Abstract
The genus Phaeoacremonium is associated with opportunistic human infections, as well as stunted growth and die-back of various woody hosts, especially grapevines. In this study, Phaeoacremonium species were isolated from necrotic woody tissue of Prunus spp. (plum, peach, nectarine and apricot) from different stone fruit growing areas in South Africa. Morphological and cultural characteristics as well as DNA sequence data (5.8S rDNA, ITS1, ITS2, β-tubulin, actin and 18S rDNA) were used to identify known, and describe novel species. From the total number of wood samples collected (257), 42 Phaeoacremonium isolates were obtained, from which 14 species were identified. Phaeoacremonium scolyti was most frequently isolated, and present on all Prunus species sampled, followed by Togninia minima (anamorph: Pm. aleophilum) and Pm. australiense. Almost all taxa isolated represent new records on Prunus. Furthermore, Pm. australiense, Pm. iranianum, T. fraxinopennsylvanica and Pm. griseorubrum represent new records for South Africa, while Pm. griseorubrum, hitherto only known from humans, is newly reported from a plant host. Five species are newly described, two of which produce a Togninia sexual state. Togninia africana, T. griseo-olivacea and Pm. pallidum are newly described from Prunus armeniaca, while Pm. prunicolum and Pm. fuscum are described from Prunus salicina.
Keywords: Diaporthales, molecular systematics, pathogenicity, Togninia, Togniniaceae
INTRODUCTION
The genus Phaeoacremonium was established 12 years ago (Crous et al. 1996) to accommodate cephalosporium-like fungi known from grapevine (Petri 1912) and human infections (Ajello et al. 1974). In spite of the exclusion of Pm. chlamydospora (now Phaeomoniella chlamydospora, Crous & Gams 2000), the number of known species increased quickly. The genus Togninia was confirmed as teleomorph (Mostert et al. 2003), and their phylogenetic position clarified within the Togniniaceae in the Diaporthales (Réblová et al. 2004). Presently 23 Phaeoacremonium species have been described, of which 10 have been linked to Togninia teleomorphs (Mostert et al. 2006a, Réblová & Mostert 2007). Togninia species known to date have been shown to have either a homo- or heterothallic mating strategy (Mostert et al. 2003, 2006a, Rooney-Latham et al. 2005). In species where the mating strategy has been studied in more detail, like T. minima, both mating types have been found to occur in the same field, and even the same grapevine (Mostert et al. 2003, Pascoe et al. 2004, Rooney-Latham et al. 2005). Recent research has focused on the development of genus- and species-specific primers to facilitate early detection (Mostert et al. 2006a, Aroca & Raposo 2007), and the development of an online polyphasic identification system (www.cbs.knaw.nl/phaeoacremonium/biolomics.aspx).
Phaeoacremonium species have been associated with human infections, often skin- or nail-infections, so-called phaeohyphomycoses (Ajello et al. 1974, Guarro et al. 2003, Hemashettar et al. 2006), as well as disease symptoms of a number of woody hosts worldwide (Rumbos 1986, Di Marco et al. 2004, Kubátová et al. 2004), especially with grapevine diseases such as Petri disease and esca (Pascoe et al. 2004, Rooney-Latham et al. 2005, Whiting et al. 2005, Mostert et al. 2006b, Aroca & Raposo 2007). Only two Phaeoacremonium species are known from Prunus. Phaeoacremonium aleophilum (T. minima) was reported on Prunus pennsylvanica in Canada (Hausner et al. 1992) and P. armeniaca in South Africa (Mostert et al. 2006a). Phaeoacremonium parasiticum (T. parasitica) was isolated from wilting trees of P. armeniaca in Tunisia (Hawksworth et al. 1976) and from P. avium in Greece (Rumbos 1986). Rumbos (1986) described Pm. parasiticum as causal agent of a serious dieback disease of cherry trees in different locations in Greece in the 1980s. Several cherry cultivars were found to be susceptible to the disease, which caused leaf drop, wilting and wood discolouration. In one orchard, were the fungus was closely associated with bark beetles (Scolytidae) and metallic wood-boring beetles (Buprestidae), the majority of the trees were affected and died. Phaeoacremonium parasiticum caused xylem lesions in cherry, apricot, olive and peach trees (Rumbos 1986).
In South Africa, stone fruit orchards are often established in close proximity of vineyards. Thirteen Phaeoacremonium species have been reported from Vitis vinifera, eight of which are known from South Africa, where T. minima was found on Vitis and Prunus (Mostert et al. 2006a). It is possible that this pathogen disseminates from one host to another. This phenomenon may be more common among phytopathogenic ascomycetes than previously accepted, as several species of Botryosphaeriaceae, Cryphonectriaceae and Valsaceae have been dispersed from branches and stems of fruit trees to other woody hosts in the vicinity (Adams et al. 2005, Crous et al. 2006b, Gryzenhout et al. 2006, Damm et al. 2007).
The comparatively slow-growing and, until recently, relatively unknown species of Phaeoacremonium were probably often excluded from surveys of fungi on woody plants in South Africa, and subsequently only Phaeomoniella chlamydospora is listed in the most recent compilation of phytopathogenic fungi from South Africa (Crous et al. 2000). Because of the highly diverse, endemic vegetation and different climatic regions, more than 200 000 species of fungi have been estimated to occur in South Africa (Crous et al. 2006a), which was acknowledged by the authors as rather conservative. As a recent study on Botryosphaeriaceae has shown (Damm et al. 2007), Prunus represents a rich catch-crop for many of these fungi, and thus would also be a good host to sample for novel species of Phaeoacremonium. Therefore, the aim of the present study is to determine the diversity of Phaeoacremonium species on Prunus wood in South Africa and to describe five new species isolated from P. armeniaca and P. salicina. A further aim of this study was to determine which species of Phaeoacremonium, formerly known from grapevines, would have Prunus spp. as alternate hosts.
MATERIAL AND METHODS
Isolates
Branches with wood symptoms (e.g. die-back, canker, necrosis) were sampled from plum (Prunus salicina), peach (P. persica), nectarine (P. persica var. nucipersica) and apricot (P. armeniaca) orchards in the Western Cape and the Limpopo province of South Africa. Wood pieces with necrosis symptoms were prepared according to Damm et al. (2007) and incubated on potato-dextrose agar (2 % PDA; Biolab, Midrand, South Africa, supplemented with 100 mg/L streptomycin sulphate and 100 mg ampicillin) and synthetic nutrient-poor agar medium (SNA; Nirenberg 1976) supplemented with 100 mg penicillin G, 50 mg streptomycin sulphate, 10 mg chlortetracycline hydrochloride (pH 6), under cool fluorescent white light at 25 °C. Single-conidial isolates were obtained from all strains for further study. Reference strains are maintained in the culture collection of the Department of Plant Pathology, University of Stellenbosch (STE-U), Stellenbosch, South Africa, and the Centraalbureau voor Schimmelcultures (CBS) Utrecht, The Netherlands. Isolates used for morphological and sequence analysis are presented in Table 1.
Table 1.
Names, accession numbers and collection details of isolates studied.
| Species | Accession No.1 | Host | Location | Pathotest2 | GenBank accessions
|
|||
|---|---|---|---|---|---|---|---|---|
| ITS | TUB | ACT | SSU | |||||
| Phaeoacremonium australiense | STE-U 5960 | Prunus salicina | Paarl, Western Cape, South Africa | EU128021 | EU128069 | EU128111 | ||
| STE-U 5961 | P. salicina | Paarl, Western Cape, South Africa | x | EU128022 | EU128070 | EU128112 | ||
| STE-U 5839 | P. salicina | Paarl, Western Cape, South Africa | x | EU128023 | EU128071 | EU128113 | ||
| STE-U 5838 | P. salicina | Paarl, Western Cape, South Africa | EU128024 | EU128072 | EU128114 | EU128055 | ||
| STE-U 5959, CBS 120861 | P. salicina | Stellenbosch, Western Cape, South Africa | EU128025 | EU128073 | EU128115 | EU128054 | ||
| Pm. fuscum | STE-U 5969, CBS 120856* | P. salicina | Mookgopong, Limpopo, South Africa | x | EU128050 | EU128098 | EU128141 | EU128059 |
| STE-U 6366 | P. salicina | Mookgopong, Limpopo, South Africa | EU128051 | EU128199 | EU128140 | |||
| Pm. griseorubrum | STE-U 5957, CBS 120860 | P. salicina | Paarl, Western Cape, South Africa | x | EU128026 | EU128074 | EU128116 | |
| STE-U 5958 | P. salicina | Paarl, Western Cape, South Africa | x | EU128027 | EU128075 | EU128117 | ||
| Pm. iranianum | STE-U 6092 | P. armeniaca | Robertson, Western Cape, South Africa | x | EU128028 | EU128076 | EU128118 | |
| STE-U 6179 | P. armeniaca | Montagu, Western Cape, South Africa | x | EU128029 | EU128077 | EU128119 | ||
| STE-U 6091, CBS 120864 | P. armeniaca | Robertson, Western Cape, South Africa | EU128030 | EU128078 | EU128120 | |||
| Pm. pallidum | STE-U 6104, CBS 120862* | P. armeniaca | Bonnievale, Western Cape, South Africa | x | EU128053 | EU128103 | EU128144 | EU128061 |
| Pm. prunicolum | STE-U 5967, CBS 120858* | P. salicina | Mookgopong, Limpopo, South Africa | x | EU128047 | EU128095 | EU128137 | EU128056 |
| STE-U 5968 | P. salicina | Mookgopong, Limpopo, South Africa | x | EU128048 | EU128096 | EU128138 | EU128057 | |
| Pm. scolyti | STE-U 5955, CBS121755 | P. persica var. nucipersica | Mookgopong, Limpopo, South Africa | x | EU128034 | EU128082 | EU128124 | |
| STE-U 6095, CBS 121438 | P. armeniaca | Robertson, Western Cape, South Africa | EU128035 | EU128083 | EU128125 | |||
| STE-U 6096 | P. armeniaca | Bonnievale, Western Cape, South Africa | EU128036 | EU128084 | EU128126 | |||
| STE-U 6097 | P. persica | Modimolle, Limpopo, South Africa | EU128037 | EU128085 | EU128127 | |||
| STE-U 6098, CBS121756 | P. persica | Modimolle, Limpopo, South Africa | EU128038 | EU128086 | EU128128 | |||
| STE-U 6099 | P. persica | Modimolle, Limpopo, South Africa | EU128039 | EU128087 | EU128129 | |||
| STE-U 6100 | P. persica | Modimolle, Limpopo, South Africa | EU128040 | EU128088 | EU128130 | |||
| STE-U 5834 | P. salicina | Stellenbosch, Western Cape, South Africa | x | EU128041 | EU128089 | EU128131 | ||
| STE-U 5954, CBS 121439 | P. salicina | Paarl, Western Cape, South Africa | EU128042 | EU128090 | EU128132 | |||
| STE-U 5956 | P. salicina | Mookgopong, Limpopo, South Africa | EU128043 | EU128091 | EU128133 | |||
| Pm. subulatum | STE-U 6094, CBS 120866 | P. armeniaca | Robertson, Western Cape, South Africa | x | EU128044 | EU128092 | EU128134 | |
| Togninia africana | STE-U 6177, CBS 120863* | P. armeniaca | Montagu, Western Cape, South Africa | x | EU128052 | EU128100 | EU128142 | EU128060 |
| STE-U 6364 | P. armeniaca | Montagu, Western Cape, South Africa | EU128101 | EU128143 | ||||
| STE-U 6365 | P. armeniaca | Montagu, Western Cape, South Africa | EU128102 | |||||
| T. fraxinopennsylvanica | STE-U 6101, CBS 120865 | P. salicina | Franschhoek, Western Cape, South Africa | x | EU128031 | EU128079 | EU128121 | |
| (Pm. mortoniae) | STE-U 6102 | P. salicina | Franschhoek, Western Cape, South Africa | x | EU128032 | EU128080 | EU128122 | |
| T. griseo-olivacea | STE-U 5966, CBS 120857* | P. armeniaca | Mookgopong, Limpopo, South Africa | x | EU128049 | EU128097 | EU128139 | EU128058 |
| T. minima (Pm. aleophilum) | STE-U 6088 | P. armeniaca | Robertson, Western Cape, South Africa | EU128014 | EU128062 | EU128104 | ||
| STE-U 6089, CBS 121434 | P. armeniaca | Bonnievale, Western Cape, South Africa | EU128015 | EU128063 | EU128105 | |||
| STE-U 6090 | P. armeniaca | Bonnievale, Western Cape, South Africa | EU128016 | EU128064 | EU128106 | |||
| STE-U 5836, CBS 121435 | P. salicina | Paarl, Western Cape, South Africa | EU128017 | EU128065 | EU128107 | |||
| STE-U 5962 | P. salicina | Paarl, Western Cape, South Africa | EU128018 | EU128066 | EU128108 | |||
| STE-U 5963 | P. salicina | Paarl, Western Cape, South Africa | x | EU128019 | EU128067 | EU128109 | ||
| STE-U 5964, CBS 121436 | P. persica | Paarl, Western Cape, South Africa | x | EU128020 | EU128068 | EU128110 | ||
| T. parasitica (Pm. parasiticum) | STE-U 6093, CBS 121437 | P. armeniaca | Montagu, Western Cape, South Africa | x | EU128033 | EU128081 | EU128123 | |
| T. viticola (Pm. viticola) | STE-U 5965, CBS 121440 | P. salicina | Paarl, Western Cape, South Africa | x | EU128045 | EU128093 | EU128135 | |
| STE-U 6180 | P. salicina | Franschoek, Western Cape, South Africa | x | EU128046 | EU128094 | EU128136 | ||
1 STE-U: Culture collection of the Department of Plant Pathology, University of Stellenbosch, South Africa; CBS: Culture collection of the Centraalbureau voor Schimmelcultures, Fungal Biodiversity Centre, Utrecht, The Netherlands.
2 Isolates studied in the pathogenicity test, * ex-type cultures.
Morphology
The Phaeoacremonium anamorphs were morphologically characterised on malt extract plates (MEA; 2 % malt extract, Oxoid Ltd., England; 1.5 % agar, Difco, USA) incubated at 25 °C in the dark for 2–3 wks as described in Mostert et al. (2006a). Teleomorph structures were described from PDA and SNA plates incubated for 3 or 2 mo at 25 °C in the laboratory under diffuse daylight. Vertical sections through perithecia and photographs of characteristic structures were made as described in Damm et al. (2007). Colony characters and pigment production on MEA, PDA and oatmeal agar (OA; Gams et al. 2007) incubated at 25 °C were noted after 8 and 16 d. Colony colours were determined using the colour charts of Rayner (1970). Cardinal temperatures for growth were determined by incubating MEA plates in the dark at temperatures ranging from 5 to 40 °C in 5 °C intervals, also including 37 °C, emulating human body temperature. Radial growth was measured after 8 d at 25 °C.
DNA isolation, amplification and analysis
Genomic DNA of all isolates was isolated from fungal mycelium grown on PDA plates, placed in a 1.5 mL tube with glass beads and 600 μL hexadecyltrimethyl ammonium bromide (CTAB) extraction buffer (0.2 M Tris, 1.4 M NaCl, 20 mM EDTA, 0.2 g/L CTAB) and crushed 3 min at 30 vibrations per second in a Retsch Mixer Mill MM301 (Retsch, Haan, Germany). Before adding 400 μL chloroform : isoamylalcohol (24 : 1), the tube was placed in a 65 °C water bath for 15 min. The fungal matrix was spun down for 5 min at 15 800 x g. The watery supernatant was transferred into a new centrifuge tube and cold ammonium acetate solution (final concentration 2.5 M) and 600 μL cold isopropanol were added. After 15 min incubation at room temperature, the precipitate was spun down for 5 min at 15 800 x g and the supernatant discarded. One millilitre cold 70 % ethanol was added to the pellet, spun down for 5 min at 15 800 x g and the supernatant discarded. The DNA pellet was dried and resuspended in 100 μL ddH2O.
The 5.8S ribosomal gene with the two flanking internal transcribed spacers (ITS1 and ITS2), the β-tubulin gene (TUB), the actin gene (ACT) and a partial sequence of the 18S rDNA gene (SSU) were amplified and sequenced using the primer pairs ITS-1F (Gardes & Bruns 1993) + ITS-4 (White et al. 1990), primers T1 (O’Donnell & Cigelnik 1997) + Bt2b (Glass & Donaldson 1995), ACT-512F + ACT-783R (Carbone & Kohn 1999) and NS1 + NS8 (White et al. 1990), according to the conditions and protocols explained in Mostert et al. (2006a). Additional primers used for sequencing the SSU were: NS2, NS3, NS4, NS5 (White et al. 1990). The ITS region was sequenced for preliminary identification of the fungi isolated from Prunus wood. Even though the ITS region has shown not to be robust for all species determination in the genus Phaeoacremonium (Groenewald et al. 2001, Mostert et al. 2005), we did found it valuable information for future ITS comparisons and lodged it in GenBank (Table 1).
The sequences generated in this study and additional sequences obtained from GenBank (www.ncbi.nlm.gov) were manually aligned using Sequence Alignment Editor v. 2.0a11 (Rambaut 2002). Pleurostomophora richardsiae (CBS 270.33) and Wuestneia molokaiensis (CBS 114877) were used as outgroup in the TUB and ACT phylogenies, while Cochliobolus sativus (U42479) and Pleospora betae (U3466) were used as outgroup in the SSU phylogeny. Two introns, only present in the outgroups (sequence positions 205–267, 388–421) were excluded from the SSU analysis. Phylogenetic analyses were performed using PAUP (Phylogenetic Analysis Using Parsimony) v. 4.0b10 (Swofford 2003). The TUB and ACT data were analysed for each region separately, as well as with a combined data set. Alignment gaps in all analyses were treated as missing data and all characters were unordered and of equal weight. Maximum parsimony analysis was performed using the heuristic search option with 100 random sequence additions and tree bisection and reconstruction (TBR) as the branch-swapping algorithm. The robustness of the trees obtained was evaluated by 1 000 bootstrap replications with 100 random sequence additions (Hillis & Bull 1993). Tree length, consistency index (CI), retention index (RI), rescaled consistency index (RC) and homoplasy index (HI) were calculated for the resulting tree. A partition homogeneity test with the same search criteria was conducted in PAUP to examine the possibility of a joint analysis of the TUB and ACT data sets. Sequences derived in this study were lodged at GenBank (Table 1) and the alignments in TreeBASE.
Pathogenicity tests
Preliminary pathogenicity tests were conducted with 14 taxa on detached apricot (cv. ‘Belida’, 4-year-old trees) and plum (cv. ‘Southern Bell’, 5-year-old trees) shoots. Depending on strain availability, one or two isolates per taxon were used and treated as sub-samples in the statistical analysis. Fresh vegetative shoots were collected from the trees shortly after harvest, cut into 12 cm pieces (5–8 mm diam), treated and inoculated with colonised agar plugs from 2-wk-old PDA cultures according to Damm et al. (2007), except for the surface sterilisation (40 s in 0.1 % solution of a patented didecyldimethylammonium chloride formulation, Sporekill, ICA International Chemicals Pty. Ltd., Stellenbosch, South Africa). Acremonium strictum (STE-U 6296) and uncolonised PDA plugs were used as negative controls. Shoots were incubated at 25 °C in moist chambers (> 93 % RH) for 2 wks, after which the bark was peeled off and lesions visible on the xylem tissue were measured. Each treatment combination consisted of one shoot, which was replicated four times in each of three blocks (= moist chambers). Re-isolations were made from the leading edges of lesions and the resulting cultures identified. The layout of the trial was a randomised block design. Lesion length data were subjected to analyses of variance using SAS v. 8.1 (SAS Institute, Cary, North Carolina USA) and Student’s t-test for Least Significant Difference was calculated at the 5 % significance level to compare the treatment means for the different taxa.
RESULTS
Phylogenetic analysis
Five SSU sequences produced in this study were added to the 32 sequences obtained from GenBank comprising an alignment of 2266 characters including the gaps, of which 244 characters were parsimony-informative, 80 variable (parsimony-uninformative) and 1942 constant. The heuristic search of the SSU data resulted in 12 most parsimonious trees (Length = 591 steps, CI = 0.633, RI = 0.772, RC = 0.489, HI = 0.367), of which one is shown in Fig. 1. The clades represent eight classes or families, respectively, within the Sordariomycetes. The unknown Phaeoacremonium species found on Prunus trees grouped with species of Togniniaceae, Togninia minima, T. novae-zealandiae and T. viticola, forming the Togniniaceae-subclade (87 % bootstrap support). This subclade grouped with other Diaporthales (65 %) forming a sister clade to the Calosphaeriales.
Fig. 1.
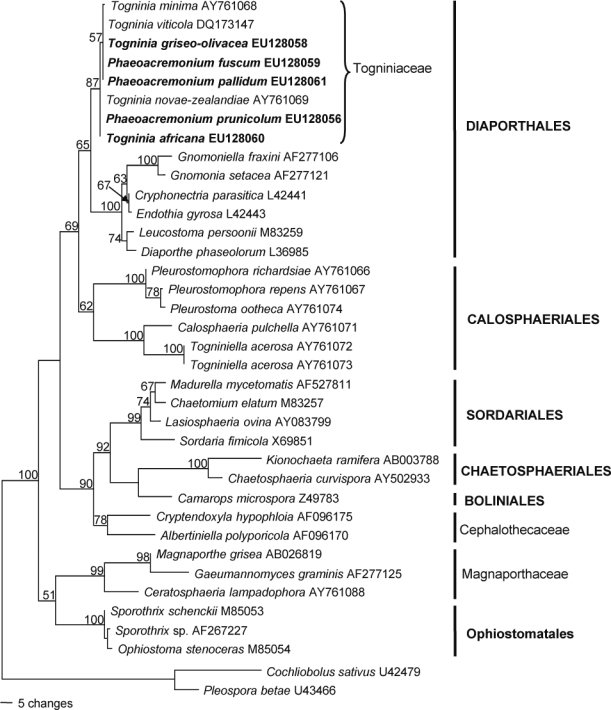
One of 12 most parsimonious trees obtained from heuristic searches of the SSU gene sequences (Length = 591 steps, CI = 0.633, RI = 0.772, RC = 0.489, HI = 0.367). Bootstrap support values (1 000 replicates) above 50 % are shown at the nodes. Cochliobolus sativus U42479 and Pleospora betae U3466 were used as outgroups. Isolates analysed in this study are emphasised in bold.
The partition homogeneity test (p-value = 0.192) led us to combine the TUB and ACT data sets (633 characters in data set 1, 298 in data set 2). A selection of 40 isolates was used for the phylogenetic analysis, with further 25 sequences being added from GenBank. The data set contained 931 characters including the gaps, of which 431 were parsimony-informative, 107 were variable and parsimony-uninformative, and 393 were constant. After a heuristic search, 312 most parsimonious trees with the same overall topology (differences only within species) were retained (Length = 1807 steps, CI = 0.522, RI = 0.833, RC = 0.435, HI = 0.478), of which one is shown in Fig. 2. The majority of the isolates grouped with known species of Togniniaceae: 10 of the isolates with Pm. scolyti (97 % bootstrap support), two with Pm. griseorubrum (99 %), one with Pm. subulatum (100 %), five with Pm. australiense (99 %), one with T. parasitica (100 %), one with T. fraxinopennsylvanica (100 %), two with T. viticola (97 %), seven with T. minima (100 %) and three with Pm. iranianum (100 %). A further eight isolates did not group with any known species. Isolates STE-U 6177 and 6364, as well as 5967 and 5968, formed two clades with 100 % bootstrap support that formed a sister group (90 %) to T. novae-zealandiae, T. fraxinopennsylvanica and T. argentinensis (94 %). Isolate STE-U 5966 grouped with these species (98 %) but formed a separate lineage. Isolate STE-U 6104 also formed a separate lineage in a clade (97 %) with Pm. angustius, T. viticola, T. austroafricana and Pm. theobromatis. Two isolates, STE-U 5969 and 6366, formed a sister group next to Pm. venezuelense (100 %).
Fig. 2.
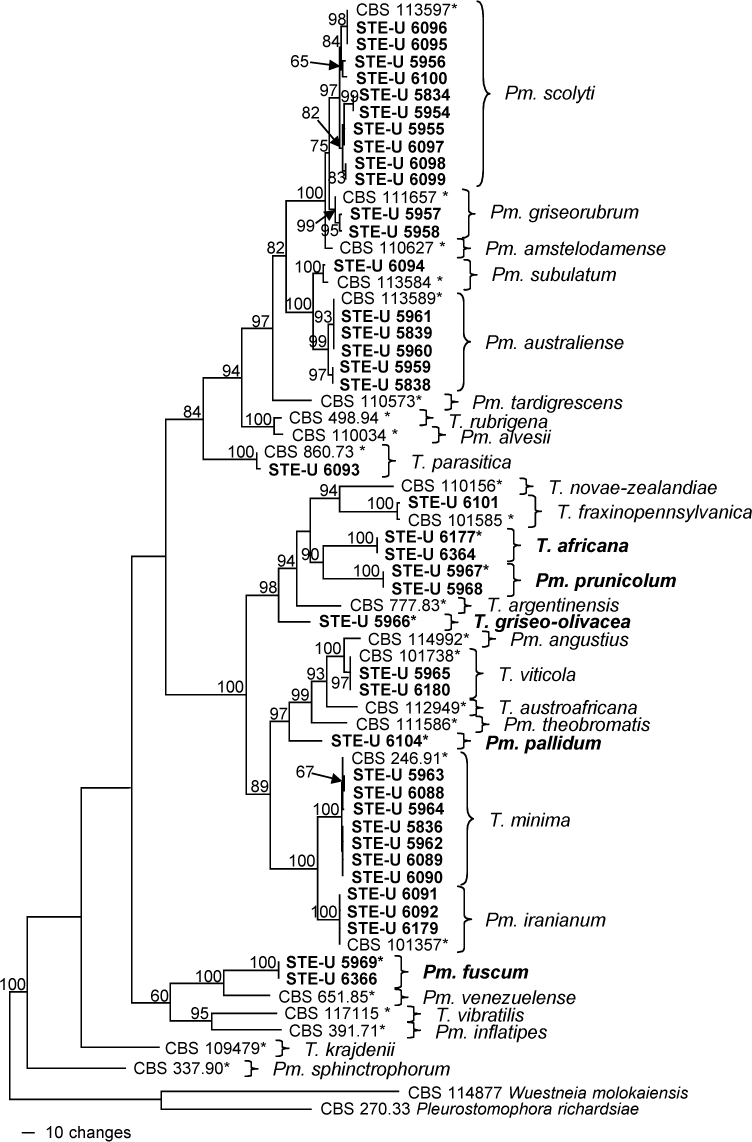
One of 312 most parsimonious trees obtained from heuristic searches of a combined alignment of the TUB and ACT gene sequences (Length = 1807 steps, CI = 0.522, RI = 0.833, RC = 0.435, HI = 0.478). Bootstrap support values (1 000 replicates) above 60 % are shown at the nodes. Pleurostomophora richardsiae and Wuestneia molokaiensis were used as outgroups. Isolates analysed in this study are emphasised in bold. Ex-type strains are indicated with asterisks.
Taxonomy
The 42 strains of Phaeoacremonium isolated from stone fruit wood (Table 1) could be assigned to 14 species based on the DNA sequence data generated and their morphology. Five species proved distinct from known species and are newly described below. Mostert et al. (2006a) developed a polyphasic, online identification system for species recognition (www.cbs.knaw.nl/phaeoacremonium/biolomics.aspx). The latter key has been updated to include all taxonomic novelties described in this study.
Phaeoacremonium fuscum L. Mostert, Damm & Crous, sp. nov. — MycoBank 505140; Fig. 3
Fig. 3.
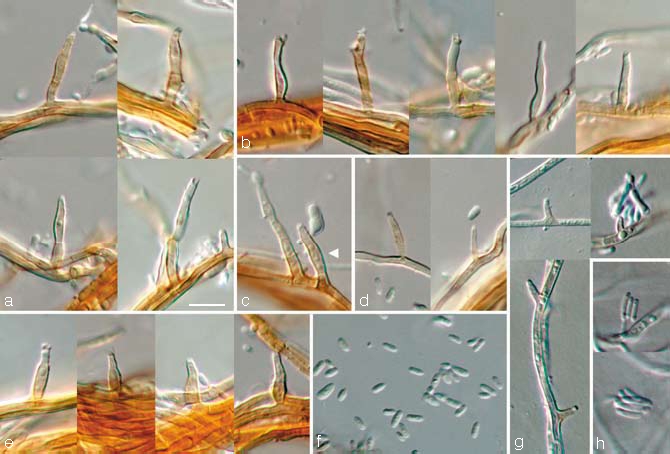
Phaeoacremonium fuscum. a–f. Aerial structures on MEA; a. conidiophores; b. type III phialides; c. conidiophore and type II phialide (indicated by arrow head); d. type I phialides; e. type II phialides; f. conidia. — g, h. Structures on the surface of and in MEA; g. adelophialides; h. conidia; all from CBS H-19944 (holotype); a–h: DIC. — Scale bar: a = 10 μm, applies to a–h.
Phaeoacremonio venezuelensi simile, sed coloniis in cultura (OA) fuscisnigris vel isabellinis, conidiophoris brevioribus et phialibus typorum I et II.
Etymology. Named after its dark brown colonies (fuscus Lat. = dark brown).
Aerial structures — Mycelium consisting of branched, septate hyphae that occur singly or in bundles of up to 10; hyphae tuberculate with warts up to 2 μm diam, verruculose, orange-brown and 1–2 μm wide. Conidiophores short and usually unbranched, up to 3-septate, bearing 1–2 terminal phialides, sometimes showing percurrent rejuvenation, (14–)17–28(–40) (av. 23) μm long and (1.5–)2(–2.5) (av. 2) μm wide. Phialides terminal or lateral, mostly monophialidic, sometimes polyphialidic, sparsely tuberculate to verruculose, orange-brown, sometimes hyaline; collarettes, slightly flaring, 1–2 μm long and 2–2.5 μm wide; type I phialides cylindrical, occasionally widened at the base, tapering towards the apex, (2–)4–7(–8) × 1–1.5(–2) (av. 5 × 1.5) μm; type II phialides subcylindrical or navicular, some elongate-ampulliform and attenuated at the base, tapering towards the apex, (7–)9–11(–12) × 1.5–2(–2.5) (av. 10 × 2) μm; type III phialides mostly subcylindrical, some navicular, 13–17(–20) × (1.5–)2(–2.5) (av. 15 × 2) μm, gradually tapering towards the apex. Type I and II phialides most common. Conidia hyaline, oblong-ellipsoidal some reniform, (3.5–)4–5 × (1–)1.5(–2) (av. 4 × 1.5) μm, L/W = 3.4.
On surface or submerged in the agar — Phialides pale orange-brown or hyaline, cylindrical, 1–4(–7) × 1–1.5(–2) (av. 3 × 1) μm. Conidia hyaline, cylindrical or allantoid, (4–)5–7(–8) × (1–)1.5–2 (av. 6 × 2) μm, L/W = 3.3.
Cultural characteristics — Colonies reaching a radius of 13.5–14 mm after 8 d at 25 °C. Minimum temperature for growth 10 °C, optimum 30 °C, maximum 37 °C. Colonies on MEA flat, mostly felty with a few woolly tufts, with entire margin; after 8 d and 16 d colonies dark mouse-grey (13″″′k) to greyish sepia (15″″I) becoming buff (19″d) towards the margin above, reverse same. Colonies on PDA flat, felty to powdery, with entire margin; after 8 d and 16 d fawn (13″′i) to vinaceous-buff (17″i), similar in reverse, becoming sepia (15″m) to fawn (13″′i) after 16 d. Colonies on OA flat, felty to woolly, with entire margin; after 8 d brown-vinaceous (5″′m) to isabelline (17″i), after 16 d fuscous-black (7″″k) to isabelline (17″i) above. A pale brown pigment produced after 16 d on PDA.
Specimen examined. South Africa, Limpopo province, Mookgopong, from small dark brown central V-shaped necrosis close to canker developing from old pruning wound in wood of Prunus salicina, 31 Aug. 2004, U. Damm, CBS H-19944 holotype, culture ex-type CBS 120856 = STE-U 5969.
Notes — The various species that have grey-brown colonies and a growth rate that falls in the range of Pm. fuscum include Pm. inflatipes, Pm. iranianum, Pm. krajdenii, Pm. sphinctrophorum and Pm. venezuelense (Mostert et al. 2006a). Of these, Pm. venezuelense also has orange-brown mycelium, but can be distinguished by the predominance of type III phialides and hyaline phialides on and in the agar, in comparison with the predominance of phialide type I and II and often pale orange-brown phialides of Pm. fuscum. Furthermore, the maximum growth temperature of Pm. fuscum was at 37 °C, in comparison with 40 °C in the case of Pm. venezuelense.
Phaeoacremonium pallidum Damm, L. Mostert & Crous, sp. nov. — MycoBank 505141; Fig. 4
Fig. 4.
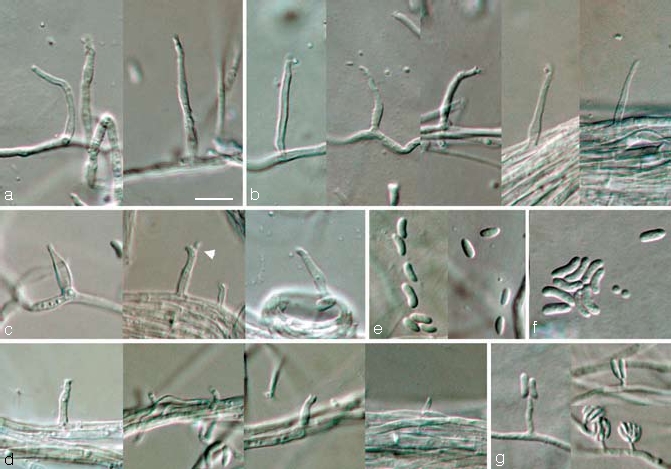
Phaeoacremonium pallidum. a–e. Aerial structures on MEA; a. conidiophores; b. type III phialides; c. type II phialides (arrow head indicates polyphialide); d. type I phialides; e. conidia. — f, g. Structures on the surface of and in MEA; f. conidia; g. adelophialides with conidia; all from CBS H-19945 (holotype). a–g: DIC. — Scale bar: a = 10 μm, applies to a–g.
Phaeoacremonio angustio simile, sed conidiis latioribus, in cultura (OA) coloniis albis, ad 20 °C optime crescentibus.
Etymology. Named after its pale colonies (pallidus Lat. = pale).
Aerial structures — Aerial mycelium sparse, consisting of branched, septate hyphae that occur singly or in bundles of up to 21; hyphae tuberculate with warts up to 2 μm diam, verruculose to smooth, hyaline and 1–2.5 μm wide. Conidiophores short and usually unbranched, up to 3-septate, bearing one terminal phialide, (14–)17–36(–40) (av. 27) μm long and 1.5–2 (av. 2) μm wide. Phialides terminal or lateral, mostly monophialidic, sometimes polyphialidic, sparsely tuberculate to verruculose, hyaline; collarettes slightly flaring, 1–1.5 μm long and 1 μm wide; type I phialides most predominant, cylindrical, occasionally widened at the base, tapering towards the apex, (1.5–)3–6(–7) × 1–1.5 (av. 4 × 1) μm; type II phialides, subcylindrical or navicular, tapering towards the apex, (7–)9–11(–12) × 1.5–2 (av. 10 × 1.5) μm; type III phialides cylindrical or subcylindrical, 16–19 × 1 (av. 18 × 1) μm, gradually tapering towards the apex. Conidia hyaline, oblong-ellipsoidal or allantoid to reniform, (3.5–)4–6(–7) × 1.5–2 (av. 5 × 2) μm, L/W = 2.6.
On surface or submerged in the agar — Phialides hyaline, cylindrical to subcylindrical, (1–)2–6(–10) × 1–1.5 (av. 4 × 1) μm. Conidia hyaline, allantoid or cylindrical, (4–)6–8(–11) × (1.5–)2(–2.5) (av. 7 × 2) μm, L/W = 3.6.
Cultural characteristics — Colonies reaching a radius of 7.5–8.5 mm after 8 d at 25 °C. Minimum temperature for growth 10 °C, optimum 20 °C, maximum 30 °C. Colonies on MEA flat, mostly felty with very little aerial mycelium, appearing yeast-like, with entire margin; after 8 d and 16 d colonies buff (19″d), similar in reverse. Colonies on PDA flat, felty, with entire to lobate margin; after 8 d buff (19″d), reverse same. Colonies on OA flat, felty with entire margin; after 8 d and 16 d white above.
Specimen examined. South Africa, Western Cape province, Bonnievale, from irregular necrosis with dark brown annual rings close to pruning wound in wood of Prunus armeniaca, 23 Aug. 2005, U. Damm, CBS H-19945 holotype, culture ex-type CBS 120862 = STE-U 6104.
Notes — Colonies do not have a distinct colour ranging from buff (on MEA and PDA) to white (on OA). Of the various pale-coloured species, Pm. pallidum resembles Pm. angustius, especially in the predominance of the type I phialide and the shape of the type II phialides that are subcylindrical or navicular (Mostert et al. 2006a). Phaeoacremonium pallidum and T. vibratilis can be distinguished by their optimum growth temperature at 20 °C (Réblová & Mostert 2007). Phaeoacremonium pallidum can be distinguished by the presence of all three phialide types in comparison with Phaeoacremonium vibratilis having type I and type II phialides.
Phaeoacremonium prunicolum L. Mostert, Damm & Crous, sp. nov. — MycoBank 505139; Fig. 5
Fig. 5.
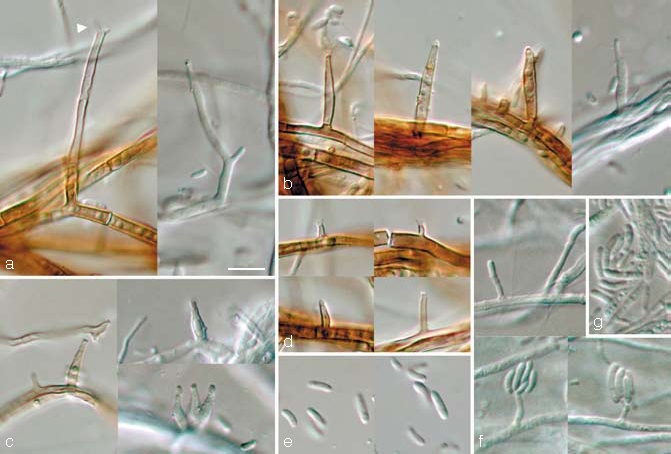
Phaeoacremonium prunicolum. a–e. Aerial structures on MEA; a. conidiophores (arrow head indicates polyphialide); b. type III phialides; c. type II phialides; d. type I phialides; e. conidia. — f, g. Structures on the surface of and in MEA; f. adelophialides with conidia; g. conidia; all from CBS H-19943 (holotype); a–g: DIC. — Scale bar: a = 10 μm, applies to a–g.
Phaeoacremonio novae-zealandiae simile, sed conidiis longioribus, in cultura (OA) pigmento flavido nullo.
Etymology. Named after its host, Prunus.
Aerial structures — Mycelium consisting of branched, septate hyphae that occur singly or in bundles of up to 11; hyphae tuberculate with warts up to 2 μm diam, verruculose, medium brown and 1–2 μm wide. Conidiophores short and usually unbranched, up to 2-septate, mostly bearing one terminal phialide, 14–37(–70) (av. 26) μm long and 1.5–3 (av. 2) μm wide. Phialides terminal or lateral, sometimes polyphialidic, sparsely tuberculate to verruculose or smooth, pale brown to subhyaline; collarettes slightly flaring, 1–1.5 μm long and 1–1.5 μm wide; type I phialides cylindrical, occasionally widened at the base, tapering towards the apex, (2–)3–6(–9) × 1–1.5 (av. 5 × 1) μm; type II phialides elongate-ampulliform and attenuated at the base, or navicular, tapering towards the apex, (7–)9–12(–13) × 1.5–2(–2.5) (av. 11 × 2) μm; type III phialides subcylindrical to navicular, (13–)14–18(–21) × (1.5–)2(–3) (av. 16 × 2) μm, gradually tapering towards the apex. Type I and III phialides most common. Conidia hyaline, oblong-ellipsoidal, cylindrical or allantoid, 5–7(–8) × 1–1.5(–2) (av. 6 × 1.5) μm, L/W = 3.9.
On surface or submerged in the agar — Phialides hyaline, cylindrical, (1–)2–6(–10) × 1(–1.5) (av. 4 × 1) μm. Conidia hyaline, cylindrical or allantoid, 6–8(–10) × 1.5(–2) (av. 7 × 1.5) μm, L/W = 4.6.
Cultural characteristics — Colonies reaching a radius of 8.5–10 mm in 8 d at 25 °C. Minimum temperature for growth 10 °C, optimum 25 °C, maximum 30 °C. Colonies on MEA flat, mostly felty to short woolly, with entire margin; after 8 d and 16 d colonies olivaceous grey (21″″′i) to buff (19″d), similar in reverse. Colony characters on PDA similar to those on MEA. Colonies on OA flat, felty with woolly tufts, with entire margin; after 8 d pale buff (19″f), after 16 d olivaceous green (23″′) to pale buff (19″f).
Specimens examined. South Africa, Limpopo province, Mookgopong, from irregularly roundish, reddish to greenish brown necrosis in wood of Prunus salicina close to pruning wound, 31 Aug. 2004, U. Damm, CBS H-19943 holotype, culture ex-type CBS 120858 = STE-U 5967; Mookgopong, from reddish brown V-shaped necrosis in wood of Prunus salicina close to pruning wound, 31 Aug. 2004, U. Damm, STE-U 5968.
Notes — Phaeoacremonium prunicolum can be distinguished by having olive-grey colonies on MEA, PDA as well as OA. Similar species such as Pm. novae-zealandiae also have olive-grey colonies on MEA, but can be differentiated by its ability to produce a yellow pigment on OA (Mostert et al. 2006a), whereas this is absent in Pm. prunicolum.
Togninia africana Damm, L. Mostert & Crous, sp. nov. — MycoBank 505138; Fig. 6
Fig. 6.

Togninia africana teleomorph and anamorph states. a. Perithecia on SNA; b. peridium; c. ascospores; d. longitudinal section through perithecium; e. asci attached to ascogenous hyphae and paraphyses; f. asci; g. asci attached to ascogenous hyphae and paraphyses (remnant bases indicated by arrow head). — h–m. Aerial structures on MEA; h, i. conidiophores; j. type III phialides; k. type II phialides; l. type I phialides; m. conidia. — n, o. Structures on the surface of and in MEA; n. adelophialides; o. conidia; all from CBS H-19942 (holotype); a: DM, b–o: DIC. — Scale bars: a = 500 μm; b = 20 μm; c = 2.5 μm; d = 200 μm; f = 5 μm; e, g, h = 10 μm; h applies to h–o.
Anamorph. Phaeoacremonium sp.
Togniniae viticolae similis, sed peritheciis majoribus et ascosporis guttulatis.
Etymology. Named after the continent of origin, Africa.
Ascomata — Perithecia formed on SNA containing the pieces of necrotic wood after 2 mo of incubation; non-stromatic, solitary, superficial to semi-immersed, subglobose to obpyriform, (215–) 270–395(–440) μm diam, basal part (270–)315–440(–460) μm tall. Wall consisting of two regions of textura angularis: outer region dark brown, individual cells hardly visible, 15–25 μm thick; inner region pale brown becoming hyaline towards the centre, 5–7 cell layers and 10–15 μm thick. Surface covered with brown, septate hyphal appendages that become hyaline towards the tip. Perithecial neck curved, 1 per perithecium, 550–1000 (av. 720) μm long, 70–130 μm wide at the base, 35–65 μm wide at the tip. Paraphyses hyaline, septate, unbranched, cylindrical with round tips, slightly constricted at septa, 30–130 (av. 80) μm long, narrowing from 3–6.5 μm at the base to 1.5–4 μm at the apex, persistent. Asci arising in acropetal succession from sympodially proliferating ascogenous hyphae that appear spicate when mature, hyaline, clavate, with bluntly rounded apex and base, (16–)20–25.5(–26) × (3.5–)4–5(–5.5) (av. 22.5 × 4.5) μm. Ascogenous hyphae hyaline, branched, smooth-walled, 15–28 × 1.5–2.5 μm, remnant bases 4–6.5 × 2–3.5 μm. Ascospores aseptate, hyaline, smooth-walled, ellipsoidal to subcylindrical with rounded ends, sometimes slightly bent, containing small guttules at the ends, biseriate, (2.5–)3.5–4.5(–5.5) × 1.5–2(–2.5) (av. 4 × 1.8) μm.
Aerial structures — Mycelium consisting of branched, septate hyphae that occur singly or in bundles of up to 22; hyphae tuberculate, with warts up to 2 μm diam, verruculose to smooth, mostly hyaline, some pale brown, 1–3 μm wide. Conidiophores short and usually unbranched, up to 2-septate, sometimes constricted at septa, bearing one terminal phialide, sometimes showing percurrent rejuvenation, (13–)15–23(–24) (av. 19) μm long and 2 μm wide. Phialides terminal or lateral, mostly monophialidic, sometimes polyphialidic, sparsely tuberculate to verruculose, hyaline, sometimes subhyaline; collarettes slightly flaring, 1–2 μm long and 1 μm wide; type I phialides most predominant, cylindrical, occasionally widened at the base, tapering towards the apex, (2–)3–5(–7) × 1–1.5(–2) (av. 4 × 1) μm; type II phialides subcylindrical, some elongate-ampulliform and attenuated at the base, (4–)7–11(–12) × (1–)1.5–2(–3) (av. 9 × 2) μm; type III phialides subcylindrical or navicular, 13–20(–28) × (1.5–)2(–2.5) (av. 16 × 2) μm. Type II and III phialides often strongly tapered towards the apex. Conidia hyaline, cylindrical or allantoid, (4.5–)5–8(–12) × 1.5–2 (av. 7 × 1.5) μm, L/W = 4.
On surface or submerged in the agar — Phialides hyaline, cylindrical, 1–7(–18) × 1–1.5(–2) (av. 4 × 1) μm. Conidia hyaline, cylindrical or allantoid, (5–)6–9(–12) × 1.5–2 (av. 8 × 2) μm, L/W = 4.3.
Cultural characteristics — Colonies reaching a radius of 9–9.5 mm after 8 d at 25 °C. Minimum temperature for growth 10 °C, optimum 25 °C, maximum 30 °C. Colonies on MEA flat, mostly felty with a few woolly tufts, with entire margin; after 8 d and 16 d colonies buff (19″d), similar in reverse. Colonies on PDA flat, felty, with entire margin; after 8 d buff (19″d), similar in reverse; after 16 d vinaceous-buff (15″′d) becoming buff (19″d) with straw undertone, reverse honey (19″b) to buff (19″d). Colonies on OA flat, felty to woolly, with entire margin; after 8 d and 16 d olivaceous-buff (21″′d) to buff (19″d) with straw (21′d) undertone above. Prominent yellow pigment produced on MEA, OA and after 16 d also on PDA.
Specimen examined. South Africa, Western Cape province, Montagu, from greenish brown V-shaped necrosis in wood under canker developing from a broken-off twig of Prunus armeniaca, 23 Aug. 2005, U. Damm, CBS H-19942 holotype, culture ex-type CBS 120863 = STE-U 6177.
Notes — Togninia africana can be distinguished by its buff coloured colonies together with the production of a yellow pigment on MEA. Phaeoacremonium angustius is similar to the Phaeoacremonium anamorph of T. africana in the pale coloured colonies, predominance of the type I phialide and production of yellow pigment on OA. However, Phaeoacremonium angustius forms yellow-white to grey-red colonies on OA (Mostert et al. 2006a) in comparison with olivaceous-buff colonies of T. africana. The type II and III phialides taper sharply towards the apex, similar to the subulate phialides found in Pm. subulatum. Only T. viticola has both perithecia that often exceed 300 μm diam and mainly ellipsoidal ascospores as found in T. africana. However, T. viticola has one to three necks per perithecium and ascospores that can also be curved, whereas T. africana has one neck per perithecium and ascospores that are predominantly straight.
Togninia griseo-olivacea Damm, L. Mostert & Crous, sp. nov. — MycoBank 505137; Fig. 7
Fig. 7.
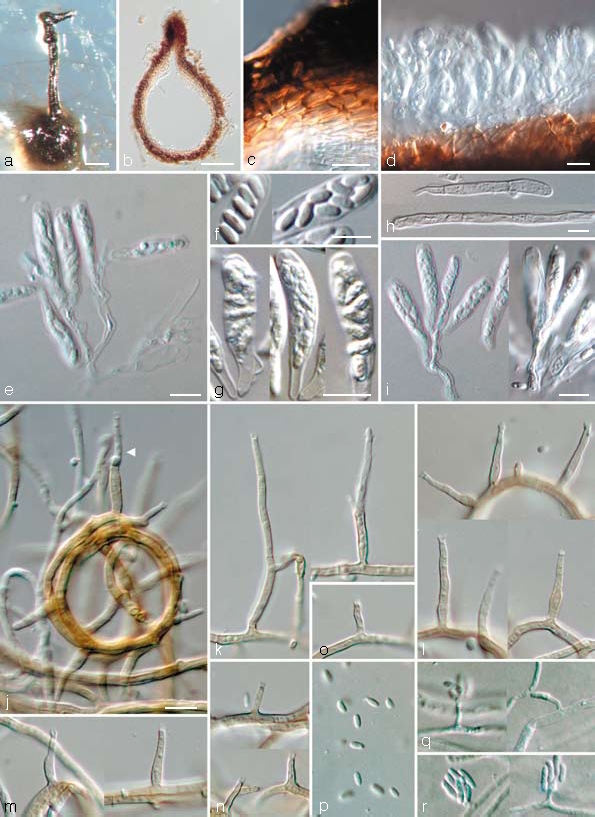
Togninia griseo-olivacea teleomorph and anamorph states. a. Perithecium on PDA; b. longitudinal section through perithecium; c. peridium; d, e. asci attached to ascogenous hyphae; f. ascospores; g. asci; h. paraphyses; i. asci attached to ascogenous hyphae. — j–p. Aerial structures on MEA; j. ring-like growth of mycelium with conidiophore (indicated by arrow head); k. conidiophores; l. type III phialides; m. type II phialides; n, o. type I phialides; p. conidia. — q, r. Structures on the surface of and in MEA; q. adelophialides with conidia; r. conidia; all from CBS H-19941 (holotype). a: DM, b–r: DIC. — Scale bars: a = 100 μm; b = 50 μm; c–i = 5 μm; j = 10 μm; j applies to j–r.
Anamorph. Phaeoacremonium sp.
Togniniae fraxinopennsylvanicae similis, sed peritheciis minoribus cum collo breviore.
Etymology. Named after its greyish olivaceous colonies (griseolus-olivaceus Lat. = greyish olivaceous).
Ascomata — Single-conidial isolates gave rise to perithecia on PDA after 3 mo; non-stromatic, solitary, superficial, globose to subglobose, dark brown, (150–)225 μm diam, (150–)200 μm tall (dimensions of only one mature perithecium available; measurements of immature perithecia in brackets). Wall consisting of two regions of textura angularis: outer region dark brown, 4–7 cells and 5–15 μm thick; inner region pale brown becoming hyaline towards the centrum, 5–6 cell layers and 10–20 μm thick. Surface covered with brown, septate hyphal appendages. Perithecial neck black, straight, 1 per perithecium, (200–)460 μm long, (40–)50 μm wide at the base, (25–)37 μm wide at the tip, dividing into two near the tip. Paraphyses hyaline, septate, unbranched, cylindrical with rounded tips, slightly constricted at septa, 30–70 (av. 45) μm long, 2–4.5 μm wide at the base. Asci arising in acropetal succession from sympodially proliferating ascogenous hyphae that appear spicate when mature, hyaline, clavate, rounded apex and base, 15–16 × 3–4 (av. 15.5 × 3.5) μm. Ascogenous hyphae hyaline, branched, smooth-walled, 13–23 × 2–3 μm, remnant bases only occasionally observed, 6–9(–12) × 3–4(–5) μm. Ascospores aseptate, hyaline, smooth-walled, ellipsoidal to reniform with rounded ends, sometimes containing small guttules at the ends, biseriate, 3–5(–6) × 1.5–2 (av. 3.5 × 1.5) μm.
Aerial structures — Mycelium consisting of branched, septate hyphae that occur singly or in bundles of up to 17; hyphae tuberculate with warts up to 2 μm diam, verruculose to verrucose, medium brown, 1–2 μm wide. Conidiophores short and usually unbranched, up to 2-septate, bearing one terminal phialide, (20–)22–35(–42) (av. 29) μm long and 1.5–2 (av. 2) μm wide. Phialides terminal or lateral, mostly monophialidic, sometimes polyphialidic, sparsely tuberculate to verruculose, medium to pale brown, sometimes hyaline; collarettes slightly flaring, 1 μm long and 1–2 μm wide; type I phialides predominant, cylindrical, occasionally widened at the base, tapering towards the apex, (2–)3–6(–9) × 1–1.5(–2) (av. 4 × 1) μm; type II phialides, elongate-ampulliform and attenuated at the base, or subcylindrical, tapering towards the apex, (7–)9–12(–13) × 1.5–2(–2.5) (av. 11 × 2) μm; type III phialides mostly subcylindrical, some navicular, (13–)14–18(–20) × (1.5–)2 (av. 16 × 2) μm, gradually tapering towards the apex. Conidia subhyaline, oblong-ellipsoidal or reniform, (3.5–)4–5(–6) × 1.5–2 (av. 5 × 1.5) μm, L/W = 2.8.
On surface or submerged in the agar — Phialides hyaline, cylindrical, (1.5–)2–6(–10) × 1(–1.5) (av. 4 × 1) μm. Conidia hyaline, cylindrical, reniform or allantoid, (5–)6–7(–8) × 1.5–2 (av. 6 × 2) μm, L/W = 3.6.
Cultural characteristics — Colonies reaching a radius of 9–9.5 mm in 8 d at 25 °C. Minimum temperature for growth 10 °C, optimum 25 °C, maximum 30 °C. Colonies on MEA flat, mostly felty, becoming woolly with age, with entire margin; after 8 d colonies buff (19″d) above, in reverse olivaceous (21″k) to buff (19″d); after 16 d mouse-grey (13″″′i) to buff (19″d) above, similar in reverse. Colonies on PDA flat, felty with a few woolly tufts, with entire margin; after 8 d buff (19″d) with a few olivaceous (21″k) spots above, similar in reverse; after 16 d olivaceous (21″k) ring in centre becoming buff (19″d) towards margin, similar in reverse. Colonies on OA flat, felty with woolly tufts and entire margin; after 8 d and 16 d isabelline (17″i) to buff (19″d) above.
Specimen examined. South Africa, Limpopo province, Mookgopong, from reddish brown to greenish irregular necrosis with darker discoloured annual rings in wood of Prunus armeniaca close to pruning wound with resin exudation, 31 Aug. 2004, U. Damm, CBS H-19941 holotype, culture ex-type CBS 120857 = STE-U 5966.
Notes — The various Togninia and Phaeoacremonium species that have brownish coloured colony centres with a broad buff ring towards the margin on MEA include Pm. australiense, T. novae-zealandiae and T. parasitica. Togninia griseo-olivacea can be distinguished from Pm. australiense and T. novae-zealandiae by not producing a yellow pigment in OA and, additionally, from T. novae-zealandiae by forming shorter asci. Togninia griseo-olivacea differs from T. parasitica in the shape of the ascospores that are usually allantoid in T. parasitica and ellipsoidal to reniform in T. griseo-olivacea. The Phaeoacremonium anamorph of T. griseo-olivacea does not have such long conidiophores as T. parasitica (av. length 47 μm), prominent warts (up to 3 μm diam) or the predominant type III phialides found in T. parasitica (Mostert et al. 2006a). The Togninia species that have relatively short asci and short oblong-ellipsoidal ascospores with ranges that overlap with that of T. griseo-olivacea include T. argentinensis, T. austroafricana, T. fraxinopennsylvanica and T. novae-zealandiae. The size ranges of the asci and ascospores mostly resemble that of T. fraxinopennsylvanica. Togninia griseo-olivacea can be distinguished by having on average smaller perithecia and a shorter neck length.
Pathogenicity
All the isolates had been obtained from discoloured wood inside living branches of trees of different Prunus species. In cross-section, the symptomatic wood had either irregularly shaped or V-shaped necrotic lesions and were situated close to old pruning wounds and/or cankers, sometimes also associated with gummosis. In plum wood, such lesions were often reddish brown in the centre and greenish towards the margin. Phaeoacremonium species were mostly isolated from these lesions in combination with other fungi, for example Botryosphaeriaceae and Schizophyllum commune.
Analyses of variance of the lesion length data on apricot and plum cane sections indicated a significant treatment effect (P < 0.0001; Anova tables not shown). All species, except Pm. fuscum and Pm. pallidum, caused lesions in the xylem of plum shoots that were significantly longer than the controls (Table 2). The re-isolation frequencies from plum were between 70 % and 100 % for all fungi, except Pm. australiense and Pm. pallidum. Five species caused lesions on apricot shoots that were significantly longer than the controls: T. parasitica, Pm. ira-nianum, Pm. subulatum, Pm. griseorubrum and T. africana. Additionally, T. parasitica, Pm. iranianum and Pm. griseorubrum also caused lesions that were visible on the bark surface of more than half of the apricot canes; mostly dark brown rings around the inoculation site. Other species formed surface lesions on apricot canes less frequently. On plum, surface lesions on the bark were only occasionally observed. Most of the species were re-isolated from apricot wood in frequencies below 50 %. No Phaeoacremonium species were isolated from the negative controls. Togninia parasitica was the species that induced the longest lesions on plum and apricot wood.
Table 2.
Means of lesion lengths caused by different Phaeoacremonium species on detached green plum and apricot shoots, and mean re-isolation frequencies of these species from observed lesions.
| Fungal species | Mean of lesion length (mm)1 |
Mean of re-isolation frequency (%) |
||
|---|---|---|---|---|
| Plum | Apricot | Plum | Apricot | |
| Togninia parasitica | 55.0 a | 63.2 a | 75 | 8 |
| Phaeoacremonium iranianum | 36.1 bc | 56.5 ab | 80 | 38 |
| Pm. subulatum | 33.5 bc | 57.7 ab | 83 | 8 |
| Pm. griseorubrum | 32.8 bc | 57.2 ab | 70 | 55 |
| T. africana | 33.1 bc | 53.9 ab | 75 | 50 |
| T. griseo-olivacea | 39.4 b | 38.8 bcd | 92 | 50 |
| T. minima | 37.8 bc | 45.7 abc | 75 | 29 |
| T. viticola | 35.8 bc | 47.6 abc | 84 | 8 |
| Pm. prunicolum | 35.2 bc | 33.4 cd | 84 | 25 |
| T. fraxinopennsylvanica | 33.9 bc | 45.7 abc | 80 | 13 |
| Pm. scolyti | 33.2 bc | 50.6 abc | 80 | 17 |
| Pm. australiense | 33.0 bc | 44.2 abcd | 54 | 38 |
| Pm. pallidum | 31.5 bcd | 38.7 bcd | 25 | 0 |
| Pm. fuscum | 25.1 cd | 39.3 bcd | 100 | 8 |
| Acremonium strictum | 19.8 de | 32.4 cd | 25 | 0 |
| Agar plug | 12.6 e | 24.8 d | – | – |
| LSD (P < 0.05) | 12.0 | 19.9 | ||
1 Means followed by the same letter are not significantly different (P < 0.05).
DISCUSSION
Although Phaeoacremonium species have previously been relatively unknown from stone fruit trees, this study reveals these hosts to harbour a broad diversity (14 species) and abundance (present in 33 of the 257 specimens, with 3 specimens occupied by more than one Phaeoacremonium species) of this genus from Prunus trees in South Africa. Most species were found in plum (8 species) and apricot (8 species) wood, while peach and nectarine were rarely colonised by Phaeoacremonium (2 species). However, there were no Phaeoacremonium species known on Prunus salicina, P. persica and P. persica var. nucipersica before the onset of this study. Most species found on P. armeniaca also represent new reports on Prunus, except for T. parasitica and T. minima (Hawksworth et al. 1976, Mostert et al. 2006a). We also observed regional differences. While the species found in the Cape Winelands (Paarl, Stellenbosch, Franschhoek) comprised only known species, three of the four species found in the Limpopo province were new to science. Reasons for this could be the relative remoteness of the area compared to the Western Cape province (hosts not previously sampled for microfungi), and the different climate (summer-rainfall area vs winter-rainfall area).
The dominant species on stone fruit trees were Pm. scolyti and T. minima. Togninia minima is known as one of the causal organisms of Petri disease and esca on grapevines, and has previously been found on V. vinifera in South Africa (Groenewald et al. 2001, Mostert et al. 2003). In this study, T. minima was found on three Prunus species in the Western Cape province of South Africa. Phaeoacremonium scolyti is also known on V. vinifera in South Africa (Mostert et al. 2003). In our study, the fungus had the broadest host range and was found on all Prunus species sampled. According to Mostert et al. (2006b), Pm. scolyti could be dispersed between woody hosts by bark beetles, as it has previously been isolated from beetles (Kubátová et al. 2004). Rumbos (1986) assumed Pm. parasiticum to be spread throughout a cherry orchard by bark and wood-boring beetles. Phaeoacremonium scolyti was also the only species that occurred in different orchards in the Western Cape and Limpopo provinces of South Africa.
Notwithstanding the new taxa, several known species were also found on Prunus, four of which comprise new reports for South Africa. Phaeoacremonium australiense, Pm. iranianum and T. fraxinopennsylvanica had been previously reported from grapevines in other countries (Groenewald et al. 2001, Mostert et al. 2006a, Gramaje et al. 2007). This study shows, however, that these fungi also occur on Prunus species in South Africa. Phaeoacremonium australiense was even quite common in one orchard in the Western Cape. Phaeoacremonium griseorubrum, which was previously known only from human infections in Japan and the United States (Mostert et al. 2005), was found here to also occur in wood of Prunus salicina in South Africa.
All Phaeoacremonium spp. were associated with wood decay symptoms on Prunus trees. According to the pathogenicity test, most species were shown to be potentially pathogenic to plum, while only a few species were shown to be potentially pathogenic to apricot. The species most commonly isolated from Prunus wood, Pm. scolyti, was not the most virulent species. Three of the species, Pm. subulatum, T. parasitica and T. viticola, had been tested on grapevines in greenhouse and field experiments (Halleen et al. 2007). While in our study, T. parasitica was the most virulent Phaeoacremonium species on apricot and plum wood, it was less virulent on grapevines trunks than T. viticola and Pm. subulatum (Halleen et al. 2007). The relevance of T. parasitica in die-back disease on Prunus species is uncertain, since only one isolate was obtained. Rumbos (1986) showed Pm. parasiticum to be pathogenic on cherry, apricot, olive and peach. Togninia minima and T. parasitica also caused discolorations in wood of potted kiwifruit vines (Di Marco et al. 2004). Associated field symptoms and the pathogenicity test indicate a possible pathogenic relationship of these Phaeoacremonium species and Prunus trees.
Only one of the five new Phaeoacremonium species, namely Pm. fuscum, had a maximum growth temperature of 37 °C in comparison with 30 °C for the other species. The ability to grow at 37 °C suggests that it has the potential to survive at human body temperature, while the other species appear to be strictly plant-associated taxa.
Phaeoacremonium species are commonly isolated from healthy (Halleen et al. 2003) and symptomatic grapevines (Mostert et al. 2006a). In grapevines they occur in association with other fungi, namely Phaeomoniella chlamydospora, Fomitiporia species and to a lesser extent, Stereum hirsutum (Larignon & Dubos 1997, Mugnai et al. 1999, Fischer 2002). In this study, Phaeoacremonium species have mostly been found in combination with other fungi. Because Phaeoacremonium species that had frequently been isolated from diseased vines only gave a weak host response in pathogenicity trials on grapevines, Halleen et al. (2007) assumed that they might not be able to cause disease on their own, but required synergism with other fungi of this disease complex. Some of these fungi have been shown to be associated with stress-related diseases (Ferreira et al. 1999), and Halleen et al. (2007) only observed a clear disease expression in a field-trial monitored over a longer period.
Except for Pm. griseorubrum, all known Phaeoacremonium species found on Prunus, had previously been isolated from Vitis vinifera (Crous et al. 1996, Groenewald et al. 2001, Mostert et al. 2005, 2006a). Phaeoacremonium species are known as causal organisms of Petri disease, destructive grapevine trunk disease (decline, die-back), and young grapevine decline (Scheck et al. 1998). Petri disease is considered as a major reason for the death of vines in nurseries and young vineyards in the Western Cape province of South Africa (Halleen et al. 2003). Togninia minima is well-known on Vitis from South Africa (Mostert et al. 2003), and T. minima, T. parasitica, T. viticola and Pm. subulatum have been shown to be true wood colonisers and vascular pathogens of grapevines (Sparapano et al. 2001, Halleen et al. 2007). Based on the results obtained in the present study on different Prunus species, Phaeoacremonium species seem to lack host-specificity. Since a number of fungi, which have previously been reported to be pathogenic to grapevines were isolated from the wood of Prunus spp., stone fruit orchards should be considered as potential inoculum sources of grapevine trunk disease pathogens. Pathogenic or saprobic survival of these grapevine trunk disease pathogens in stone fruit orchards could have serious implications for disease management practices employed on farms where vineyards are planted adjacent to fruit tree orchards.
Acknowledgments
The authors acknowledge the University of Stellenbosch, National Research Foundation, THRIP, Winetech and the Deciduous Fruit Producer’s Trust for financial support. Prof. Uwe Braun, Martin-Luther-Universität Halle-Wittenberg, Institut für Geobotanik und Botanischer Garten, is kindly thanked for providing the Latin diagnoses.
REFERENCES
- Adams GC, Wingfield MJ, Common R, Roux J. 2005. Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus. Studies in Mycology 52: 1 – 146 . [Google Scholar]
- Ajello L, Georg LK, Steigbigel RT, Wang CJK. 1974. A case of phaeohyphomycosis caused by a new species of Phialophora. Mycologia 66: 490 – 498 . [PubMed] [Google Scholar]
- Aroca A, Raposo R. 2007. PCR-based strategy to detect and identify species of Phaeoacremonium causing grapevine diseases. Applied and Environmental Microbiology 73: 2911 – 2918 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553 – 556 . [Google Scholar]
- Crous PW, Gams W. 2000. Phaeomoniella chlamydospora gen. et comb. nov., a causal organism of Petri grapevine decline and esca. Phytopathologia Mediterranea 39: 112 – 118 . [Google Scholar]
- Crous PW, Gams W, Wingfield MJ, van Wyk PS. 1996. Phaeoacremonium gen. nov. associated with wilt and decline diseases of woody hosts and human infections. Mycologia 88: 786 – 796 . [Google Scholar]
- Crous PW, Phillips AJL, Baxter AP. 2000. Phytopathogenic fungi from South Africa Department of Plant Pathology Press, University of Stellenbosch printers; Stellenbosch, South Africa: . [Google Scholar]
- Crous PW, Rong IH, Wood A, Lee S, Glen H, Botha W, Slippers B, Beer WZ de, Wingfield MJ, Hawksworth DL. 2006a. How many species of fungi are there at the tip of Africa? Studies in Mycology 55: 13 – 33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ. 2006b. Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235 – 253 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Crous PW, Fourie PH. 2007. Botryosphaeriaceae as potential pathogens of Prunus species in South Africa, with descriptions of Diplodia africana and Lasiodiplodia plurivora spp. nov.. Mycologia 99: 664 – 680 . [DOI] [PubMed] [Google Scholar]
- Ferreira JHS, Wyk PS van, Calitz FJ. 1999. Slow dieback of grapevine in South Africa: stress-related predisposition of young vines for infection by Phaeoacremonium chlamydosporum. South African Journal of Enology and Viticulture 20: 43 – 46 . [Google Scholar]
- Fischer M. 2002. A new wood-decaying basidiomycete species associated with esca of grapevine: Fomitiporia mediterranea (Hymenochaetales). Mycological Progress 1: 315 – 324 . [Google Scholar]
- Gams W, Verkley GJM, Crous PW. (eds). 2007. CBS course of mycology Centraalbureau voor Schimmelcultures, Utrecht, the Netherlands: . [Google Scholar]
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113 – 118 . [DOI] [PubMed] [Google Scholar]
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323 – 1330 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaje D, Alaniz S, Pérez-Sierra A, Abad-Campos P, García-Jiménez J, Armengol J. 2007. First report of Phaeoacremonium mortoniae causing Petri disease of grapevine in Spain. Plant Disease 91: 1206 . [DOI] [PubMed] [Google Scholar]
- Groenewald M, Kang JC, Crous PW, Gams W. 2001. ITS and beta-tubulin phylogeny of Phaeoacremonium and Phaeomoniella species. Mycological Research. 105: 651 – 657 . [Google Scholar]
- Gryzenhout M, Myburg H, Hodges CS, Wingfield BD, Wingfield MJ. 2006. Microthia, Holocryphia and Ursicollum, three new genera on Eucalyptus and Coccoloba for fungi previously known as Cryphonectria. Studies in Mycology 55: 35 – 52 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarro J, Alves SH, Gené J, Grazziotin NA, Muzzuco R, Dalmagro C, Capilla J, Zaror L, Mayayo E. 2003. Two cases of subcutaneous infection due to Phaeoacremonium spp. Journal of Clinical Microbiology 41: 1332 – 1336 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleen F, Crous PW, Petrini O. 2003. Fungi associated with healthy grapevine cuttings in nurseries, with special reference to pathogens involved in the decline of young vines. Australasian Plant Pathology 32: 47 – 52 . [Google Scholar]
- Halleen F, Mostert L, Crous PW. 2007. Pathogenicity testing of lesser-known vascular fungi of grapevines. Australasian Plant Pathology 36: 277 – 285 . [Google Scholar]
- Hausner G, Eyjólfsdóttir GG, Reid J, Klassen GR. 1992. Two additional species of the genus Togninia. Canadian Journal of Botany 70: 724 – 732 . [Google Scholar]
- Hawksworth DL, Gibson IAS, Gams W. 1976. Phialophora parasitica associated with disease conditions in various trees. Transactions of the British Mycological Society. 66: 427 – 431 . [Google Scholar]
- Hemashettar BM, Siddaramappa B, Munjunathaswamy BS, Pangi AS, Pattan J, Andrade AT, Padhye AA, Mostert L, Summerbell RC. 2006. Phaeoacremonium krajdenii, a cause of white grain eumycetoma. Journal of Clinical Microbiology 44: 4619 – 4622 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis DM, Bull JJ. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182 – 192 . [Google Scholar]
- Kubátová A, Kolarik M, Pazoutová S. 2004. Phaeoacremonium rubrigenum – hyphomycete associated with bark beetles found in Czechia. Folia Microbiology 49: 99 – 104 . [DOI] [PubMed] [Google Scholar]
- Larignon P, Dubos B. 1997. Fungi associated with esca disease in grapevine. European Journal of Plant Pathology 103: 147 – 157 . [Google Scholar]
- Marco S Di, Calzarano F, Osti F, Mazzullo A. 2004. Pathogenicity of fungi associated with a decay of kiwifruit. Australasian Plant Pathology 33: 337 – 342 . [Google Scholar]
- Mostert L, Crous PW, Groenewald JZ, Gams W, Summerbell RC. 2003. Togninia (Calosphaeriales) is confirmed as teleomorph of Phaeoacremonium by means of morphology, sexual compatibility, and DNA phylogeny. Mycologia 95: 646 – 659 . [DOI] [PubMed] [Google Scholar]
- Mostert L, Groenewald JZ, Summerbell RC, Gams W, Crous PW. 2006a. Taxonomy and pathology of Togninia (Diaporthales) and its Phaeoacremonium anamorphs. Studies in Mycology 54: 1 – 115 . [Google Scholar]
- Mostert L, Groenewald JZ, Summerbell RC, Robert V, Sutton DA, Padhye AA, Crous PW. 2005. Species of Phaeoacremonium associated with infections in humans and environmental reservoirs in infected woody plants. Journal of Clinical Microbiology 43: 1752 – 1767 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostert L, Halleen F, Fourie P, Crous PW. 2006b. A review of Phaeoacremonium species involved in Petri disease and esca of grapevines. Phytopathologia Mediterranea 45: 12 – 29 . [Google Scholar]
- Mugnai L, Graniti A, Surico G. 1999. Esca (black measles) and brown wood-streaking: two old and elusive diseases of grapevines. Plant Disease 83: 404 – 416 . [DOI] [PubMed] [Google Scholar]
- Nirenberg HI. 1976. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem 169: 1 – 117 . [Google Scholar]
- O’Donnell K, Cigelnik E. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103 – 116 . [DOI] [PubMed] [Google Scholar]
- Pascoe IG, Edwards J, Cunnington JH, Cottral E. 2004. Detection of the Togninia teleomorph of Phaeoacremonium aleophilum in Australia. Phytopathologia Mediterranea 43: 51 – 58 . [Google Scholar]
- Petri L. 1912. Osservazioni sopra le alterazioni del legno della vite in seguito a ferite. Le Stazioni Sperimentali Agrarie Italiane 45: 501 – 547 . [Google Scholar]
- Rambaut A. 2002. Sequence Alignment Editor. Version 2.0 University of Oxford, Oxford: . [Google Scholar]
- Rayner RW. 1970. A mycological colour chart Commonwealth Mycological Institute and British Mycological Society, Kew, Surrey, UK: . [Google Scholar]
- Réblová M, Mostert L. 2007. Romellia is congeneric with Togninia, and description of Conidiotheca gen. nov. for one species of this genus with polysporous asci. Mycological Research 111: 299 – 307 . [DOI] [PubMed] [Google Scholar]
- Réblová M, Mostert L, Gams W, Crous PW. 2004. New genera in Calosphaeriales: Togniniella and its anamorph Phaeocrella, and Calosphaeriophora as anamorph of Calosphaeria. Studies in Mycology 50: 533 – 550 . [Google Scholar]
- Rooney-Latham S, Escalen A, Gubler WD. 2005. Teleomorph formation of Phaeoacremonium aleophilum, cause of esca and grapevine decline in California. Plant Disease 89: 177 – 184 . [DOI] [PubMed] [Google Scholar]
- Rumbos IC. 1986. Phialophora parasitica, causal agent of cherry dieback. Journal of Phytopathology 117: 283 – 287 . [Google Scholar]
- Scheck HJ, Vasquez SJ, Gubler WD. 1998. First report of three Phaeoacremonium spp. causing young grapevine decline in California. Plant Disease 82: 590 . [DOI] [PubMed] [Google Scholar]
- Sparapano L, Bruno G, Graniti A. 2001. Three-year observation of grapevines cross-inoculated with esca-associated fungi. Phytopathologia Mediterranea 40: 369 – 375 . [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods). Version 4 Sinauer Associates, Sunderland, MA, USA: . [Google Scholar]
- White TJ, Bruns T, Lee J, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–322 Academic Press, San Diego, California, USA: . [Google Scholar]
- Whiting E, Cunha MG, Gubler WD. 2005. Phaeomoniella chlamydospora and Phaeoacremonium species distinguished through cultural characters and ribosomal DNA sequence analysis. Mycotaxon 92: 351 – 360 . [Google Scholar]


