Abstract
Angiotensin II (Ang II) acutely stimulates thick ascending limb (TAL) NO via an unknown mechanism. In endothelial cells, activation of Ang II type 2 receptor (AT2) stimulates NO. Akt1 activates NOS3 by direct phosphorylation. We hypothesized that Ang II stimulates TAL NO production via AT2-mediated Akt1 activation, which phosphorylates NOS3 at serine 1177. We measured NO production by fluorescence microscopy. In isolated TALs, Ang II (100 nm) increased NO production by 1.1 ± 0.2 fluorescence units/min (p < 0.01). Ang II increased cGMP accumulation by 4.9 ± 1.3 fmol/μg (p < 0.01). Upon adding the AT2 antagonist PD123319 (1 μm), Ang II failed to stimulate NO (0.1 ± 0.1 fluorescence units/min; p < 0.001 versus Ang II); adding the AT1 antagonist losartan (1 μm) resulted in Ang II stimulating NO by 0.9 ± 0.1 fluorescence units/min. Akt inhibitor (5 μm) blocked Ang II-stimulated NO (−0.1 ± 0.2 fluorescence units/min versus inhibitor alone). Phospho-Akt1 increased by 72% after 5 min (p < 0.006), returning to basal after 10 min. Phospho-Akt2 did not change after 5 min but increased by 115 and 163% after 10 and 15 min (p < 0.02). Phospho-Akt3 did not change. An AT2 agonist increased pAkt1 by 78% (p < 0.02), PI3K inhibition blocked this effect. In TALs transduced with dominant negative Akt1, Ang II failed to stimulate NO (0.1 ± 0.2 fluorescence units/min versus 1.2 ± 0.2 for controls; p < 0.001). Ang II increased phospho-NOS3 at serine 1177 by 130% (p < 0.01) and 150% after 5 and 10 min (p < 0.02). Ang II increased phosphoNOS3 at serine 633 by 50% after 5 min (p < 0.01). Akt inhibition prevented NOS3 phosphorylation. We concluded that Ang II enhances TAL NO production via activation of AT2 and Akt1-dependent phosphorylation of NOS3 at serines 1177 and 633.
Keywords: Akt PKB, Cyclic GMP (cGMP), Kidney, Nitric Oxide, Nitric-oxide Synthase, Dominant Negative, Thick Ascending Limb
Introduction
The thick ascending limb (TAL)2 of the loop of Henle is the diluting segment of the renal nephron. It reabsorbs ∼30% of filtered NaCl and generates the osmotic gradient necessary for water reabsorption by the collecting duct. Enhanced Na+ reabsorption by this segment has been implicated in the development of hypertension (1, 2).
The TAL cells produce NO. Endogenously produced NO inhibits Na+ reabsorption by the TAL (3, 4). Inappropriate NO bioavailability can lead to enhanced TAL Na+ reabsorption, Na+ retention, and hypertension. Thus, understanding the signaling cascade leading to NO production by TALs could help understand the pathophysiology of hypertension and identify new targets for its treatment.
In the TAL, NO is stimulated by a number of factors including angiotensin II (Ang II) (5). Ang II can activate two different receptors: type 1 (AT1) or type 2 (AT2). In the vasculature, activation of AT1 leads to vasoconstriction, resulting in increased renal vascular resistance (6). In contrast, activation of AT2 in endothelial cells stimulates NO release (7). Similarly, in the kidney, activation of AT1 mediates the salt-retaining and prohypertensive actions of Ang II (8–12), whereas activation of AT2 leads to natriuresis via activation of the NO/cGMP signaling pathway (13, 14). To our knowledge, the receptor that mediates the stimulatory actions of Ang II on NO production by the TAL has not yet been identified.
In the TAL, NO is produced by nitric-oxide synthase 3 (NOS3 or eNOS) (15, 16). NOS3 can be activated by several signaling pathways, including those involving Ca2+/calmodulin (17) and Akt-dependent phosphorylation of NOS3 (18, 19). In endothelial cells, both pathways are important. However, in epithelial cells, activation of Akt appears to be the main mechanism (20). Three Akt isoforms have been identified: Akt1, -2, and -3. In endothelial cells, Akt1 directly phosphorylates NOS3 at serine 1177, heightening enzyme activity and NO production (18, 21–25). In the TAL, activation of Akt has been shown to participate in the activation of NOS3 by different stimuli (16, 20, 26, 27, 28). However, the mechanism by which Ang II stimulates NO in the TAL has not been investigated to our knowledge. We hypothesized that Ang II stimulates TAL NO production via activation of AT2 and Akt1, phosphorylating NOS3 at serine 1177 and enhancing NO production.
EXPERIMENTAL PROCEDURES
Animals
Male Sprague-Dawley rats (Charles River, Kalamazoo, MI), were fed a diet containing 0.22% Na+ and 1.1% K+ (Purina, Richmond, IN) for at least 7 days. On the day of the experiment, animals were anesthetized with ketamine (100 mg/kg body weight, intraperitoneally) and xylazine (20 mg/kg body weight, intraperitoneally). All protocols were carried out in accord with the guidelines of the Institutional Animal Care and Use Committee.
Measurement of NO Production by Fluorescence Microscopy
TALs were isolated from rats weighing 100–135 g and held between glass pipettes at 4 °C in a chamber designed for live cell imaging on the stage of an inverted microscope (Eclipse TE-2000-U, Nikon) as done routinely in our laboratory (29). The bath was started at 0.6 ml/min, and the chamber was warmed to 37.0 ± 0.5 °C. TALs were bathed for 15 min in a solution containing: 130 mm NaCl, 2.5 mm NaH2PO4, 4 mm KCl, 1.2 mm MgSO4, 6 mm alanine, 1 mm Na3 citrate, 5.5 mm glucose, 2 mm Ca2+ (lactate)2, and 10 mm HEPES (pH 7.4) (solution A) containing 5 μm 4,5-diaminofluorescein diacetate (EMD Biosciences, Gibbstown, NJ) and washed for 25 min with solution A containing 50 μm l-arginine, the substrate for NOS. At this concentration, l-arginine supports but does not stimulate NO production (30). TALs were imaged using a 100× oil-immersion objective (numerical aperture, 1.3), and the dye was excited with an argon laser at 488 nm. The fluorescence emitted by NO-bound dye (>500 nm) was measured using a laser-scanning confocal microscope equipped with data acquisition and analysis software (VisiTech International). Measurements were recorded once every 30 s for a 5-min control period, and then either 100 nm Ang II (EMD Biosciences, San Diego, CA) or vehicle (0.005% acetic acid) was added to the bath. Fluorescence was measured once every 30 s during the 15-min experimental period. For experiments testing the effect of Akt inhibitor VIII (EMD Biosciences, 5 μm), the AT1 antagonist losartan (Merck, Rahway, NJ; 1 μm) or the AT2 antagonist PD123319 (Parke-Davis, Ann Arbor, MI; 1 μm), the drug was added to the bath during the last 5 min of the washing period and maintained in all solutions throughout the experiment. Measurements were performed in the absence of luminal flow because we have found that flow stimulates NO production by the TAL (27). Rates of NO production were calculated as the slope of the initial rate of increase in 4,5-diaminofluorescein fluorescence after adding Ang II (or vehicle) minus the slope during the control period. Data are expressed as fluorescence units/min.
Medullary TAL Suspensions
TAL suspensions were obtained from rats weighing 150–220 g as described previously (31). This procedure yields a 92% pure suspension of TALs (32), so that contamination by other types of cells in our preparation (if any) was minimal.
Protein Content Determination
Total protein content was measured using Coomassie Plus reagent (Pierce), based on Bradford's colorimetric method.
Measurements of cGMP by Enzyme-linked Immunosorbent Assay
TAL suspensions were divided into two 2-ml centrifuge tubes and resuspended in 1 ml solution A containing 1 mm 3-isobutyl-1-methylxanthine (Sigma) with vehicle (0.005% acetic acid), 100 nm Ang II or 200 μm spermine NONOate (Cayman, Ann Arbor, MI). TALs were incubated for 15 min at 37 °C, gassing with 100% oxygen and inverting every 5 min. Reaction was stopped by adding 1 ml ice-cold solution A plus 3-isobutyl-1-methylxanthine and leaving tubes on ice for 5 min. After centrifugation, 100 μl of solution A plus 3-isobutyl-1-methylxanthine was added to the TALs following addition of 100 μl ice-cold methanol. Tubes were centrifuged at 16,000 × g for 15 min, and supernatants were dried overnight by centrifugation under high vacuum. The pellets were used to measure total protein content. cGMP was measured by using a commercially available enzyme-linked immunosorbent assay kit from R&D Systems (Minneapolis, MN). The lower limit of detectability for this assay is 0.21 pmol/sample. Results are expressed as fmol cGMP/μg protein.
Measurements of Akt and NOS3 Phosphorylation by Western Blot
TAL suspensions were divided into four 2-ml centrifuge tubes and incubated for 15 min in 500 μl of solution A containing 50 μm l-arginine at 37 °C. Next, 500 μl of solution A containing either vehicle, Ang II, or the AT2 agonist CGP-42112A (Sigma) was added to each tube. Final concentrations were as follows: tube 1: 0.005% acetic acid (vehicle) and tubes 2, 3, and 4: 100 nm Ang II. Tubes were incubated at 37 °C and gassed with 100% oxygen every 5 min. After incubation for either 5 (tubes 1 and 2), 10 (tube 3), or 15 min (tube 4), suspensions were cooled by adding 1 ml of ice-cold solution A to each tube. Control experiments showed that phosphorylation of Akt and NOS3 did not change with incubation time (vehicle tubes). When studying the effect of the AT2 agonist CGP-42112A (Sigma), it was used at the final concentration of 10 nm for 10 min. When studying the effect of Akt or PI3K inhibition, the same procedure was followed, except we added the Akt inhibitor VIII (5 μm) or wortmannin (150 nm, Sigma) 5 min prior addition of either Ang II or CPG-42112A. TALs were centrifuged and lysed by vortexing them in 100 μl of a buffer containing 20 mm HEPES (pH 7.4), 2 mm EDTA, 300 mm sucrose, 1.0% Nonidet P-40, 0.1% sodium dodecyl sulfate, 5 μg/ml antipain, 10 μg/ml aprotinin, 5 μg/ml leupeptin, 4 mm benzamidine, 5 μg/ml chymostatin, 5 μg/ml pepstatin A, and 0.105 m 4(2-aminoethyl)-benzene sulfonyl fluoride (Sigma). A 1:100 dilution of phosphatase inhibitor mixture II (EMD Biosciences) was added to the buffer prior to use. Samples were centrifuged at 6,000 × g for 5 min at 4 °C, and protein content in the supernatant was measured. For total NOS3 and phosphorylated proteins, 5 and 50 μg of total protein, respectively, were loaded into each lane of an 8% SDS-polyacrylamide gel, separated by electrophoresis, and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA). The membrane was incubated in blocking buffer containing 20 mm Tris, 137 mm NaCl, 5% nonfat dried milk, and 0.1% Tween 20 for 60 min and then with a 1:1000 dilution of a NOS3-specific monoclonal antibody (BD Transduction Laboratories, San Diego, CA), a 1:1000 dilution of a monoclonal antibody against NOS3 phosphorylated at serine 1177 (BD Transduction Laboratories), or a 1:500 dilution of a polyclonal antibody against NOS3 phosphorylated at serine 633 (Upstate, Lake Placid, NY) in blocking buffer for 2 h at room temperature. For phosphorylated Akt, the process was similar except for the following. 1) a 1:2000 dilution of a polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used for 1 h at room temperature to detect Akt1 phosphorylated at serine 473. 2) A 1:500 dilution of a polyclonal antibody (Abcam, Cambridge, MA) was used overnight at 4 °C to detect phosphorylated Akt2 at serine 474. And 3) a 1:500 dilution of a polyclonal antibody (Abcam) was used for 1 h at room temperature to detect Akt3 phosphorylation at serine 472. The membrane was washed in a buffer containing 20 mm Tris, 137 mm NaCl, and 0.1% Tween 20 and incubated with a 1:1000 dilution of a secondary antibody against the appropriate IgG conjugated to horseradish peroxidase (Amersham Biosciences). The reaction products were detected using a chemiluminescence kit (Amersham Biosciences) and by exposure to Fuji RX film. Bands were quantified by densitometry. Total NOS3 was used as a loading control. When necessary, membranes were stripped and reprobed for total NOS3. Changes in phosphorylation (% from vehicle) were normalized to total NOS3. Data are expressed as the ratio phosphorylated protein/total NOS3 in arbitrary units (a.u.). Only freshly isolated TALs from a single rat were used in each experiment. n indicates the number of rats used for each data set.
Dominant Negative Akt1
The dominant negative Akt1 (dn-Akt1) plasmid was kindly provided by Dr. Kenneth Walsh at Boston University School of Medicine. The dominant negative is a mutant form of Akt1 (T308A, S473A) that cannot be activated by phosphorylation (33). dn-Akt1 was subcloned into a shuttle vector containing the CMV promoter. A scrambled DNA (control) was subcloned into a shuttle vector containing the H1 mouse RNA polymerase promoter. Plasmids were sent to ViraQuest (North Liberty, IA) for viral production.
In Vivo Gene Delivery of Dominant Negative Akt1
TALs were transduced in vivo with recombinant replication-deficient adenoviruses expressing 1) dn-Akt1 or 2) a scrambled DNA sequence as we reported previously (34). Briefly, the left kidney of a 95–105 g rat was exposed via a flank incision, and the renal artery and vein were clamped. Four 20-μl virus injections (1 × 1012 particles/ml) were made along the longitudinal axis at a flow rate of 20 μl/min. The renal vessels were unclamped; the kidney was returned to the abdominal cavity, the muscle incision was sutured, and the skin was clipped. We previously found that maximum expression occurred 3–5 days after injection of the adenovirus (16). Thus, all experiments were performed within these time points.
Statistics
Results are expressed as mean ± S.E. All statistical analyses were performed by the Biostatistics Department at Henry Ford Hospital. Data were analyzed by t tests with corrections for multiple testing (Hochberg's method) when appropriate. p < 0.05 was considered significant for single comparisons. For multiple testing, the ordered p values were < 0.0167, 0.0250, and 0.0500.
RESULTS
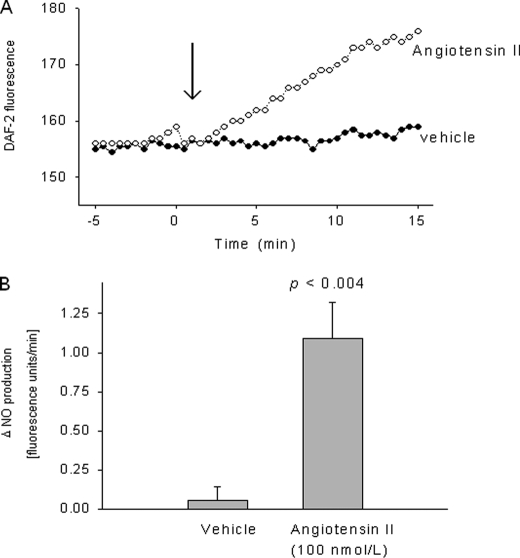
We first measured changes in DAF2 fluorescence to investigate whether Ang II increases NO. In isolated rat TALs, 100 nm Ang II increased NO production by 1.1 ± 0.2 fluorescence units/min, whereas vehicle (0.005% acetic acid) had no effect (0.1 ± 0.1 fluorescence units/min) (p < 0.004; n = 5; Fig. 1). The increase in NO production normally reached a plateau ∼12 min after adding Ang II. In the absence of Ang II, there was no change in emitted fluorescence, ruling out photobleaching of the dye. These data indicate that Ang II acutely stimulates NO production in isolated rat TALs.
FIGURE 1.
Effect of 100 nm angiotensin II on NO production by isolated thick ascending limbs. A, representative experiment for an angiotensin II- and a vehicle-stimulated tubule, respectively. B, mean data. p < 0.004 versus vehicle (n = 5).
Next, we investigated the effect of Ang II on cGMP accumulation by TALs. In TAL suspensions incubated with vehicle, cGMP levels were undetectable. The calculated limit of detectability of the assay, when normalizing by the amount of protein used, is <0.8 ± 0.1 fmoles/μg. After 15 min with 100 nm Ang II, cGMP increased to 4.9 ± 1.3 fmol/μg (p < 0.01; n = 6). In addition, 15 min with the NO donor spermine NONOate increased cGMP content by 10.2 ± 2.6 fmol/μg (p < 0.02, n = 6). These data suggest that Ang II stimulates cGMP generation in the TAL and that this stimulation is about half of that produced by an NO donor.
We next studied which receptor mediates the stimulatory effect of Ang II on NO production. In isolated TALs, 100 nm Ang II stimulated NO production by 0.9 ± 0.1 fluorescence units/min (p < 0.001; n = 5). When we added the AT2 antagonist PD123319 (1 μm), the ability of Ang II to stimulate NO production was abolished (0.1 ± 0.1 fluorescence units/min; p < 0.001 versus Ang II alone; n = 6). However, on adding the AT1 antagonist losartan (1 μm), Ang II stimulated NO production by 0.9 ± 0.1 fluorescence units/min (p < 0.001; n = 5) (Fig. 2). These data suggest that Ang II stimulates TAL NO production via activation of AT2.
FIGURE 2.
Effect of the angiotensin type 1 (AT1) blocker losartan and the angiotensin type 2 (AT2) blocker PD 123319 on angiotensin II-induced NO production by isolated thick ascending limbs. p < 0.001 versus vehicle or losartan (n = 5–6).
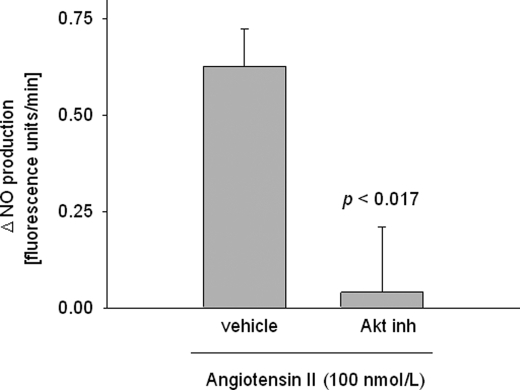
To investigate whether Ang II stimulates TAL NO production via Akt, we used Akt inhibitor VIII. In isolated TALs, 100 nm Ang II increased NO production by 0.63 ± 0.09 fluorescence units/min (p < 0.002; n = 5). After adding the Akt inhibitor (5 μm), this increase only reached 0.04 ± 0.17 fluorescence units/min (p < 0.017 versus Ang II alone; n = 5) (Fig. 3), representing 93% inhibition. Inhibitor alone did not change basal fluorescence (0.07 ± 0.10 fluorescence units/min; not significant). These data suggest that Ang II stimulates TAL NO production by activating Akt.
FIGURE 3.
Effect of 5 μm Akt inhibitor (inh) VIII on angiotensin II-stimulated NO production by isolated thick ascending limbs. p < 0.017 versus vehicle (n = 5).
There are three Akt isoforms. To pinpoint which Akt isoform mediates the effects of Ang II, we investigated the effect of Ang II on Akt1, -2, and -3 phosphorylation. In TAL suspensions, Ang II (100 nm) increased phosphorylation of Akt1 at serine 473 by 0.7 ± 0.2 arbitrary units (a.u.) after 5 min (p < 0.006; n = 6), returning to basal levels after 10 and 15 min of incubation (change: 0.6 ± 0.3 and 0.3 ± 0.4 a.u. for 5 and 10 min; n.s; n = 6) (Fig. 4A). Ang II (100 nm) had no effect on phosphorylation of Akt2 at serine 474 after 5 min (change: 0.3 ± 0.1 a.u., n = 7), but it increased by 1.1 ± 0.4 (p < 0.02; n = 7) and 1.6 ± 0.5 a.u. (p < 0.01; n = 7) after 10 and 15 min, respectively (Fig. 4B). Ang II had no effect on phosphorylation of Akt3 at serine 472 (Fig. 4C) at any time point. Thus, Ang II acutely stimulated phosphorylation of Akt1 at serine 473 after 5 min and Akt2 at serine 474 after 10 and 15 min.
FIGURE 4.
Time-dependent effect of 100 nm angiotensin II on phosphorylation of Akt1 (A), Akt2 (B), and Akt3 (C) (n = 6 for A and C and n = 7 for B). Right, representative Western blots for each panel.
Because the time course of phosphorylation of Akt1 most closely matched the action of NO production, we next studied the effect of the Akt inhibitor on Akt1 phosphorylation. Incubation of TALs with the Akt inhibitor (5 μm) alone decreased basal Akt1 phosphorylation from 1 to 0.17 ± 0.02 a. u., an inhibition of 83% (p < 0.004 versus control; n = 4). In the presence of the Atk inhibitor, Ang II (100 nm) for 5 min did not stimulate Akt1 phosphorylation (change: 0.03 ± 0.05 versus inhibitor alone; n = 4) (Fig. 5A). Thus, the Akt inhibitor prevented phosphorylation of Akt1 in the TAL under both basal and stimulated (Ang II) conditions.
FIGURE 5.
A, effect of the Akt inhibitor VIII (Akt inh, 5 μm) on basal and Ang II-stimulated phosphorylation of Akt1 at serine 473 (n = 4). B, effect of the AT2 receptor agonist (AT2 ago) GCP-42112A on Akt1 phosphorylation. Effect of the PI3K inhibitor wortmannin (wortm, 150 nm) on basal and AT2-stimulated Akt1 phosphorylation (n = 6). Right, representative Western blot for each panel.
To identify the signaling cascade upstream of Akt1, we investigated: a) the effect of an AT2 receptor agonist to stimulate Akt1 phosphorylation and b) the ability of the PI3K inhibitor wortmannin to block its effect. Incubation of TAL suspensions for 10 min with the AT2 agonist CGP-42112A (10 nm), increased Akt1 phosphorylation by 0.8 ± 0.3 a.u. (p < 0.02; n = 6). Wortmannin alone significantly reduced basal Akt1 phosphorylation by 0.7 ± 0.2 a.u. (p < 0.01; n = 6). In the presence of wortmannin, the AT2 agonist failed to stimulate Akt1 phosphorylation (change: 0.1 ± 0.2 a.u.; n = 6) (Fig. 5B). Thus, the PI3K inhibitor prevented phosphorylation of Akt1 in the TAL under both basal and stimulated (AT2 agonist) conditions.
Next, we examined the effect of dn-Akt1 on Ang II-stimulated NO production. In isolated TALs expressing scrambled DNA, Ang II (100 nm) stimulated NO production by 1.2 ± 0.2 fluorescence units/min (p < 0.002; n = 5); but it was significantly reduced in TALs expressing dn-Akt (0.1 ± 0.2 fluorescence units/min; p < 0.003 versus scrambled; n = 6) (Fig. 6). These data indicate that Ang II stimulates TAL NO production by activating Akt1.
FIGURE 6.
Effect of 100 nm angiotensin II on NO production by thick ascending limbs transduced in vivo with an adenovirus expressing a scrambled DNA sequence (scr-DNA) or dn-Akt1. p < 0.003 versus scrambled DNA (n = 5–6).
To determine whether Ang II stimulates NO production by phosphorylating NOS3, we measured its effect on NOS3 phosphorylation by Western blot. In TAL suspensions, Ang II (100 nm) increased NOS3 phosphorylation at serine 1177 by 1.3 ± 0.3 a.u. after 5 min (p < 0.01; n = 6) and 1.5 ± 0.4 a.u. after 10 min (p < 0.015; n = 6), returning to basal levels by 15 min (change: 0.1 ± 0.5; n.s; n = 6) (Fig. 7A, dark bars). Ang II stimulated NOS3 phosphorylation at serine 633 by 0.5 ± 0.1 a.u. after a 5-min incubation (p < 0.007; n = 6), returning to basal levels at 10 and 15 min (change: 0.3 ± 0.1 a.u. and 0.5 ± 0.2 a.u. for 10 and 15 min; n.s; n = 6) (Fig. 7B, dark bars). These data indicate that Ang II acutely phosphorylates TAL NOS3 at serines 1177 and 633.
FIGURE 7.
Top, time-dependent effect of 100 nm Ang II on NOS3 phosphorylation at serines 1177 (A) and 633 (B) in the absence (dark bars) or presence of 5 μm Akt inhibitor VIII (light bars) (n = 6). Bottom, representative Western blot for each panel.
To ascertain whether Ang II enhances phosphorylation NOS3 at serines 1177 and 633 via Akt activation, we measured the effect of the Akt inhibitor VIII on Ang II-stimulated NOS3 phosphorylation. After adding the Akt inhibitor VIII (5 μm), the ability of Ang II to phosphorylate NOS3 at serine 1177 was blocked (change: 0.2 ± 0.2 and 0.8 ± 0.2 a.u. after 5 and 10 min; n.s; n = 4) (Fig. 7A, light bars). The Akt inhibitor alone significantly reduced NOS3 phosphorylation at serine 1177 by 66 ± 6% (p < 0.002; n = 4). In addition, the ability of Ang II to phosphorylate NOS3 at serine 633 after 5 min was abolished by the Akt inhibitor (change: 0.1 ± 0.2 a.u.) (Fig. 7B, light bars). These data suggest that Ang II acutely enhances NOS3 phosphorylation at serines 1177 and 633 via Akt activation. Additionally, Akt appears to regulate basal phosphorylation of NOS3.
DISCUSSION
We hypothesized that Ang II stimulates TAL NO production by activating AT2 and Akt1, which phosphorylates NOS3 at serine 1177. To test this hypothesis, we first directly measured the effect of Ang II on NO production by the rat TAL and the receptor involved. Using fluorescence microscopy, we found that Ang II stimulated TAL NO production within 4 min after 100 nm Ang II was added to the basolateral bath. In a different set of experiments, 100 nm Ang II stimulated cGMP accumulation in TAL suspensions, about half of the increase caused by 200 μm spermine NONOate.
To investigate which angiotensin receptor mediates the effect of Ang II on TAL NO production, we tested the ability of angiotensin receptor antagonists to block the stimulatory effect of Ang II. We found that the AT2 antagonist PD 123319 blocked the ability of Ang II to stimulate TAL NO production, whereas the AT1 antagonist losartan had no effect on Ang II-induced NO. This suggests that Ang II binds AT2 and stimulates NO production.
Our conclusion that AT2 receptors mediate the stimulatory effect of Ang II on NO production is in agreement with others. Batenburg et al. (7) found that activation of AT2 in microarteries (though not in large vessels) stimulated NO production and vasodilatation. Hiyoshi et al. (35) reported that in rats with 2-kidney 1-clip hypertension, up-regulation of AT2 resulted in stimulation of Akt1, NOS3 activation, and NO production in the thoracic aorta. Similarly, Yayama et al. (36) reported that in a model of aortic banding, AT2 mediated up-regulation of phosphorylated NOS3, resulting in enhanced cyclic GMP and vasodilatation. In contrast, our data contradict findings from Saito et al. (37), showing that in cultured bovine aortic endothelial cells, activation of AT1 mediated the stimulatory actions of Ang II on NO production. They are also at odds with data showing that AT1 activation stimulates NO in the macula densa (38). Although the explanation for the disparate results is presently unclear, it could be that activation of AT1, which leads to increased intracellular calcium (38), results in NOS3 activation via a calcium/calmodulin-mediated mechanism.
After showing that Ang II stimulated NO production via activation of AT2, we addressed the mechanism involved. We have previously shown that several other stimuli act via Akt to enhance NO in TALs. Thus, we tested whether Ang II stimulates NO production via this signaling cascade. To do this, we first studied the ability of Ang II to increase TAL NO production in the presence of an Akt inhibitor. Pharmacological inhibition of Akt using the Akt inhibitor completely blocked the stimulatory effect of Ang II on NO production. This suggested that activation of Akt is required for Ang II to stimulate NO production in the TAL.
The Akt family consists of three isoforms: Akt1, Akt2, and Akt3, all serine-threonine kinases (39). Each is activated by phosphorylation at a specific amino acid. Thus we next studied whether Ang II alters phosphorylation of the three isoforms at their respective positive regulatory sites. We found that Ang II acutely stimulated phosphorylation of Akt1 at serine 473. Phosphorylation of Akt1 was transient, peaking 5 min after addition of Ang II and returning to basal levels after 10 min. Ang II also increased phosphorylation of Akt2, but only after 10 min. Ang II had no effect on Akt3 phosphorylation. These data indicate that both Akt1 and -2 are activated by Ang II but with different time courses.
Because the increase in NO production caused by Ang II occurred almost immediately and phosphorylation of Akt1 occurred sooner than that of Akt2, we reasoned that activation of Akt1 is likely the main mediator of the effect of Ang II on NOS3 stimulation. To test this, we measured the effect of the Akt inhibitor on Akt1 phosphorylation and the ability of dn-Akt1 to block stimulatory actions of Ang II. We found that the Akt inhibitor blocked Akt1 phosphorylation as would be required to prevent Ang II-induced stimulation of NO. We also found that Ang II-stimulated NO production was blocked in TALs expressing dn-Akt1. This effect was specific because transduction with a scrambled DNA sequence did not affect the ability of Ang II to stimulate NO in control TALs. These data indicate that activation of Akt1 is necessary for Ang II to stimulate TAL NO production.
Our data regarding Akt1 indicate that activation of this kinase is necessary for Ang II to stimulate NO production, but they do not necessarily rule out the involvement of Akt2. Our experiments showed that NO production reaches a plateau ∼12 min after Ang II is added and remains elevated. At this time, Akt1 activity has already returned to baseline, but Akt2 activity is increasing. These data suggest that both are necessary for the sustained increase in NO production and that possibly Akt1 activation results in the stimulation of Akt2.
One of the most studied mechanisms for Akt1 activation is its phosphorylation by PI3K (18, 21–25). To study the signaling cascade upstream Akt1, we investigated the ability of the PI3K inhibitor wortmannin to block the stimulatory effect of AT2 activation on Akt1 phosphorylation. We found that pharmacologic stimulation of the AT2 agonist enhanced Akt1 phosphorylation and wortmannin abolished this effect. These data not only confirmed the involvement of the AT2 receptor in the Ang II-stimulated NO but also outline the signaling pathway whereby Akt1 is activated. Angiotensin receptors are of the G protein-coupled type. The exact signaling events leading to PI3K activation downstream the AT2 receptor are unknown. Recent reports suggest that the Gβγ subunit of G proteins can directly interact with certain PI3K isoforms promoting their translocation to the plasma membrane, a prerequisite for PI3K activation. Depending on the isoform, this process may or may not require participation of Ras-GTP (40). The mechanism whereby AT2 activation leads to increased PI3K activity in the TAL needs to be further explored.
The involvement of PI3K in the activation of NOS3 and NO production by the TAL is in agreement with other reports. In this regard, luminal flow, endothelin-1, ATP and clonidine all stimulate NO production in the TAL, and this effect was blocked by the PI3K inhibitor wortmannin (16, 26–28). Thus, Ang II stimulates PI3K to phosphorylate Akt1 which in turn, phosphorylates NOS3 to stimulate NO production.
We have previously shown that NOS3 is responsible for flow-, ATP- and endothelin-induced NO production in TALs. Thus, we studied whether Ang II enhances NOS3 activity by measuring phosphorylation of NOS3 at serines 1177 and 633, positive regulators of enzyme activity (18, 19). Our data showed that Ang II increased phosphorylation of NOS3 at serine 1177 1.5-fold after 5 min. The increase was sustained after 10 min but returned to basal levels at 15 min. In addition, phosphorylation of NOS3 at serine 633 increased 0.5-fold after 5 min of incubation with Ang II and returned to basal levels after 10 min. Because NO production is already stimulated by 5 min after adding Ang II, phosphorylation of NOS3 at both regulatory sites is likely required for full NOS3 activation. The fact that phosphoserine 1177 remained elevated at 10 min, whereas phosphoserine 633 was already back to basal levels, may indicate that phosphorylation at serine 633 is required during early stages of the signaling event. The fact that Ang II enhances phosphorylation of NOS3 at positive regulatory sites in conjunction with our previous data (16, 28), indicate that NOS3 is the NOS isoform activated by Ang II.
Because Akt activation mediated the stimulatory effect of Ang II on NO production, we tested whether phosphorylation of NOS3 also depended on Akt activity. We found that pharmacological inhibition of Akt blocked Ang II-induced NOS3 phosphorylation at serine 1177. These findings are in agreement with studies showing that Akt directly phosphorylates NOS3 at this residue, enhancing NO production (18). In addition, we found that Akt inhibition prevented Ang II-stimulated phosphorylation of NOS3 at serine 633. This was surprising because most of the literature supports the notion that phosphorylation of NOS3 at serine 633 occurs primarily via protein kinase A (41, 42). Thus it is possible that Akt-dependent phosphorylation at serine 1177 promotes phosphorylation at serine 633 by a non-Akt-dependent pathway.
We found that Ang II increased NO production in the TAL by activating NOS3 via Akt. Two distinct mechanisms have been shown to regulate NOS3 activation: Ca2+-dependent and Ca2+-independent pathways. In endothelial cells, both mechanisms are important; however, in epithelial cells of the TAL, Akt-dependent activation of NOS3 appears to be the main route (16, 20, 26, 27). We recently reported that endothelin-1 activates NOS3 via activation of Akt and phosphorylation of NOS3 (16, 43). Clonidine, an α-2 adrenergic agonist, stimulates TAL NO production without measurable changes in intracellular Ca2+, but it also requires activation of the PI3K/Akt pathway (26). In addition, luminal flow activates the PI3K/Akt pathway, stimulating NO production in isolated TALs (27). More recently, it has been reported that ATP stimulates TAL NO production upon activation of Akt1 (28). From these studies and the current work, we concluded that Akt1 is the primary mechanism by which Ang II activates NOS3 to stimulate NO production in the TAL. Although Akt has been shown to participate in the activation of NOS3 in endothelial cells, stimulation of Ca2+/calmodulin and protein kinase A appear to be the main mediators. Unlike endothelial cells, the PI3K/Akt-1 pathway appears to be the primary mechanism for activation of NOS3 in epithelial cells of the TALs.
Our data showed that Ang II acutely stimulated Akt1 to phosphorylate NOS3 and increase NO production. These increases were transient, and NO returned to basal levels very rapidly (15 min); thus, one may question the physiological significance of such an acute effect. However, there are several explanations for these transient responses. First, under our experimental conditions, the cofactors required for NOS3 activity might have been consumed. This is not expected in vivo where cofactors are produced endogenously. Second, the effect of Ang II on NO production may depend on the dose; the amount we used may have overwhelmed the system, leading to activation of parallel mechanisms that would terminate the stimulatory effect of Ang II on NO.
Previous data from this laboratory showed that long term exposure to Ang II reduces TAL NOS3 expression. The signaling mechanism whereby this occurs has been shown to be both NO and O2−-dependent (44). Thus, Ang II acutely stimulates TAL NO; however, long term exposure of TALs to elevated NO results in decreased NOS3 expression likely due to the formation of peroxinitrite after NO and O2− react with each other.
In summary, we discovered the following. 1) Ang II acutely stimulated NO production and cGMP accumulation by isolated rat TALs. 2) An AT2 antagonist blocked Ang II-stimulated NO. 3) Akt inhibition prevented the Ang II-induced increase in NO production. 4) Ang II increased phosphorylation of Akt1 at serine 473, and this was blocked by Akt inhibition. 5) AT2 activation stimulated Akt1 phosphorylation, and this was blocked by the PI3K inhibitor wortmannin. 6) dn-Akt1 blocked the effect of Ang II on NO production. And 7) Ang II increased phosphorylation of NOS3 at serine 1177 and 633, whereas Akt inhibition blocked these effects. We concluded that Ang II activates PI3K/Akt1 via the AT2 receptors, inducing phosphorylation of NOS3 at serines 1177 and 633, resulting in increased production of NO by the TAL. NO has been shown to buffer the actions of Ang II within the renal medulla (5, 45–49). In the TAL, NO inhibits (15, 50), reabsorption whereas O2− stimulates Na+ reabsorption (51). Ang II-stimulated NO production is likely to buffer the stimulatory effects of Ang II on O2− production and Na+ reabsorption in the TAL. Although Ang II has been shown to enhance TAL Na+ transport and blood pressure in vivo (52), data investigating the direct effect of Ang II on Na+ transport in vitro are controversial (53, 54), and the explanation for the discrepancy is still unclear.
Acknowledgment
We thank Dr. Kenneth Walsh, Boston University School of Medicine for providing the dominant negative Akt.
This work was supported, in whole or in part, by NHLBI, National Institutes of Health Grants PO1HL-28982, PO1HL-90550, and RO1HL-70985 (to J. L. G.).
- TAL
- thick ascending limb
- Ang II
- angiotensin II
- PI3K
- phosphatidylinositol 3-kinase
- NOS
- nitric-oxide synthase
- a.u.
- arbitrary units.
REFERENCES
- 1.Roman R. J., Kaldunski M. L. (1991) Hypertension 17, 1018–1024 [DOI] [PubMed] [Google Scholar]
- 2.García N. H., Plato C. F., Stoos B. A., Garvin J. L. (1999) Hypertension 34, 508–513 [DOI] [PubMed] [Google Scholar]
- 3.Plato C. F., Stoos B. A., Wang D., Garvin J. L. (1999) Am. J. Physiol. 276, F159–163 [DOI] [PubMed] [Google Scholar]
- 4.Herrera M., Ortiz P. A., Garvin J. L. (2006) Am. J. Physiol. Renal Physiol. 290, F1279–1284 [DOI] [PubMed] [Google Scholar]
- 5.Dickhout J. G., Mori T., Cowley A. W., Jr. (2002) Circ. Res. 91, 487–493 [DOI] [PubMed] [Google Scholar]
- 6.Kost C. K., Jr., Jackson E. K. (1993) Hypertension 21, 420–431 [DOI] [PubMed] [Google Scholar]
- 7.Batenburg W. W., Garrelds I. M., Bernasconi C. C., Juillerat-Jeanneret L., van Kats J. P., Saxena P. R., Danser A. H. (2004) Circulation 109, 2296–2301 [DOI] [PubMed] [Google Scholar]
- 8.Cervenka L., Wang C. T., Navar L. G. (1998) Am. J. Physiol. 274, F940–945 [DOI] [PubMed] [Google Scholar]
- 9.Harris P. J., Navar L. G., Ploth D. W. (1984) Clinical Science 66, 541–544 [DOI] [PubMed] [Google Scholar]
- 10.Harris P. J., Navar L. G. (1985) Am. J. Physiol. 248, F621–630 [DOI] [PubMed] [Google Scholar]
- 11.Welch W. J., Wilcox C. S. (2001) Kidney Int. 59, 1257–1263 [DOI] [PubMed] [Google Scholar]
- 12.Hall J. E., Guyton A. C., Trippodo N. C., Lohmeier T. E., McCaa R. E., Cowley A. W., Jr. (1977) Am. J. Physiol. 232, F538–F544 [DOI] [PubMed] [Google Scholar]
- 13.Siragy H. M., Carey R. M. (1997) J. Clin. Invest. 100, 264–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padia S. H., Howell N. L., Siragy H. M., Carey R. M. (2006) Hypertension 47, 537–544 [DOI] [PubMed] [Google Scholar]
- 15.Plato C. F., Shesely E. G., Garvin J. L. (2000) Hypertension 35, 319–323 [DOI] [PubMed] [Google Scholar]
- 16.Herrera M., Hong N. J., Ortiz P. A., Garvin J. L. (2009) J. Biol. Chem. 284, 1454–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt H. H., Pollock J. S., Nakane M., Förstermann U., Murad F. (1992) Cell Calcium. 13, 427–434 [DOI] [PubMed] [Google Scholar]
- 18.Fulton D., Gratton J. P., McCabe T. J., Fontana J., Fujio Y., Walsh K., Franke T. F., Papapetropoulos A., Sessa W. C. (1999) Nature 399, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boo Y. C., Sorescu G. P., Bauer P. M., Fulton D., Kemp B. E., Harrison D. G., Sessa W. C., Jo H. (2003) Free Radic. Biol. Med. 35, 729–741 [DOI] [PubMed] [Google Scholar]
- 20.Pollock J. S., Carmines P. K. (2006) Hypertension 47, 19–21 [DOI] [PubMed] [Google Scholar]
- 21.Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A. M. (1999) Nature 399, 601–605 [DOI] [PubMed] [Google Scholar]
- 22.Mineo C., Yuhanna I. S., Quon M. J., Shaul P. W. (2003) J. Biol. Chem. 278, 9142–9149 [DOI] [PubMed] [Google Scholar]
- 23.Symons J. D., McMillin S. L., Riehle C., Tanner J., Palionyte M., Hillas E., Jones D., Cooksey R. C., Birnbaum M. J., McClain D. A., Zhang Q. J., Gale D., Wilson L. J., Abel E. D. (2009) Circ. Res. 104, 1085–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernández-Hernando C., Ackah E., Yu J., Suárez Y., Murata T., Iwakiri Y., Prendergast J., Miao R. Q., Birnbaum M. J., Sessa W. C. (2007) Cell Metab. 6, 446–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Q., Sheibani N. (2008) Am. J. Physiol. Cell Physiol. 295, C1647–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plato C. F., Garvin J. L. (2001) Am. J. Physiol. Renal Physiol. 281, F679–686 [DOI] [PubMed] [Google Scholar]
- 27.Ortiz P. A., Hong N. J., Garvin J. L. (2004) Am. J. Physiol. Renal Physiol. 287, F281–288 [DOI] [PubMed] [Google Scholar]
- 28.Silva G. B., Garvin J. L. (2009) Am. J. Physiol. Renal Physiol. 297, F646–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garvin J. L., Burg M. B., Knepper M. A. (1988) Am. J. Physiol. 255, F57–65 [DOI] [PubMed] [Google Scholar]
- 30.Ortiz P. A., Garvin J. L. (2002) Hypertension 39, 591–596 [DOI] [PubMed] [Google Scholar]
- 31.Herrera M., Garvin J. L. (2005) Am. J. Physiol. Renal Physiol. 288, F58–64 [DOI] [PubMed] [Google Scholar]
- 32.Herrera M., Garvin J. L. (2004) Am. J. Physiol. Renal Physiol. 287, F231–235 [DOI] [PubMed] [Google Scholar]
- 33.Luo Z., Fujio Y., Kureishi Y., Rudic R. D., Daumerie G., Fulton D., Sessa W. C., Walsh K. (2000) J. Clin. Invest. 106, 493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortiz P. A., Hong N. J., Wang D., Garvin J. L. (2003) Hypertension 42, 674–679 [DOI] [PubMed] [Google Scholar]
- 35.Hiyoshi H., Yayama K., Takano M., Okamoto H. (2005) Hypertension 45, 967–973 [DOI] [PubMed] [Google Scholar]
- 36.Yayama K., Hiyoshi H., Imazu D., Okamoto H. (2006) Hypertension 48, 958–964 [DOI] [PubMed] [Google Scholar]
- 37.Saito S., Hirata Y., Emori T., Imai T., Marumo F. (1996) Hypertens Res. 19, 201–206 [DOI] [PubMed] [Google Scholar]
- 38.Liu R., Persson A. E. (2004) Hypertension 43, 649–653 [DOI] [PubMed] [Google Scholar]
- 39.Franke T. F. (2008) Oncogene 27, 6473–6488 [DOI] [PubMed] [Google Scholar]
- 40.Kurig B., Shymanets A., Bohnacker T., Prajwal, Brock C., Ahmadian M. R., Schaefer M., Gohla A., Harteneck C., Wymann M. P., Jeanclos E., Nürnberg B. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 20312–20317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boo Y. C., Hwang J., Sykes M., Michell B. J., Kemp B. E., Lum H., Jo H. (2002) Am. J. Physiol. Heart Circ Physiol. 283, H1819–1828 [DOI] [PubMed] [Google Scholar]
- 42.Michell B. J., Harris M. B., Chen Z. P., Ju H., Venema V. J., Blackstone M. A., Huang W., Venema R. C., Kemp B. E. (2002) J. Biol. Chem. 277, 42344–42351 [DOI] [PubMed] [Google Scholar]
- 43.Ortiz P. A., Hong N. J., Garvin J. L. (2004) Am. J. Physiol. Renal Physiol. 287, F274–280 [DOI] [PubMed] [Google Scholar]
- 44.Ramseyer V. D., Garvin J. L. (2009) Hypertension 53, 313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szentiványi M., Jr., Zou A. P., Mattson D. L., Soares P., Moreno C., Roman R. J., Cowley A. W., Jr. (2002) Am. J. Physiol. Regul. Integr. Comp Physiol. 283, R266–272 [DOI] [PubMed] [Google Scholar]
- 46.Helle F., Hultström M., Skogstrand T., Palm F., Iversen B. M. (2009) Am. J. Physiol. Renal Physiol. 296, F78–86 [DOI] [PubMed] [Google Scholar]
- 47.Majid D. S., Nishiyama A., Jackson K. E., Castillo A. (2005) Am. J. Physiol. Renal Physiol. 288, F412–419 [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z., Rhinehart K., Solis G., Pittner J., Lee-Kwon W., Welch W. J., Wilcox C. S., Pallone T. L. (2005) Am. J. Physiol. Heart Circ Physiol. 288, H29–36 [DOI] [PubMed] [Google Scholar]
- 49.Ikenaga H., Fallet R. W., Carmines P. K. (1996) Am. J. Physiol. 271, F365–373 [DOI] [PubMed] [Google Scholar]
- 50.Ortiz P. A., Hong N. J., Garvin J. L. (2001) Am. J. Physiol. Renal Physiol. 281, F819–825 [DOI] [PubMed] [Google Scholar]
- 51.Ortiz P. A., Garvin J. L. (2002) Am. J. Physiol. Renal Physiol. 283, F957–962 [DOI] [PubMed] [Google Scholar]
- 52.Silva G. B., Garvin J. L. (2008) Hypertension 52, 1091–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amlal H., LeGoff C., Vernimmen C., Soleimani M., Paillard M., Bichara M. (1998) Am. J. Physiol. 274, C1047–1056 [DOI] [PubMed] [Google Scholar]
- 54.Lerolle N., Bourgeois S., Leviel F., Lebrun G., Paillard M., Houillier P. (2004) Am. J. Physiol. Renal Physiol. 287, F404–410 [DOI] [PubMed] [Google Scholar]