Abstract
During a survey of Prunus wood from South Africa, isolations were made of three presumably Calosphaerialean fungi that formed hyphomycetous, phialidic anamorphs in culture. In order to reveal the phylogenetic relationship of these fungi, they were characterised on a morphological and molecular (LSU and ITS rDNA) basis. Two isolates that formed a teleomorph in culture are newly described as Calosphaeria africana sp. nov. Although asci of Calosphaeria are characterised by having non-amyloid apical rings, two functional wall layers were observed in asci of C. africana, which has hitherto not been observed in any member of the Calosphaeriaceae. However, Calosphaeriaceae (Calosphaeriales, Sordariomycetes) are not closely related to other bitunicate fungi like Dothideomycetes, Chaetothyriales and bitunicate lichens. Possession of two separating wall layers is considered to be a result of both inherited abilities and convergent evolution under a strong selection pressure of the environmental conditions that favour an extension of the ascus. The other two species represented a separate lineage within Calosphaeriaceae, and formed phialophora-like anamorphs. By obtaining the teleomorph in culture, one of them could be identified as a species of Jattaea, described here as Jattaea prunicola sp. nov., while the second, which only produced the anamorph, is named as Jattaea mookgoponga sp. nov. These findings suggest that some species of Jattaea are true members of the Calosphaeriaceae, though the phylogenetic relation of the type, J. algeriensis, remains unknown. Furthermore, it also represents the first report of Jattaea on Prunus wood, and from South Africa.
Keywords: apical ring, ascospore discharge, ascus dehiscence, beta-tubulin, Calosphaeria, Calosphaeriaceae, ITS, Jattaea, LSU, Phialophora, Prunus, SSU, systematics
INTRODUCTION
In 1993, Barr et al. revised the Calosphaeriaceae (Calosphaeriales), and recognised five genera. The latter were circumscribed as having papillate, short or elongate, beaked ascomata, forming 1-celled or delicately septate ascospores in clavate and short-stipitate, or oblong and sessile, octosporous or polysporous asci. This initial treatment was followed by several additions and revisions, namely reinstating Wegelina (Barr 1998), recognising Erostella and Romellia as synonyms of Togninia (anamorph: Phaeoacremonium) (Hausner et al. 1992, Mostert et al. 2003, Réblová & Mostert 2007), and recognising Pleurostoma (anamorph: Pleurostomophora) as a separate genus (Vijaykrishna et al. 2004). Furthermore, Enchnoa was placed in the Nitschkiaceae (Barr 1994), Pleurostoma in the Pleurostomataceae, and Togninia in the Togniniaceae (Réblová et al. 2004). The Calosphaeriaceae currently comprises five genera, namely Calosphaeria (anamorph: Calosphaeriophora), Jattaea, Pachytrype, Togniniella (anamorph: Phaeocrella) and Wegelina (Mostert et al. 2006). Réblová et al. (2004) referred to the genus Pachytrype as having affinities to the Diaporthales due to the development of a stroma, the presence of oblong, short-stipitate asci with a round base, and a cytospora-like anamorph. The remaining four non-stromatic genera were described as having asci arranged in fascicles, ascogenous hyphae with short branches, and producing a sympodial succession of lateral and terminal cells, each of which gives rise to an ascus.
Only a few species of the Calosphaeriaceae have thus far been studied in culture, and hence data pertaining to their respective anamorphs and DNA phylogeny remain sparse. Samuels & Candoussau (1996) were the first to cultivate a species of Calosphaeria. They described C. fagi with ramichloridium- and sporothrix-like synanamorphs (but see Arzanlou et al. 2007 for the phylogeny of Ramichloridium, and Roets et al. 2006 for Sporothrix). Similar anamorphs have recently been described for Barbatosphaeria barbirostris (syn. C. barbirostris) that has been removed from the Calosphaeriales based on LSU sequence analysis (Réblová 2007). Phialidic anamorphs have been observed in cultures of C. pulchella and Togniniella acerosa (Réblová et al. 2004). By analysing their LSU and SSU sequences, they have been shown to be closely related to each other and to Pleurostoma/Pleurostomophora spp., and to form a separate clade adjacent to the Diaporthales (Sordariomycetes), establishing the basis for a molecular phylogeny of the Calosphaeriales (Réblová et al. 2004). Conidiophores of Phaeocrella, the anamorph of Togniniella, are erect, pigmented, regularly branched and have prominent constrictions at their septa (Réblová et al. 2004). Calosphaeriophora, the anamorph of Calosphaeria forms mostly unbranched conidiophores, often adelophialides, and hyaline phialides with a pigmented apical region and deep, flaring collarettes. Conidia in both genera are hyaline and aseptate. Pleurostomophora, the anamorph of the closely related genus Pleurostoma (Calosphaeriales, Pleurostomataceae), looks similar and includes two former Phialophora species (Vijaykrishna et al. 2004). Additionally, Jattaea villosa is known to form spermatogonia together with perithecia (Mostert et al. 2006). Calosphaeria pulchella and Togniniella acerosa are presently the only Calosphaeriaceae species having sequences available in GenBank, and a phylogenetic relationship confirmed by sequence data. However, there are currently 107 records for Calosphaeria listed in Index Fungorum (http://www.indexfungorum.org), 14 records for Jattaea, one for Togniniella and eight for Wegelina. Exept for C. pulchella, C. fagi and T. acerosa, these species are only known from their teleomorph states on natural substrates.
Calosphaeriaceae are typical inhabitants of wood and bark of a broad spectrum of trees and shrubs worldwide, including Prunus wood (Barr 1985). Calosphaeria vasculosa is reported on P. spinosa from France (Mostert et al. 2006), C. pulchella on decayed branches of P. persica from South Carolina (Adaskaveg et al. 1993) and on P. avium from France (Réblová et al. 2004). Calosphaeria kriegeriana is known to occur on branches of P. spinosa from Germany (Saccardo 1899), and C. princeps on P. avium and P. cerasi from Belgium, Finland, France, Germany, Great Britain, Italy, Switzerland and North America (Saccardo 1882a). Reports from South Africa include C. princeps on P. armeniaca and P. persica, and C. cylindrica on dead wood and branches of Solanum auriculatum (Doidge 1950).
In a survey of alternative hosts of grapevine trunk disease pathogens, three fungi were isolated from Prunus wood that appeared to belong to the Calosphaeriaceae based on sequence data derived from their rDNA ITS region. All three fungi produced hyphomycetous, phialidic anamorphs, and two species produced a teleomorph in culture. The aim of the present study was to resolve the phylogenetic relationship of these species and clarify their taxonomy. We also focused on the ascospore release and ascus dehiscence of the species described.
MATERIAL AND METHODS
Isolates
Fungi were isolated from branches of plum (Prunus salicina), nectarine (P. persica var. nucipersica) and apricot (P. armeniaca) with necroses in the wood. The branches were sampled in orchards in the Western Cape and the Limpopo Province of South Africa according to the method described in Damm et al. (2007). Single-conidial isolates were obtained for further molecular and taxonomic study. Reference strains are maintained in the culture collection of the Department of Plant Pathology, University of Stellenbosch (STE-U) in Stellenbosch, South Africa, and the Centraalbureau voor Schimmelcultures (CBS) Utrecht, the Netherlands (Table 1).
Table 1.
Names, accession numbers and collection details of isolates studied.
| Species | Accession No.1 | Host | Location | GenBank accessions |
|||
|---|---|---|---|---|---|---|---|
| ITS | LSU | SSU | TUB | ||||
| Calosphaeria africana | STE-U 6182, CBS 1208702 | Prunus armeniaca | Robertson, Western Cape, South Africa | EU367444 | EU367454 | EU367460 | EU367464 |
| STE-U 6181 | P. armeniaca | Robertson, Western Cape, South Africa | EU367445 | EU367455 | EU367461 | EU367465 | |
| Jattaea prunicola | STE-U 6201, CBS 1208712 | P. salicina | Franschhoek, Western Cape, South Africa | EU367446 | EU367456 | EU367462 | EU367466 |
| STE-U 6399 | P. salicina | Franschhoek, Western Cape, South Africa | EU367447 | EU367457 | |||
| STE-U 6400 | P. salicina | Franschhoek, Western Cape, South Africa | EU367448 | ||||
| J. mookgoponga | STE-U 6184, CBS 1208672 | P. persica var. nucipersica | Mookgopong, Limpopo, South Africa | EU367449 | EU367458 | EU367463 | EU367467 |
| STE-U 6401 | P. persica var. nucipersica | Mookgopong, Limpopo, South Africa | EU367450 | EU367459 | |||
1 STE-U: Culture collection of the Department of Plant Pathology, University of Stellenbosch, South Africa; CBS: Culture collection of the Centraalbureau voor Schimmelcultures, Fungal Biodiversity Centre, Utrecht, The Netherlands.
2 Ex-type cultures.
Morphology
Fungi were morphologically characterised according to Damm et al. (2007) on synthetic nutrient-poor agar medium (SNA, Nirenberg 1976) and on malt extract plates (MEA, 2 % malt extract, Oxoid Ltd., England; 1.5 % agar, Difco, USA) incubated at 25 °C in the dark for 2 wk (anamorphs) or on SNA amended with pieces of double autoclaved grapevine wood incubated for 2–3 mo (teleomorphs). Microscopic preparations were made in clear lactic acid or water, with 30 measurements per structure where possible (Damm et al. 2007), and observed with a Nikon SMZ800 dissecting microscope (DM) or with a Nikon Eclipse E600 microscope using bright field (BF), differential interference contrast (DIC) or phase contrast (PC) illumination. The reaction of apical tips with iodine was tested using Melzer’s reagent as described in Mostert et al. (2006). Colony characters and pigment production on MEA and potato-dextrose agar (2 % PDA, Biolab, Midrand, South Africa), incubated in a growth chamber in the dark at 25 °C and in the laboratory under diffuse daylight, were noted after 2 wk. Colony colours were determined using the colour charts of Rayner (1970). Growth characteristics were studied on MEA plates incubated in the dark at temperatures ranging from 5–35 °C, in 5° intervals.
DNA isolation, amplification and analyses
Genomic DNA of isolates was extracted using the CTAB-based method of Damm et al. (in press) where the mycelium was crushed in a mixer with glass beads. The 5.8S ribosomal RNA gene with the two flanking internal transcribed spacers (ITS1 and ITS2), a partial sequence of the 28S rRNA gene (LSU), the β-tubulin gene (TUB) and a partial sequence of the 18S rRNA gene (SSU) were amplified and sequenced using the primer pairs ITS1F (Gardes & Bruns 1993) and ITS4 (White et al. 1990), NL1 and NL4 (O’Donnell 1993), T1 (O’Donnell & Cigelnik 1997) and Bt2b (Glass & Donaldson 1995) and NS1 and NS8 (White et al. 1990), according to the conditions and protocols explained in Mostert et al. (2006). Additional primers used for sequencing the SSU were: NS2, NS3, NS4, NS5 (White et al. 1990). The LSU and ITS sequences were added to the outgroup sequences (LSU: Xylaria hypoxylon U47841, ITS: Togninia minima AF266647) and sequences obtained from GenBank (http://www.ncbi.nlm.gov). The alignment was manually assembled and manually adjusted using Sequence Alignment Editor v. 2.0a11 (Rambaut 2002). Phylogenetic analyses were performed using PAUP (Phylogenetic Analysis Using Parsimony) v. 4.0b10 (Swofford 2003). Ambiguously aligned data positions 78–144 were excluded from the analyses of the ITS region. Alignment gaps in all analyses were treated as missing data and all characters were unordered and of equal weight. Maximum parsimony analysis was performed using the heuristic search option with 100 random sequence additions and tree bisection and reconstruction (TBR) as the branch-swapping algorithm. The robustness of the trees obtained was evaluated by 1000 bootstrap replications with 100 random sequence additions (Hillis & Bull 1993). Tree length, consistency index (CI), retention index (RI), rescaled consistency index (RC) and homoplasy index (HI) were calculated for the resulting tree. Since there were no close matches in the BlastSearch with ITS and LSU sequences of the two Jattaea species, beta-tubulin and SSU genes were sequenced additionally and used in BlastSearches (not shown). These data were not used for the calculation of phylogenies, but added as additional information. Sequences derived in this study were deposited in GenBank, and the alignments in TreeBASE.
RESULTS
Phylogenetic analysis
The LSU analysis included 39 taxa and 1356 characters. Of these characters, 336 were parsimony-informative, 225 variable and 795 constant. The heuristic search resulted in 3 equally most parsimonious trees (Length = 1300 steps, CI = 0.583, RI = 0.727, RC = 0.424, HI = 0.417) of which one is shown in Fig. 1. The main clades represent orders and families in the Sordariomycetidae (Sordariomycetes), including Calosphaeriales (73 % bootstrap support) with Calosphaeriaceae (100 %) and Pleurostomataceae (100 %). All six isolates from Prunus cluster within the Calosphaeriaceae clade. Isolates STE-U 6181 (Genbank EU367455) and STE-U 6182 (Genbank EU367454) group with Calosphaeria pulchella AY761075 (100 %). The other four isolates form two groups (99 % and 100 %) and form a sister-clade (92 %) to Calosphaeria and Togniniella (100 %). The three trees only differ in the order of the three species within the Calosphaeria subclade.
Fig. 1.
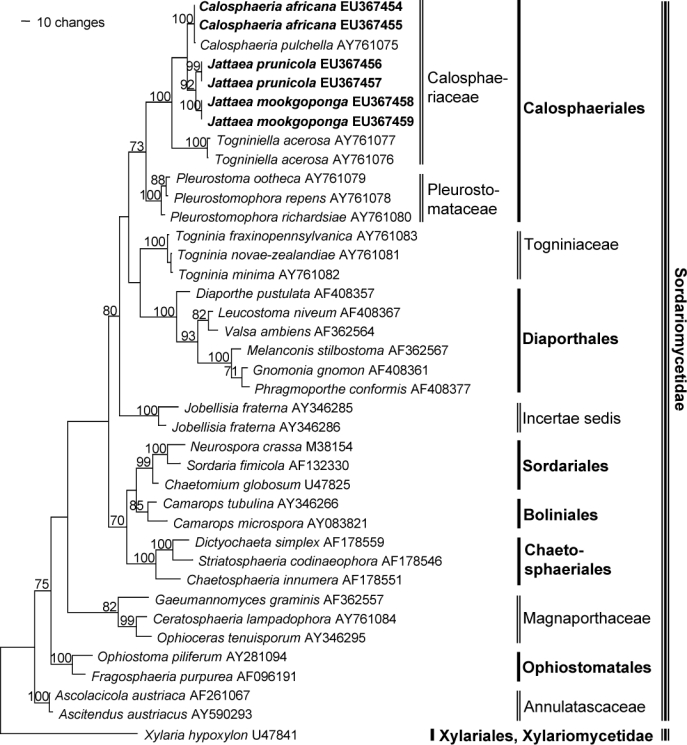
One of three equally parsimonious trees obtained from heuristic searches of LSU gene sequences (Length = 1300 steps, CI = 0.583, RI = 0.727, RC = 0.424, HI = 0.417). Bootstrap support values (1000 replicates) above 70 % are shown at the nodes. Xylaria hypoxylon U47841 was used as outgroup. Isolates analysed in this study are emphasised in bold.
The ITS alignment comprised 14 sequences and, after excluding ambiguously aligned positions 78–144, 571 characters including the gaps remained, of which 122 characters were parsimony-informative, 42 variable and parsimony-uninformative, and 407 constant. The heuristic search resulted in a single most parsimonious tree (Length = 262 steps, CI = 0.813, RI = 0.857, RC = 0.697, HI = 0.187), which is shown in Fig. 2. The clades represent genera in Calosphaeriaceae and Pleurostomataceae (96 %). Isolates STE-U 6181 (GenBank EU367445) and STE-U 6182 (GenBank EU367444) group with Calosphaeria pulchella (GenBank EU367451; 100 %) and form an adjacent clade to it (84 %). The other five isolates form a clade next to Togniniella (91 %) with two subclades, STE-U 6201 (GenBank EU367446), STE-U 6399 (GenBank EU367447) and STE-U 6400 (GenBank EU367448) (100 %) as well as STE-U 6184 (GenBank EU367449) and STE-U 6401 (GenBank EU367450) (100 %).
Fig. 2.
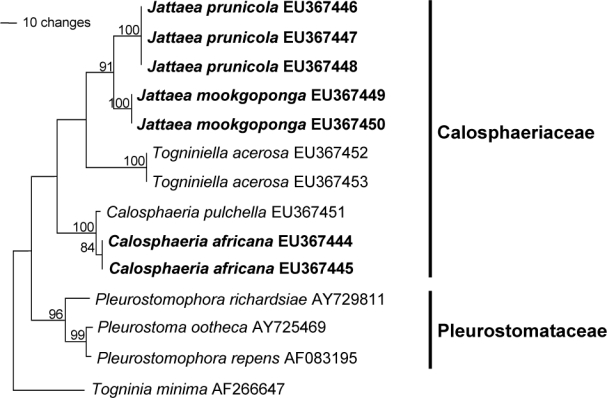
Single most parsimonious tree obtained from heuristic searches of ITS gene sequences (Length = 262 steps, CI = 0.813, RI = 0.857, RC = 0.697, HI = 0.187). Bootstrap support values (1000 replicates) above 70 % are shown at the nodes. Togninia minima AF266647 was used as outgroup. Isolates analysed in this study are emphasised in bold.
Taxonomy
One novel species of Calosphaeria, and two species of Jattaea were collected in the present study. These species appear to be new to science, and are described below.
Calosphaeria Africana Damm & Crous, sp. nov. — MycoBank MB511428; Fig. 3, 4
Fig. 3.
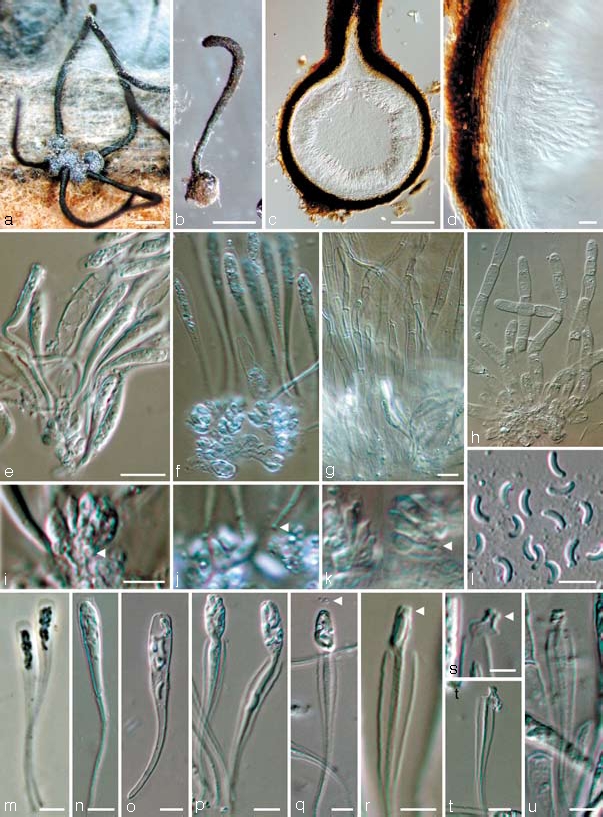
Calosphaeria africana. a–b. Perithecia formed on grapevine wood; c. longitudinal section through perithecium; d. peridium with asci attached; e–g. asci and paraphyses attached to ascogenous hyphae; h. paraphyses; i–k. ascogenous hyphae with ovoid to ellipsoidal cells (arrow heads in i and k indicate croziers, arrow head in j indicates basis of ascus); l. ascospores; m–u. asci in different stages of dehiscence (arrow heads indicate apical rings); all from CBS H-19988 (holotype); a–b: DM, c–l, n–u: DIC, m. PC. — Scale bars: a–b = 500 μm; c = 100 μm; d, e, g = 10 μm; e applies to e & f; g applies to g & h; i, l–u = 5 μm; i applies to i–k.
Fig. 4.
Calosphaeriophora anamorph of Calosphaeria africana. a. Conidiophores and conidia; b–h. conidiophores (arrow heads indicate brown pigment on apical parts); i, j. conidia; all from CBS H-19988 (holotype); a–h, j: SNA medium, i: MEA medium, a–c, f–j: DIC, d–e: BF. — Scale bars: a = 5 μm; a applies to a–j.
Anamorph. Calosphaeriophora sp.
Calosphaeriae pulchellae similis, sed ascis et ascosporis minoribus, (27–)32–46(–51) × (3.5–)4–5(–5.5) μm et (3–)3.5–4.5(–5) × (0.5–)1–1.5 μm.
Etymology. Named after the continent of origin, Africa.
Perithecia non-stromatic, formed in dense clusters under the bark of grapevine wood pieces in culture after 3 mo, black, venter globose to subglobose, 300–500 μm diam, 250–400 μm tall. Peridium leathery, consisting of 12–16 layers (30–45 μm) of textura angularis, outer region brown with cells smaller and more rounded than those of the inner layer, hyaline and more flattened towards the centrum, surface glabrous or covered with brown, septate hyphal appendages. Perithecial necks central, black, smooth 1.2–3 mm long, 80–120 μm wide at the base, 120–170 μm wide at the widest part in the upper third. Ascogenous hyphae discrete, hyaline, smooth, with short branches, 5–15 × 1–2 μm, producing a sympodial sequence of hyaline, ovoid to ellipsoidal cells, derived from croziers, often with mucronate apex, in dense clusters, each giving rise to an ascus, 2.5–6 × 1.5–3 μm. Paraphyses persistent, abundant, hyaline, unbranched, septate, cylindrical to clavate, apex round, apically free, much longer than asci, 80–250 μm long, in water 4–8 μm wide at the widest part (av. 150 × 6 μm) and restricted at septae (Fig. 3h), in lactic acid 3–5 μm wide and not restricted at septae (Fig. 3g), pores in septa visible. Asci unitunicate (but see discussion), 8-spored, clavate with obtuse or rounded apex, tapering towards a long, filiform, stipitate base, more intensely tapering below the sporiferous portion, with a nodule at the base, in fascicles, floating freely within the centrum at maturity, (27–)32–46(–51) × (3.5–)4–5(–5.5) μm, (av. 40 × 4.5 μm), pars sporifera 11–20 μm long, apical region 0.5–2(–3) μm thick, everted apical rings (non-amyloid) often visible after ascospore release, ascospores released with a fissitunicate dehiscence mechanism (explained below). Ascospores biseriate to multiseriate, in the upper third of the ascus, aseptate, hyaline, allantoid, smooth, (3–)3.5–4.5(–5) × (0.5–)1–1.5 μm (av. 4 × 1.2 μm), L/W ratio = 3.3. Spermatogonia not observed. Vegetative hyphae on SNA hyaline, 1.5–4 μm wide, chlamydospores absent. Conidiophores micronematous, arising from aerial or submerged hyphae, erect, rarely branched, 1-septate, 10–20 × 2–3 μm, but mainly reduced to conidiogenous cells. Conidiogenous cells enteroblastic, hyaline, though brown in apical part, smooth, single, generally intercalary, necks cylindrical, 2–8 × 1–2 μm, discrete phialides ampulliform to elongate ampulliform, 8–25 × 2–3 μm, pigmented at the apical region (well visible in BF, Fig. 4d, e), collarettes distinct, cylindrical to funnel-shaped, < 0.5–2 μm long, 0.5–2 μm wide, opening 0.5–1.5 μm wide. Conidia aggregated in heads, hyaline, aseptate, cylindrical, allantoid to reniform, both ends rounds or one end attenuated, (2.5–)3–4.5(–6) × 1–1.5(–2) μm, mean±SD = 3.8 ± 0.8 × 1.4 ± 0.2 μm, L/W ratio = 2.7. Vegetative hyphae on MEA hyaline, 1.5–6 μm wide, chlamydospores absent. Conidiophores micronematous, arising from aerial or submerged hyphae, mainly reduced to conidiogenous cells. Conidiogenous cells enteroblastic, hyaline, single, generally intercalary, necks cylindrical, 5–7 × 1–3 μm, discrete phialides elongate-ampulliform, 10 × 2–3 μm. Conidia aggregated in heads, hyaline, aseptate, variable in shape, cylindrical, oval, allantoid, subglobose to lemon-shaped, verruculose, (2.5–)3.5–6.5(–10) × (1–)1.5–3.5(–4.5) μm, mean±SD = 5.0 ± 1.7 × 2.5 ± 1 μm, L/W ratio = 2.
Cultural characteristics — Colonies on PDA flat and spreading, entire edge, sometimes folded towards and wrinkled in the centre, appressed, sparse aerial mycelium, pale luteous to ochreous, reverse same colour, 31 mm in 2 wk (25 °C). Colonies on MEA flat, erose, sometimes wrinkled in the centre, devoid of aerial mycelium, ochreous, cinnamon to dark fulvous, reverse same colour, 18 mm in 2 wk (25 °C). Conditions for growth: min 10 °C, max > 35 °C, opt 30 °C.
Specimens examined. South Africa, Western Cape Province, Robertson, from V-shaped necrosis in wood of Prunus armeniaca with canker close to old pruning wound, 23 Aug. 2005, U. Damm, CBS H-19988 holotype, culture ex-type CBS 120870 = STE-U 6182; Robertson, from necrosis in wood of P. armeniaca, 23 Aug. 2005, U. Damm, STE-U 6181.
Notes — Calosphaeria species with similar ascospore size to C. africana are C. ciliatula, C. cyclospora, C. cylindrica, C. fagi, C. fallax, C. lantanae, C. micromeria, C. pirellifera, C. pulchella, and C. rimicola. However, asci of C. cylindrica are shorter and broader (18 × 8 μm), asci and spores of C. ciliatula have a different shape (Saccardo 1882b, c), asci of C. pirellifera are shorter (Kirschstein 1941), ascospores of C. cyclospora are more strongly curved (semi-circular to circular), and asci (pars sporifera) are longer (Petrak 1924), ascospores of C. fagi are septate, and it has ramichloridium- and sporothrix-like synanamorphs (Samuels & Candoussau 1996). Asci of C. fallax are larger than those of C. africana, and polysporous (Saccardo 1891), while asci of C. lantanae are smaller, ascoma are formed in a stroma, and no paraphyses were observed (Tilak & Nagre 1964). Ascospores of C. micromeria (syn. Diatrype micromeria) measuring 4–6 × 1–1.5 μm are less bent than those of C. africana, and subhyaline (Berlese 1900). Ascomata of C. rimicola are ovate-globose and collapse often (Ellis & Everhart 1892), while those of C. africana are globose and do not collapse. Calosphaeria africana can be distinguished from the closely related C. pulchella by the on average smaller, more bent ascospores with a lower L/W ratio, namely 3.3 compared to 4.8 in C. pulchella and smaller asci, 40 × 4.5 μm, compared to > 50 × 5.4 μm in C. pulchella (Réblová et al. 2004). Asci in C. pulchella also differ in shape: they taper uniformly towards the base, resulting in elongate, wedge-shaped asci, compared to the more clavate shape of the asci in C. africana. Although supposed to be synonymous to C. pulchella, ascospore sizes given for C. princeps differ from C. pulchella, as well as from C. africana, measuring 8–10 × 1.5 μm (Saccardo 1878), 6 × 1.5 μm (Saccardo 1882a) and 6 × 1 μm (Berlese 1900). There is no ascospore size given in the descriptions of C. abieticola and C. rosarum, but the ascomata of both species are much larger, measuring 1–2 mm and 3–4 mm, respectively (Saccardo 1882d, e).
Jattaea prunicola Damm & Crous, sp. nov. — MycoBank MB511429; Fig. 5, 6
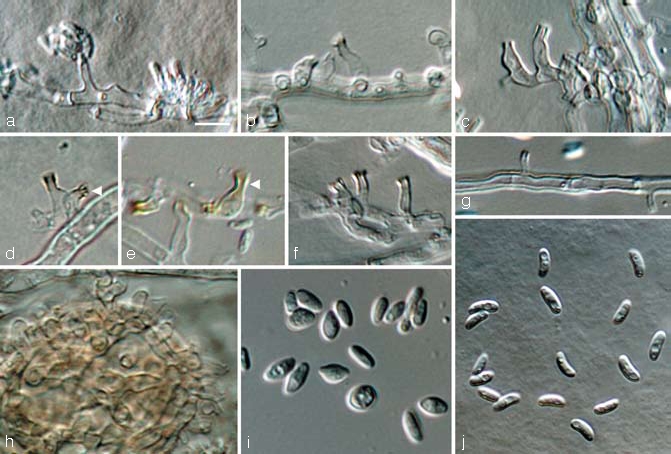
Fig. 5.
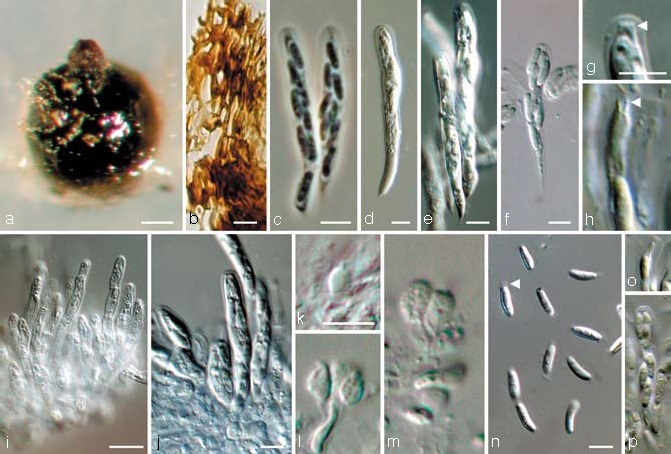
Jattaea prunicola. a. Perithecium formed on SNA medium; b. peridium; c–f. asci with ascospores; g–h. apical region of asci in different developmental stages (arrow heads indicate apical rings); i. paraphyses; j. asci attached to ascogenous hyphae; k–m. ascogenous hyphae with ovoid to ellipsoidal cells; n–p. ascospores (arrow head indicates mucoid appendages); all from CBS H-19987 (holotype); a: DM, b, d–p: DIC, c: PC. — Scale bars: a = 50 μm; b–g, i–k, n = 5 μm; g applies to g & h; k applies to k–m; n applies to n–p.
Fig. 6.
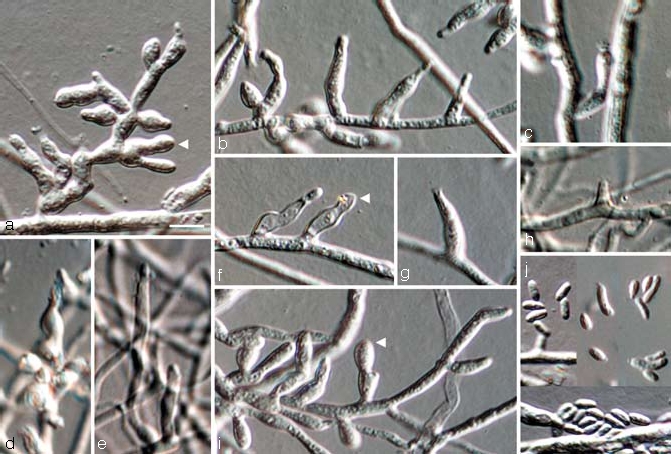
Jattaea prunicola, anamorph. a–i. Conidiophores (arrow heads indicate sterile inflated cells); j. conidia; all from CBS H-19987 (holotype); a–j. SNA medium, a–j: DIC. — Scale bars: a = 5 μm; a applies to a–j.
Anamorph. Phialophora-like sp.
Jattaeae microthecae similis, sed ascis angustioribus, 25–48 × 4–5.5 μm.
Etymology. Named after its host, Prunus.
Perithecia formed after 2 mo on SNA medium, solitary, in small groups, non-stromatic, dark-brown to black, globose to subglobose, with a short neck, superficial or immersed in the agar medium, dimensions of the only mature perithecium: 210 μm diam, central neck 110 μm long and 60 μm wide, glabrous, surface covered with brown, septate, hyphal appendages. Peridium consisting of 6–8 layers of brown textura angularis (20–25 μm), inner layer flattened, elongate, and paler than the outer cells. Ascogenous hyphae hyaline, smooth, delicate, rarely visible, producing ovoid to ellipsoidal cells, often mucronate at the apex, arranged alongside each other, each giving rise to an ascus, 3–5 × 2–4 μm. Paraphyses persistent, hyaline, branched near the base, septate, apically free, cylindrical to subulate with round apex, smooth-walled, 15–45 × 2.5–5 μm (av. 32 × 3.5 μm). Asci unitunicate (but see discussion), 8-spored, either subcylindrical and abruptly tapering towards the acicular, sometimes bristle-like base, or clavate and gradually tapering to the base, apex truncate to broadly rounded, in fascicles, floating freely within the centrum at maturity, 25–48 × 4–5.5 μm (av. 34 × 4.5 μm), pars sporifera 19–32 μm long, apical region c. 1.5 μm thick, apical ring visible, ascospores are released through the apical ring (Fig. 5h, o). Ascospores biseriate to crowded, hyaline to very pale yellow-brown, aseptate, cylindrical to suballantoid with obtuse ends, often two tiny polar droplets, smooth to fine verruculose, (4–)5–7.5(–11) × 1.5–2 μm (av. 6.3 × 2), L/W ratio = 3.2, terminal mucoid appendages on both ends, 1–1.5 μm long. Spermatogonia not observed. Vegetative hyphae on SNA hyaline to yellow-brown, 0.5–3 μm wide, septate, smooth, chlamydospores absent. Conidiophores hyaline to yellow-brown, multiple branches, often not terminating in phialides, but with sterile, irregularly shaped, mostly inflated cells, 15–40 μm long. Conidiogenous cells enteroblastic, discrete phialides dominating in the aerial mycelium, single or in conidiophores, subcylindrical, elongate-ampulliform or ampulliform with constricted base, 6–25 × 2–3 μm, adelophialides occasionally in the aerial mycelium, but submerged in medium dominating, 2.5–6 × 2–3 μm, collarettes distinct, funnel-shaped, 1–3 μm long, 1.5–2 μm wide, opening 1 μm wide. Conidia aggregated in heads, hyaline, aseptate, cylindrical to allantoid, smooth-walled, containing several droplets, 2–3.5(–5) × 1–1.5(–2) μm, mean±SD = 2.7 ± 0.6 × 1.3 ± 0.3 μm, L/W ratio = 2. Vegetative hyphae on MEA yellow-brown, 1.5–3 μm wide, septate, smooth, combined to compact hyphal strands, chlamydospores absent. Conidiophores hyaline to yellow-brown, simple or branches, sometimes ending in sterile inflated cells. Conidiogenous cells enteroblastic with periclinal wall thickening, discrete phialides and adelophialides in the aerial mycelium, discrete phialides cylindrical, elongate-ampulliform or ampulliform with constricted base, 5–15 × 2–3 μm, adelophialides 2.5–6 × 2–3 μm, collarettes distinct, funnel-shaped, 1–2.5 μm long, 1.5–2 μm wide, opening 1 μm wide. Conidia aggregated in heads, hyaline, aseptate, ellipsoidal, oval, cylindrical to allantoid, smooth-walled, containing two or more droplets, (2–)3–5(–9) × (1–)1.5–2(–3) μm, mean±SD = 4.1 ± 1.1 × 1.8 ± 0.3 μm, L/W ratio = 2.3.
Cultural characteristics — Colonies on PDA flat, with entire edge, sometimes folded towards the centre, aerial mycelium floccose to villose, margin white, smoke-grey, buff, hazel, olivaceous-grey to dark green, reverse similar colours, sometimes scarlet pigment released into the medium, 15 mm in 2 wk (25 °C). Colonies on MEA flat, with entire edge, folded towards the centre, aerial mycelium short floccose-felty, margin white, glaucous sky blue, greenish olivaceous, fawn to dark brick reverse grey-olivaceous, dull green to fuscous-black, 16 mm in 2 wk (25 °C). Conditions for growth: min 10 °C, max 30 °C, opt 25 °C.
Specimens examined. South Africa, Western Cape Province, Franschhoek, from brown necrosis in wood of Prunus salicina close to old pruning wound, 10 June 2004, U. Damm, CBS H-19987 holotype, culture ex-type CBS 120871 = STE-U 6201; Franschhoek, from the same specimen, STE-U 6399; Franschhoek, from the same specimen, STE-U 6400.
Notes — Jattaea species and short-necked Calosphaeria species with ascospore sizes similar to that of J. prunicola are J. brevirostris, J. microtheca, C. alpina (Berlese 1900), J. spermatozoides (Berlese 1900, Saccardo 1902) and J. stachybotryoides (Romero & Samuels 1991). However, J. stachybotryoides has shorter asci and long-beaked perithecia (Romero & Samuels 1991). Ostioles of J. spermatozoides are thick and 3–4-sulcate or -tubercular, and asci are clavate with a long, filliform stipe (Berlese 1900), while J. prunicola has short beaks without furrows or tubercles and subcylindrical asci. The asci of J. brevirostris and J. microtheca are wider (Berlese 1900). Calosphaeria alpina has a similar neck length, but the ascomata are surrounded by a dense, almost stromatic hyphal layer, and the asci are shorter and clavate, gradually tapering towards the base (Berlese 1900).
Jattaea mookgoponga Damm & Crous, sp. nov. — MycoBank MB511430; Fig. 7
Fig. 7.
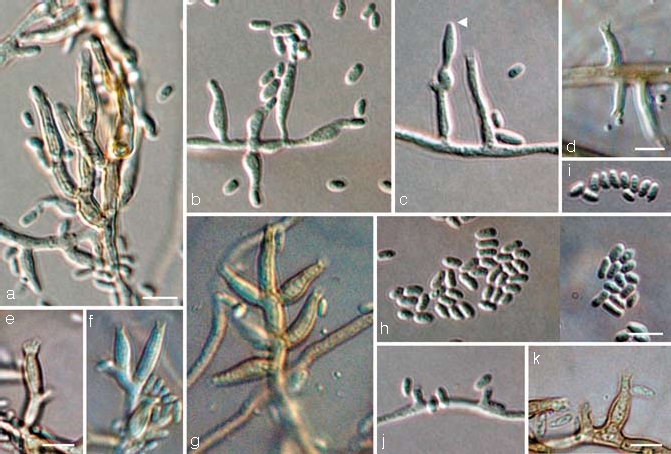
Jattaea mookgoponga, anamorph. a–g, j, k. Conidiophores and conidia (arrow head indicates sterile inflated cell); h, i. conidia; all from CBS H-19986 (holotype); a–c, e–k. SNA medium, d. PDA medium, a–c, h–j: DIC, d–g, k: BF. — Scale bars: a, d, e, h, k = 5 μm; a applies to a–c; e applies to e–g; h applies to h–j.
Jattaeae prunicolae similis, sed conidiis non allantoidibus, in cultura (PDA) coloniis citioribus crescentibus.
Etymology. Named after the town, Mookgopong, where it was collected.
Vegetative hyphae on SNA hyaline to yellow-brown, 1–3 μm wide, septate, smooth, fine granular contents, sometimes appearing to be covered with slime, chlamydospores absent. Conidiophores hyaline to yellow-brown, multiple branched, up to 35 μm tall, sometimes ending with sterile, irregularly inflated cells. Conidiogenous cells enteroblastic, discrete phialides dominant, single or in conidiophores, subcylindrical, elongate-ampulliform or ampulliform with constricted base, 2–15 × 1.5–3 μm, occasionally adelophialides present, cylindrical or conical, 2–4 × 1.5–3 μm; collarettes distinct, funnel-shaped to narrowly so, 1–2 μm long, 1.5–2 μm wide, opening 1 μm wide. Conidia aggregated in heads, hyaline, aseptate, cylindrical, ellipsoidal or oval, both ends obtuse, smooth-walled, with several droplets, 2–3.5(–5) × 1–1.5(–2) μm, mean±SD = 2.7 ± 0.6 × 1.3 ± 0.3 μm, L/W ratio = 2. Vegetative hyphae on MEA yellow-brown, 1.5–3.5 μm wide, septate, smooth to verrucose, partly combined to hyphal strands, chlamydospores absent. Conidiophores hyaline to yellow-brown, simple or branched, up to 50 μm tall, sometimes ending with sterile cells. Conidiogenous cells enteroblastic with periclinical wall thickening, discrete phialides and adelophialides, discrete phialides subcylindrical to elongate-ampulliform with constricted base, 8–20 × 2–3 μm, adelophialides cylindrical to conical, 2–8 × 1.5–3 μm; collarettes distinct, narrowly funnel-shaped, 1–2 μm long, 1.5–2 μm wide, opening 1 μm wide. Conidia aggregated in heads, hyaline, aseptate, cylindrical to ellipsoidal, both ends obtuse, smooth-walled, sometimes with droplets, (2.5–)3.5–4.5(–7) × (1–)1.5–2(–3) μm, mean±SD = 4 ± 0.7 × 1.6 ± 0.2 μm, L/W ratio = 2.4.
Cultural characteristics — Colonies on PDA flat, with fimbriate edge, sometimes folded in the centre, aerial mycelium short floccose, white, buff, honey, ochreous to fulvous, turning dark sepia with age, reverse white, luteus to umber, attaining 19 mm within 2 wk (25 °C). Colonies on MEA flat, fimbriate edge, with floccose aerial mycelium; surface rosy-buff, cinnamon to orange, turning umber with age, reverse pale luteous, orange, sienna to umber, 21 mm within 2 wk (25 °C). Conditions for growth: min 10 °C, max 30 °C, opt 25 °C.
Specimens examined. South Africa, Limpopo Province, Mookgopong (= Naboomspruit), from reddish brown, round, central necrosis in wood of Prunus persica var. nucipersica, close to an old pruning wound, 31 Aug. 2004, U. Damm, CBS H-19986 holotype, culture ex-type CBS 120867 = STE-U 6184; Mookgopong, from the same specimen, STE-U 6401.
Notes — Jattaea mookgoponga, which is presently only known from its asexual state, is distinct from J. prunicola based on its faster growth rate in culture, lack of allantoid conidia, and DNA phylogeny. No asexual states are known from any of the other Jattaea species described to date.
Ascus dehiscence and ascospore release in Calosphaeria africana
The process of ascus dehiscence and ascospore release in C. africana require the following steps:
-
0.
An ascus that did not absorb water is clavate with a very thin stipe (Fig. 3m, n). Ascospores are biseriate to crowded in the upper part (pars sporifera). The ascus wall appears broad, especially at the apex.
-
1.
When the ascus absorbs water, the inner ascus wall appears to break through the outer wall and stretches to a certain extent beyond the outer wall (Fig. 3o). In the process, the volume of the ascus increases and the ascus changes shape, becoming broader, nearly wedge-shaped in plan view. The wall layers are pressed together appearing as a single, well visible line, but slightly differing in thickness depending whether there are one or two layers. Ascospores and droplets are first moving throughout the ascus, but at separation of the wall layers locate towards the apex.
-
2.
When the ascus has reached its maximum volume, the ascus starts changing its shape again. The extended inner apex wall seems to deflate, and starts to detach from the outer wall by forming a depression close to the transition zone to the outer wall, letting the inner wall appear like a bag (Fig. 3p, q). Within a few seconds, the bag-like structure expands, the ascospores are pressed into it and the inner wall layer seems to fold around them. The ascospores are rather crowded again, comparable to ‘stage 0’. The apical ring is sometimes visible at the tip of the bag-like structure. The inner ascus wall also detaches laterally from the outer wall, while it stays attached at the base. The edges of the outer wall layer become free and usually fold inside.
-
3.
After the bag stretches to its maximum size, sometimes leading to a tube-like shape, the ascospores are released. Ascospore release could have happened either trough the apical ring (Fig. 3r) or by the disintegration of the inner ascus wall. Although ascospore release through the apical ring has hardly been observed directly, regular tremors of the rehydrated hymenium resulting in an increase of released ascospores and everted and obviously punctured apical rings observed at the apex of empty endoasci suggest ascospore release occurring through the apical ring. However, the stretched endoascus looks rather deflated than being under high hydrostatic pressure.
-
4.
After the ascospores are released, the inner ascus contracts or just collapses and the everted apical ring is visible, while the outer wall retains the same shape (Fig. 3s–u). The empty asci quickly disintegrate.
In our observations, the asci were detached from the ascoma and the rest of the centrum. Additional pressure from paraphyses and peridium on the asci is missing that might alter the process. Under the dissecting microscope we observed an enormous volume increase of the centrum in water which is also due to the swelling of the paraphyses. Paraphyses mounted in water were wider than when mounted in lactic acid, they were restricted at the septa, cells sometimes even rupture longitudinally, otherwise stretched out. Within an intact ascoma there would be a build-up of hydrostatic pressure throughout the centrum. Thus not only the turgor of the asci increases, but also the pressure in its environment. This pressure is also necessary to transport the ascospores through the neck and thus usually outside the bark/wood where they can be disseminated by wind, water or insects and infect new host tissue. By placing perithecia in a water drop or with the venter in wet filter paper, we sometimes observed ascospores extruding out of the neck. If the neck was cut off a perithecium close to the venter, without placing a cover slip over the preparation, asci in different stages of dehiscence were visible emerging from the ascoma directly into the water drop that was placed under the light microscope. This shows that the process is a result of a turgor pressure due to rehydration. However, asci from mature perithecia often failed to release ascospores; they obviously needed to dry out to a certain degree (not estimated) before being rehydrated.
DISCUSSION
During our survey, several grapevine trunk disease pathogens and related species in the Botryosphaeriaceae and Togniniaceae were encountered that had previously been reported on Prunus trees (Damm et al. 2007, Damm et al. in press). In this study, we report on species of presumably Calosphaerialean fungi that are not known as grapevine pathogens. All three species isolated during our survey are new to science and were described here as Calosphaeria africana, Jattaea prunicola and J. mookgoponga. In the LSU phylogeny, these species grouped close to Calosphaeria and Togniniella species and therefore could be confirmed to belong to Calosphaeriaceae.
Calosphaeria africana could easily be assigned to the genus Calosphaeria based on its DNA sequence similarity to C. pulchella, as well as anamorph and teleomorph morphology. Sequences of the other two species showed affinities to the Calosphaeriaceae but did not result in a close match with sequences from GenBank. However, by obtaining the teleomorph of one of these strains, it could be identified as a Jattaea species using the key in Mostert et al. (2006). Jattaea prunicola fits well in the generic concept of Jattaea by having small, globose, short-beaked ascomata that are covered by short hyphae and appear in small groups, unitunicate, 8-spored, fasciculate asci, broad, elongate paraphyses, and hyaline to slightly pigmented, allantoid, aseptate ascospores (Barr 1985). According to Berlese’s drawing (Berlese 1900), the asci of the type species, J. algeriensis, are similar in shape to those of J. africana, being rather cylindrical-fusiform and not conspicuously divided into pars sporifera and stipe as in C. pulchella. The morphology of J. africana did not match any of the 14 Jattaea species presently known, nor any of the Calosphaeria species described as having a short neck.
This is the first time that teleomorphs of species of Calosphaeriaceae have been obtained in culture. Most species of Jattaea have not been collected since their original description, and all reports and descriptions in this genus are based on teleomorph structures in vivo, none of which have ever been cultured. Therefore, neither DNA sequence data, nor any anamorph association was known prior to this study. Jattaea was originally placed in the Calosphaeriaceae (Munk 1957). Réblová et al. (2004) regarded taxa in the Calosphaeriales as probably being polyphyletic, because of the diversity in teleomorph morphology and anamorphs. The genera Togninia and Jobellisia, for example, have been shown to be closer to the Diaporthales based on DNA sequence analysis, in spite of being similar to the Calosphaeriales (Réblová et al. 2004). Additionally, this assumption has recently been substantiated by recollecting C. barbirostris (now Barbatosphaeria barbirostris) that has ramichloridium- and sporothrix-like synanamorphs and is not closely related to Calosphaeriales (Réblová 2007). In this study, two species were recognised as belonging to the genus Jattaea, which is closely related to Calosphaeria and Togniniella as shown in the phylogenies obtained here. Presumed that J. prunicola and J. mookgoponga are true species of the genus Jattaea, our results confirm that the genus Jattaea belongs to the Calosphaeriaceae (Calosphaeriales). However, this needs to be verified once a sequence is available for J. algeriensis, the type species of the genus. Since we sequenced only two species and the species described in the literature are morphologically quite diverse, the genus Jattaea may well be polyphyletic. While there are reports of Calosphaeria (Doidge 1950) on Prunus in South Africa, this is the first report for Jattaea from this host and country.
While C. africana produced an anamorph in culture similar to that of C. pulchella, Jattaea prunicola and J. mookgoponga produced phialophora-like anamorphs that resembled Phaeocrella, the anamorph of Togniniella (Réblová et al. 2004). Conidiophores of the phialophora-like anamorph of Jattaea prunicola often end in inflated sterile cells. Similar structures have been observed in Ramophialophora vesiculosa (Calduch et al. 2004). However, while sterile end-cells in R. vesiculosa occur regularly at the terminal ends of conidiophores and have a distinct shape, those in J. prunicola are irregular both in occurrence and shape, often looking like phialides without openings. Comparison of the ITS sequences confirmed R. vesiculosa to not be closely related to Jattaea (data not shown).
Non-amyloid apical rings were described in C. rhododendri, C. transversa, C. tumidula, C. vasculosa and J. villosa (Mostert et al. 2006). Apical rings were also observed in J. prunicola and C. africana that were described in this study. However, while in J. prunicola ascospores appear to be passing through apical rings and probably are discharged in turn as it is known from other pyrenomycetes like Sordaria and Xylaria (Webster & Weber 2007), ascospores in C. africana are not directly discharged from the complete ascus. Ascus dehiscence and ascospore release require separate steps. Firstly, ascospores are released in a bag-like structure, after which they are discharged from the ascus in a subsequent step. Even if the process as described here does not reflect exactly the process in nature, it shows that in C. africana, which is supposed to have unitunicate asci, two functional ascus wall layers are active in ascospore discharge. The inner and the outer ascus wall layer differ in their ability to extend, which corresponds with the definition of a bitunicate ascus (Luttrell 1951) and they almost completely detach from each other with the endoascus extruding out of the exoascus, which is described as fissitunicate ascus dehiscence (Bellemère 1994) or ‘jack-in-box’ (Ingold 1933).
As demonstrated by means of electron microscopy (Bellemère 1994), the ascus wall structure is primarily characterised by the presence or absence and thickness of up to four distinguishable layers that can be divided in sublayers. The formation of endo- and exoascus is rather secondary, and the layers that are combined are variable. The ascus wall of unitunicate pyrenomycetes is often composed of two tunicae, a thin single- or double-layered exoascus, and a thicker endoascus (Parguey-Leduc & Janex-Favre 1984). Compared to bitunicate fungi sensu stricto, the endoascus has no or only little fibrillar waves and both layers are thinner (Parguey-Leduc & Janex-Favre 1984) and usually remain attached during ascospore discharge (Webster & Weber 2007). According to Bellemère (1994), who emphasised that there is no strict correlation between apex structure and dehiscence type, C. africana might be considered as having unitunicate asci with apical rings and fissitunicate ascus dehiscence. However, in fissitunicate ascus dehiscence the layers forming the endoascus are different from the ones for example in Dothideomycetes, and comparatively thin. In the fissitunicate ascospore release demonstrated here for C. africana the inner ascus wall apparently does not stretch much in length, which could be due to differences in structure such as less fibrillar waves or endo- and exoascus formed by different layers than those of bitunicate Dothideomycetes.
The presence of asci with two functional wall layers is a typical feature of Dothideomycetes (Schoch et al. 2006, Crous et al. 2007). However, it has also been found in other taxa. The Coryneliales had been regarded as representative of unitunicate ascomycetes (Müller & von Arx 1973), having a thin-walled, evanescent ascus wall (Fitzpatrick 1920), until Johnston & Minter (1989) scrutinised asci of different ages microscopically. They observed the ectotunica to break already in an early stage of ascus development, before spore liberation, asci without apical rings and passive ascospore release, which differs from typical bitunicate asci found in the Dothidiomycetes, and as shown in this study, from C. africana. The Coryneliales as then being recognised as representative of bitunicate fungi, had been regarded as an order of uncertain position within the Pezizomycotina (Eriksson 2006). Schoch et al. (2006) demonstrated by DNA sequence analyses that Coryneliales as well as Chaetothyriales and Verrucariales (also bitunicate) belong to the Eurotiomycetes.
Within the lichen-forming Lecanoromycetes, there is a variety of unitunicate and bitunicate/fissitunicate ascus types as well as transitional stages (Honegger 1982). The Peltigera-ascus type (Peltigerales) is similar to that of C. africana in having both fissitunicate ascus dehiscence and apical rings that evert after extension of the endoascus (Honegger 1978). Unlike C. africana, apical rings in Peltigeraceae are iodine-positive (amyloid), that means they have a different chemical composition, and the endoascus is still laterally connected to the exoascus. There are also fissitunicate dehiscence mechanisms described in unitunicate pyrenomycetes. In Melanamphora spiniferum (current name: Melogramma spiniferum, Sordariomycetes), the ascospores are discharged individually through the apical ring after the endoascus becomes free at the apex (Laflamme 1975), as is possibly the case in C. africana.
Some other taxa in Calosphaeriales and Togniniaceae have been found to have the tendency of separating outer and inner ascus walls. The ascal base of Pleurostoma contains a thin appendage after ascus dehiscence, that is regarded as a remnant of the inner ascus layer of the functionally unitunicate ascus wall (Réblová et al. 2004). Hair-like structures at the ascus base have been observed in Calosphaeria and Jattaea species (Mostert et al. 2006, and this study). In the genus Togninia, that is closely related to the Calosphaeriales, basal parts of asci, so called remnant bases, often remain on the ascogenous hyphae after detachment of mature asci (Réblová et al. 2004, Mostert et al. 2006). Since free-floating asci are usually undamaged, this can only be explained as being parts of an ectotunica, similar to the frills at the bases of asci in Coryneliales.
The type of ascus dehiscence observed here, appears to be the same as described for a number of species in Diaporthales and Xylariales, and even in Calosphaeria princeps (Calosphaeriales, all Sordariales) as ‘pseudo-Jack-in-box’ (Parguey-Leduc & Chadefaud 1963, Parguey-Leduc 1977) or pseudofissitunicate dehiscence (Eriksson 1981). What extrudes is considered as only the uppermost part of the epiplast of the ascus surrounded by plasmalemma, while the two layers of the ascus wall stay attached and rupture as a unit. In the pseudofissitunicate dehiscence as well as in C. africana, the apical ring usually turns out with the bag-like structure (Parguey-Leduc 1977, fig. 47, 48). However, our figures clearly show there is a continuum between the apical ring and the layer that turns out with the epiplast (Fig 3r–u). Thus both must have the same origin and, since the apical ring is formed as an inward extension of the apical wall (Parguey-Leduc 1977, Bellemère 1994, Webster & Weber 2007), both must be part of the ascus wall. According to Bellemère (1994), rings develop in the (inner) c and d layers of the ascus wall. On the other hand, in Botryosphaeria gregaria (current name: Glomerella cingulata, Sordariomycetes) and Venturia rumicis (Dothideomycetes) the dehiscence is described as fissitunicate with an endoascus that is diffluent; it dissolves in the surrounding water after extending from the exoascus (Parguey-Leduc 1977). That could be an explanation for the emergence of the apical ring together with the epiplasm in the pseudofissitunicate dehiscence type. If this is the case, it would mean these fungi would actually be fissitunicate.
In pyrenomycetes, which have ostiolate fruit bodies, ascus types that elongate at discharge are an advantage and usually enable the tip of the ascus to protrude from the ostiole of the perithecium at spore discharge, and a well-defined apical apparatus ensures the ascospores not to discharge laterally (Sherwood 1981). These adaptations are probably even more important for fungi with long-necked ascomata, like Calosphaeria species, compared to Jattaea species with short necks. If spores have a longer route until they reach the outside environment of the ascoma, more pressure needs to be built up from the hymenium and the asci need to get out of the hymenium quickly. Although asci of both C. africana and J. prunicola are usually detached before discharge, asci with a long stalk that can even extend might be a further advantage. However, we observed the fissitunicate discharge mechanism only in one Calosphaeria species, while Parguey-Leduc & Chadefaud 1963 observed a pseudofissitunicate ascus discharge in C. princeps.
Luttrell (1951) emphasised pyrenomycetes having ascolocular development as also having bitunicate asci, and combined them in the loculoascomycetes (Luttrell 1955), hence assuming them to be closely related, and opposing unitunicate pyrenomycetes. However, using molecular data, loculoascomycetes have been shown to be polyphyletic (Berbee 1996, Lindemuth et al. 2001), and are currently classified in Chaetothyriomycetidae and Dothideomycetes (Eriksson 2006, Spatafora et al. 2006). Lumbsch & Huhndorf (2007) discussed how fungal classification has changed by using molecular data, and features that were used to combine fungi even on order and class level like ascoma and ascus type, are recognised as being convergent developments. According to Sherwood (1981), convergent evolution of fungal structures in diverse taxa can be a result of strong selection pressure in habitats that are unfavourable for fungi. As a result, fissitunicate ascus mechanisms were found in different classes of ascomycetes. In our study, a fissitunicate ascus mechanism was described in a fungus belonging to Calosphaeriaceae (Calosphaeriales, Sordariomycetes) for the first time.
Honegger (1982) assumes the functionally unitunicate ascus types within the lichenised ascomycetes have evolved from the bitunicate ones. Hawksworth (1982) goes further and regards the bitunicate Peltigeraceae as a probably very ancient lineage of ascomycetes where different groups of ascomycetes, lichenised and non-lichenised, might have derived from, either by loss of the iodine-positive ring (bitunicate fungi), by loss or fusion of the exoascus (unitunicate fungi) or by reduction of the expansible inner layer and converting the ring to a broad dome (rostrate Lecanorales). Bellemère (1994) emphasised that the ascus dehiscent type often differs among taxa of the same systematic group and that there is no correlation between apex structure or ascus type and dehiscent mechanism. Therefore, he considers the ascus wall structure as a feature of high systematic value and regards the dehiscent type rather as a result of functional adaptation of structures to the conditions in the environment. However, the ability of two layers of the ascus wall to detach and the inner ascus to extend requires morphological features such as fibrillar structures (Parguey-Leduc & Janex-Favre 1984). It is possible that, in spite of the reduction of the ascus wall layers, some features that enable the fissitunicate ascus dehiscence might have persisted in the genes of more recent groups like Calosphaeriaceae, and function again when the genes are recombined in a particular way, and might be an advantage for the fungi under their specific conditions.
Acknowledgments
The authors acknowledge the University of Stellenbosch (US), National Research Foundation, THRIP, Winetech and the Deciduous Fruit Producer’s Trust for financial support. Prof dr David L. Hawksworth (Departamiento de Biologia Vegetal II, Facultad de Farmacia, Universidad Cornplutense, Madrid, Spain) is kindly thanked for constructive comments on the manuscript and Dr Lizel Mostert (US) for providing the ITS sequences of Calosphaeria pulchella and Togniniella acerosa.
REFERENCES
- Adaskaveg JE, Miller RW, Gilbertson RL. 1993. Wood decay, lignicolous fungi, and decline of peach trees in South Carolina. Plant Disease 77: 707 – 710 . [Google Scholar]
- Arzanlou M, Groenewald JZ, Gams W, Braun U, Shin H-D, Crous PW. 2007. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58: 57 – 93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr ME. 1985. Notes on the Calosphaeriales. Mycologia 77: 549 – 565 . [Google Scholar]
- Barr ME. 1994. Notes on ascomycete systematics – No. 1812. Systema Ascomycetum 13, 2: 192 . [Google Scholar]
- Barr ME. 1998. Wegelina a reinstated genus in the Calosphaeriales. Cryptogamie, Bryologie-Lichénologie 19, 1–2: 169 – 173 . [Google Scholar]
- Barr ME, Rogers JD, Ju Y-M. 1993. Revisionary studies in the Calosphaeriales. Mycotaxon 48: 529 – 535 . [Google Scholar]
- Bellemère A. 1994. Asci and ascospores in ascomycete systematics. In: Hawksworth DL. (ed), Ascomycete systematics: problems and perspectives in the nineties: 111–126 Plenum Press, New York, USA: . [Google Scholar]
- Berbee ML. 1996. Loculoascomycete origins and evolution of filamentous ascomycete morphology based on 18S rRNA gene sequence data. Molecular Biology and Evolution 13: 462 – 470 . [DOI] [PubMed] [Google Scholar]
- Berlese AN. 1900. Icones Fungorum omnium hucusque cognitorum. Vol.III (reprint 1968) Cramer, Lehre, Germany: . [Google Scholar]
- Calduch M, Gené J, Stchigel AM, Cano JF, Guarro J. 2004. Ramophialophora, a new anamorphic genus of Sordariales. Studies in Mycology 50: 83 – 88 . [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. 2007. Mycosphaerella is polyphyletic. Studies in Mycology 58: 1 – 32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Crous PW, Fourie PH. 2007. Botryosphaeriaceae as potential pathogens of Prunus species in South Africa, with descriptions of Diplodia africana and Lasiodiplodia plurivora spp. nov. Mycologia 99: 664 – 680 . [DOI] [PubMed] [Google Scholar]
- Damm U, Crous PW, Fourie PH . In press. Novel Phaeoacremonium species associated with necrotic wood of Prunus trees. Mycological Research . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doidge EM. 1950. The South African fungi and lichens. Bothalia Vol. V: 1 – 1094 Pretoria, South Africa: . [Google Scholar]
- Ellis JB, Everhart BM. 1892. The North American Pyrenomycetes: 1–793 Newfield, New Jersey, USA: . [Google Scholar]
- Eriksson OE. 1981. The families of bitunicate ascomycetes. Opera Botanica 60: 1 – 220 . [Google Scholar]
- Eriksson OE. (ed). 2006. Outline of Ascomycota – 2006. Myconet 12: 1 – 82 . [Google Scholar]
- Fitzpatrick HM. 1920. Monograph of Coryneliaceae. Mycologia 12: 206 – 267 . [Google Scholar]
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113 – 118 . [DOI] [PubMed] [Google Scholar]
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323 – 1330 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausner G, Eyjólfsdóttir GG, Reid J, Klassen GR. 1992. Two additional species of the genus Togninia. Canadian Journal of Botany 70: 724 – 732 . [Google Scholar]
- Hawksworth DL. 1982. Co-evolution and the detection of ancestry in lichens. Journal of the Hattori Botanical Laboratory 52: 323 – 329 . [Google Scholar]
- Hillis DM, Bull JJ. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182 – 192 . [Google Scholar]
- Honegger R. 1978. The ascus apex in lichenized fungi. I. The Lecanora-, Peltigera-, and Teloschistes-types. Lichenologist 10: 47 – 67 . [Google Scholar]
- Honegger R. 1982. Ascus structure and function, ascospore delimitation, and phycobiont cell wall types associated with the Lecanorales (Lichenized Ascomycetes). Journal of the Hattori Botanical Laboratory 52: 417 – 429 . [Google Scholar]
- Ingold CT. 1933. Spore discharge in the ascomycetes I. Pyrenomycetes. New Phytologist 32: 175 – 196 . [Google Scholar]
- Johnston PR, Minter DW. 1989. Structure and taxonomic significance of the ascus in the Coryneliaceae. Mycological Research 92: 422 – 430 . [Google Scholar]
- Kirschstein W. 1941. De plerisque novis ascomycetibus et paucis novis fungis imperfectis. Hedwigia 80: 119 – 137 . [Google Scholar]
- Laflamme G. 1975 (publ. 1976). Les genres Melogramma Fries et Melanamphora gen. nov., Sphaeriales. Sydowia 28: 237 – 274 . [Google Scholar]
- Lindemuth R, Wirtz N, Lumbsch HT. 2001. Phylogenetic analysis of nuclear and mitochondrial rDNA sequences supports the view that loculoascomycetes (Ascomycota) are not monophyletic. Mycological Research 105: 1176 – 1181 . [Google Scholar]
- Lumbsch HT, Huhndorf SM. 2007. Whatever happened to the pyrenomycetes and loculoascomycetes? Mycological Research 111: 1064 – 1074 . [DOI] [PubMed] [Google Scholar]
- Luttrell ES. 1951. Taxonomy of the pyrenomycetes. University of Missouri Studies 24, 3: 1 – 120 . [Google Scholar]
- Luttrell ES. 1955. The ascostromatic ascomycetes. Mycologia 47: 511 – 532 . [Google Scholar]
- Mostert L, Crous PW, Groenewald JZ, Gams W, Summerbell RC. 2003. Togninia (Calosphaeriales) is confirmed as teleomorph of Phaeoacremonium by means of morphology, sexual compatibility, and DNA phylogeny. Mycologia 95: 646 – 659 . [DOI] [PubMed] [Google Scholar]
- Mostert L, Groenewald JZ, Summerbell RC, Gams W, Crous PW. 2006. Taxonomy and pathology of Togninia (Diaportales) and its Phaeoacremonium anamorphs. Studies in Mycology 54 Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands: . [Google Scholar]
- Müller E, von Arx JA. 1973. Pyrenomycetes: Meliolales, Coronophorales, Sphaeriales. In: Ainsworth GC, Sparrow FK, Sussman AS. (eds), The Fungi: 87–132 Academic Press, New York, London: . [Google Scholar]
- Munk A. 1957. Danish Pyrenomycetes. A preliminary flora. Dansk Botanisk Arkiv 17, 1: 1 – 491 . [Google Scholar]
- Nirenberg HI. 1976. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem 169: 1 – 117 . [Google Scholar]
- O’Donnell K. 1993. Fusarium and its relatives. In: Reynolds DR, Taylor JW. (eds), The fungal holomorph: mitotic, meiotic, and pleomorphic speciation in fungal systematics: 225–233 CAB International, Wallingford, UK: . [Google Scholar]
- O’Donnell K, Cigelnik E. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103 – 116 . [DOI] [PubMed] [Google Scholar]
- Parguey-Leduc A. 1977. Les asques des Pyrénomycètes. Revue de Mycologie 41: 281 – 338 . [Google Scholar]
- Parguey-Leduc A, Chadefaud M. 1963. Les asques du Cainia incarcerata (Desm.) von Arx et Müller et la position systématique du genre Cainia. Revue de Mycologie 28: 200 – 234 . [Google Scholar]
- Parguey-Leduc A, Janex-Favre MC. 1984. La paroi des asques ches les pyrénomycètes: Étude ultrastructurale. II. Les asques unituniqués. Cryptogamie, Mycologie 5: 171 – 187 . [Google Scholar]
- Petrak F. 1924. Mykologische Notizen. VII. Annales Mycologici 22, 1–2: 1 – 182 . [Google Scholar]
- Rambaut A. 2002. Sequence Alignment Editor. Version 2.0 Department of Zoology, University of Oxford, Oxford, UK: . [Google Scholar]
- Rayner RW. 1970. A mycological colour chart Commonwealth Mycological Institute and British Mycological Society, Kew, Surrey, UK: . [Google Scholar]
- Réblová M. 2007. Barbatosphaeria gen. et comb. nov., a new genus for Calosphaeria barbirostris. Mycologia 99: 723 – 732 . [PubMed] [Google Scholar]
- Réblová M, Mostert L. 2007. Romellia is congeneric with Togninia, and description of Conidiotheca gen. nov. for one species of this genus with polysporous asci. Mycological Research 111: 299 – 307 . [DOI] [PubMed] [Google Scholar]
- Réblová M, Mostert L, Gams W, Crous PW. 2004. New genera in Calosphaeriales: Togniniella and its anamorph Phaeocrella, and Calosphaeriophora as anamorph of Calosphaeria. Studies in Mycology 50: 533 – 550 . [Google Scholar]
- Roets F, Beer ZW de, Dreyer LL, Zipfel R, Crous PW, Wingfield MJ. 2006. Multi-gene phylogeny for Ophiostoma spp. reveals two new species from Protea infructescences. Studies in Mycology 55: 199 – 212 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero AI, Samuels GJ. 1991. Studies on xylophilous fungi from Argentina. VI. Ascomycotina on Eucalyptus viminalis (Myrtaceae). Sydowia 43: 228 – 248 . [Google Scholar]
- Saccardo PA. 1878. Fungi Italici Autographice Delineati Italy, Patavii, tab. 476 . [Google Scholar]
- Saccardo PA. 1882a. Sylloge Fungorum Omnium hucosque cognitorum. I: 95 (C. princeps) . [Google Scholar]
- Saccardo PA. 1882b. Sylloge Fungorum Omnium hucosque cognitorum. I: 96 (C. ciliatula) . [Google Scholar]
- Saccardo PA. Sylloge Fungorum. 1882c. Omnium hucosque cognitorum. I: 98 (C. cylindrica) [Google Scholar]
- Saccardo PA. 1882d. Sylloge Fungorum Omnium hucosque cognitorum. I: 104 (C. abieticola) . [Google Scholar]
- Saccardo PA. 1882e. Sylloge Fungorum Omnium hucosque cognitorum. I: 105 (C. rosarum) . [Google Scholar]
- Saccardo PA. 1891. Sylloge Fungorum Omnium hucosque cognitorum. Supplementum universale IX: 448 . [Google Scholar]
- Saccardo PA. 1899. Sylloge Fungorum Omnium hucosque cognitorum. Supplementum universale XIV: 479 . [Google Scholar]
- Saccardo PA. 1902. Sylloge Fungorum Omnium hucosque cognitorum. Supplementum universale XVI: 421 . [Google Scholar]
- Samuels GJ, Candoussau F. 1996. Heterogeneity in the Calosphaeriales: a new Calosphaeria with Ramichloridium- and Sporothrix-like synanamorphs. Nova Hedwigia 62: 47 – 60 . [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW. 2006. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041 – 1052 . [DOI] [PubMed] [Google Scholar]
- Sherwood M.A. 1981. Convergent evolution in discomycetes from bark and wood. Botanical Journal of the Linnean Society 82: 15 – 34 . [Google Scholar]
- Spatafora JW, Sung G-H, Johnson D, Hesse C, O’Rourke B, Serdani M, Spotts R, Lutzoni F, Hofstetter V, Miadlikowska J, Reeb V, Gueidan C, Fraker E, Lumbsch T, Lücking R, Schmitt I, Hosaka K, Aptroot A, Roux C, Miller AN, Geiser DM, Hafellner J, Hestmark G, Arnold AE, Büdel B, Rauhut A, Hewitt D, Untereiner WA, Cole MS, Scheidegger C, Schultz M, Sipman H, Schoch CL. 2006. A five-gene phylogeny of Pezizomycotina. Mycologia 98: 1018 – 1028 . [DOI] [PubMed] [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods). Version 4 Sinauer Associates, Sunderland, MA, USA: . [Google Scholar]
- Tilak ST, Nagre RS. 1964. A new species of Calosphaeria from India. Mycopathologia 22: 341 – 342 . [Google Scholar]
- Vijaykrishna D, Mostert L, Jeewon R, Gams W, Hyde KW, Crous PW. 2004. Pleurostomophora, an anamorph of Pleurostoma (Calosphaeriales), a new anamorph genus morphologically similar to Phialophora. Studies in Mycology 50: 387 – 395 . [Google Scholar]
- Webster J, Weber R. 2007. Introduction to fungi 3d Edn University Press, Cambridge, UK: . [Google Scholar]
- White TJ, Bruns T, Lee J, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–322 Academic Press, San Diego, California, USA: . [Google Scholar]


