding of domain A results in the global unfolding of domain B, as discussed above, no evidence was found for the reverse pathway; global unfolding of domain B as sensed by a decrease in class I protection factors does not cause a measurable change in the global unfolding of domain A (Figs. 6 and 8). Whereas this result is largely based on the H/D exchange profiles presented here and measured under native conditions (Fig. 6), the observation that the global unfolding of domain A is not significantly affected by domain B is also consistent with previous investigations of urea-induced unfolding monitored through intrinsic tryptophan fluorescence (33). Considering that the tryptophan residues in RIα (i.e. Trp-188, -222, and -260) are largely confined to domain A with Trp-222 contributing the most to the intrinsic fluorescence of RIα, these measurements sense predominantly the unfolding of CBD-A and indicate that the ΔGunfolding of domain A measured at [cAMPfree] = 149 μm remains within error unchanged at 9.5 ± 0.2 kcal/mol in both the wild-type RIα and the Δ(260–379) deletion mutant (33). Moreover, the ΔGunfolding values independently measured for the CBD-A construct RIα-(119–244) using capillary electrophoresis (34) converge again to a value of 9.5 kcal/mol when scaled to a concentration of free cAMP = 149 μm, as observed for the longer constructs of RIα (33). The absence of significant variations for the ΔGunfolding of domain A in wild-type RIα and Δ(260–379) RIα as well as RIα-(119–244) further confirms that the addition of domain B to domain A does not cause a significant stabilization of CBD-A against global unfolding. Overall, the combined analysis of H/D exchange under native conditions and urea-induced unfolding supports the absence of a significant B-to-A pathway at the level of global unfolding.
The remarkable asymmetry between the A-to-B and B-to-A interdomain pathways at the level of global unfolding is in agreement with previous theoretical predictions on unidirectional allosteric pathways proposed for other globular proteins (35), and it is likely to reflect the asymmetric nature of the interdomain contacts in RIα. Specifically, the structure of RIα-(91–379)·cAMP2 shows that the two domains contribute different β-subdomain residues to the interdomain interface. A comparative solvent-accessible surface area analysis (Fig. 1e, supplemental Fig. S5) reveals that although in CBD-A the β-barrel residues contacting domain B are confined to residues in the outer β5,6 strands, which belong to a large extent to the intermediate H/D exchange class II, in CBD-B the β-barrel residues contacting domain A and the lid A region also include inner β-strands that belong to the slow H/D exchange class I (Fig. 1e). As a result, if CBD-A unfolds, it affects highly buried CBD-B residues that are subject to global unfolding exchange pathways, but if CBD-B unfolds, it perturbs mostly more exposed CBD-A residues that can access alternative local unfolding exchange pathways in the native state. The asymmetric nature of the interdomain interface, therefore, provides a basis to understand why the B-to-A interdomain cross-talk is limited to local as opposed to global unfolding.
The B-to-A coupling at the level of local unfolding causes perturbations at the PBC-B site to propagate not only to the hinge and N3A regions of domain B but also to the hinge of domain A (Fig. 8). A simple explanation of this PCB-B to hinge-A,B pathway relies again on the tandem transdomain lid model discussed above to rationalize the reverse pathway (i.e. PCB-A to hinge-A,B). Specifically, our chemical shift and PF maps (Figs. 4, b and c, and 6) are consistent with the release and/or perturbation of cAMP at site B causing a shift toward a C-bound-like structure in the conformational equilibria which pertain to both the hinge-B and the adjacent N3A-B. Considering that the N3A-B contains the lid of domain A and is covalently linked to the hinge-A, this cAMP-dependent conformational shift provides an effective mechanism to propagate signals from PBC-B to the hinge regions of both domains even in the absence of the C-subunit.
Proposed Physiological Significance of the B-to-A Interdomain Communication Pathways
The biological relevance of the B-to-A interdomain cross-talk discussed above is best appreciated in the context of the activation mechanism of PKA, which has been recently elucidated (7). Although it is clear that CBD-B plays a pivotal gatekeeper role in the activation of PKA due to its control of cAMP access to site A, recent evidence from small-angle x-ray scattering (36) as well as the similarity in the R·C KD values for the RIα-(91–244) and RIα-(91–379) constructs (37) consistently point to CBD-B as being only a marginal contributor to the stability of the R·C complex. Taking into account the flexibility of the C-helix connecting domains A and B (38), the picture that emerges from these analyses is that of a labile CBD-B/C-subunit interface that is at least transiently disrupted by the dynamic on/off exchange of CBD-B occurring even in the absence of raised cAMP levels.
The dynamic nature of the CBD-B/C-subunit interface entails at least two major implications for the activation mechanism of PKA. First, it implies that CBD-B must function as the CBD-A gatekeeper even when not bound to C to preserve an effective cAMP-dependent activation of PKA. Second, it means that even when the CBD-B/C interface is transiently severed in the absence of cAMP, CBD-A should still bind tightly to C to maintain an effective inhibition of the kinase function under resting conditions. Although the first requirement is well met by the presence of the B-to-A coupling at the level of local unfolding as it ensures that PBC-B also controls the N3A-B region, which contains the lid of CBD-A, even when CBD-B is not bound to C, the second condition is best fulfilled by the absence of B-to-A coupling at the level of global unfolding. This can be appreciated by considering that when CBD-B does not contact the C-subunit in the absence of cAMP, it is at least partially but globally unfolded. If the global unfolding of CBD-B resulted in the global unfolding of CBD-A, an excessive entropic penalty would be imposed on the CBD-A/C-subunit interaction, promoting the premature dissociation of the R·C complex even before cAMP levels are raised as a result of external signals. Overall, it appears, therefore, that the specific patterns of interdomain communication in RIα both at the level of local and global unfolding have evolved to ensure optimal PKA inhibition in the absence of cAMP as well as optimal PKA activation in the presence of cAMP, despite the dynamic nature of the CBD-B/C interface within the RIα·C complex.
Universal Versus Domain-specific cAMP-dependent Allosteric Networks
The aforementioned discussion illustrates that the two homologous CBDs of RIα differ not only in terms of their binding properties (e.g. faster cAMP off rate in site A than B; CBD-B/C interface more dynamic than the CBD-A/C interaction) but also in terms of their cAMP-dependent allosteric patterns. In particular, the asymmetry in the A-to-B versus B-to-A global unfolding pathways indicates that although Arg-209 in CBD-A is a critical nucleation point for the interaction networks of both domains, the corresponding arginine in CBD-B, i.e. Arg-333, appears to control a more limited region that does not span domain A in its entirety. This striking asymmetry between the A-to-B and the B-to-A pathways at the level of global unfolding is just one aspect of a more general set of differences between the cAMP-dependent allosteric networks of CBD-A and CBD-B that have emerged from our comparative NMR analyses of wild-type, R209K, and R333K. Specifically, both H/D and chemical shift data point to a tighter N3A/PBC intradomain coupling in CBD-B than in CBD-A, whereas the hydrophobic N terminus of the β2-3 loop is more tightly coupled to the adjacent PBC in CBD-A than in CBD-B despite the high degree of local sequence and structure homology between these two domains (Fig. 1c). In general, these considerations suggest that although the main structural elements involved in the allosteric networks (i.e. PBC, BBR, β2-3 loop, hinge, and N3A) are quite conserved among different eukaryotic CBDs (12), the nature of their couplings may vary from one CBD to another more than previously anticipated, even when the two domains are highly homologous both in terms of sequence and structure, such as CBD-A and B of RIα. It is anticipated that these domain-specific allosteric profiles that are starting to emerge from the comparative high resolution analyses of eukaryotic CBDs in solution (15, 17–19) may provide the foundation for the design of novel allosteric effectors that are selective for specific cAMP-regulated protein systems.
Conclusions
The comparative NMR analysis of wild-type RIα-(119–379) and the two domain silencing mutants, i.e. R209K and the corresponding R333K, have unveiled at an unprecedented level of detail a picture of both intra- and interdomain allosteric networks in two integral CBDs. Although the main CBD allosteric hot spots (i.e. PBC, hinge, N3A, β2-3 loop) are conserved in the two homologous domains, their responses to the R/K perturbations, which mimic cAMP release, exhibit marked differences especially at the level of the β2-3 loop. Furthermore, our data reveal that the tandem CBD disposition and the transdomain lid of CBD-A, embedded within the N3A motif of CBD-B, ensure that cAMP binding to either PBC affects the hinge regions of both domains, resulting in a fully bidirectional interdomain coupling at the level of local unfolding. On the contrary, the interdomain coupling at the level of global unfolding is strikingly unidirectional. Although both domains are stabilized with respect to global unfolding by cAMP binding, such stabilization propagates from CBD-A to B but not from B to A. This remarkably asymmetric interdomain pathway at the level of global unfolding has evolved to ensure robustness in both PKA activation and deactivation. The stability of CBD-A irrespective of CBD-B ensures that the activation of PKA is only minimally susceptible to the dynamic release of apoCBD-B from C. The instability of CBD-B as a result of the global unfolding of CBD-A ensures that the complete deactivation of PKA occurs promptly with cAMP released from both sites A and B before new external signals need to be transduced into biological responses.
Acknowledgments
We are grateful to Rajeevan Selvaratnam and Madoka Akimoto for helpful discussions.
This work was supported by the Canadian Institute of Health Research.
This paper is dedicated to the memory of Captain George A. McNicholl.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Experimental Procedures and Figs. S1–S5.
- PKA
- cAMP-dependent protein kinase A
- BBR
- base binding region
- CBD
- cAMP binding domain
- H/D
- hydrogen/deuterium exchange
- HSQC
- heteronuclear single-quantum coherence
- PBC
- phosphate binding cassette
- PF
- protection factor
- R
- regulatory subunit of PKA
- C
- catalytic subunit of PKA
- RIα
- isoform Iα of the R subunit of PKA
- R2
- dimeric regulatory subunit of PKA
- TROSY
- transverse relaxation optimized spectroscopy
- NOESY
- two-dimensional nuclear Overhauser effect spectroscopy.
REFERENCES
- 1.Berman H. M., Ten Eyck L. F., Goodsell D. S., Haste N. M., Kornev A., Taylor S. S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shabb J. B. (2001) Chem. Rev. 101, 2381–2411 [DOI] [PubMed] [Google Scholar]
- 3.Dodge K. L., Khouangsathiene S., Kapiloff M. S., Mouton R., Hill E. V., Houslay M. D., Langeberg L. K., Scott J. D. (2001) EMBO J. 20, 1921–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopperud R., Krakstad C., Selheim F., Døskeland S. O. (2003) FEBS Lett. 546, 121–126 [DOI] [PubMed] [Google Scholar]
- 5.Taylor S. S., Kim C., Vigil D., Haste N. M., Yang J., Wu J., Anand G. S. (2005) Biochim. Biophys. Acta 1754, 25–37 [DOI] [PubMed] [Google Scholar]
- 6.Scott J. D. (2006) Biochem. Soc. Trans. 34, 465–467 [DOI] [PubMed] [Google Scholar]
- 7.Kim C., Cheng C. Y., Saldanha S. A., Taylor S. S. (2007) Cell 130, 1032–1043 [DOI] [PubMed] [Google Scholar]
- 8.Su Y., Dostmann W. R., Herberg F. W., Durick K., Xuong N. H., Ten Eyck L., Taylor S. S., Varughese K. I. (1995) Science 269, 807–813 [DOI] [PubMed] [Google Scholar]
- 9.Wu J., Jones J. M., Nguyen-Huu X., Ten Eyck L. F., Taylor S. S. (2004) Biochemistry 43, 6620–6629 [DOI] [PubMed] [Google Scholar]
- 10.Wu J., Brown S., Xuong N. H., Taylor S. S. (2004) Structure 12, 1057–1065 [DOI] [PubMed] [Google Scholar]
- 11.Kim C., Xuong N. H., Taylor S. S. (2005) Science 307, 690–696 [DOI] [PubMed] [Google Scholar]
- 12.Rehmann H., Prakash B., Wolf E., Rueppel A., de Rooij J., Bos J. L., Wittinghofer A. (2003) Nat. Struct. Biol. 10, 26–32 [DOI] [PubMed] [Google Scholar]
- 13.Kornev A. P., Taylor S. S., Ten Eyck L. F. (2008) PLoS Comput. Biol. 4, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das R., Melacini G. (2007) J. Biol. Chem. 282, 581–593 [DOI] [PubMed] [Google Scholar]
- 15.Das R., Esposito V., Abu-Abed M., Anand G. S., Taylor S. S., Melacini G. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abu-Abed M., Das R., Wang L., Melacini G. (2007) Proteins 69, 112–124 [DOI] [PubMed] [Google Scholar]
- 17.Mazhab-Jafari M. T., Das R., Fotheringham S. A., SilDas S., Chowdhury S., Melacini G. (2007) J. Am. Chem. Soc. 129, 14482–14492 [DOI] [PubMed] [Google Scholar]
- 18.Das R., Mazhab-Jafari M. T., Chowdhury S., SilDas S., Selvaratnam R., Melacini G. (2008) J. Biol. Chem. 283, 19691–19703 [DOI] [PubMed] [Google Scholar]
- 19.Das R., Chowdhury S., Mazhab-Jafari M. T., Sildas S., Selvaratnam R., Melacini G. (2009) J. Biol. Chem. 284, 23682–23696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kannan N., Wu J., Anand G. S., Yooseph S., Neuwald A. F., Venter J. C., Taylor S. S. (2007) Genome Biology 8, R264.1–R264.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herberg F. W., Taylor S. S., Dostmann W. R. G. (1996) Biochemistry 35, 2934–2942 [DOI] [PubMed] [Google Scholar]
- 22.Englander J., Cohen L., Arshava B., Estephan R., Becker J. M., Naider F. (2006) Biopolymers 84, 508–518 [DOI] [PubMed] [Google Scholar]
- 23.Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 24.Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 25.Goddard T. D., Kneller D. G. (2006) SPARKY 3, University of California, San Francisco, CA [Google Scholar]
- 26.Neal S., Nip A. M., Zhang H., Wishart D. S. (2003) J. Biomol. NMR 26, 215–240 [DOI] [PubMed] [Google Scholar]
- 27.Eghbalnia H. R., Wang L., Bahrami A., Assadi A., Markley J. L. (2005) J. Biomol. NMR 32, 71–81 [DOI] [PubMed] [Google Scholar]
- 28.Das R., Abu-Abed M., Melacini G. (2006) J. Am. Chem. Soc. 128, 8406–8407 [DOI] [PubMed] [Google Scholar]
- 29.Tollinger M., Skrynnikov N. R., Mulder F. A., Forman-Kay J. D., Kay L. E. (2001) J. Am. Chem. Soc. 123, 11341–11352 [DOI] [PubMed] [Google Scholar]
- 30.Herberg F. W., Dostmann W. R., Zorn M., Davis S. J., Taylor S. S. (1994) Biochemistry 33, 7485–7494 [DOI] [PubMed] [Google Scholar]
- 31.Rehmann H., Wittinghofer A., Bos J. L. (2007) Nat. Rev. Mol. Cell Biol. 8, 63–73 [DOI] [PubMed] [Google Scholar]
- 32.Esposito V., Sjoberg T., Das R., Brown S., Taylor S. S., Melacini G. (2006) J. Biomol. NMR 36, 64. [DOI] [PubMed] [Google Scholar]
- 33.Cànaves J. M., Leon D. A., Taylor S. S. (2000) Biochemistry 39, 15022–15031 [DOI] [PubMed] [Google Scholar]
- 34.Seguí-Lines G., Gavina J. M., D'Amaral J. C., Britz-McKibbin P. (2007) Analyst 132, 741–744 [DOI] [PubMed] [Google Scholar]
- 35.Luque I., Leavitt S. A., Freire E. (2002) Annu. Rev. Biophys. Biomol. Struct. 31, 235–256 [DOI] [PubMed] [Google Scholar]
- 36.Cheng C. Y., Yang J., Taylor S. S., Blumenthal D. K. (2009) J. Biol. Chem. 284, 35916–35925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anand G., Taylor S. S., Johnson D. A. (2007) Biochemistry 46, 9283–9291 [DOI] [PubMed] [Google Scholar]
- 38.Vigil D., Blumenthal D. K., Taylor S. S., Trewhella J. (2005) J. Biol. Chem. 280, 35521–35527 [DOI] [PubMed] [Google Scholar]