Abstract
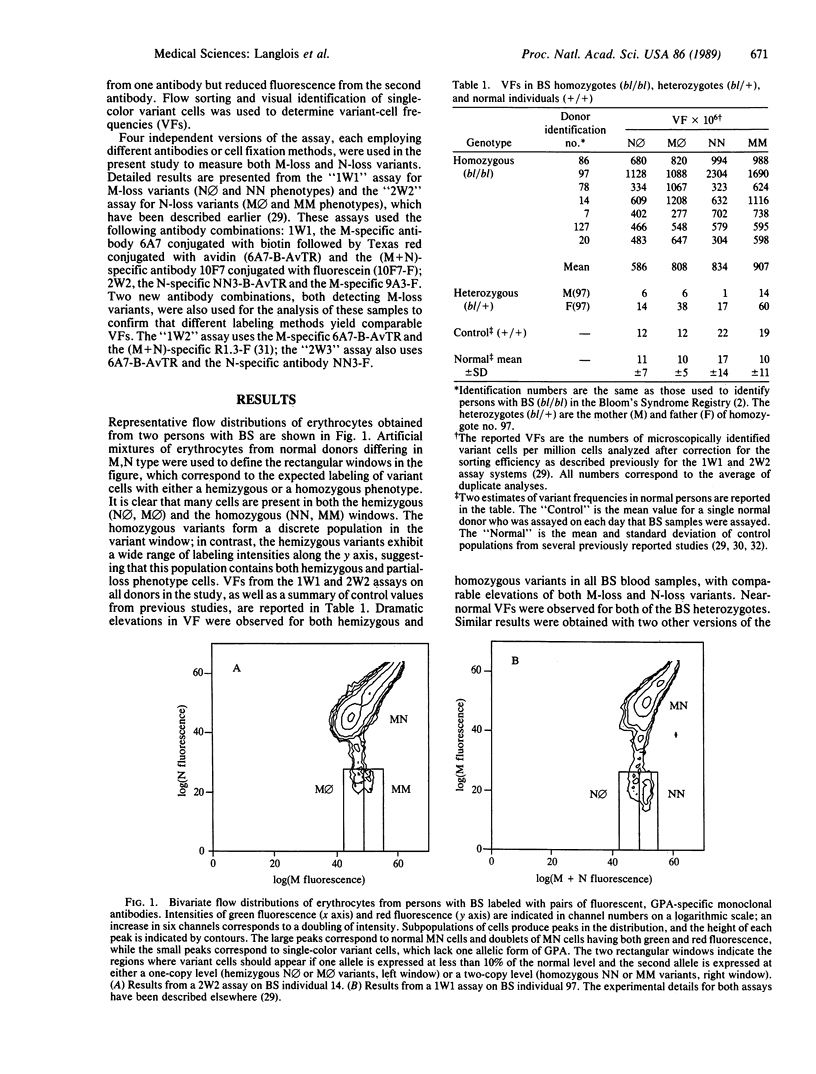
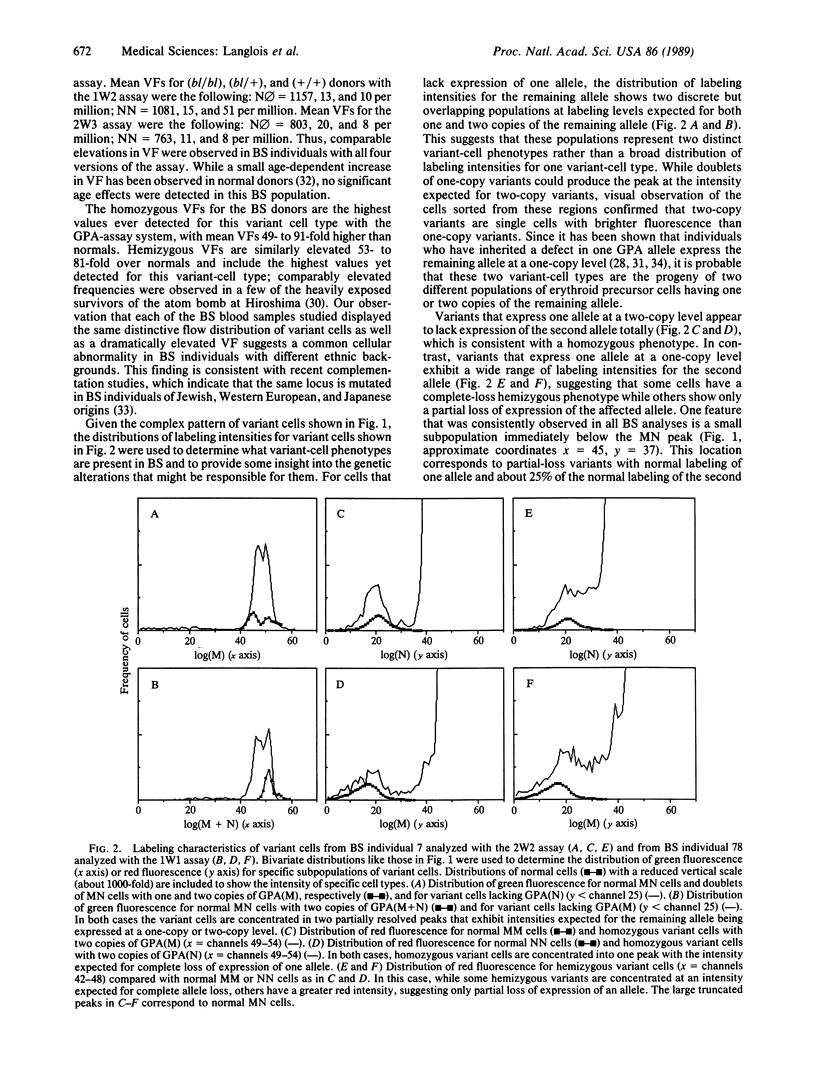
The glycophorin A assay was used to estimate the frequency of mutations that accumulate in vivo in somatic cells of persons with Bloom's syndrome (BS). This assay measures the frequency in persons of blood type MN of variant erythrocytes that lack the expression of one allelic form of glycophorin A, presumably due to mutational or recombinational events in erythroid precursor cells. Samples of blood from persons with BS showed dramatic 50- to 100-fold increases in the frequency of variants of three types, those with a hemizygous phenotype, those with a homozygous phenotype, and those with what appears to be partial loss of the expression of one locus. The high frequency of homozygous variants, genetic evidence for altered allelic segregation of a specific biochemical locus, provides evidence for increased somatic crossing-over in vivo in BS. An increased generation of functional hemizygosity and homozygosity in their somatic cells may play an important role in the extreme cancer risk of persons with BS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Lidereau R., Theillet C., Callahan R. Reduction to homozygosity of genes on chromosome 11 in human breast neoplasia. Science. 1987 Oct 9;238(4824):185–188. doi: 10.1126/science.3659909. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson S. A., Cohen T., Voss R. Background allelic variants in normal hemopoietic cells and Bloom's syndrome erythrocytes and the possible implication of somatic crossingover. Cancer Genet Cytogenet. 1985 Feb 15;15(3-4):237–242. doi: 10.1016/0165-4608(85)90167-0. [DOI] [PubMed] [Google Scholar]
- Bubley G. J., Schnipper L. E. Effects of Bloom's syndrome fibroblasts on genetic recombination and mutagenesis of herpes simplex virus type 1. Somat Cell Mol Genet. 1987 Mar;13(2):111–117. doi: 10.1007/BF01534691. [DOI] [PubMed] [Google Scholar]
- Cairney A. E., Andrews M., Greenberg M., Smith D., Weksberg R. Wilms tumor in three patients with Bloom syndrome. J Pediatr. 1987 Sep;111(3):414–416. doi: 10.1016/s0022-3476(87)80469-9. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Chaganti R. S., Schonberg S., German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. Y., Becker F. F., German J., Ray J. H. Altered DNA ligase I activity in Bloom's syndrome cells. Nature. 1987 Jan 22;325(6102):357–359. doi: 10.1038/325357a0. [DOI] [PubMed] [Google Scholar]
- Cook P. J., Noades J. E., Lomas C. G., Buckton K. E., Robson E. B. Exclusion mapping illustrated by the MNSs blood group. Ann Hum Genet. 1980 Jul;44(Pt 1):61–73. doi: 10.1111/j.1469-1809.1980.tb00946.x. [DOI] [PubMed] [Google Scholar]
- Dahr W., Beyreuther K., Moulds J., Unger P. Hybrid glycophorins from human erythrocyte membranes. I. Isolation and complete structural analysis of the hybrid sialoglycoprotein from Dantu-positive red cells of the N.E. variety. Eur J Biochem. 1987 Jul 1;166(1):31–36. doi: 10.1111/j.1432-1033.1987.tb13479.x. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Rapaport J. M., Epstein J., Goorin A. M., Weichselbaum R., Koufos A., Cavenee W. K. Chromosome 13 homozygosity in osteosarcoma without retinoblastoma. Am J Hum Genet. 1986 Jan;38(1):59–66. [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Adams A. C., Clarkson J. M., German J. Chromosome aberrations and unscheduled DNA synthesis in X- and UV-irradiated lymphocytes from a boy with Bloom's syndrome and a man with xeroderma pigmentosum. Cytogenet Cell Genet. 1978;20(1-6):124–140. doi: 10.1159/000130844. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Feinberg A. P., Hamilton S. H., Vogelstein B. Loss of genes on the short arm of chromosome 11 in bladder cancer. 1985 Nov 28-Dec 4Nature. 318(6044):377–380. doi: 10.1038/318377a0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Hamilton S. R., Vogelstein B. Clonal analysis of human colorectal tumors. Science. 1987 Oct 9;238(4824):193–197. doi: 10.1126/science.2889267. [DOI] [PubMed] [Google Scholar]
- Festa R. S., Meadows A. T., Boshes R. A. Leukemia in a black child with Bloom's syndrome: somatic recombination as a possible mechanism for neoplasia. Cancer. 1979 Oct;44(4):1507–1510. doi: 10.1002/1097-0142(197910)44:4<1507::aid-cncr2820440448>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Frorath B., Schmidt-Preuss U., Siemers U., Zöllner M., Rüdiger H. W. Heterozygous carriers for Bloom syndrome exhibit a spontaneously increased micronucleus formation in cultured fibroblasts. Hum Genet. 1984;67(1):52–55. doi: 10.1007/BF00270558. [DOI] [PubMed] [Google Scholar]
- Furthmayr H. Structural comparison of glycophorins and immunochemical analysis of genetic variants. Nature. 1978 Feb 9;271(5645):519–524. doi: 10.1038/271519a0. [DOI] [PubMed] [Google Scholar]
- GERMAN J., ARCHIBALD R., BLOOM D. CHROMOSOMAL BREAKAGE IN A RARE AND PROBABLY GENETICALLY DETERMINED SYNDROME OF MAN. Science. 1965 Apr 23;148(3669):506–507. doi: 10.1126/science.148.3669.506. [DOI] [PubMed] [Google Scholar]
- GERMAN J. CYTOLOGICAL EVIDENCE FOR CROSSING-OVER IN VITRO IN HUMAN LYMPHOID CELLS. Science. 1964 Apr 17;144(3616):298–301. doi: 10.1126/science.144.3616.298. [DOI] [PubMed] [Google Scholar]
- German J., Crippa L. P., Bloom D. Bloom's syndrome. III. Analysis of the chromosome aberration characteristic of this disorder. Chromosoma. 1974;48(4):361–366. doi: 10.1007/BF00290993. [DOI] [PubMed] [Google Scholar]
- German J., Passarge E. Bloom's syndrome. XII. Report from the Registry for 1987. Clin Genet. 1989 Jan;35(1):57–69. doi: 10.1111/j.1399-0004.1989.tb02905.x. [DOI] [PubMed] [Google Scholar]
- German J., Schonberg S., Louie E., Chaganti R. S. Bloom's syndrome. IV. Sister-chromatid exchanges in lymphocytes. Am J Hum Genet. 1977 May;29(3):248–255. [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S., Goldstein S. Diphtheria toxin resistance in human fibroblast cell strains from normal and cancer-prone individuals. Mutat Res. 1980 Dec;73(2):331–338. doi: 10.1016/0027-5107(80)90198-0. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr, Strong L. C. Mutation and cancer: a model for Wilms' tumor of the kidney. J Natl Cancer Inst. 1972 Feb;48(2):313–324. [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Copeland N. G., Jenkins N. A., Lampkin B. C., Cavenee W. K. Loss of heterozygosity in three embryonal tumours suggests a common pathogenetic mechanism. Nature. 1985 Jul 25;316(6026):330–334. doi: 10.1038/316330a0. [DOI] [PubMed] [Google Scholar]
- Kuhn E. M. Effects of X-irradiation in G1 and G2 on Bloom's Syndrome and normal chromosomes. Hum Genet. 1980;54(3):335–341. doi: 10.1007/BF00291579. [DOI] [PubMed] [Google Scholar]
- Kuhn E. M., Therman E. No increased chromosome breakage in three Bloom's syndrome heterozygotes. J Med Genet. 1979 Jun;16(3):219–222. doi: 10.1136/jmg.16.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Jensen R. H. Flow cytometric characterization of normal and variant cells with monoclonal antibodies specific for glycophorin A. J Immunol. 1985 Jun;134(6):4009–4017. [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Jensen R. H. Measurements of the frequency of human erythrocytes with gene expression loss phenotypes at the glycophorin A locus. Hum Genet. 1986 Dec;74(4):353–362. doi: 10.1007/BF00280485. [DOI] [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Kyoizumi S., Nakamura N., Bean M. A., Akiyama M., Jensen R. H. Evidence for increased somatic cell mutations at the glycophorin A locus in atomic bomb survivors. Science. 1987 Apr 24;236(4800):445–448. doi: 10.1126/science.3563520. [DOI] [PubMed] [Google Scholar]
- Merry A. H., Hodson C., Thomson E., Mallinson G., Anstee D. J. The use of monoclonal antibodies to quantify the levels of sialoglycoproteins alpha and delta and variant sialoglycoproteins in human erythrocyte membranes. Biochem J. 1986 Jan 1;233(1):93–98. doi: 10.1042/bj2330093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor S. L., Johnson B. E., Minna J. D., Sakaguchi A. Y. Loss of heterozygosity of chromosome 3p markers in small-cell lung cancer. Nature. 1987 Oct 1;329(6138):451–454. doi: 10.1038/329451a0. [DOI] [PubMed] [Google Scholar]
- Passarge E., Bartram C. R. Somatic recombination as possible prelude to malignant transformation. Birth Defects Orig Artic Ser. 1976;12(1):177–180. [PubMed] [Google Scholar]
- Raizis A. M., Becroft D. M., Shaw R. L., Reeve A. E. A mitotic recombination in Wilms tumor occurs between the parathyroid hormone locus and 11p13. Hum Genet. 1985;70(4):344–346. doi: 10.1007/BF00295375. [DOI] [PubMed] [Google Scholar]
- Rosin M. P., German J. Evidence for chromosome instability in vivo in Bloom syndrome: increased numbers of micronuclei in exfoliated cells. Hum Genet. 1985;71(3):187–191. doi: 10.1007/BF00284570. [DOI] [PubMed] [Google Scholar]
- Shiraishi Y., Sandberg A. A. The relationship between sister chromatid exchanges and chromosome aberrations in Bloom's syndrome. Cytogenet Cell Genet. 1977;18(1):13–23. doi: 10.1159/000130744. [DOI] [PubMed] [Google Scholar]
- Siebert P. D., Fukuda M. Molecular cloning of a human glycophorin B cDNA: nucleotide sequence and genomic relationship to glycophorin A. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6735–6739. doi: 10.1073/pnas.84.19.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therman E., Kuhn E. M. Cytological demonstration of mitotic crossing-over in man. Cytogenet Cell Genet. 1976;17(5):254–267. doi: 10.1159/000130721. [DOI] [PubMed] [Google Scholar]
- Therman E., Otto P. G., Shahidi N. T. Mitotic recombination and segregation of satellites in Bloom's syndrome. Chromosoma. 1981;82(5):627–636. doi: 10.1007/BF00285772. [DOI] [PubMed] [Google Scholar]
- Tsuji H., Heartlein M. W., Latt S. A. Disparate effects of 5-bromodeoxyuridine on sister-chromatid exchanges and chromosomal aberrations in Bloom syndrome fibroblasts. Mutat Res. 1988 Mar;198(1):241–253. doi: 10.1016/0027-5107(88)90061-9. [DOI] [PubMed] [Google Scholar]
- Vijayalaxmi, Evans H. J., Ray J. H., German J. Bloom's syndrome: evidence for an increased mutation frequency in vivo. Science. 1983 Aug 26;221(4613):851–853. doi: 10.1126/science.6879180. [DOI] [PubMed] [Google Scholar]
- Wahlström J., Kyllerman M., Hansson A., Taranger J. Unequal mitotic sister chromatid exchange and different length of Y chromosomes. Hum Genet. 1985;70(2):186–188. doi: 10.1007/BF00273081. [DOI] [PubMed] [Google Scholar]
- Warren S. T., Schultz R. A., Chang C. C., Wade M. H., Trosko J. E. Elevated spontaneous mutation rate in Bloom syndrome fibroblasts. Proc Natl Acad Sci U S A. 1981 May;78(5):3133–3137. doi: 10.1073/pnas.78.5.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksberg R., Smith C., Anson-Cartwright L., Maloney K. Bloom syndrome: a single complementation group defines patients of diverse ethnic origin. Am J Hum Genet. 1988 Jun;42(6):816–824. [PMC free article] [PubMed] [Google Scholar]
- Willis A. E., Lindahl T. DNA ligase I deficiency in Bloom's syndrome. Nature. 1987 Jan 22;325(6102):355–357. doi: 10.1038/325355a0. [DOI] [PubMed] [Google Scholar]
- Willis A. E., Weksberg R., Tomlinson S., Lindahl T. Structural alterations of DNA ligase I in Bloom syndrome. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8016–8020. doi: 10.1073/pnas.84.22.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]



