Abstract
Species of Mycosphaerella and Teratosphaeria represent important foliicolous pathogens of Proteaceae. Presently approximately 40 members of these genera (incl. anamorphs) have been recorded from Proteaceae, though the majority are not known from culture, and have never been subjected to DNA sequence analysis. During the course of this study, epitypes were designated for several important species, namely Batcheloromyces leucadendri, B. proteae, Catenulostroma macowanii, Mycosphaerella marksii, Teratosphaeria bellula, T. jonkershoekensis, T. parva, and T. proteae-arboreae. Several species were also newly described, namely Batcheloromyces sedgefieldii, Catenulostroma wingfieldii, Dissoconium proteae, Teratosphaeria persoonii, T. knoxdavesii, and T. marasasii. Although accepted as being highly host specific, some species were shown to have wider host ranges, such as M. communis (Eucalyptus, Protea), M. konae (Leucospermum, Eucalyptus), M. marksii (Eucalyptus, Leucadendron), T. associata (Eucalyptus, Protea), and T. parva (Eucalyptus, Protea), which in most cases were found to co-occur with other species of Mycosphaerella or Teratosphaeria on Proteaceae. Furthermore, earlier records of T. jonkershoekensis on Proteaceae in Australia were shown to be representative of two recently described species, T. associata and T. maxii. A phenomenon of underdeveloped, or micro-ascospores was also newly observed in asci of T. maculiformis and T. proteae-arboreae. The exact purpose of asci with two distinct types of ascospores remains to be clarified, as both types were observed to germinate on agar.
Keywords: ITS, Leucadendron, Leucospermum, Mycosphaerella, Protea, Teratosphaeria
INTRODUCTION
Several genera of South African Proteaceae, especially Protea, Leucospermum and Leucadendron are routinely cultivated for the local and export cut-flower industry. Due to popular demand, these flowers are also now being cultivated in various countries around the world (Crous et al. 2004a). In spite of the popularity of these crops, fungal pathogens still represent a serious impediment to their cultivation. Several groups of fungal pathogens of Proteaceae have in recent years been characterised morphologically as well as phylogenetically, such as the Botryosphaeriaceae stem cankers (Denman et al. 1999, 2000, 2003, Crous et al. 2006b), Armillaria and Cylindrocladium root rot (Schoch et al. 1999, Crous 2002, Coetzee et al. 2003), Elsinoë scab disease (Swart et al. 2001) and Phomopsis cankers (Mostert et al. 2001a, b). However, this is generally not true for the pathogens associated with leaf diseases, as most have not been studied in culture.
Species of Mycosphaerella and Teratosphaeria are widespread on Proteaceae, and cause leaf spots and blights on numerous plant hosts in this family (Swart et al. 1998, Crous & Palm 1999, Crous et al. 2000a, Taylor & Crous 2000). In the compendium of Proteaceae diseases, Crous et al. (2004a) listed 13 species of Mycosphaerella (incl. Teratosphaeria), and 18 associated anamorph species, while Crous & Groenewald (2006a, b) recently described a further two Teratosphaeria spp. from Protea. Although several studies have focused on the distribution of Mycosphaerella spp. of Proteaceae in native and exotic habitats (Crous et al. 2000b, 2004a, Taylor & Crous 2000, Taylor et al. 2001a, b), their phylogenetic relationships have remained largely unresolved (Taylor et al. 2003).
The genus Mycosphaerella includes more than 3 000 names (Aptroot 2006), which together with names in associated anamorph genera probably represent close to 10 000 names (Crous et al. 2000a, 2001, 2004a, b, 2006a, b, c, 2007a, b, c, Crous & Braun 2003, Arzanlou et al. 2007). Although previous phylogenetic studies based on the ITS rDNA region have suggested Mycosphaerella to be monophyletic (Crous et al. 2000a, 2001, Goodwin et al. 2001), recent studies employing LSU sequence data have refuted this (Hunter et al. 2006), and split off several genera such as Davidiella (Davidiellaceae, Braun et al. 2003, Schoch et al. 2006, Crous et al. 2007b, Schubert et al. 2007), Schizothyrium (Schizothyriaceae, Batzer et al. 2008), and Teratosphaeria (Teratosphaeriaceae, Crous et al. 2007a). Although Crous et al. (2004a) listed eight species of Mycosphaerella from Proteaceae in South Africa, Crous et al. (2007a) have recently placed several of these in Teratosphaeria.
The genus Teratosphaeria is separated from Mycosphaerella s.str. based on several characters such as the presence of superficial stromatic tissue, ascospores that darken in their asci, remnants of the hamathecial tissue, ascospores that are frequently covered by a mucoid sheath, asci with a multi-layered endotunica, and the presence of ostiolar periphyses (Crous et al. 2007a). Presently 12 anamorph genera have been linked to Teratosphaeria (see Crous et al. 2007a for key), with the majority being quite distinct from those found in Mycosphaerella s.str. Although the genus Teratosphaeria was initially established for species occurring on Proteaceae, the genus remains poorly understood, as many of the taxa are not known from culture, and their phylogenetic position remains uncertain. The aim of the present study was thus to recollect these taxa from Proteaceae, and designate epitype specimens for many of the older names, thereby enabling us to clarify their phylogeny.
MATERIALS AND METHODS
Isolates
Proteaceae leaves bearing ascomata, or with leaf spots were chosen for study. Excised lesions were soaked in water for approximately 2 h, after which they were placed in the bottom of Petri dish lids, with the top half of the dish containing 2 % malt extract agar (MEA; Oxoid, Hampshire, England). Ascospore germination patterns were examined after 24 h, and single ascospore and conidial cultures established as described by Crous (1998). Colonies were sub-cultured onto 2 % potato-dextrose agar (PDA), synthetic nutrient-poor agar (SNA), MEA, and oatmeal agar (OA) (Gams et al. 2007), and incubated under continuous near-ultraviolet light at 25 °C to promote sporulation. All cultures obtained in this study are maintained in the culture collection of the CBS (Table 1). Nomenclatural novelties, descriptions and trace files of the ITS DNA barcodes were deposited in MycoBank (www.MycoBank.org).
Table 1.
Details of isolates included for morphological and / or molecular examination in this study. The GenBank accession numbers of isolates for which ITS sequences were generated for the first time are printed in bold face.
| Teleomorph | Anamorph | Accession number1 | Host | Country | Collector | GenBank Accession number |
|---|---|---|---|---|---|---|
| Mycosphaerella buckinghamiae | CBS 111996; CPC 3006* | Buckinghamia sp. | Australia | P.W. Crous & B. Summerell | EU707855 | |
| CBS 112175; CPC 3360 | Buckinghamia sp. | – | – | EU707856 | ||
| M. communis | Dissoconium commune | CBS 112889; CPC 3359 | Protea magnifica | Australia | P.W. Crous | AY725539 |
| CBS 114238; CPC 10440* | Eucalyptus globulus | Spain | J.P. Mansilla | AY725541 | ||
| M. holualoana | CBS 110698; CPC 2126* | Leucospermum sp. | USA: Hawaii | P.W. Crous & M.E. Palm | AY260087 | |
| M. konae | Pseudocercospora sp. | CBS 111261; CPC 2123* | Leucadendron sp. | USA: Hawaii | P.W. Crous & M.E. Palm | AY260086 |
| CBS 111028; CPC 2125 | Leucadendron sp. | USA: Hawaii | P.W. Crous & M.E. Palm | AY260085 | ||
| CBS 120748; CPC 13469 | E. camaldulensis | Thailand | W. Himaman | EF394842 | ||
| M. marksii | CBS 110942; CPC 982* | E. botryoides | Australia | A.J. Carnegie | AF309589 | |
| CBS 110974; CPC 984 | E. botryoides | Australia | A.J. Carnegie | – | ||
| CBS 115501; CPC 5358 | Leucadendron tinctum | Madeira Islands | S. Denman | DQ302979 | ||
| M. stromatosa | Pseudocercospora stromatosa | CBS 101953; CPC 1731* | Protea sp. | South Africa | S. Denman | EU167598 |
| M. waimeana | Stenella sp. | CBS 110697; CPC 2179* | Leucospermum sp. | USA: Hawaii | P.W. Crous & M.E. Palm | AY260083 |
| Teratosphaeria alistairii | Batcheloromyces sp. | CBS 120035; CPC 12730* | P. repens | South Africa | P.W. Crous & A. Smith | DQ885901 |
| T. associata | CBS 112224; CPC 3116 | P. lepidocarpodendron | Australia | P.W. Crous & B. Summerell | DQ302968 | |
| CBS 112627; CPC 3115 | P. lepidocarpodendron | Australia | P.W. Crous & B. Summerell | EU707857 | ||
| CBS 114165; CPC 3117 | P. lepidocarpodendron | Australia | P.W. Crous & B. Summerell | EU707858 | ||
| CBS 120730; CPC 13119* | Corymbia henryii | Australia | A.J. Carnegie | EF394826 | ||
| CBS 120731; CPC 13128 | C. variegata | Australia | A.J. Carnegie | EF394827 | ||
| CBS 120732; CPC 13108 | E. dunnii | Australia | A.J. Carnegie | EF394824 | ||
| T. bellula | CBS 111699; CPC 1816 | Leucospermum sp. | South Africa | J.E. Taylor | EU707859 | |
| CBS 111700; CPC 1821 | P. eximia | South Africa | J.E. Taylor | EU019301 | ||
| CBS 114145; CPC 2795 | Leucadendron sp. | South Africa | L. Swart | EU707860 | ||
| CPC 14908 | Protea sp. | South Africa | P.W. Crous | EU707861 | ||
| T. fibrillosa | CBS 121707; CPC 13960* | Protea sp. | South Africa | P.W. Crous & L. Mostert | EU707862 | |
| CPC 1876 | P. nitida | South Africa | J.E. Taylor | AY260094 | ||
| CPC 13969 | Protea sp. | South Africa | P.W. Crous | EU707863 | ||
| T. jonkershoekensis | CBS 122897; CPC 13984* | Protea sp. | South Africa | P.W. Crous & L. Mostert | EU707864 | |
| T. knoxdavesii | CBS 122898; CPC 14960* | Protea sp. | South Africa | P.W. Crous & M. Crous | EU707865 | |
| CPC 14905 | Protea sp. | South Africa | P.W. Crous & M. Crous | EU707866 | ||
| T. maculiformis | No culture available | Protea sp. | South Africa | P.W. Crous & K.L. Crous | EU707867 | |
| T. marasasii | CBS 122899; CPC 14889* | Protea sp. | South Africa | P.W. Crous & M. Crous | EU707868 | |
| T. maxii | CBS 112231; CPC 3321 | Protea sp. | Australia | P.W. Crous & B. Summerell | EU707869 | |
| CBS 112232; CPC 3323 | Protea sp. | Australia | P.W. Crous & B. Summerell | EU707870 | ||
| CBS 112496; CPC 3322 | Protea sp. | Australia | P.W. Crous & B. Summerell | EU707871 | ||
| CBS 120137; CPC 12805* | P. repens | South Africa | M. Crous & P.W. Crous | DQ885899 | ||
| CPC 12943 | P. repens | South Africa | P.W. Crous | DQ885898 | ||
| T. microspora | Catenulostroma microsporum | CBS 101951; CPC 1960* | P. cynaroides | South Africa | S. Denman & J.E. Taylor | EU707872 |
| CBS 110890; CPC 1832 | P. cynaroides | South Africa | L. Swart | AY260097 | ||
| CBS 111031; CPC 1848 | P. cynaroides | South Africa | J.E. Taylor | AY260098 | ||
| CBS 111697; CPC 1597 | P. cynaroides | South Africa | P.W. Crous | EU707873 | ||
| T. parva | CBS 114761; CPC 1217 | P. repens | South Africa | P.W. Crous | EU707874 | |
| CBS 122892; CPC 12421* | E. globulus | Australia | I. Smith | EU707875 | ||
| CBS 122893; CPC 14898 | P. repens | South Africa | L. Mostert | EU707876 | ||
| CBS 122894; CPC 13896 | P. nitida | South Africa | P.W. Crous & L. Mostert | EU707877 | ||
| CPC 2120 | P. repens | South Africa | G. Matthews | AY260091 | ||
| CPC 12418 | E. globulus | Australia | I. Smith | EU707878 | ||
| CPC 12419 | E. globulus | Australia | I. Smith | EU707879 | ||
| T. persoonii | CBS 122895; CPC 13972* | Protea sp. | South Africa | P.W. Crous & L. Mostert | EU707880 | |
| CBS 122896; CPC 14846; STE-U 6389 | Euchaetis meridionalis | South Africa | A.R. Wood | EU707881 | ||
| T. proteae-arboreae | CPC 12952* | P. nitida | South Africa | M.K. Crous & P.W. Crous | EU707882 | |
| CPC 12954* | P. nitida | South Africa | M.K. Crous & P.W. Crous | EU707883 | ||
| CPC 14963 | Protea sp. | South Africa | P.W. Crous & M. Crous | EU707884 | ||
| Teratosphaeria sp. | CPC 13917 | P. nitida | South Africa | P.W. Crous & L. Mostert | EU707885 | |
| Teratosphaeria sp. | CPC 13963 | P. nitida | South Africa | P.W. Crous & L. Mostert | EU707886 | |
| Teratosphaeria sp. | CPC 13981 | P. repens | Portugal | M.F. Moura | EU707887 | |
| Teratosphaeria sp. | CPC 14957 | Protea sp. | South Africa | P.W. Crous & M. Crous | EU707888 | |
| Batcheloromyces leucadendri | CBS 110892; CPC 1837 | Leucadendron sp. | South Africa | L. Swart | AY260100 | |
| CBS 111577; CPC 1838* | Leucadendron laureolum | South Africa | L. Swart | AY260101 | ||
| CBS 111937; CPC 2822 | Leucadendron sp. | South Africa | L. Swart | EU707889 | ||
| CBS 114024; CPC 2794 | Leucadendron sp. | South Africa | L. Swart | EU707890 | ||
| CBS 114144; CPC 2823 | Leucadendron sp. | South Africa | L. Swart | EU707891 | ||
| CBS 114146; CPC 2820 | Leucadendron sp. | South Africa | L. Swart | EU707892 | ||
| CBS 119344; CMW 20456; PREM 58041 | Leucadendron salignum | South Africa | S. Lee | EU552103 | ||
| CPC 1839 | Leucadendron sp. | South Africa | L. Swart | – | ||
| CPC 1840 | Leucadendron gandogeri | South Africa | L. Swart | – | ||
| B. proteae | CBS 110696; CPC 1518* | P. cynaroides | South Africa | L. Swart | AY260099 | |
| CPC 1833 | P. cynaroides | South Africa | L. Swart | – | ||
| CPC 1834 | P. repens | South Africa | L. Swart | – | ||
| CPC 1835 | Protea sp. | South Africa | L. Swart | – | ||
| CPC 1836 | P. neriifolia | South Africa | L. Swart | – | ||
| B. sedgefieldii | CBS 112119; CPC 3026* | P. repens | South Africa | J.E. Taylor | EU707893 | |
| Catenulostroma elginense | CBS 111030; CPC 1958 | P. grandiceps | South Africa | J.E. Taylor & S. Denman | AY260093 | |
| C. macowanii | CBS 110756; CPC 1872 | P. nitida | South Africa | J.E. Taylor | AY260095 | |
| CBS 111029; CPC 1488 | P. nitida | South Africa | P.W. Crous | AY260096 | ||
| CBS 122901; CPC 13899* | P. nitida | South Africa | P.W. Crous & L. Mostert | EU707894 | ||
| CPC 13966 | P. nitida | South Africa | P.W. Crous & L. Mostert | EU707895 | ||
| C. wingfieldii | CBS 112163; CPC 2944* | P. nitida | South Africa | J.E. Taylor | EU707896 | |
| Dissoconium proteae | CBS 122900; CPC 13853* | Protea sp. | Canary Islands: | |||
| Tenerife | P.W. Crous | EU707897 | ||||
| Phaeothecoidea proteae | CBS 114129; CPC 2831* | P. repens | South Africa | S. Denman | EU707898 | |
| Pseudocercospora protearum | ||||||
| var. leucadendri | CPC 1869 | Leucadendron sp. | South Africa | P.W. Crous & S. Denman | AY260089 | |
| Ramularia proteae | CBS 112161; CPC 3075* | P. longifolia | Australia | A. Macfadyen | EU707899 | |
| Readeriella guyanensis | CBS 117550; MUCL 46082 | Leaf litter | French Guiana | – | EU707900 | |
| Septoria protearum | CBS 778.97; ATCC 201159; | |||||
| CPC 1470; IMI 375230 | P. cynaroides | South Africa | L. Viljoen | AY260081 | ||
| CPC 5212 | Protea sp. | Canary Islands: | ||||
| Tenerife | S. Denman | AY260082 |
1 ATCC: American Type Culture Collection, Virginia, USA; CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CMW: Culture collection of Mike Wingfield, housed at FABI, Pretoria, South Africa; CPC: Culture collection of Pedro Crous, housed at CBS; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, UK; MUCL: Mycotheque de l’ Université Catholique de Louvain, Louvainla-Neuve, Belgium; PREM: National Collection of Fungi, Pretoria, South Africa; STE-U: Culture collection of the Department of Plant Pathology, University of Stellenbosch, South Africa.
* Ex-type cultures.
DNA phylogeny
Fungal colonies were established on agar plates, and genomic DNA was isolated following the CTAB-based protocol described in Gams et al. (2007). The primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990) were used to amplify part (ITS) of the nuclear rDNA operon spanning the 3′ end of the 18S rRNA gene, the first internal transcribed spacer (ITS1), the 5.8S rRNA gene, the second ITS region and the 5′ end of the 28S rRNA gene. The primer ITS4 (White et al. 1990) was used in combination with primer V9G for sequencing to ensure good quality overlapping sequences were obtained. The PCR conditions, sequence alignment and subsequent phylogenetic analysis followed the methods of Crous et al. (2006b). The ITS1, ITS2 and 5.8S rRNA gene were sequenced only for those isolates for which these data were not available. Gaps longer than 10 bases were coded as single events for the phylogenetic analyses; the remaining gaps were treated as missing data. Sequence data were deposited in GenBank (Table 1) and the alignment and trees in TreeBASE (www.treebase.org).
Taxonomy
Wherever possible, 30 measurements (× 1 000 magnification) were made of structures mounted in lactic acid, with the extremes of spore measurements given in parentheses. Colony colours (surface and reverse) were assessed after 2–8 wk on MEA, OA and PDA at 25 °C in the dark, using the colour charts of Rayner (1970).
RESULTS
DNA phylogeny
Amplicons of approximately 1 700 bases were obtained for the isolates listed in Table 1. The ITS sequences were used to obtain additional sequences from GenBank, which were added to the alignment. The manually adjusted ITS alignment contained 151 sequences (including the outgroup sequence) and 973 characters including alignment gaps (available in TreeBASE). Of the 540 characters used in the phylogenetic analysis, 279 were parsimony-informative, 55 were variable and parsimony-uninformative, and 206 were constant. Neighbour-joining analyses using three substitution models on the sequence alignment yielded trees with identical topologies to one another, except for the position of Capnobotryella renispora, which differed when the uncorrected ‘p’ substitution model was compared with the Kimura 2-parameter and HKY85 models (it is placed basal to Teratosphaeria and Mycosphaerella in the latter two models). The neighbour-joining trees support the same clades as obtained from the parsimony analysis, but with a different arrangement at the deep nodes, for example the position of the Dissoconium and Phaeotheca clades. Because of the large number of different strain associations, for example in the Mycosphaerella marksii, Pseudocercospora and Teratosphaeria parva clades (as evident from the strict consensus branches shown in Fig. 1), only the first 10 000 equally most parsimonious trees (TL = 1725 steps; CI = 0.386; RI = 0.870; RC = 0.336) were saved, one of which is shown in Fig. 1. The phylogenetic results obtained are discussed where applicable in the descriptive notes below.
Fig. 1.
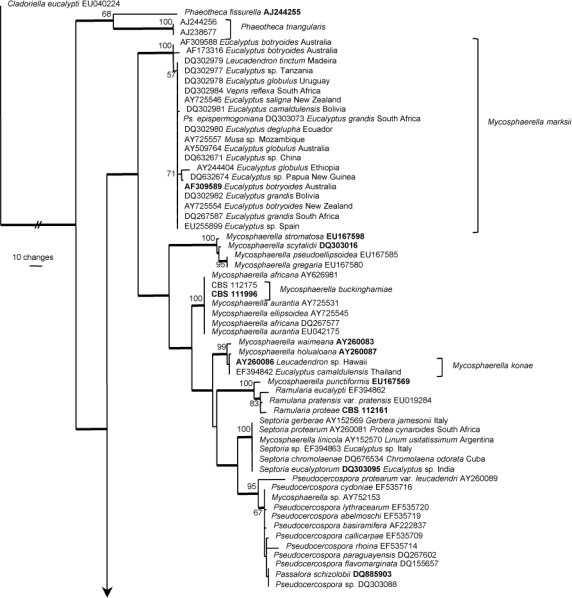

One of 10 000 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the ITS sequence alignment using PAUP v. 4.0b10. The scale bar shows 10 changes and bootstrap support values from 10 000 000 fast stepwise replicates are shown at the nodes. Thickened lines indicate the strict consensus branches and ex-type sequences are printed in bold face. The tree was rooted to a sequence obtained from GenBank (Cladoriella eucalypti EU040224).
Taxonomy
Although numerous species of Mycosphaerella, Teratosphaeria and associated anamorphs have been described from Proteaceae (Crous & Wingfield 1993, Crous & Braun 1994, 1996, Swart et al. 1998, Crous & Palm 1999, Crous et al. 2000b, Taylor & Crous 2000, Taylor et al. 2001a, b, c, 2003, Sivanesan & Shivas 2002), only those known from culture are discussed below.
Batcheloromyces leucadendri P.S. van Wyk, Marasas & Knox-Dav., S. African J. Bot. 51: 344. 1985 — Fig. 2
Fig. 2.
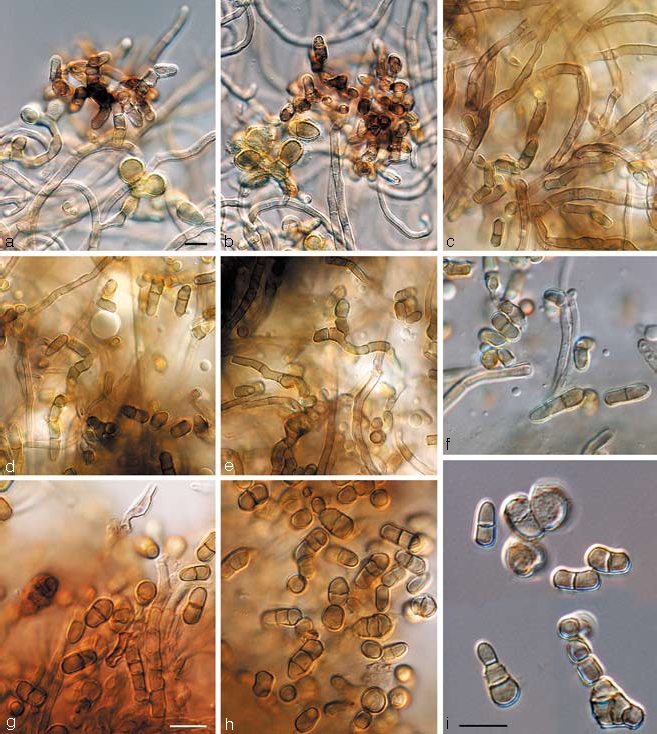
Batcheloromyces leucadendri and B. proteae. a–f. Batcheloromyces leucadendri (CBS 110892). a, b. Sporodochia formed in culture; c–e. chains of conidia formed in aerial mycelium; f. conidia. — g–i. Batcheloromyces proteae (CBS 110696). g, h. conidia formed in aerial mycelium; i. catenulate conidia (note percurrent proliferation on solitary conidiogenous cell). — Scale bars = 10 μm.
Descriptions — Taylor et al. (1999), Crous et al. (2004a).
Cultural characteristics — Colonies on MEA iron-grey, reverse olivaceous-black, smooth with irregular margins; aerial mycelium sparse and grey or lacking; moderately slow growing, up to 40 mm in diam after 2 mo.
Specimens examined. South Africa, Western Cape Province, Betty’s Bay, living leaves of Leucadendron gandogeri, 27 Mar. 1984, P.S. van Wyk, holotype PREM 47423; Cape Town, Kirstenbosch Botanical Gardens, on a living leaf of Leucadendron coniferum, 1996, L. Swart, F54, PREM 55954, CPC 1840; Porterville, Osdam Farm, on a living leaf of Leucadendron sp., 15 Jan. 1998, J.E. Taylor, JT84, PREM 55940; Stellenbosch, Helderberg Nature Reserve, on a living leaf of Leucadendron sp., 19 Jan. 1998, J.E. Taylor, JT107, PREM 55941; Stellenbosch, J.S. Marais Nature Reserve, on a living leaf of Leucadendron sp., 1996, L. Swart, F46, CPC 1837 = CBS 110892; Stellenbosch, Protea Heights farm, on a living leaf of Leucadendron laureolum, 1996, L. Swart, epitype designated here PREM 55949, cultures ex-epitype, CPC 1838 = CBS 111577; ibid, Leucadendron cultivar ‘Pisa’, F49, PREM 55950; Stellenbosch, J.S. Marais Park, on a living leaf of Leucadendron salicifolium, 1996, L. Swart, F50, PREM 55951; ibid, Leucadendron sp., F51, PREM 55952, CPC 1839; ibid, Leucadendron elimense, F52, PREM 55953; Western Cape Province, Leucadendron sp., 27 May 1999, L. Swart, CPC 2820 = CBS 114146; ibid, CPC 2823 = CBS 114144; ibid, CPC 2794 = CBS 114024; ibid, CPC 2822 = CBS 111937; Jonkershoek Nature Reserve, 6 June 2000, leaf litter of Leucadendron salignum, S. Marincowitz, S.L.130, PREM 58041, cultures CBS 119344 = CMW 20456.
Batcheloromyces proteae Marasas, P.S. van Wyk, & Knox-Dav., J. S. African Bot. 41: 43. 1975 — Fig. 2
≡ Stigmina proteae (Marasas, P.S. van Wyk, & Knox-Dav.) B. Sutton & Pascoe, Mycol. Res. 92: 214. 1989.
Descriptions — Taylor et al. (1999), Crous et al. (2004a).
Cultural characteristics — Colonies olivaceous, the same in reverse, smooth with irregular margins, sectored; aerial mycelium lacking; slow-growing, approximately 10–18 mm diam after 2 mo.
Specimens examined. South Africa, Western Cape Province, Stellenbosch, on living leaves of Protea cynaroides, 15 Aug. 1973, P.S. van Wyk, holotype PREM 44850; Betty’s Bay, Harold Porter Botanical Garden, on a living leaf of P. magnifica, 1997, L. Swart, F44, PREM 55947; ibid, P. neriifolia, F45, PREM 55948, CPC 1836; Stellenbosch, J.S. Marais Park, on a living leaf of P. cynaroides, 28 Feb. 1998, J.E. Taylor, JT119, PREM 55942; ibid, 1996, L. Swart, F42, PREM 55946; ibid, 26 Aug. 1996, L. Swart, F25, PREM 55943, CPC 1833; ibid, P. repens, F40, PREM 55944, CPC 1834; ibid, Protea sp., F41, PREM 55945, CPC 1835; Stellenbosch, Devon Valley, on a living leaf of P. cynaroides, 30 Aug. 1996, L. Swart, epitype designated here CBS H-20087, culture ex-epitype CPC 1518 = CBS 110696.
Notes — Although morphologically distinct, B. proteae could not be distinguished phylogenetically from B. leucadendri in the present study (Fig. 1).
Batcheloromyces sedgefieldii Crous, sp. nov. — MycoBank MB506591; Fig. 3
Fig. 3.

Batcheloromyces sedgefieldii in vivo (CBS H-20088). a. Sporodochia on leaf; b. sporodochium; c–e. conidiogenous cells giving rise to conidia; f. verruculose conidia. — Scale bars = 10 μm.
Batcheloromycetis proteae similis, sed in agaro (MEA) coloniis tarde crescentibus, usque ad 5 mm diam post 30 dies 25 °C.
Etymology. Named after Sedgefield, a town in the Southern Cape of South Africa, from where this fungus was collected.
Leaf spots containing black sporodochial plates, not extending through leaf lamina, on both sides of leaf surface, irregular, non-necrotic, becoming somewhat erumpent, up to 7 mm diam. Conidiomata sporodochial, composed of a single layer of radiating, septate, branched, dark brown, thick-walled hyphae which are formed from a stroma within the substomatal cavity, forming pulvinate plates, 100–120 μm diam; hyphae radiating from sporodochial plates and adhering closely to the host surface or growing into the stomata. Conidiophores erect or ascending, short lateral branches on the superficial hyphae, simple, brown, effuse, but occurring mainly towards the centre of the sporodochial plates, above the stomata, terminating to produce conidiogenous cells. Conidiogenous cells mainly subcylindrical, but occasionally doliiform, proliferating percurrently resulting in up to three irregular, ragged annellides, 5–10 × 3–5 μm. Conidia arising singly from blown out ends of the conidiogenous cells, solitary, unicellular, but often remaining in fragile chains of 2 conidia, ellipsoidal, oblong, with thick, verrucose walls, and a basal marginal frill, (5–)6.5–7(–10) × (3.5–)4–5 μm.
Cultural characteristics — Colonies on MEA olivaceous, with smooth margins, lacking aerial mycelium; extremely slow-growing, up to 5 mm diam after 2 mo.
Specimen examined. South Africa, Western Cape Province, Southern Cape, Sedgefield, on leaves of Protea repens, 10 Aug. 1999, J.E. Taylor, holotype CBS H-20088, culture ex-type CPC 3026 = CBS 112119.
Notes — Although phylogenetically distinct, B. sedgefieldii is morphologically similar to B. proteae. The two species can be distinguished in culture, however, as colonies of B. proteae are relatively fast growing compared to those of B. sedgefieldii, which only reach 5 mm diam after 2 mo.
Catenulostroma elginense (Joanne E. Taylor & Crous) Crous & U. Braun, Stud. Mycol. 58: 16. 2007
Basionym. Trimmatostroma elginense Joanne E. Taylor & Crous, Mycol. Res. 104: 633. 2000.
Descriptions — Taylor & Crous (2000), Crous et al. (2004a).
Specimen examined. South Africa, Western Cape Province, Elgin, Molteno Brothers Farm, on a living leaf of Protea grandiceps Tratt., 21 July 1998, J.E. Taylor & S. Denman, holotype PREM 56208, culture ex-type CPC 1958 = CBS 111030.
Catenulostroma macowanii (Sacc.) Crous & U. Braun, Stud. Mycol. 58: 17. 2007 — Fig. 4
Fig. 4.
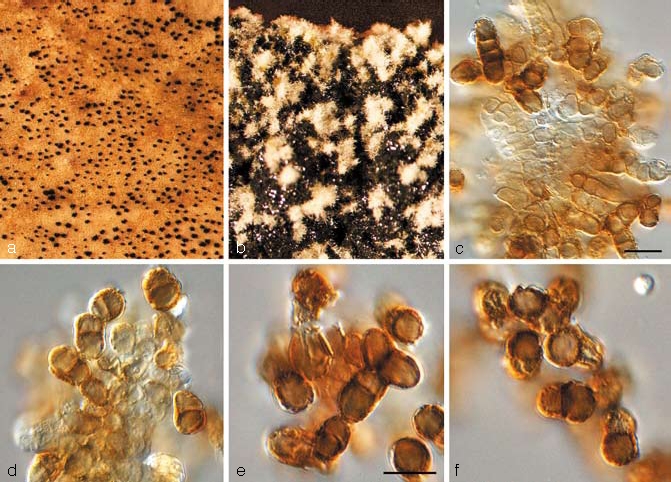
Catenulostroma macowanii (CBS 122901). a. Sporodochia on leaf; b. sporulation on MEA; c, d. conidiogenous cells giving rise to conidia; e, f. conidia. — Scale bars = 10 μm.
Basionym. Coniothecium macowanii Sacc., Syll. Fung. 4: 512. 1886. nom. nov., based on Coniothecium punctiforme G. Winter, Hedwigia 24: 33. 1885, non C. punctiforme Corda, Icon. Fungorum (Corda) 1: 2. 1837.
≡ Trimmatostroma macowanii (Sacc.) M.B. Ellis, More Dematiacous Hyphomycetes: 29. 1976.
Descriptions — Taylor & Crous (1998f), Crous et al. (2004a).
Cultural characteristics — Colonies on MEA erumpent, spreading, margins irregular, smooth, surface with sparse pale olivaceous-grey aerial mycelium, interrupted by black conidial masses bursting through the layer of aerial mycelium; reaching 4 mm diam after 2 wk.
Specimens examined. South Africa, Western Cape Province, Table Mountain, on living leaves of Protea grandiflora, P. MacOwan, holotype JE (of Coniothecium punctiforme G. Winter); Cederberge, on living leaves of P. nitida, 10 May 1998, J.E. Taylor, CPC 1872 = CBS 110756; Hermanus, on a living leaf of P. nitida, 13 Aug. 1996, P.W. Crous, CPC 1488 = CBS 111029; Jonkershoek, S33°59′11.2″ E18°57′14.7″, on living leaves of P. nitida, 1 Apr. 2007, P.W. Crous & L. Mostert, epitype designated here CBS H-20089, cultures ex-epitype CPC 13899, 13901, 13900 = CBS 122901; ibid, CPC 13966–13968.
Catenulostroma wingfieldii Crous, sp. nov. — MycoBank MB506592; Fig. 5
Fig. 5.
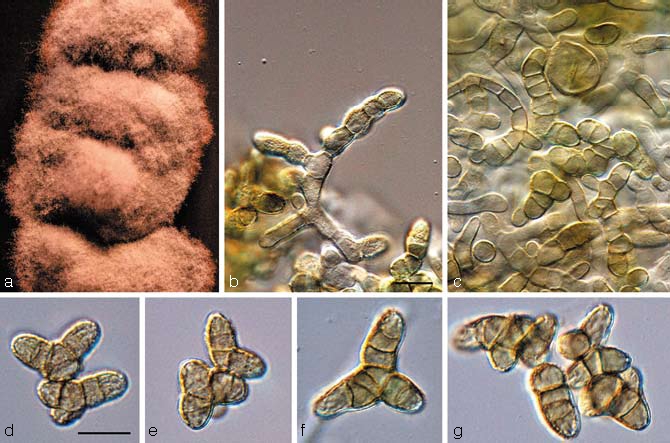
Catenulostroma wingfieldii (CBS 112163). a. Colony growing on MEA; b, c. conidia forming on aerial mycelium; d–g. conidia. — Scale bars = 10 μm.
Catenulostromatis macowanii simile, sed conidiis longioribus, (13–)20–26 (–35) × (5–)6–8(–12) μm.
Etymology. Named in honour of Prof. M.J. Wingfield, who has made a significant contribution to our knowledge of the fungi occurring on Proteaceae.
Conidiomata sporodochial, pulvinate, punctiform, brown-black, 40–80 μm diam. Conidiophores emerging as fascicles through the stomata, hyaline to pale brown, smooth or verruculose, cylindrical. Conidiogenous cells holoblastic, basipetal, forming an unconnected chain of conidia delimited by a septum followed by diffuse wall-building below the previous conidium to form the next conidium which is delimited retrogressively, conidia separating by schizolytic secession. Conidia formed in simple and branched chains, dry, highly variable in shape, ellipsoidal to subcylindrical, Y-shaped, curved or straight, with rounded apices, and truncate bases, frequently with a prominent marginal frill, transversely 1- to multi-septate, at times with oblique septa, pale to medium-brown, verrucose, (13–)20–26(–35) × (5–)6–8(–12) μm.
Cultural characteristics — Colonies on MEA erumpent, with smooth, regular margins, and sparse aerial mycelium, pale olivaceous-grey; colonies reaching 7 mm diam after 2 wk on MEA; on OA erumpent with smooth to feathery margins, sparse to moderate aerial mycelium, pale olivaceous-grey to olivaceous-grey; colonies reaching 7 mm diam after 2 wk at 25 °C.
Specimen examined. South Africa, Western Cape Province, Kirstenbosch Botanical Gardens, on living leaves of Protea nitida, 15 Aug. 1999, J.E. Taylor, holotype CBS H-20090, cultures ex-type CPC 2944 = CBS 112163, CPC 2945–2946.
Notes — Catenulostroma wingfieldii is similar to C. macowanii, but differs in that colonies are pale olivaceous-grey on MEA, while those of C. macowanii are iron-grey to black, and lack aerial mycelium. Furthermore, conidia of C. wingfieldii are somewhat larger, being (13–)20–26(–35) × (5–)6–8(–12) μm, while those of C. macowanii are (10–)15–17(–23) × (6–)6.5–7(–9) μm.
Dissoconium proteae Crous, sp. nov. — MycoBank MB506593; Fig. 6
Fig. 6.
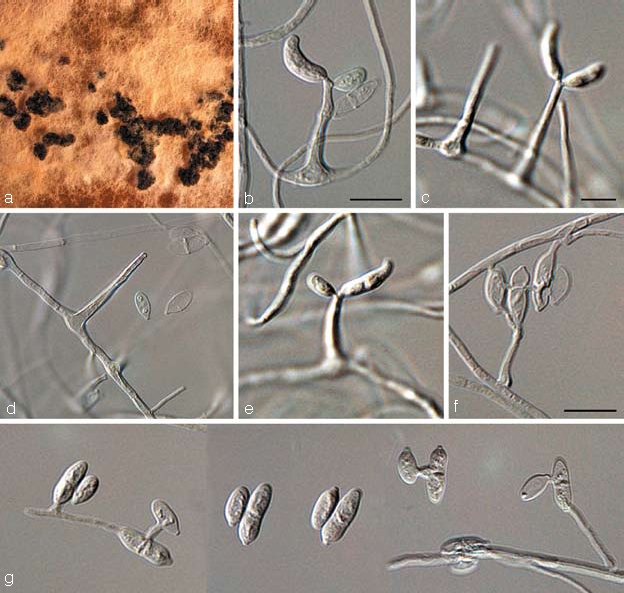
Dissoconium proteae (CBS 122900). a. Sclerotia forming on MEA; b–f. solitary conidiophores giving rise to primary and secondary conidia; g. anastomosing primary and secondary conidia. — Scale bars = 10 μm.
Dissoconio aciculari simile, sed conidiis primariis minoribus, (9–)10–11(–12) × (3–)3.5(–4) μm, conidiis secondariis quoque minoribus, 7–8(–10) × (3–)3.5(–4) μm.
Etymology. Named after the host on which it occurs, Protea.
Mycelium internal and external, consisting of branched, septate, smooth, hyaline to pale brown hyphae, 1.5–2 μm wide. Conidiophores separate, arising from hyphae, subcylindrical, subulate or lageniform, tapering to a bluntly rounded or truncate apex, straight to gently curved, smooth, hyaline, becoming medium brown with age, aseptate, 10–30 × 3–5 μm; loci terminal and lateral, visible as slightly thickened, darkened scars, 0.5 μm wide. Conidia (9–)10–11(–12) × (3–)3.5(–4) μm, solitary, straight to somewhat curved, hyaline to pale olivaceous, smooth, ellipsoid, not to slightly constricted at median septum, apex obtuse, base obconic-truncate, tapering pronounced at somewhat protruding hilum, unthickened, not darkened, 1 μm wide. Secondary conidia developing adjacent to primary coni- dia, hyaline to subhyaline, aseptate, ellipsoid, tapering prominently towards a protruding, truncate base, 7–8(–10) × (3–)3.5(–4) μm; anastomosing with primary conidia after active discharge (in some cases the secondary conidia were observed to germinate, which has never been observed in species with smaller, pyriform secondary conidia).
Cultural characteristics — Colonies on OA spreading, with sparse aerial mycelium, and irregular margins; surface sienna, with patches of white and cinnamon; forming clusters of black sclerotia (remaining infertile) on OA, MEA and PDA; reaching 10 mm diam after 1 mo on OA.
Specimen examined. Canary Islands, Tenerife, on leaves of Protea sp., 1 Mar. 2007, P.W. Crous, holotype CBS H-20091, culture ex-type CPC 13853 = CBS 122900.
Notes — Of the Dissoconium species known to date (Crous et al. 2004b, Zhang et al. 2007, Arzanlou et al. 2008), D. proteae is most similar to D. eucalypti (primary conidia, 8–14 × 4.5–6 μm, and secondary conidia, 4–7 × 2.5–3 μm), and D. aciculare (primary conidia, 12–25 × 3.5–6 μm, and secondary conidia, 7.5–12 × 3.5–6 μm), but has smaller and narrower primary and secondary conidia (9–12 × 3–4 μm, and 7–10 × 3–4 μm, respectively), than both.
Mycosphaerella buckinghamiae Crous & Summerell, Australas. Pl. Pathol. 29: 272. 2000
Description — Crous et al. (2000b).
Specimen examined. Australia, New South Wales, Mangrove Mountain, on leaves of Buckinghamia sp., Aug. 1999, P.W. Crous & B. Summerell, holotype DAR 74865, cultures ex-type CPC 3006 = CBS 111996.
Notes — Although the ITS DNA sequence of M. buckinghamiae is identical to that of M. africana (ascospores 7–11 × 2–3 μm, darkening and distorting upon germination), M. buckinghamiae has larger ascospores (9–13 × 2.5–3.5 μm), which do not darken at germination, and colonies that contain rose and off-white sectors, which are lacking in M. africana, which again has black colonies, forming a brown pigment in MEA (Crous 1998, Crous et al. 2000b).
Mycosphaerella communis Crous & Mansilla, Stud. Mycol. 50: 203. 2004
Anamorph. Dissoconium commune Crous & Mansilla, Stud. Mycol. 50: 203. 2004.
Description — Crous et al. (2004b).
Specimens examined. Spain, Pontevedra, Lourizán, Areeiro, on leaves of Eucalyptus globulus, Dec. 2002, J.P. Mansilla, holotype of M. communis and D. commune CBS H-9900, culture ex-type CBS 114238 = CPC 10440. – Australia, New South Wales, Mount Tomah Botanic Garden, on leaves of Protea magnifica, 2002, P.W. Crous, CPC 3359 = CBS 112889.
Mycosphaerella holualoana Crous, Joanne E. Taylor & M.E. Palm, Mycotaxon 78: 458. 2001
Descriptions — Taylor et al. (2001b), Crous et al. (2004a).
Specimen examined. USA, Hawaii, Kona district, Holualoa, on a living leaf of Leucospermum sp., 17 Nov. 1998, P.W. Crous & M.E. Palm, holotype PREM 56926, culture ex-type CPC 2126 = CBS 110698.
Mycosphaerella konae Crous, Joanne E. Taylor & M.E. Palm, Mycotaxon 78: 459. 2001
Anamorph. Pseudocercospora sp.
Descriptions — Taylor et al. (2001b), Crous et al. (2004a).
Specimens examined. USA, Hawaii, Kona district, Holualoa, on a living leaf on Leucadendron ‘Safari Sunset’, 17 Nov. 1998, P.W. Crous & M.E. Palm, holotype PREM 56921, cultures ex-type CPC 2123 = CBS 111261, CPC 2125 = CBS 111028. – Thailand, Thatakiab District, Chachoengsao Province, on leaves of Eucalyptus camaldulensis, 12 Oct. 2006, W. Himaman, CPC 13469 = CBS 120748, CPC 13470.
Mycosphaerella marksii Carnegie & Keane, Mycol. Res. 98: 414. 1994
Description — Carnegie & Keane (1994), Crous (1998).
Specimens examined. Australia, Victoria, Nowa Nowa, on leaves of Eucalyptus botryoides, 11 Nov. 1990, A.J. Carnegie, holotype IMI 353731; Tostaree, on leaves of E. botryoides, Oct. 1994, A.J. Carnegie, epitype designated here PREM 51932, cultures ex-epitype CPC 982 = CBS 110942, CPC 984 = CBS 110974. – Madeira Islands, Florialis Estate, on leaves of Leucadendron tinctum, 1 Apr. 2000, S. Denman, CPC 5358 = CBS 115501.
Mycosphaerella stromatosa Joanne E. Taylor & Crous, Mycol. Res. 104: 625. 2000
Anamorph. Pseudocercospora stromatosa Joanne E. Taylor & Crous, Mycol. Res. 104: 625. 2000.
Description — Taylor & Crous (2000), Crous et al. (2004a).
Specimen examined. South Africa, Kwazulu-Natal, Drakensberg, Dragon’s Peak, on a living leaf of Protea sp., Jan. 1998, S. Denman, holotype PREM 56204, culture ex-type CPC 1731 = CBS 101953.
Mycosphaerella waimeana Crous, Joanne E. Taylor & M.E. Palm, Mycotaxon 78: 463. 2001
Anamorph. Stenella sp.
Descriptions — Taylor et al. (2001b), Crous et al. (2004a).
Specimen examined. USA, Hawaii, Kona district, Waimea, on a living leaf of Leucospermum hybrid 24, 17 Nov. 1998, P.W. Crous & M.E. Palm, holotype PREM 56950, culture ex-type CPC 2179 = CBS 110697.
Phaeothecoidea proteae Crous, sp. nov. — MycoBank MB506594; Fig. 7
Fig. 7.

Phaeothecoidea proteae (CBS 114129). a. Colony on OA; b–e. hyphae with endoconidia visible; f–i. released endoconidia become brown and verruculose. — Scale bars = 10 μm.
Phaeothecoideae eucalypti similis, sed conidiis majoribus, (6–)8–10(–13) × (4–)5–6(–11) μm.
Etymology. Named after the host from which it was collected, Protea.
Hyphae in vitro creeping, brown, verruculose, branched, septate, 3–5 μm wide, becoming swollen, up to 15 μm wide, verruculose, dark brown, or forming a mucoid capsule filled with endoconidia which are former hyphal cells that turn brown and thick-walled; end cells dividing into several endoconidia, which are released upon rupture of the cell wall. Endoconidia medium to dark brown, verruculose to verrucose to warty, thick-walled, ellipsoid to obovoid or obclavate, (6–)8–10(–13) × (4–)5–6(–11) μm; after liberation swelling, becoming transversely 1-septate, or with several oblique septa, again forming endoconidia, becoming warty with age, with the outer layer peeling off once endoconidia are released.
Cultural characteristics — Colonies on MEA slimy, erumpent, lacking aerial mycelium, irregular, folded, with smooth, regular margin; surface iron-grey, reverse fuscous-black; colonies reaching 7 mm diam after 2 wk; on OA lacking aerial mycelium, erumpent with smooth margins, black, reaching 6 mm diam after 2 wk; fertile.
Specimen examined. South Africa, Western Cape Province, Stellenbosch, Elsenburg Farm, on leaves of Protea repens, 23 July 1999, S. Denman, holotype CBS H-20092, cultures ex-type CPC 2828–2830, 2831 = CBS 114129.
Notes — The present species clusters close to the type of the genus Phaeothecoidea, P. eucalypti (Crous et al. 2007d), and as it shares the feature of brown, verruculose endoconidia, we chose to describe it as a new species of this genus. It is interesting to note that it was originally isolated as a coelomycete, and based on its yeast-like growth in culture, identified as Coniothyrium leucospermi (Swart et al. 1998, Taylor & Crous 2001), which has subsequently been allocated to a new genus, Coniozyma (Marincowitz et al. 2008). Unfortunately, the original herbarium specimen could not be located, and thus only the cultural synanamorph can be described here.
Pseudocercospora protearum var. leucadendri (Cooke) U. Braun & Crous, Mycol. Progr. 1: 22. 2002
Basionym. Cercospora protearum Cooke var. leucadendri Cooke, Grevillea 12: 39. 1883.
≡ Stigmina protearum var. leucadendri (Cooke) M.B. Ellis, Mycol. Pap. 131: 7. 1972.
≡ Cercostigmina protearum var. leucadendri (Cooke) U. Braun & Crous, Sydowia 46: 206. 1994.
= Passalora protearum Kalchbr. & Cooke, Grevillea 19: 6. 1890.
Description — Crous et al. (2004a).
Specimens examined. South Africa, Cape Province, Cape of Good Hope, Table Mountain, Leucadendron argenteum, MacOwan, No 1457, holotype K; Stellenbosch, Devon Valley, Protea Heights, on leaves of Leucadendron sp., 3 Apr. 1998, P.W. Crous & S. Denman, culture CPC 1869.
Ramularia proteae Crous & Summerell, Australas. Pl. Pathol. 29: 277. 2000
Description — Crous et al. (2000b).
Specimen examined. Australia, Tasmania, Royal Tasmanian Botanical Gardens, Hobart, on leaves of Protea longifolia, Aug. 1999, A. Macfadyen, holotype DAR 74883, culture ex-type CPC 3075 = CBS 112161.
Septoria protearum Viljoen & Crous, S. Afr. J. Bot. 64: 144. 1998
Description — Crous et al. (2004a).
Specimens examined. South Africa, Gauteng Province, Pretoria, leaves of Protea cynaroides, Sept. 1996, L. Viljoen, holotype PREM 55353, culture ex-type CPC 1470 = IMI 375230 = ATCC 201159 = CBS 778.97. – Canary Islands, Tenerife, leaves of Protea sp., 1 Apr. 2000, S. Denman, CPC 5212.
Teratosphaeria alistairii (Crous) Crous & U. Braun, Stud. Mycol. 58: 9. 2007
Basionym. Mycosphaerella alistairii Crous, in Crous & Groenewald, Fungal Planet, No. 4. 2006.
Anamorph. Batcheloromyces sp.
Description — Crous & Groenewald (2006a).
Specimen examined. South Africa, Western Cape Province, Hermanus, Rotary Road, close to the Vodacom tower, on leaves of Protea repens, 31 Dec. 2005, P.W. Crous & A. Smith, holotype CBS H-19765, cultures ex-type CPC 12730 = CBS 120035, CPC 12731–12732.
Notes — Teratosphaeria alistairii resembles T. jonkershoekensis in symptomatology on the host, but is distinct in having smaller ascospores, (9–)10–12(–13) × (2.5–)3–4 μm, and a Batcheloromyces anamorph (Crous & Groenewald 2006a).
Teratosphaeria associata (Crous & Carnegie) Crous & U. Braun, Stud. Mycol. 58: 9. 2007
Basionym. Mycosphaerella associata Crous & Carnegie, Fung. Diversity 26: 159. 2007.
Description — Crous et al. (2007d).
Specimens examined. Australia, New South Wales, South Grafton, Grafton City Council Landfill Plantation, E152°54′38″, S29°46′21″, on leaves of Corymbia henryii, 16 Feb. 2006, A.J. Carnegie, holotype CBS-H 19833, isotype DAR 78031, cultures ex-type CPC 13119 = CBS 120730, CPC 13120 (occurring with Lembosina sp.); NSW, Bungawalbin, Robertson Plantation, E153°15′39″, S29°5′34″, on leaves of Corymbia variegata, 23 Jan. 2005, A.J. Carnegie, DAR 78032, cultures CPC 13128 = CBS 120731, CPC 13129–13130 (occurring with Lembosina sp.); NSW, Bungawalbin, Robertson Plantation, E153°15′39″, S29°5′34″, on leaves of Eucalyptus dunnii, 14 Feb. 2006, A.J. Carnegie, cultures CPC 13108 = CBS 120732, CPC 13109–13110, 13113–13114 (occurring with M. suberosa); NSW, Mount Tomah Botanic Gardens, on leaves of Protea lepidocarpodendron, Aug. 1999, P.W. Crous & B. Summerell, JT 993, DAR 74867, cultures CPC 3115 = CBS 112627, CPC 3116 = CBS 112224, CPC 3117 = CBS 114165.
Notes — Teratosphaeria associata was recently described from Eucalyptus in Australia (Crous et al. 2007d), where it occurred in association with several other species (hence the name, associata). Crous et al. (2000b) reported the presence of T. jonkershoekensis from Protea spp. in Australia, where this species was observed to be an important primary pathogen (suggesting Eucalyptus is not a primary host of T. associata). Based on the results obtained here, it appears that the Protea isolates were incorrectly identified, and belong to the recently named T. associata. Furthermore, the recent description of T. alistairii and T maxii from this host, suggest that there could be several more species that resemble T. jonkershoekensis in morphology and symptomatology, but which could represent distinct species.
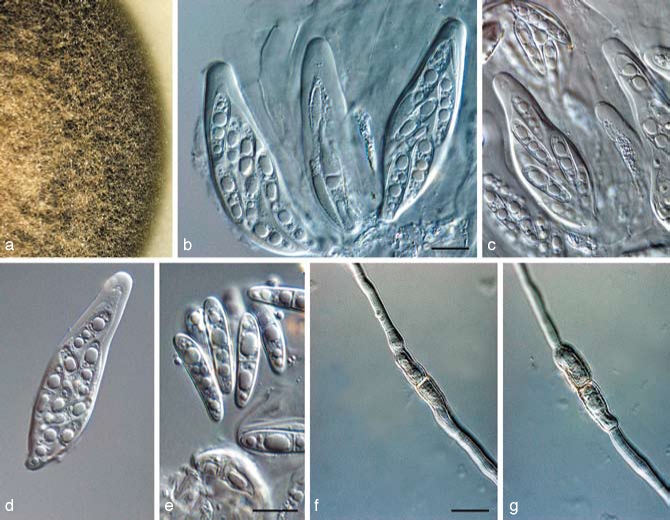
Teratosphaeria bellula (Crous & M.J. Wingf.) Crous & U. Braun, Stud. Mycol. 58: 10. 2007 — Fig. 8
Fig. 8.
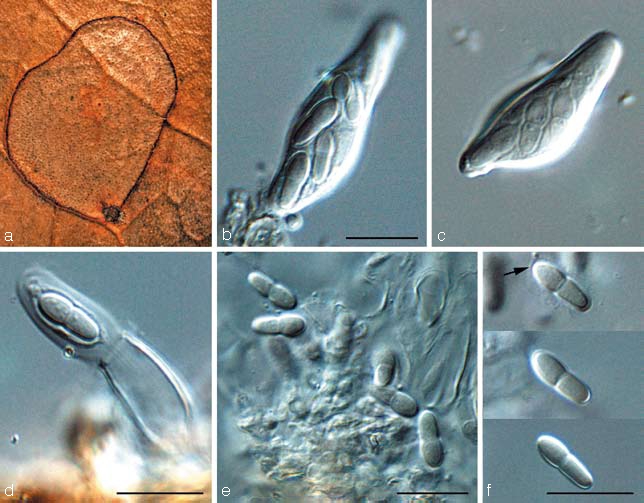
Teratosphaeria bellula (CBS 111699). a. Leaf spot on Protea eximia; b, c. asci; d. ascus with jack-in-the-box release of inner sack, showing mucilaginous sheath around ascospore; e, f. ascospores (sheath indicated by arrow). — Scale bars = 10 μm.
Basionym. Mycosphaerella bellula Crous & M.J. Wingf., Mycotaxon 46: 20. 1993.
Descriptions — Crous & Wingfield (1993), Taylor & Crous (1998b), Crous et al. (2004a).
Specimens examined. South Africa, Western Cape Province, Stellenbosch, Stellenbosch Mountain, living leaves of Protea repens, 30 Aug. 1991, P.W. Crous, holotype PREM 51028; Western Cape Province, Stellenbosch, Protea Heights, Devon Valley, on Leucospermum sp., 6 Mar. 1998, J.E. Taylor, CBS H-20093, CPC 1815, 1816 = CBS 111699; Western Cape Province, Stellenbosch, J.S. Marais Botanical Garden, on leaves of Protea eximia, Apr. 1998, J.E. Taylor, epitype designated here CBS H-20094, culture ex-epitype CPC 1821 = CBS 111700; Western Cape Province, Leucadendron sp., 27 May 1999, L. Swart, CPC 2795 = CBS 114145; Western Cape Province, Kirstenbosch Botanical Gardens, on leaves of Protea sp., 1 Jan. 2008, P.W. Crous, CPC 14908.
Notes — Teratosphaeria bellula is characterised by having ascospores that are strongly constricted at the median septum, with small guttules, and surrounded by a prominent sheath when mounted in water. Attempts to recollect T. bellula for the purpose of epitypification have revealed it to be a species complex. As the morphology of the strains is quite similar, more isolates from other hosts in the Proteaceae need to be collected to clarify if strains from Leucadendron and Leucospermum represent T. bellula s.str.
Teratosphaeria fibrillose Syd. & P. Syd., Ann. Mycol. 10: 40. 1912 — Fig. 9
Fig. 9.
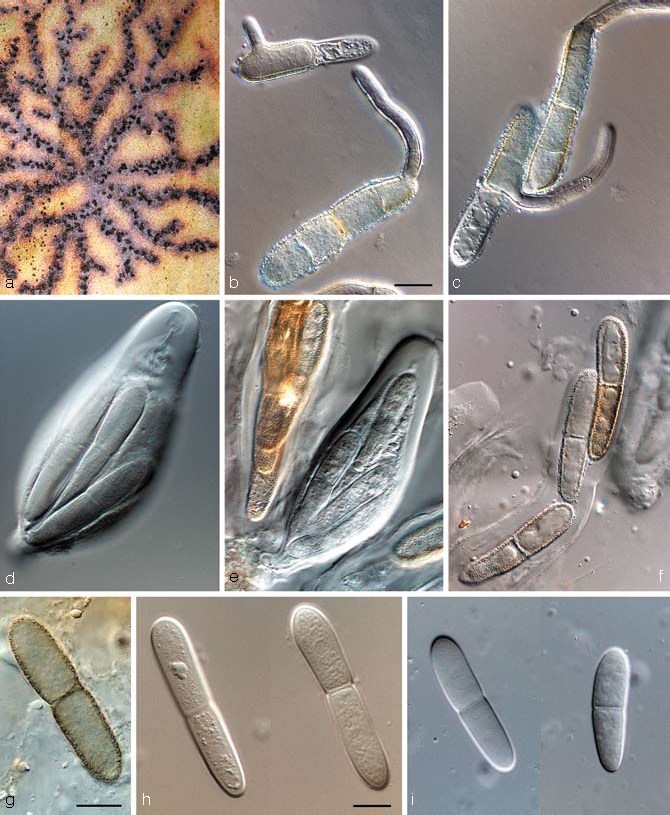
Teratosphaeria fibrillosa in vivo (CBS H-19913). a. Ascomata on leaf surface, linked by stromatic tissue; b, c. germinating ascospores; d–f. asci with darkening ascospores; g–i. ascospores. — Scale bars = 10 μm.
≡ Mycosphaerella fibrillosa (Syd. & P. Syd.) Joanne E. Taylor & Crous, Mycol. Res. 107: 657. 2003.
Descriptions — Taylor & Crous (1998d), Crous et al. (2004a, 2007a).
Specimens examined. South Africa, Western Cape Province, Bains Kloof near Wellington, on living leaves of Protea grandiflora, 26 Feb. 1911, E.M. Doidge, holotype PREM; Stellenbosch, Jonkershoek valley, S33°59′44.7″, E18°58′50.6″, on leaves of Protea sp., 1 Apr. 2007, P.W. Crous & L. Mostert, epitype CBS H-19913, culture ex-epitype CBS 121707 = CPC 13960; Cederberg, on leaves of P. nitida, J.E. Taylor, CPC 1876.
Notes — Teratosphaeria fibrillosa is the type species of Teratosphaeria (Teratosphaeriaceae), and is characterised by having subepidermal ascomata linked by means of stromatic tissue, apical periphyses, asci with a multi-layered endotunica, ascospores that have a sheath and turn brown and verruculose while still in their asci (Crous et al. 2007a).
Teratosphaeria jonkershoekensis (P.S. van Wyk, Marasas & Knox-Dav.) Crous & U. Braun, Stud. Mycol. 58: 10. 2007 — Fig. 10
Fig. 10.
Teratosphaeria jonkershoekensis (CBS 122897). a. Colony on OA; b–d. asci; e. ascospores; f, g. germinating ascospores. — Scale bar = 10 μm.
Basionym. Mycosphaerella jonkershoekensis P.S. van Wyk, Marasas & Knox-Dav., J. S. African Bot. 41: 234. 1975.
Descriptions — Van Wyk et al. (1975), Taylor & Crous (1998a), Crous et al. (2004a).
Cultural characteristics — Colonies on MEA erumpent with feathery margins and radiating superficial ridges; aerial mycelium sparse, fuscous-black (surface and reverse); on OA with smooth, regular margins and moderate pale olivaceous-grey aerial mycelium; outer margin olivaceous-black; reaching 30 mm diam after 1 mo on OA at 25 °C; sterile.
Specimens examined. South Africa, Western Cape Province, Jonkershoek, on living leaves of Protea repens, 9 Sept. 1971, P.S. van Wyk, holotype PREM 44830; Jonkershoek, S33°59′4.2″ E18°57′16.1″, on living leaves of Protea sp., 1 Apr. 2007, P.W. Crous & L. Mostert, epitype designated here CBS H-20095, culture ex-epitype CBS 122897 = CPC 13984 (occurring on leaf spots in association with T. persoonii).
Notes — No anamorph has thus far been observed for this species. Germ tubes grow parallel to the long axis of the spore, but after 48 h several germ tubes have been produced and germination is irregular. Germinating ascospores become brown, verruculose and constricted at the septum. Optimal ascospore germination occurs at 15 °C, and it is hypothesised that this low temperature requirement is necessary for successful germination and infection of leaf tissue (Swart et al. 1998, Taylor & Crous 1998a). Isolates reported from Australia as representative of T. jonkershoekensis (Crous et al. 2000b), are in fact representative of two morphologically similar species that were recently described in the complex, namely T. asso-ciata and T. maxii (Fig. 1).
Teratosphaeria knoxdavesii Crous, sp. nov. — MycoBank MB506595; Fig. 11
Fig. 11.
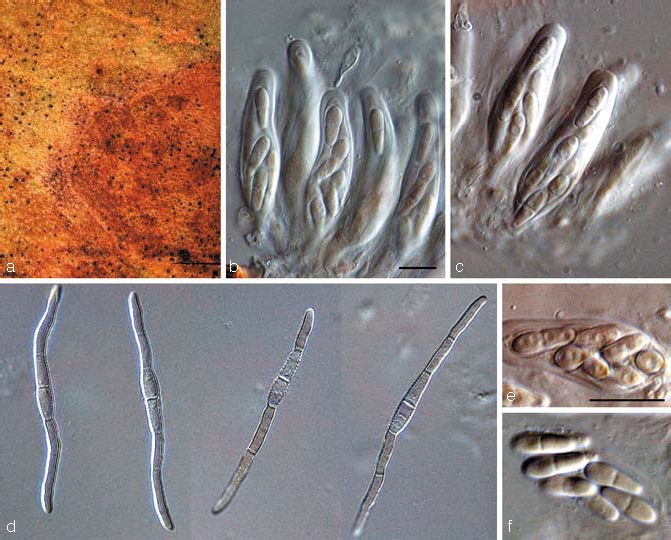
Teratosphaeria knoxdavesii (CBS 122898). a. Leaf spot on Protea sp.; b, c. asci; d. germinating ascospores; e, f. close-up of ascospores in asci. — Scale bars = 10 μm.
Teratosphaeriae bellulae similis, sed ascosporis cum tubis germinalibus parallelis ad axem longum sporae.
Etymology. Named in honour of Prof. P.S. Knox-Davies, who dedicated a large part of his career to studying fungal pathogens of Proteaceae.
Leaf spots amphigenous, irregular to subcircular, 5–12 mm diam, medium brown with a raised border, and thin, red-purple margin. Ascomata amphigenous, black, immersed, substomatal, up to 100 μm diam; wall consisting of 2–3 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, obovoid to broadly ellipsoid, straight to slightly curved, 8-spored, 35–45 × 8–12 μm. Ascospores tri- to multi-seriate, overlapping, hyaline, non-guttulate, thick-walled, straight, fusoid-ellipsoidal with obtuse ends, widest in middle of apical cell, prominently constricted at the septum, tapering towards both ends, but more prominently towards the lower end, (8.5–)10–11(–12) × (3–)3.5(–4) μm; germinating ascospores on MEA become brown and verruculose, germinating from both polar ends, with germ tubes parallel to the long axis of the spore, constricted at septum, but not distorting, 3.5–4 μm wide.
Cultural characteristics — Colonies on MEA erumpent, spreading, folded, with moderate, pale olivaceous-grey aerial mycelium, and smooth, catenulate, olivaceous-grey margins; reverse olivaceous-grey; colonies reaching 10 mm diam on MEA after 1 mo; on OA erumpent, spreading, grey-olivaceous, with moderate aerial mycelium and even margins; sterile.
Specimens examined. South Africa, Western Cape Province, Kirstenbosch Botanical Garden, on living leaves of Protea sp., 6 Jan. 2008, P.W. Crous & M. Crous, holotype CBS H-200104, cultures ex-type CBS 122898 = CPC 14960, 14961, 14962 (occurring on leaf spots in association with Coleroa senniana); Kirstenbosch Botanical Garden, on living leaves of Protea sp., 6 Jan. 2008, P.W. Crous & M. Crous, CPC 14905–14907.
Notes — Although several small-spored species of Teratosphaeria are known from Proteaceae, T. knoxdavesii is distinct in having a very characteristic ascospore germination pattern, germinating from both polar ends, with spores becoming constricted, brown, and verruculose, germ tubes growing parallel to the long axis of the spore.
Teratosphaeria maculiformis (G. Winter) Joanne E. Taylor & Crous, IMI Descriptions of Fungi and Bacteria No. 1346. 1998 — Fig. 12
Fig. 12.
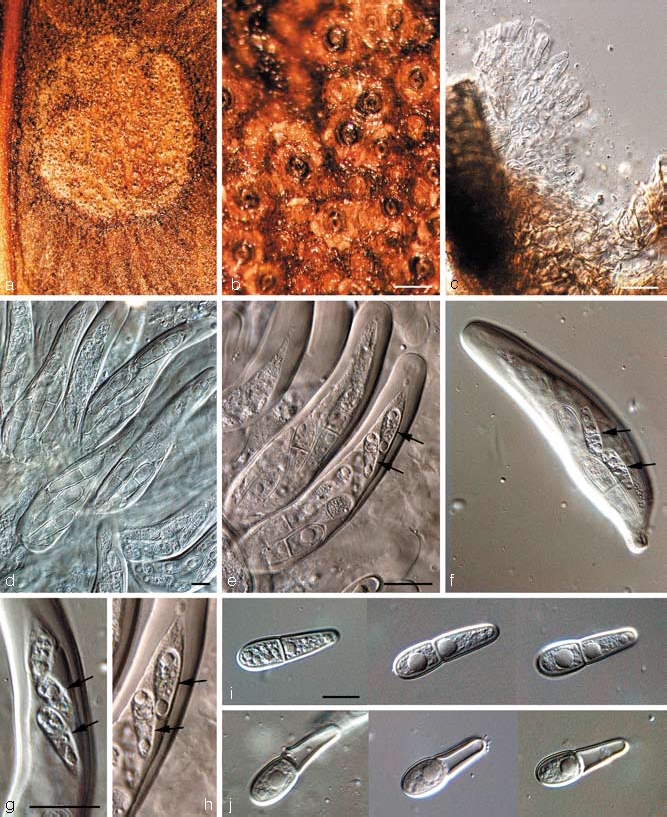
Teratosphaeria maculiformis in vivo (CBS H-20096). a. Leaf spot on Protea sp.; b. close-up of substomatal ascomata; c. periphysoids; d–h. asci with ascospores (microascospores arrowed); i. mature ascospores; j. disarticulating ascospores. — Scale bars = 10 μm.
Basionym. Didymella maculiformis G. Winter, Hedwigia 23: 169. 1884.
≡ Oligostroma maculiformis (G. Winter) Doidge, Bothalia 1: 31. 1921.
= Oligostroma proteae Syd., Ann. Mycol. 12: 265. 1914.
≡ Mycosphaerella proteae (Syd.) Arx, Beitr. Kryptogamenfl. Schweiz 11(2): 357. 1962.
= Euryachora maculiformis Nel, Annals of the University of Stellenbosch 20 Ser. A (2): 11. 1942.
Descriptions — Taylor & Crous (1998c), Crous et al. (2004a).
Cultural characteristics — Colonies black and erumpent, devoid of aerial mycelium, slow-growing; 3 mm diam after 6 mo at 25 °C on PDA; colonies did not survive preservation, and numerous subsequent attempts have been unsuccessful in cultivating it again. Germinating ascospores died on OA, MEA and PCA. Ascospores become brown at germination on PDA, and produce germ tubes parallel to the long axis of the ascospore, but the spore wall does not become verruculose.
Specimens examined. South Africa, Kentani, on Protea flanaganii, 17 July 1912, Pegler, 5163 deposited in PREM (type of Oligostroma proteae); Cape Town, Protea grandiflora, June 1884, MacOwan (Winter, Fungi Eur. Extraeur. Exs. 3056, type of Didymella maculiformis); Western Cape Province, Knysna, S34°1′49.9″, E23°1′6.0″, on leaves of Protea sp., 4 Jan. 2008, P.W. Crous & K.L. Crous, CBS H-20096 (used to harvest ascospores for DNA isolation).
Notes — Ascospores were observed to disarticulate at the septum, with each cell frequently becoming 1-septate and germinating (on host tissue) (Crous et al. 2004a). Although the spores are thick-walled, they also seem to get punctured quite easily, in which case cytoplasm leaks out of the damaged ascospore cell, and the spore sheds this cell by disarticulating relatively easily at the septum. In some cases, this separation was observed even in the absence of ascospore cell damage. This interesting mechanism has not been observed in this group of fungi before, and may be linked to the relatively large ascospore size of T. maculiformis. Ascospores often adhere to leaf hairs, with the resulting hyphae growing superficially until they infect the host. Upon germination ascospores become brown and produce germ tubes parallel to the long axis of the ascospore, but the spore wall does not become verruculose. A further interesting phenomenon observed in T. maculiformis is the fact that asci frequently have one or two microascospores that appear completely underdeveloped, and much smaller than the ‘normal’ ascospores. As far as we could establish, these microascospores are viable, and germinate along with the normal, larger ascospores.
Ascospores on the leaf surface are frequently hyperparasitised by a species of Cladosporium. A similar Cladosporium species (a member of the C. cladosporioides species complex) was also observed to grow on exuding ascospore masses of T. proteae-arboreae. Due to the extremely slow growth of T. maculiformis, this species could not be deposited in the CBS culture collection. Ascospores germinate on PDA (though they fail to do so upon MEA, OA or SNA), but although germ tubes elongate and branch, they never form mycelium, and stay recognisable as single germinating ascospores, even 6–8 mo after they started germinating. At a certain point (3–5 mo after the onset of germination), all growth ceases, though the spore and the hyphae do not dissolve, but still appear viable, though they enter a dormant phase. The DNA sequence provided in this study was obtained from harvesting a mass of discharged, germinated ascospores (4 mo after ascospore discharge), and extracting their DNA using a commercial DNA isolation kit (E.Z.N.A. Forensic DNA Isolation Kit, Omega Bio-Tek).
Teratosphaeria marasasii Crous, sp. nov. — MycoBank MB506596; Fig. 13
Fig. 13.
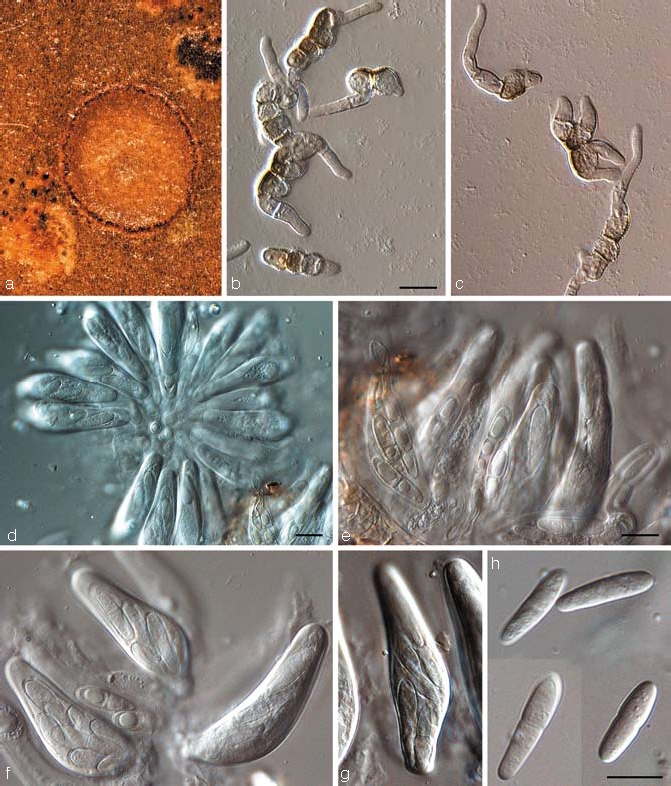
Teratosphaeria marasasii (CBS 122899). a. Leaf spot on Protea sp.; b, c. germinating ascospores; d. fasciculate asci viewed from above; e. asci with darkening ascospores; f, g. asci; h. ascospores. — Scale bars = 10 μm.
Teratosphaeriae jonkershoekensis similis, sed maculis minoribus et ascosporis brevioribus, (15–)16–18(–22) × (3.5–)4(–5) μm.
Etymology. Named in honour of Prof. W.F.O. Marasas, who was instrumental in naming T. jonkershoekensis, which this species closely resembles in morphology and symptomatology.
Leaf spots amphigenous, circular, 2–3 mm diam, pale brown to grey-brown, with a thin, raised, dark brown border. Ascomata amphigenous, black, immersed, substomatal, up to 120 μm diam; wall consisting of 2–3 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, obovoid to broadly ellipsoid, straight to slightly curved, 8-spored, 35–50 × 11–15 μm; with a well-developed ocular chamber, and multi-layered endotunica. Ascospores tri- to multi-seriate, overlapping, hyaline, guttulate, thick-walled, straight, fusoid-ellipsoidal with obtuse ends, widest in middle of apical cell, not to slightly constricted at the septum, tapering towards both ends, but more prominently towards the lower end, (15–)16–18(–22) × (3.5–)4(–5) μm, becoming brown and verruculose in the asci; germinating ascospores on MEA become brown and verruculose, distorting, with 2–4 germ tubes, growing irregular to the long axis, 6–10 μm wide.
Cultural characteristics — Colonies after 1 mo on MEA erumpent, spreading, with sparse aerial mycelium, and smooth, entire margins; surface olivaceous-grey in middle, iron-grey in outer region; reverse greenish black, 10–15 mm diam; on OA with moderate aerial mycelium, and smooth, regular margins, olivaceous-grey; sterile.
Specimen examined. South Africa, Western Cape Province, Kirstenbosch Botanical Garden, on living leaves of Protea sp., 6 Jan. 2008, P.W. Crous & M. Crous, holotype CBS H-20105, cultures ex-type CBS 122899 = CPC 14889, 14890, 14891 (occurring on leaf spots in association with Coleroa senniana).
Notes — Teratosphaeria marasasii closely resembles T. jonkershoekensis in morphology (ascospores 15–23 × 4–6 μm), and symptomatology. It is distinct, however, by producing smaller leaf spots and having shorter ascospores.
Teratosphaeria maxii (Crous) Crous & U. Braun, Stud. Mycol. 58: 10. 2007
Basionym. Mycosphaerella maxii Crous, in Crous & Groenewald, Fungal Planet No. 6. 2006.
Description — Crous & Groenewald (2006b).
Specimens examined. Australia, New South Wales, Mount Tomah Botanic Gardens, on leaves of Protea sp., Aug. 1999, P.W. Crous & B. Summerell, DAR 74870, CBS H-20097, cultures CPC 3321 = CBS 112231, CPC 3322 = CBS 112496, CPC 3323 = CBS 112232. – South Africa, Western Cape Province, Bettie’s Bay, Harold Porter Botanical Garden, on leaves of Protea repens, 4 Jan. 2006, M. Crous & P.W. Crous, holotype CBS H-19774, cultures ex-type CPC 12805 = CBS 120137, CPC 12806–12807; Hermanus, Rotary road on top of mountain, on leaves of P. repens, 31 Dec. 2005, P.W. Crous, CPC 12943–12945.
Notes — Teratosphaeria maxii is associated with leaf spot symptoms reminiscent of those of T. alistairii, T. bellula and T. jonkershoekensis. It is distinct from T. alistairii and T. bellula in its larger ascospores, (15–)17–19(–22) × 4–5(–6) μm, which closely resemble those of T. jonkershoekensis in size. However, ascospores of T. maxii do not darken during germination, and colonies have a peculiar, thick-walled, budding aerial mycelium, which eventually form clumps of orange crystals, which has never been observed in T. jonkershoekensis (Crous & Groenewald 2006b). It is interesting to note, however, that several strains reported as ‘Mycosphaerella jonkershoekensis’ from Australia (Crous et al. 2000b), are in fact T. maxii.
Teratosphaeria microspore Joanne E. Taylor & Crous, Mycol. Res. 104: 631. 2000
≡ Mycosphaerella microspora (Joanne E. Taylor & Crous) Joanne E. Taylor & Crous, Mycol. Res. 107: 657. 2003.
Anamorph. Catenulostroma microsporum (Joanne E. Taylor & Crous) Crous & U. Braun, Stud. Mycol. 58: 10. 2007.
Basionym. Trimmatostroma microsporum Joanne E. Taylor & Crous, Mycol. Res. 104: 631. 2000.
Descriptions — Taylor & Crous (2000), Crous et al. (2004a).
Specimens examined. South Africa, Western Cape Province, Somerset West, Hilly Lands Farm, on a living leaf of a Protea cynaroides, 21 July 1998, S. Denman & J.E. Taylor, PREM 56207a, holotype of teleomorph, culture ex-type CPC 1960 = CBS 101951; PREM 56207b, holotype of anamorph; Stellenbosch, J.S. Marais Nature Reserve, on a living leaf of P. cynaroides, July 1998, L. Swart, CPC 1832 = CBS 110890; Somerset West, on a living leaf of P. cynaroides, July 1998, J.E. Taylor, CPC 1848 = CBS 111031; Stellenbosch, J.S. Marais Nature Reserve, on leaves of P. cynaroides, 30 Aug. 1996, P.W. Crous, CPC 1597 = CBS 111697.
Notes — Teratosphaeria microspora is presently the only known teleomorph connection for species of Catenulostroma (Crous et al. 2007a). Catenulostroma microsporum is part of a species complex that resembles C. abietis, which is known from needles of various species of Gymnospermae. Ascospores of T. microspora germinate on MEA from both cells, and germ tubes grow parallel to the long axis of the spore. There is no constriction at the septum and the spores do not darken or become verruculose upon germination. Isolate CBS 111697 is listed here under T. microspora, but has somewhat larger conidia, and probably represents a cryptic species. More collections are required to fully elucidate the variation present in this species complex.
Teratosphaeria parva (R.F. Park & Keane) Crous & U. Braun, Stud. Mycol 58: 10. 2007 — Fig. 14
Fig. 14.
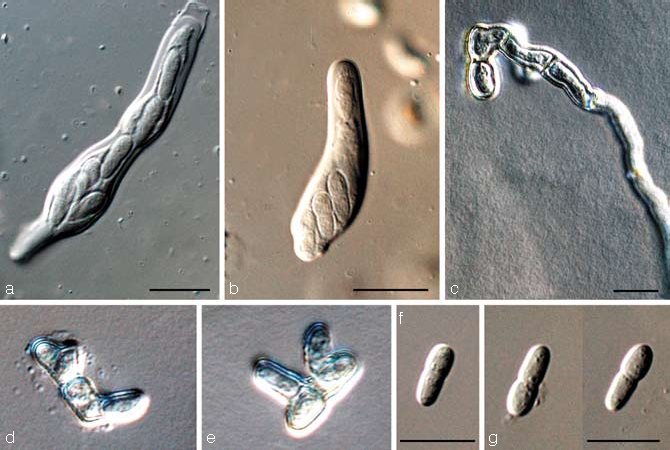
Teratosphaeria parva (CBS 122892). a. Jack-in-the-box separation of layers in bitunicate ascus; b. ascus; c–e. germinating ascospores; f, g. ascospores. — Scale bars = 10 μm.
Basionym. Mycosphaerella parva R.F. Park & Keane, Trans. Brit. Mycol. Soc. 79: 99. 1982.
= Mycosphaerella grandis Carnegie & Keane, Mycol. Res. 98: 414. 1994.
Descriptions — Carnegie & Keane (1994), Crous (1998).
Specimens examined. Australia, Victoria, Nowa Nowa, on leaves of Eucalyptus globulus, July 1981, R.F. Park, holotype IMI 263258 (published as 263358); Victoria, Otway Ranges, (near Gellibrand), latitude: -38.568412, longitude: 143.539586, elevation: 175 m, on leaves of E. globulus, Sept. 2005, I. Smith, epitype designated here CBS H-20098, cultures ex-epitype CPC 12421 = CBS 122892, CPC 12422, 12423. – South Africa, Western Cape Province, Stellenbosch, Stellenbosch Mountain, on leaves of Protea repens, 20 Sept. 1995, P.W. Crous, CPC 1217 = CBS 114761; Western Cape Province, on leaves of P. repens, 12 Nov. 1998, G. Matthews, CBS H-20099, CPC 2118–2120; Western Cape Province, Botmaskop, on leaves of P. repens, Nov. 2007, L. Mostert, CBS H-20101, CPC 14898 = CBS 122893, CPC 14899, 14900; Western Cape Province, Jonkershoek, S33°59′11.2″ E18°57′14.7″, on living leaves of P. nitida, 1 Apr. 2007, P.W. Crous & L. Mostert, CPC 13896 = CBS 122894, CPC 13897, 13898.
Teratosphaeria persoonii Crous & L. Mostert, sp. nov. — MycoBank MB506597; Fig. 15
Fig. 15.
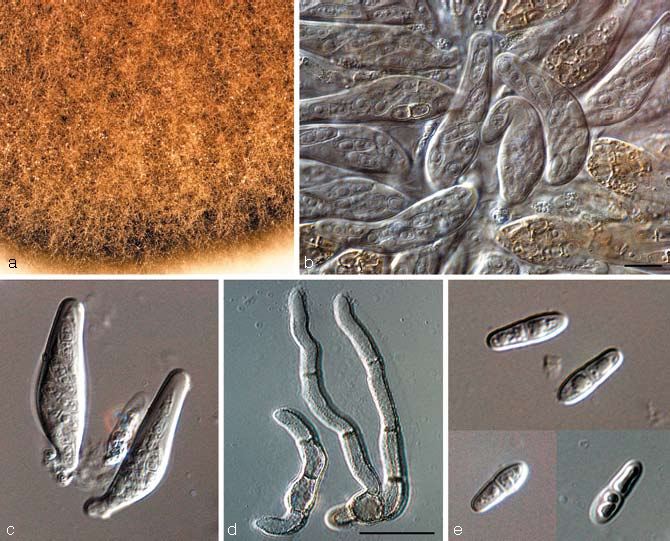
Teratosphaeria persoonii (CBS 122895). a. Colony on OA; b, c. bitunicate asci; d. germinating ascospores; e. ascospores. — Scale bars = 10 μm.
Teratosphaeriae bellulae similis, sed ascosporis sine vagina gelatinosa, ascosporis quidem in asco brunnescentibus.
Etymology. Named after Christiaan Hendrik Persoon (31 Dec. 1761 – 16 Nov. 1836) who was born in South Africa, but left for Europe at the age of 12, never to return. His most important work was Synopsis Fungorum, published in 1801, which formed the basis of modern mycology.
Leaf spots amphigenous, subcircular to circular, 2–7 mm diam, pale brown with a raised, pale to medium brown border. Ascomata amphigenous, black, immersed, substomatal, up to 120 μm diam; wall consisting of 2–3 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, obovoid to ellipsoid, straight to slightly curved, 8-spored, 23–40 × 8–11 μm. Ascospores tri- to multi-seriate, overlapping, hyaline, with 1–2 large guttules in every cell, thick-walled, straight to slightly curved, fusoid-ellipsoidal with obtuse ends, widest in middle of apical cell, prominently constricted at the septum, tapering towards both ends, but more prominently towards the lower end, (7–)8–10(–11) × 3–3.5(–4) μm; ascospores commonly observed to turn brown and verruculose in asci. Germinating ascospores on MEA become brown and verruculose, and one to several germ tubes grow at irregular angles to the long axis of the spore.
Cultural characteristics — Colonies on MEA erumpent with moderate aerial mycelium and smooth, regular margins; surface pale grey-olivaceous to grey-olivaceous; reverse grey-olivaceous, reaching 10 mm diam after 2 wk on MEA; sterile.
Specimens examined. South Africa, Western Cape Province, Jonkershoek, S33°59′4.2″ E18°57′16.1″, on living leaves of Protea sp., 1 Apr. 2007, P.W. Crous & L. Mostert, holotype CBS H-20102, cultures ex-type CPC 13972 = CBS 122895, CPC 13973, 13974 (occurring on leaf spots in association with T. jonkershoekensis); Western Cape Province, De Hoop Nature Reserve, Bredasdorp, S34°27′33″ E20°27′04″, on leaves of Euchaetis meridionalis (whole leaf turns brown, covered with brown-black mycelium; leaf and stem necrosis often starts where petiole attaches to branch; several fungi appear to be present, incl. species of Phoma and Leptosphaeria), 29 June 2006, A.R. Wood, CBS H-20103, culture STE-U 6389 = CPC 14846 = CBS 122896.
Notes — Although T. persoonii is morphologically very similar to T. bellula with regards to ascospore dimensions and symptomatology, it can be distinguished by having ascospores that have large, prominent guttules, are relatively thick-walled, commonly turn brown and verruculose while still in asci, and lack a mucoid sheath when mounted in water. No anamorph has been observed. Teratosphaeria persoonii also has a wider host range, occurring on Protea as well as Euchaetis.
Teratosphaeria proteae-arboreae P.S. van Wyk, Marasas & Knox-Dav., J. S. Afr. Bot. 41: 232. 1975 — Fig. 16
Fig. 16.
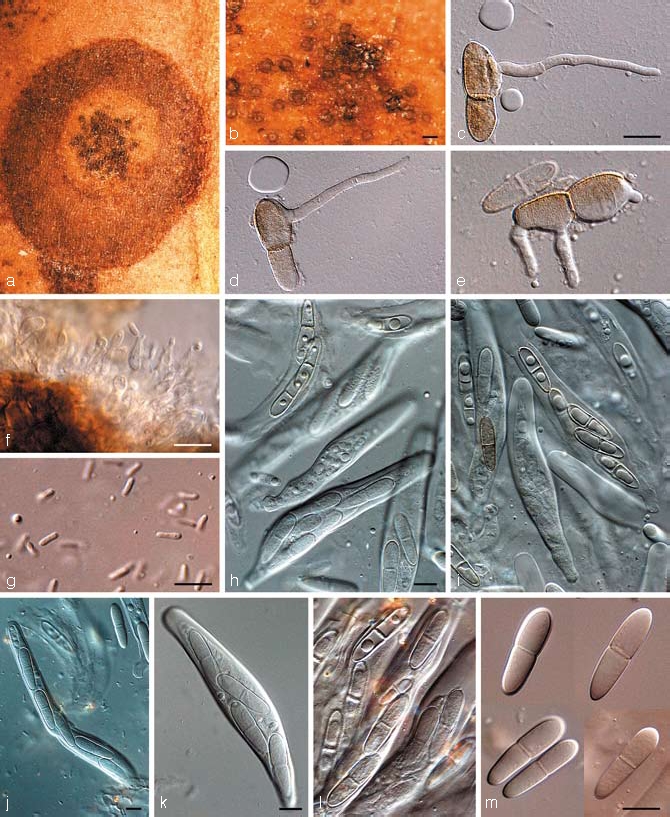
Teratosphaeria proteae-arboreae (CPC 12952). a. Leaf spot on Protea nitida; b. close-up of substomatal ascomata; c–e. germinating ascospores; f. spermatophores; g. spermatia; h–l. asci with darkening ascospores; m. ascospores. — Scale bars: b = 200 μm, all others = 10 μm.
≡ Mycosphaerella proteae-arboreae (P.S. van Wyk, Marasas & Knox- Dav.) Joanne E. Taylor & Crous, Mycol. Res. 107: 657. 2003.
Descriptions — Taylor & Crous (1998e), Crous et al. (2004a).
Leaf spots initially indistinct, chlorotic, raised, circular, with catenulate margins, with substomatal ascomata appearing as black spots in lesions; spots not extending through lamina, becoming black in centre, later extending so that the whole spot appears black, 1–3 mm diam. Ascomata epiphyllous or hypophyllous, globose, ostiolate, non-papillate, black, singular, gregarious; in section substomatal, subepidermal, globose to slightly pyriform, periphysoids lining the ostiole and the upper ascoma wall, up to 250 μm diam. Peridium consisting of three layers of compressed, brown textura angularis. Asci narrowly ellipsoid to obovoid, tapering abruptly to a small pedicel, narrowing to a rounded apex with a distinct ocular chamber, 2–4 μm diam, mainly straight, fasciculate, bitunicate with fissitunicate dehiscence, with a multi-layered endotunica, 80–120 × 14–20 μm; asci predominantly 8-spored, with two ascospores being underdeveloped. Ascospores bi- to tri-seriate, fusoid-ellisoidal, straight to slightly curved, 1-septate, prominently guttulate, with septum median or slightly supra-median, constricted, broadest in the middle of the apical cell and tapering to the lower end, hyaline, but becoming brown and verruculose in asci, (17–)25–30(–35) × (5–)6–7(–9) μm. Spermatogonia intermixed with ascomata, similar in morphology. Spermatophores producing spermatia that are bacilliform, smooth, hyaline with obtuse ends, 4–6 × 1.5 μm. Germinating ascospores on MEA after 24 h with one or several germ tubes, germinating from polar ends, parallel to the long axes, or lateral, from sides of spore; ascospores distorting, brown, verruculose, with outer, brown, verruculose layer frequently becoming separate from inner, hyaline layer.
Cultural characteristics — Colonies on MEA erumpent with sparse aerial mycelium and even, catenulate margins; centre hazel, margins isabelline to sepia; reverse fuscous-black; reaching 12 mm diam after 1 mo. On OA erumpent with sparse aerial mycelium and even, catenulate margins, pale olivaceous-grey to olivaceous-grey, reaching 10 mm diam after 1 mo at 25 °C.
Specimens examined. South Africa, Western Cape Province, Jonkershoek, Houttuyn, on living leaves of Protea arborea, 21 June 1971, P.S. van Wyk, holotype PREM 44801; Western Cape Province, Bettie’s Bay, Harold Porter Botanical Garden, on leaves of P. nitida, 4 Jan. 2006, M.K. Crous & P.W. Crous, epitype designated here CBS H-20106, cultures ex-epitype CPC 12952–12954; Western Cape Province, Kirstenbosch Botanical Garden, on living leaves of Protea sp., 6 Jan. 2008, P.W. Crous & M. Crous, CBS H-20107, cultures CPC 14963–14965.
Notes — Teratosphaeria proteae-arboreae is commonly associated with prominent leaf spots on Protea nitida. The present collection was obtained from the type location, and could subsequently be designated as epitype to clarify its phylogenetic relationship to other species of Teratosphaeria. It is interesting to note that as observed in T. maculiformis, asci frequently have one or two underdeveloped microascospores. The reason for the smaller ascospores occurring in asci along with normally developed ascospores is unknown. It is thus tempting to speculate that microascospores (which frequently aggregate at the ascal apex in T. proteae-arboreae and T. maculiformis) could have a different ecological role, being the first to be discharged. Alternatively, these could simply be weakly developed ascospores that are not viable. Further collections would be required to clarify this aspect.
Teratosphaeria sp.
Cultural characteristics — Colonies on MEA erumpent, with sparse aerial mycelium and smooth, catenulate margins; grey-olivaceous in centre, olivaceous-grey at margins and underneath; colonies reaching 7 mm diam after 2 wk at 25 °C.
Specimen examined. South Africa, Western Cape Province, Jonkershoek, S33°59′4.2″ E18°57′16.1″, on living leaves of Protea nitida, 1 Apr. 2007, P.W. Crous & L. Mostert, CPC 13917–13919.
Notes — This species, which is closely related to T. proteae-arboreae, could not be described here due to insufficient material. It was isolated from small, fusoid-ellipsoidal ascospores, 10 × 3.5 μm, that became constricted and distorted upon germination, and germinated from one end only. Based on its DNA phylogeny and ascospore germination pattern, it clearly represents yet another undescribed species of Teratosphaeria.
Teratosphaeria sp.
Cultural characteristics — Colonies on PDA erumpent, irregular, with smooth, catenulate margins and sparse aerial mycelium, iron-grey (surface), olivaceous-black (reverse); on OA spreading with smooth, regular margins; aerial mycelium sparse, iron-grey (surface); reaching 20 mm diam after 1 mo on OA at 25 °C; sterile.
Specimen examined. South Africa, Western Cape Province, Jonkershoek, S33°59′4.2″ E18°57′16.1″, on living leaves of Protea nitida, 1 Apr. 2007, P.W. Crous & L. Mostert, CPC 13963–13965.
Notes — This species, which has ascospores that distort and turn brown upon germination, occurred on spots in association with T. fibrillosa and T. proteae-arboreae. It could not be described, however, due to paucity of material.
Teratosphaeria sp.
Cultural characteristics — Colonies on MEA erumpent, folded, with sparse to moderate aerial mycelium and smooth, catenulate to feathery margins; grey-olivaceous in centre, olivaceous-grey at margins and underneath; colonies reaching 12 mm diam after 1 mo at 25 °C.
Specimen examined. South Africa, Western Cape Province, Kirstenbosch Botanical Garden, on living leaves of Protea sp., 6 Jan. 2008, P.W. Crous & M. Crous, CPC 14957–14959.
Notes — This species was isolated from ascospores that turned brown and distorted upon germination. It could not be described, however, due to paucity of material.
Teratosphaeria sp.
Cultural characteristics — Colonies on PDA erumpent with smooth margins and moderate aerial mycelium; isabelline in middle, becoming olivaceous towards margin; dark mouse-grey underneath. On OA spreading with smooth, catenulate margins and moderate olivaceous-grey aerial mycelium; iron-grey in outer region; colonies reaching 40 mm diam after 1 mo on OA at 25 °C; sterile.
Specimen examined. Portugal, on leaves of Protea repens, 1 Jan. 2007, M.F. Moura, CPC 13981.
Notes — This species was isolated from leaf spots on living leaves of Protea repens, but could not be described due to paucity of the material. Based on its cultural characteristics and DNA phylogeny, it appears to be distinct from the Teratosphaeria spp. presently known from Proteaceae
DISCUSSION
In their treatment of the Mycosphaerella diseases associated with Proteaceae, Crous et al. (2004a) listed 13 species of Mycosphaerella (incl. Teratosphaeria) and 18 associated anamorph species. Since the publication of the compendium, several species have been investigated by means of DNA molecular analyses, showing the morphological species concepts used in the past to have been too wide, obscuring the presence of several novel taxa. A good example of this was the report of M. jonkershoekensis from Australia based on symptomatology, morphology, and ascospore germination patterns (Crous et al. 2000b), which based on DNA techniques employed here, was revealed to in fact represent two species that were newly described in the T. jonkershoekensis complex, namely T. associata and T. maxii (Crous & Groenewald 2006a, b). A further significant step has been the acknowledgement of Teratosphaeriaceae as being distinct from Mycosphaerellaceae (Crous et al. 2007a), which made it essential to re-evaluate all species occurring on Proteaceae.
The present study also addressed the problem that many of the older names known from Proteaceae have never been studied in culture, and thus were omitted from previous DNA studies. These species had to be recollected, compared to the holotype specimens, and epitype specimens designated, so that ex-epitype cultures could become available for DNA analyses. This was achieved for most, but not all species, namely Batcheloromyces leucadendri, B. proteae, Catenulostroma macowanii, Mycosphaerella marksii, Teratosphaeria bellula, T. jonkershoekensis, T. parva, and T. proteae-arboreae. Several species are also newly described, namely Batcheloromyces sedgefieldii, Catenulostroma wingfieldii, Dissoconium proteae, Teratosphaeria knoxdavesii, T. marasasii and T. persoonii.
While Mycosphaerella and Teratosphaeria species are generally accepted to be host specific (Crous & Braun 2003), several species have now been shown to have wider host ranges than was commonly accepted (Burgess et al. 2007, Crous et al. 2004c, 2007d, Crous & Groenewald 2005). These include M. communis, which is known to have a wider host range, including Eucalyptus (South Africa, Spain, New Zealand), Musa (Trinidad) as well as Protea magnifica in Australia; Mycosphaerella konae (Leucospermum, Hawaii), which also occurs on Eucalyptus in Thailand (Crous et al. 2007d), M. marksii (Eucalyptus, Australia, Bolivia, China, Ecuador, Ethiopia, Papua New Guinea, New Zealand, South Africa, Spain, Tanzania, Uruguay), which also occurs on Leucadendron on the Madeira Islands, and Musa in Mozambique (Arzanlou et al. 2008). Teratosphaeria associata, an apparent opportunist on Eucalyptus in Australia, appears to be a primary pathogen on Protea in the same country (Crous et al. 2000b, 2007d). Teratosphaeria parva (on Eucalyptus in Australia, Chile, Ethiopia, Portugal, South Africa and Spain), is also found on Protea in South Africa. Wider host ranges may also be applicable to T. microspora and Septoria protearum, though species concepts in these genera are still unresolved.
Based on DNA analysis of single ascospore cultures of Mycosphaerella spp. derived from various hosts and substrates, Crous & Groenewald (2005) introduced the pogo stick hypothesis to explain the fact that well-known plant pathogenic species of Mycosphaerella (incl. Teratosphaeria) are frequently encountered on ‘non-hosts’, where they appear to colonise leaf spots of other Mycosphaerella species that are primary pathogens on these hosts, to enable them to produce a limited amount of progeny to enable onward dispersal. This ‘host jumping’ phenomenon appears to be much more common in Mycosphaerella and Teratosphaeria than generally accepted in literature, revealing these necrotrophic species to be able to grow also as saprobes on dead tissue, enabling them to disperse further in an attempt to locate their ideal hosts.
Many species of Mycosphaerella and Teratosphaeria, which were commonly accepted as host-specific necrotrophic pathogens, thus appear to also exhibit a facultative saprobic behaviour. This indicates that the definitions of ‘necrotroph’ or ‘saprobe’ do not clearly define all species of Mycosphaerella and Teratosphaeria, as some have obviously retained the ability to also grow on dead tissue when they lose the connection to their real host.
The exact mechanism that allows species of Mycosphaerella and Teratosphaeria to co-colonise the same host tissue, leading to several species co-occurring in the same leaf spot (Crous & Wingfield 1996, Crous 1998), also deserves further study.
Acknowledgments
The University of Stellenbosch is thanked for financial support to P.W.C. during a recent collecting trip to the fynbos region in South Africa. Prof. dr U. Braun (Martin-Luther-Univ., Halle, Germany) is thanked for providing the Latin diagnoses. Profs W.F.O. Marasas and M.J. Wingfield, who have been honoured in the present paper, have both been awarded the Hendrik Persoon Gold Medal by the Southern African Society for Plant Pathology. The medal was designed by Prof. P.S. Knox-Davies (deceased), also remembered here. They all love(d) fungi.
REFERENCES
- Aptroot A. 2006. Mycosphaerella and its anamorphs: 2. Conspectus of Mycosphaerella. CBS Biodiversity Series 5: 1 – 231 . [Google Scholar]
- Arzanlou M, Groenewald JZ, Fullerton RA, Abeln ECA, Carlier J, Zapater M-F, Buddenhagen IW, Viljoen A, Crous PW. 2008. Multiple gene genealogies and phenotypic characters differentiate several novel species of Mycosphaerella and related anamorphs on banana. Persoonia 20: 19 – 37 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzanlou M, Groenewald JZ, Gams W, Braun U, Shin H-D, Crous PW. 2007. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58: 57 – 93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batzer JC, Mercedes Diaz Arias M, Harrington TC, Gleason ML, Groenewald JZ, Crous PW. 2008. Four species of Zygophiala (Schizothyriaceae, Capnodiales) are associated with the sooty blotch and flyspeck complex on apple. Mycologia 100: 232 – 244 . [DOI] [PubMed] [Google Scholar]
- Braun U, Crous PW, Dugan F, Groenewald JZ, Hoog SG de. 2003. Phylogeny and taxonomy of Cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s.str. Mycological Progress 2: 3 – 18 . [Google Scholar]
- Burgess TI, Barber PA, Sufaati S, Xu D, Hardy GE StJ, Dell B. 2007. Mycosphaerella spp. on Eucalyptus in Asia; new species, new hosts and new records. Fungal Diversity 24: 135 – 157 . [Google Scholar]
- Carnegie AJ, Keane PJ. 1994. Further Mycosphaerella species associated with leaf diseases of Eucalyptus. Mycological Research 98: 413 – 418 . [Google Scholar]
- Coetzee MPA, Wingfield BD, Roux J, Crous PW, Denman S, Wingfield MJ. 2003. Discovery of two northern hemisphere Armillaria species on Proteaceae in South Africa. Plant Pathology 52: 604 – 612 . [Google Scholar]
- Crous PW. 1998. Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1 – 170 . [Google Scholar]
- Crous PW. 2002. Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera APS Press, Minnesota, USA: . [Google Scholar]
- Crous PW, Aptroot A, Kang J-C, Braun U, Wingfield MJ. 2000a. The genus Mycosphaerella and its anamorphs. Studies in Mycology 45: 107 – 121 . [Google Scholar]
- Crous PW, Braun U. 1994. Cercospora species and similar fungi occurring in South Africa. Sydowia 46: 204 – 224 . [Google Scholar]
- Crous PW, Braun U. 1996. Cercosporoid fungi from South Africa. Mycotaxon 57: 233 – 321 . [Google Scholar]
- Crous PW, Braun U. 2003. Mycosphaerella and its anamorphs. 1. Names published in Cercospora and Passalora. CBS Biodiversity Series 1: 1 – 571 . [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. 2007a. Mycosphaerella is polyphyletic. Studies in Mycology 58: 1 – 32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Schubert K, Groenewald JZ. 2007b. Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 58: 33 – 56 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Denman S, Taylor JE, Swart L, Palm ME. 2004a. Cultivation and diseases of Proteaceae: Leucadendron, Leucospermum and Protea. CBS Biodiversity Series 2: 1 – 228 . [Google Scholar]
- Crous PW, Groenewald JZ. 2005. Hosts, species and genotypes: opinions versus data. Australasian Plant Pathology 34: 463 – 470 . [Google Scholar]
- Crous PW, Groenewald JZ. 2006a. Mycosphaerella alistairii. Fungal Planet No. 4 CBS, Utrecht, Netherlands: . [Google Scholar]
- Crous PW, Groenewald JZ. 2006b. Mycosphaerella maxii. Fungal Planet No. 6 CBS, Utrecht, Netherlands: . [Google Scholar]
- Crous PW, Groenewald JZ, Mansilla JP, Hunter GC, Wingfield MJ. 2004b. Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. Studies in Mycology 50: 195 – 214 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Pongpanich K, Himaman W, Arzanlou M, Wingfield MJ. 2004c. Cryptic speciation and host specificity among Mycosphaerella spp. occurring on Australian Acacia species grown as exotics in the tropics. Studies in Mycology 50: 457 – 469 . [Google Scholar]
- Crous PW, Kang J-C, Braun U. 2001. A phylogenetic redefinition of anamorph genera in Mycosphaerella based on ITS rDNA sequence and morphology. Mycologia 93: 1081 – 1101 . [Google Scholar]
- Crous PW, Mohammed C, Glen M, Verkley GJM, Groenewald JZ. 2007c. Eucalyptus microfungi known from culture. 3. Eucasphaeria and Sympoventuria genera nova, and new species of Furcaspora, Harknessia, Heteroconium and Phacidiella. Fungal Diversity 25: 19 – 36 . [Google Scholar]
- Crous PW, Palm ME. 1999. Systematics of selected foliicolous fungi associated with leaf spots of Proteaceae. Mycological Research 103: 1299 – 1304 . [Google Scholar]
- Crous PW, Rong IH, Wood A, Lee S, Glen H, Botha W, Slippers B, Beer WZ de, Wingfield MJ, Hawksworth DL. 2006a. How many species of fungi are there at the tip of Africa? Studies in Mycology 55: 13 – 33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ. 2006b. Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235 – 253 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Mohammed C, Himaman W, Groenewald JZ. 2007d. Foliicolous Mycosphaerella spp. and their anamorphs on Corymbia and Eucalyptus. Fungal Diversity 26: 143 – 185 . [Google Scholar]
- Crous PW, Summerell BA, Taylor JE, Bullock S. 2000b. Fungi occurring on Proteaceae in Australia: selected foliicolous species. Australasian Plant Pathology 29: 267 – 278 . [Google Scholar]
- Crous PW, Wingfield MJ. 1993. Additions to Mycosphaerella in the fynbos biome. Mycotaxon 46: 19 – 26 . [Google Scholar]
- Crous PW, Wingfield MJ. 1996. Species of Mycosphaerella and their anamorphs associated with leaf blotch disease of Eucalyptus in South Africa. Mycologia 88: 441 – 458 . [Google Scholar]
- Crous PW, Wingfield MJ, Mansilla JP, Alfenas AC, Groenewald JZ. 2006c. Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology 55: 99 – 131 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman S, Crous PW, Groenewald JG, Slippers B, Wingfield BD, Wingfield MJ. 2003. Circumscription of Botryosphaeria species associated with Proteaceae based on morphology and DNA sequence data. Mycologia 95: 294 – 307 . [PubMed] [Google Scholar]
- Denman S, Crous PW, Taylor JE, Kang J-C, Pascoe I, Wingfield MJ. 2000. An overview of the taxonomic history of Botryosphaeria, and a re-evaluation of its anamorphs based on morphology and ITS rDNA phylogeny. Studies in Mycology 45: 129 – 140 . [Google Scholar]
- Denman S, Crous PW, Wingfield MJ. 1999. A taxonomic reassessment of Phyllachora proteae, a leaf pathogen of Proteaceae. Mycologia 91: 510 – 516 . [Google Scholar]
- Gams W, Verkleij GJM, Crous PW. 2007. CBS Course of Mycology, 5th ed Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: . [Google Scholar]
- Goodwin SB, Dunkley LD, Zismann VL. 2001. Phylogenetic analysis of Cercospora and Mycosphaerella based on the internal transcribed spacer region of ribosomal DNA. Phytopathology 91: 648 – 658 . [DOI] [PubMed] [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. 1998. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183 – 189 . [DOI] [PubMed] [Google Scholar]
- Hunter GC, Wingfield BD, Crous PW, Wingfield MJ. 2006. A multi-gene phylogeny for species of Mycosphaerella occurring on Eucalyptus leaves. Studies in Mycology 55: 147 – 161 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marincowitz S, Crous PW, Groenewald JZ, Wingfield MJ. 2008. Microfungi occurring on Proteaceae in the fynbos. CBS Biodiversity Series 7: 1 – 166 . [Google Scholar]
- Mostert L, Crous PW, Kang J-C, Phillips AJL. 2001a. Species of Phomopsis and a Libertella sp. occurring on grapevines with specific reference to South Africa: morphological, cultural, molecular and pathological characterization. Mycologia 93: 145 – 166 . [Google Scholar]
- Mostert L, Kang J-C, Crous PW, Denman S. 2001b. Phomopsis saccharata sp. nov., causing a canker and die-back disease of Protea repens in South Africa. Sydowia 53: 227 – 235 . [Google Scholar]
- Rayner RW. 1970. A mycological colour chart CMI and British Mycological Society, Kew, Surrey, England: . [Google Scholar]
- Schoch CL, Crous PW, Wingfield BD, Wingfield MJ. 1999. The Cylindrocladium candelabrum species complex includes four distinct mating populations. Mycologia 91: 286 – 298 . [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW. 2006. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1043 – 1054 . [DOI] [PubMed] [Google Scholar]
- Schubert K, Groenewald JZ, Braun U, Dijksterhuis J, Starink M, Hill CF, Zalar P, Hoog GS de, Crous PW. 2007. Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Studies in Mycology 58: 105 – 156 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanesan A, Shivas RG. 2002. Studies on Mycosphaerella species in Queensland, Australia. Mycological Research 106: 355 – 364 . [Google Scholar]
- Swart L, Crous PW, Denman S, Palm ME. 1998. Fungi occurring on Proteaceae. I. South African Journal of Botany 64: 137 – 145 . [Google Scholar]
- Swart L, Crous PW, Kang J-C, Mchau GRA, Pascoe IA, Palm ME. 2001. Differentiation of species of Elsinoë associated with scab disease of Proteaceae based on morphology, symptomatology, and ITS sequence phylogeny. Mycologia 93: 365 – 379 . [Google Scholar]
- Taylor JE, Cannon PF, David JC, Crous PW. 2001a. Two new Phaeophleospora species associated with leaf spots of Proteaceae. South African Journal of Botany 67: 39 – 43 . [Google Scholar]
- Taylor JE, Crous PW. 1998a. Mycosphaerella jonkershoekensis. IMI Descriptions of Fungi and Bacteria. No. 1344 . [Google Scholar]
- Taylor JE, Crous PW. 1998b. Mycosphaerella bellula. IMI Descriptions of Fungi and Bacteria. No. 1345 . [Google Scholar]
- Taylor JE, Crous PW. 1998c. Teratosphaeria maculiformis. IMI Descriptions of Fungi and Bacteria. No. 1346 . [Google Scholar]
- Taylor JE, Crous PW. 1998d. Teratosphaeria fibrillosa. IMI Descriptions of Fungi and Bacteria. No. 1347 . [Google Scholar]
- Taylor JE, Crous PW. 1998e. Teratosphaeria proteae-arboreae. IMI Descriptions of Fungi and Bacteria. No. 1348 . [Google Scholar]
- Taylor JE, Crous PW. 1998f. Trimmatostroma macowanii. IMI Descriptions of Fungi and Bacteria. No. 1349 . [Google Scholar]
- Taylor JE, Crous PW. 2000. Fungi occurring on Proteaceae. New anamorphs for Teratosphaeria, Mycosphaerella and Lembosia, and other fungi associated with leaf spots and cankers of Proteaceous hosts. Mycological Research 104: 618 – 636 . [Google Scholar]
- Taylor JE, Crous PW. 2001. Morphological variation and cultural characteristics of Coniothyrium leucospermi associated with leaf spots of Proteaceae. Mycoscience 42: 265 – 271 . [Google Scholar]
- Taylor JE, Crous PW, Palm ME. 2001b. Foliar and stem fungal pathogens of Proteaceae in Hawaii. Mycotaxon 78: 449 – 490 . [Google Scholar]
- Taylor JE, Crous PW, Swart L. 2001c. Foliicolous and caulicolous fungi associated with Proteaceae cultivated in California. Mycotaxon 78: 75 – 103 . [Google Scholar]
- Taylor JE, Crous PW, Wingfield MJ. 1999. The genus Batcheloromyces on Proteaceae. Mycological Research 103: 1478 – 1484 . [Google Scholar]
- Taylor JE, Groenewald JZ, Crous PW. 2003. A phylogenetic analysis of Mycosphaerellaceae leaf spot pathogens of Proteaceae. Mycological Research 107: 653 – 658 . [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238 – 4246 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee J, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–322 Academic Press, San Diego, California, USA: . [Google Scholar]
- Wyk PS van, Marasas WFO, Knox-Davies PS. 1975. Teratosphaeria proteae-arboreae and Mycosphaerella jonkershoekensis, two new ascomycetes on Protea in South Africa. Journal of South African Botany 41: 231 – 238 . [Google Scholar]
- Zhang R, Zhang Z, Zhang M, Sun GG, Gleason ML. 2007. A new species of Dissoconium from China colonizing apples. Mycotaxon 101: 165 – 172 . [Google Scholar]