Abstract
Phenylketonuria (PKU) is caused by hepatic phenylalanine hydroxylase (PAH) deficiency and is associated with systemic accumulation of phenylalanine (Phe). Previously we demonstrated correction of murine PKU after intravenous injection of a recombinant type 2 adeno-associated viral vector pseudotyped with type 8 capsid (rAAV2/8), which successfully directed hepatic transduction and Pah gene expression. Here, we report that liver PAH activity and phenylalanine clearance were also restored in PAH-deficient mice after simple intramuscular injection of either AAV2 pseudotype 1 (rAAV2/1) or rAAV2/8 vectors. Serotype 2 AAV vector (rAAV2/2) was also investigated, but long-term phenylalanine clearance has been observed only for pseudotypes 1 and 8. Therapeutic correction was shown in both male and female mice, albeit more effectively in males, in which correction lasted for the entire period of the experiment (>1 year). Although phenylalanine levels began to rise in female mice at about 8–10 months after rAAV2/8 injection they remained only mildly hyperphenylalaninemic thereafter and subsequent supplementation with synthetic tetrahydrobiopterin resulted in a transient decrease in blood phenylalanine. Alternatively, subsequent administration of a second vector with a different AAV pseudotype to avoid immunity against the previously administrated vector was also successful for long-term treatment of female PKU mice. Overall, this relatively less invasive gene transfer approach completes our previous studies and allows comparison of complementary strategies in the development of efficient PKU gene therapy protocols.
Introduction
Phenylketonuria (PKU) is an autosomal recessive genetic disorder with an average incidence of roughly 1 case in 10,000 Caucasian live births (OMIM 261600). It is caused primarily by deficiency of the hepatic enzyme phenylalanine hydroxylase (PAH; EC 1.14.16.1), responsible for converting phenylalanine to tyrosine, using molecular oxygen and tetrahydrobiopterin (BH4) as a necessary cofactor for its catalytic activity (Scriver and Kaufman, 2001). This conversion is the rate-limiting step in phenylalanine (Phe) catabolism in the liver. PAH deficiency due to mutations in the gene that encodes PAH leads to hyperphenylalaninemia (HPA), a dramatic increase in blood Phe concentration from <0.12 to >1.2 mM (the equivalent of <2 to >20 mg/dl). The inability to degrade Phe present in dietary protein leads to the excretion of urinary phenylalanine, phenylpyruvate, and phenylacetate. Mild forms of PKU accumulate Phe in the blood at levels of 0.6 to 1.2 mM. High levels of accumulated Phe in patients with PKU are toxic to the human body if left untreated, and are associated with an abnormal phenotype presenting with growth failure, microcephaly, seizures, and mental retardation (Donlon et al., 2008). HPA can also be caused by the absence of the BH4 cofactor due to deficiency of cofactor biosynthesis and regeneration, which leads to a diverse group of neurometabolic diseases (Blau et al., 2001; Thöny and Blau, 2006). The biochemistry and genetics of PKU are well characterized, with more than 500 disease-causing mutations identified in the human PAH gene spread over the entire 13 exon-containing gene (see also the Phenylalanine Hydroxylase Locus Knowledgebase [PAHdb], www.pahdb.mcgill.ca) (Scriver et al., 2003). Moreover, PKU has been detected in most Western countries for many decades by newborn screening programs (Guthrie, 1996).
HPA during pregnancy can produce PKU-like symptoms in genetically unaffected offspring, termed maternal PKU syndrome, which is associated with severe birth defects such as microcephaly, congenital heart disease, and low birth weight (Levy and Ghavami, 1996; Guttler et al., 2003). The current established treatment for PKU consists of life-long dietary Phe restriction, which is challenging in practice as it is demanding in schedule, unpleasant in taste, carries risk for nutritional deficiencies, and is a substantial psychosocial burden, associated with reduction in quality of life (Harding, 2000; National Institutes of Health Consensus Development Panel, 2001). Furthermore, it was reported that decline in intellectual function (Koch et al., 1984), behavioral performance (Koch et al., 2002), and severe emotional dysfunction, including attention deficit disorder (Antshel and Waisbren, 2003), depression, and anxiety (Waisbren and Levy, 1991), are often observed in noncompliant patients with PKU.
Because of these shortcomings, the focus of PKU research has shifted since the beginning of the twenty-first century to the improvement of current therapy and development of alternative options (Sarkissian et al., 2009). With the progress in gene delivery, particularly with the advent of adeno-associated virus (AAV)-based vectors (Wu et al., 2006; Buning et al., 2008; Schultz and Chamberlain, 2008; Zincarelli et al., 2008), gene therapy has emerged as an attractive alternative to meet the particularly challenging issue of long-term treatment in PKU. Successful correction of murine HPA using recombinant type 2 AAV vectors pseudotyped with serotype 2 or 5 capsid (rAAV2/2 or rAAV2/5) has been achieved in several laboratories (Laipis et al., 2004; Mochizuki et al., 2004; Oh et al., 2004). However, in these AAV experiments, extremely high doses of viral vectors (1013 to 1014 vector genomes per mouse) were necessary for efficient reduction in blood Phe. Moreover, the therapy was less effective in female mice. In one published study, efficacy was observed to last 40 weeks in male mice but only 10 weeks in females (Mochizuki et al., 2004). Gender-dependent differences in liver transduction with AAV vectors have been reported in many other studies (Davidoff et al., 2003; Grimm et al., 2003; Berraondo et al., 2006; Ogura et al., 2006; Voutetakis et al., 2007; Ho et al., 2008) and could complicate human clinical trials, with important implications for the prevention of maternal PKU. Furthermore, other factors, such as stability of the PAH protein (Harding et al., 2006a), subphysiological hepatic concentrations of tetrahydrobiopterin (BH4, an essential cofactor for PAH) (Chen et al., 2007), or differences in intestinal absorption and transport of Phe (Scriver and Waters, 1999), may also contribute to the lower Phe tolerance observed in treated PKU female mice. In this regard, experiments with nonviral vectors designed for stable liver-directed genomic integration of a PAH expression cassette (Chen and Woo, 2007) or with AAV vectors pseudotyped with serotype 8 capsid (rAAV2/8) (Ding et al., 2006b; Harding et al., 2006b) also indicated that a higher therapeutic threshold is required for long-term correction of PKU in females. Recombinant AAV2/8 has been reported to be 10 to 100 times more effective in liver than rAAV2/2 and rAAV2/5, respectively (Sarkar et al., 2004). In addition to the role of the AAV capsid in gene transfer, the number of hepatocytes that are successfully transduced, even when high doses of AAV vector are used, is highly influenced by the routes of administration for AAV delivery (Berraondo et al., 2006; Inagaki et al., 2006). Indeed, our previous study showed a more robust and longer lasting correction of hyperphenylalaninemia in both male and female PKU mice when rAAV2/8 vectors were delivered via the portal vein rather than by the tail vein (Ding et al., 2006a). In that experiment, expression of the PAH gene was driven by the constitutive or ubiquitous cytomegalovirus (CMV) enhancer–chicken β-actin (CBA) promoter. Although therapeutic corrections up to 53 weeks (for more than 1 year) have been observed for some animals, we also noticed a loss of effectiveness starting at about week 40 in female mice. Together, these observations emphasize the need to further extend the therapeutic persistence achieved with rAAV2/8 vectors and to find alternative AAV serotype vectors if repeated gene vector delivery is required.
In this study, we performed a dose–response and time course analysis of the efficiency of recombinant AAV2/1, AAV2/2, and AAV2/8 vectors, expressing the murine Pah gene from the CBA promoter, and delivered by direct intramuscular injection rather than intravenously, to mediate liver transduction and correct hyperphenylalaninemia in Pahenu2 mice, a model of human PKU. The superiority of rAAV2/1 and rAAV2/8 vectors over rAAV2/2 vector in achieving long-term therapeutic correction was reaffirmed. More importantly, a therapeutic effect was observed in both male and female treated mice, albeit more effective in males. For treated female mice in which blood Phe levels had risen again several weeks after gene transfer, supplementation with BH4 cofactor led to transient restoration of blood Phe to near normal levels. Alternatively, intramuscular injection of rAAV2/8 vector extended therapeutic correction in females previously exposed to rAAV2/1 vector.
Materials and Methods
Plasmid vectors and recombinant AAV production
The cloning strategy for the construction of plasmid pAAV2-PKU5 has been previously described (Ding et al., 2006a). Briefly, the transgene expression cassette flanked by two inverted terminal repeats in pAAV2-PKU5 was designed with the CMV enhancer–chicken β-actin (CBA) promoter, the mouse 1.4-kb Pah cDNA, the woodchuck posttranscriptional regulatory element (WPRE), and the simian virus 40 (SV40) polyadenylation signal sequence. Recombinant AAV2 vectors pseudotyped with serotype 1 or 8 capsid proteins were produced in an adenovirus-free system in HEK 293 cells, using a three-plasmid transfection protocol with the pAAV2-PKU5 plasmid, the adenovirus helper plasmid pBS-E2A-VA-E4 (provided by H. Büeler [Paterna et al., 2004]), and a pAAV packaging plasmid expressing the rep and cap genes. For pseudotyping with serotype 1 and 8 capsid proteins, plasmids p5E18RXCI and p5E18-VD2/8 were used, respectively (provided by J.M. Wilson [Gao et al., 2002]). For the production of recombinant AAV2 serotype 2 vectors, a two-plasmid transfection protocol was used with plasmids pAAV2-PKU5 and pDG, the latter carrying the AAV2 rep and cap genes, as well as adenovirus helper genes (provided by J.A. Kleinschmidt [Grimm et al., 1998]). All vectors were purified by two rounds of cesium chloride gradient centrifugation to provide a uniform purification method. After the second centrifugation, the peak fractions, as determined by semiquantitative polymerase chain reaction (PCR), were dialyzed for three rounds against sterile phosphate-buffered saline (PBS), and concentrated with Amicon Ultra filters (Millipore, Billerica, MA) (Grieger et al., 2006). The physical particle titers were determined by TaqMan analysis of the WPRE sequence with the following primers and probe (Potter et al., 2002; Grieger et al., 2006): forward primer, 5′-CCGTTGTCAGGCAACGTG-3′; reverse primer, 5′-AGCTGACAGGTGGTGGCAAT-3′; probe, 5′-FAM-TGCTGACGCAACCCCCACTGGT-TAMRA-3′. A standard curve was generated by dilution of vector plasmids in salmon sperm DNA (40 ng/ml) to increase the stability of plasmid dilutions.
Animal experiments
Animal experiments were carried out in accordance with the State Veterinary Office of Zürich (Zürich, Switzerland) and Swiss law on animal protection, the Swiss Federal Act on Animal Protection (1978), and the Swiss Animal Protection Ordinance (1981). All animal studies were approved by the Cantonal Veterinary Office (Zürich, Switzerland) and the Cantonal Committee for Animal Experiments (Zürich, Switzerland). PAH-deficient C57BL/6-Pahenu2 (“PKU”) mice were homozygous for the same Pah mutation as described for the original BTBR-Pahenu2 strain (McDonald and Charlton, 1997; Ding et al., 2006a). All mice were maintained on standard mouse chow, and 8- to 10-week-old male and female PKU mice were selected for viral injection. Fifty microliters of recombinant AAV vector suspension was injected intramuscularly through the skin into both gastrocnemius muscles in less than 1 min (about 0.025 ml/min); mice were not included in this study if blood was present in the syringe on withdrawal, or if the injected mice showed signs of bleeding. Monitoring of blood Phe collected from tail veins was carried out as described earlier (Ding et al., 2008). For cofactor administration, BH4 from Schircks Laboratories (Jona, Switzerland) was dissolved in 1% ascorbic acid, pH 7, to a concentration of 10–100 mM and administered to mice (between 16 and 32 mg of BH4 per kilogram body weight) by intraperitoneal injection, using a 27-gauge needle on a 1-ml disposable syringe. Note that when the first cohort of animals was injected with a dose of 32 mg of BH4 per kilogram body weight, we noticed moderate signs of weakness, passivity, and sluggishness in these animals; however, mice receiving 16 mg of BH4 per kilogram body weight remained asymptomatic after BH4 treatment. The amount of BH4 given here is comparable to human doses for oral loading tests, which are between 20 to 40 mg of BH4 per kilogram body weight. Tissue BH4 content was determined 24 hr after intraperitoneal injection as described (Ding et al., 2006a, 2008).
PAH enzyme assay and immunoblotting analysis
PAH enzyme activity in liver and muscle homogenates was measured according to a published method (Thöny et al., 2004) with minor modifications of the homogenization procedure. Briefly, frozen harvested tissues were ground to powder in liquid nitrogen, suspended in 1 vol (muscle) or 5 vol (liver) of cold lysis buffer, and homogenized with a DUALL Kontes glass homogenizer (Kimble Chase, Vineland, NJ). For immunoblot analysis, equal amounts of liver (20 μg) or muscle (30 μg) homogenate from wild-type or rAAV-treated Pahenu2 mice were separated by 4–12% polyacrylamide gel electrophoresis (PAGE), blotted onto nitrocellulose membrane, and probed with rabbit anti-PAH antibody (diluted 1:10,000) or mouse anti-β-actin antibody (1:1000 or 1:10,000; Sigma-Aldrich, St. Louis, MO). The blots were incubated with a secondary antibody conjugated with peroxidase (1:10,000; GE Healthcare Biosciences, Piscataway, NJ) and the bands were visualized with enhanced chemiluminescence (ECL) solution (GE Healthcare Biosciences). Quantification of Western blots was done with NIH Image software, with the amounts of PAH protein normalized to the amount of β-actin protein detected.
AAV vector biodistribution
Real-time PCR analysis of genomic DNA extracted from snap-frozen tissues was performed with the same set of primers and probe indicated in the preceding section, Plasmid Vectors and Recombinant AAV Production. Details of the procedure have been described previously (Ding et al., 2006a).
Statistical analysis
Statistical analyses were performed with GraphPad Prism software (GraphPad Software, San Diego, CA). Statistical differences between the various experimental groups were evaluated by t test. p < 0.05 was considered statistically significant.
Results
Dose response and kinetics of AAV serotype 1, 2, and 8 vector-mediated correction of murine PKU
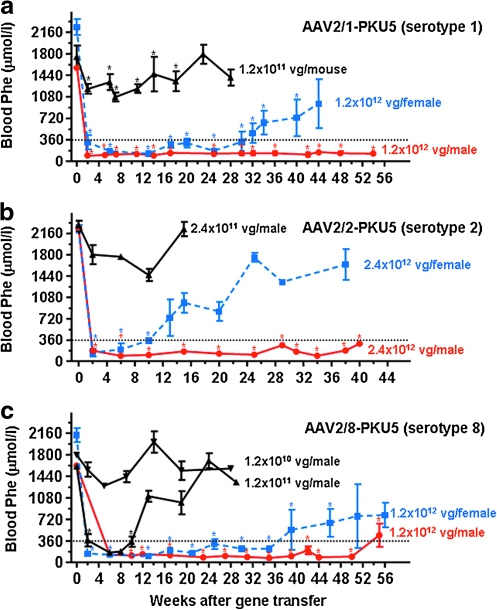
To compare the efficacy of various AAV serotypes for mediating long-term therapeutic correction of PKU in Pahenu2 mice, we generated three different recombinant AAV2 genomes packaged with either serotype 1, 2, or 8 capsid. Each of these vectors contained an identical expression cassette consisting of the murine Pah cDNA under the control of the CMV enhancer–chicken β-actin (CBA) promoter with a woodchuck posttranscriptional regulatory element (WPRE) sequence. This expression cassette, named AAV2-PKU5, was previously described in our study of liver-directed gene transfer via intraportal or tail vein injection of recombinant AAV vectors (Ding et al., 2006a). The AAV particles pseudotyped with serotype 1, 2, or 8 capsid (the vectors are named AAV2/1-PKU5, AAV2/2-PKU5, and AAV2/8-PKU5, respectively) were produced in the same manner to avoid inconsistencies due to differences in purification methodologies (Klein et al., 2008). To evaluate the influence of the various AAV capsid proteins on long-term therapeutic correction, hyperphenylalaninemic male and female Pahenu2 mice were first injected with an equivalent, high dose of viral genomes (VG) of AAV2/1-PKU5 or AAV2/8-PKU5 vector (1.2 × 1012 VG/mouse) or with a slightly higher dose of 2.4 × 1012 VG/mouse for the comparative study with AAV2/2-PKU5. For each mouse, half of the total vector dose was administered to each hindleg by direct percutaneous injection into the gastrocnemius muscles. Given the low invasiveness of intramuscular injections, this route would be more advantageous for secondary gene transfer than intraportal delivery, and thus was selected for this study. Blood Phe levels were monitored periodically for several weeks and used as a surrogate marker of transgene expression. In all treatment groups, blood Phe levels decreased significantly from levels greater than 1800 μM to less than 360 μM, the defined therapeutic value for blood Phe, by 2 weeks after vector injection, and this effect persisted in all injected mice, both males and females, for at least 10 weeks (Fig. 1). Normalization of Phe levels was also accompanied by a complete phenotypic change from brown hair to black at about 8 weeks, similar to results we (and others) have observed in previous studies (Ding et al., 2006a, 2008). The duration of correction in males was monitored for more than 1 year and the results are summarized as follows: In males injected with the highest dose of vector AAV2/1-PKU5, AAV2/2-PKU5, or AAV2/8-PKU5, blood Phe levels were stably maintained at low levels for 54, 40, and 55 weeks postinjection, respectively. Blood Phe values rose slightly above 360 μmol/liter in some mice injected with AAV2/8-PKU5 at week 55, the last time point analyzed (Fig. 1c). Similarly, side-by-side comparative analyses were performed in females and the results showed striking differences in treatment effect between males and females, and among the three vectors tested. Although the normalization of blood Phe levels in females treated with the highest doses of AAV2/1-PKU5 and AAV2/8-PKU5 were temporary, they lasted out to 30 and 35 weeks postinjection, respectively (see Fig. 1a and c). Interestingly, blood Phe levels did not return to pretreatment values (:1800 μmol/liter), and all females remained only mildly hyperphenylalaninemic with Phe concentrations ranging from 700 to 900 μmol/liter up to 1 year. In female mice, AAV2/2-PKU5 treatment was least effective (Fig. 1b), as the Phe values in this cohort of mouse began to rise 10 weeks after gene transfer and returned to levels close to pretreatment values by 30 weeks.
FIG. 1.
Serotype-, dose-, and gender-dependent changes of blood phenylalanine (Phe) content in phenylketonuria (PKU) mice after intramuscular administration of vector AAV2-PKU5. Various doses of vector AAV2-PKU5 expressing phenylalanine hydroxylase (PAH) and pseudotyped with either capsid 1, 2, or 8 were injected into the gastrocnemius muscles of both hindlegs of male and female PKU mice. Approximately every second week blood was withdrawn from tail veins for Phe determination, depicted as a function of time after injection of AAV2-PKU5. Blood Phe concentrations are represented as means ± SD. The dotted lines in each graph indicate the therapeutic threshold of blood Phe concentration for treatment of patients with PKU (9360 μmol/liter). (a) Vector AAV2-PKU5 serotype 1 was injected at a dose of 1.2 × 1012 viral genome (VG) particles per mouse in males (red circles, n = 4) and in females (blue squares, n = 3), or at a dose of 1.2 × 1011 VG particles per mouse (black triangles, 3 males plus 3 females, n = 6). (b) Vector AAV2-PKU5 serotype 2 was injected at a dose of 2.4 × 1012 VG particles per mouse in males (red circles, n = 2), and in females (blue squares, n = 2), or at a dose of 2.4 × 1011 VG particles for the lower dose–response study (black triangles, male, n = 3). (c) Vector AAV2/8-PKU5 serotype 8 was injected at three different vector doses, in 10-fold increments, ranging from 1.2 × 1010 to 1.2 × 1012 VG particles per mouse. Data for males (red circles, n = 4) and females (blue squares, n = 3) are plotted separately for the 1.2 × 1012 VG particle dose–response study, whereas vector doses of 1.2 × 1010 VG particles (black inverted triangles, n = 3) and 1.2 × 1011 VG particles (black triangles, n = 3) were used for injection of two male groups. Blood Phe levels at various time points after AAV2-PKU5 injection were compared with preinjection blood levels by Student t test. Significant differences as compared with t = 0 found for all AAV2-PKU5-treated animals are marked with asterisks, whereas at all unmarked points blood Phe was not significantly different from baseline levels (p > 0.05).
Because these comparisons were made with relatively high vector doses, we wanted also to exclude the possibility that a potential vector hierarchy had been obscured because of saturating conditions. Therefore, we proceeded to test the long-term correction efficiency of the various AAV vectors in a dose range study. At a dose of 1.2 × 1011 VG/mouse, statistically significant correction in blood Phe was seen only in animals treated with AAV2/8-PKU5 vector (Fig. 1c) and not in mice receiving either AAV2/2-PKU5 or AAV2/1-PKU5. The therapeutic effect with AAV2/8-PKU5 vector, however, was only transient; blood Phe rose again to preinjection levels by 10 weeks postinjection. Only modest decreases in blood Phe were observed after treatment with 1.2 × 1011 VG of AAV2/1-PKU5 (Fig. 1a), 2.4 × 1011 VG of AAV2/2-PKU5 (Fig. 1b), or with a lower dose (1.2 × 1010 VG/mouse) of AAV2/8-PKU5 (Fig. 1c). In all cases, this modest effect lasted only a few weeks. As was expected, the results from the dose–response study confirmed that rAAV vector pseudotyped with serotype 8 capsid was the best performing vector. Surprisingly, however, at the highest dose, AAV serotypes 1 and 8 were almost equally effective in yielding long-term correction of blood Phe levels in both male and female Pahenu2 mice.
Tissue distribution of rAAV vector DNA
We anticipated that murine liver would be the principal target for transduction and PAH expression in our experiments because of the established general hierarchy of tissue-specific transduction efficiency of the various AAV serotypes (Wu et al., 2006) and because the CBA promoter directs robust gene expression in the liver. Furthermore, our previous studies and those of others have demonstrated that tail vein administration of recombinant AAV vector particles leads to preferential transduction of the liver (Davidoff et al., 2005; Ding et al., 2006a). To confirm that hindlimb muscle injection of our vectors led to vector accumulation in the liver in addition to muscle deposition, and to further examine the influence of factors such as dose, gender, and serotype on vector biodistribution, AAV2-PKU5 vector genomes were quantified by qPCR analysis from identical amounts of genomic DNA extracted from liver, hindleg and foreleg muscles, diaphragm, heart, kidney, spleen, brain, and lung (see Table 1). In animals that had received the highest vector dose (> 1.2 × 1012 VG), AAV2-PKU5 vector DNA was detected in all tissues examined, but vector genome copies per cell (VG per cell) varied significantly between the samples. For all three AAV serotypes and in both male and female mice, most vector genomes were detected either in hindleg muscle tissue (the site of injection) or in liver. Muscle tissue from hindlegs of AAV2 serotype 1-injected mice contained the highest vector genome copy numbers per cell followed by the serotype 8- and 2-treated groups, as expected from several reports of preferential transduction of muscle tissue by rAAV2/1 vectors (Xiao et al., 1999; Chao et al., 2000; Arruda et al., 2004; Louboutin et al., 2005). Between weeks 28 and 56 postinjection of rAAV2/8 vector, the number of AAV2-PKU5 genomes measured in liver decreased roughly 8-fold whereas the number of genomes measured in the injected muscle decreased only by half. This difference may reflect the hepatocyte turnover rate and accelerated episomal vector clearance in liver in comparison with muscle. Administration of the highest dose (> 1.2 × 1012) of AAV vectors to female mice also led to efficient liver transduction, which was, however, 2- to 7-fold lower than the values found in the male cohort. The gender difference in vector genome copy numbers in liver was found to be highly significant for the AAV2 serotype 8-treated group (p = 0.0013 by Student t test). Such sex-dependent differences in transduction of murine liver by AAV has also been reported by other groups (Davidoff et al., 2003) (see also Discussion). A gender difference for muscle (hindleg) transduction with vectors serotype 1 and 8 was also observed, but this difference was much less pronounced than for liver. The gender difference in vector genome copy numbers per cell for all three AAV serotypes is also reflected in the difference for long-term Phe clearance as shown in Fig. 1. In contrast to liver, the difference in hindleg muscle transduction for both AAV2 serotype 1 and 8 vectors seemed to be slightly less pronounced between females and males, with values in females decreasing by only approximately one-third to one-fourth compared with copy number in males. Similar gender-related differences were noted at lower vector doses (Table 1). The biodistribution of these vectors in all other tissues, that is, foreleg muscle, diaphragm, heart, kidney, spleen, brain, and lung, was also influenced by the AAV capsid proteins. The overall results suggested a gender-related difference in vector distribution especially observable for the serotype 8 vector.
Table 1.
Vector Genome Copy Numbers per Cell in Selected Tissues
| AAV vector (dose; time after injection; sex) | Liver | Hindleg muscle | Foreleg muscle | Diaphragm | Heart | Kidney | Spleen | Brain | Lung |
|---|---|---|---|---|---|---|---|---|---|
| AAV2/1-PKU5 (serotype 1) | |||||||||
| 1.2 × 1012; 54 weeks; M (n = 4) | 279 ± 18 | 341 ± 18 | 0.4 ± 0.04 | 6 ± 0.2 | 20 ± 1 | 5 ± 0.3 | 3 ± 1 | 1 ± 0.2 | 3 ± 0.2 |
| 1.2 × 1012; 56 weeks; F (n = 1)a | 84 ± 6 | 232 ± 13 | <0.1 | 1 ± 0.02 | 7 ± 0.1 | 0.3 ± 0.01 | 0.4 ± 0.03 | 2 ± 0.3 | 0.2 ± 0.01 |
| 1.2 × 1011; 53 weeks; M (n = 3) | 18 ± 0.1 | 75 ± 17 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| 1.2 × 1011; 53 weeks; F (n = 2) | 10 ± 2 | 57 ± 6 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| AAV2/2-PKU5 (serotype 2) | |||||||||
| 2.4 × 1012; 55 weeks; M (n = 2) | 96 ± 10 | 57 ± 9 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| 2.4 × 1012; 44 weeks; F (n = 2) | 44 ± 10 | 49 ± 8 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| 2.4 × 1011; 38 weeks; M (n = 3) | 2 ± 0.5 | 4 ± 1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| AAV2/8-PKU5 (serotype 8) | |||||||||
| 1.2 × 1012; 28 weeks; M (n = 2) | 823 ± 80 | 184 ± 18 | 0.8 ± 0.06 | 1 ± 0.4 | 2 ± 0.05 | 0.2 ± 0.01 | <0.1 | <0.1 | 0.3 ± 0.10 |
| 1.2 × 1012; 55 weeks; M (n = 3) | 190 ± 16 | 109 ± 29 | 0.1 ± 0.5 | 0.4 ± 0.07 | 0.6 ± 0.07 | <0.1 | <0.1 | 0.6 ± 0.03 | 0.3 ± 0.14 |
| 1.2 × 1012; 56 weeks; F (n = 3) | 109 ± 6 | 79 ± 7 | 8 ± 0.3 | 1 ± 0.1 | 2 ± 0.1 | 1 ± 0.1 | 0.4 ± 0.08 | 0.2 ± 0.01 | 0.2 ± 0.01 |
| 1.2 × 1011; 34 weeks; M (n = 3) | 33 ± 2 | 9 ± 1 | 0.1 ± 0.01 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 0.2 ± 0.004 |
| 1.2 × 1010; 34 weeks; M (n = 3) | 3 ± 1 | 2 ± 1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
Abbreviations: AAV, adeno-associated virus; F, female; M, male; PCR, polymerase chain reaction; PKU, phenylketonuria; WPRE, woodchuck posttranscriptional regulatory element.
Persistence of viral genomes at various time points after intramuscular injection of AAV2-PKU5 serotypes 1, 2, and 8. Every tissue type was measured in triplicate. Genomic DNA was isolated from the indicated tissues and 100 ng of each was used as template for quantitative PCR analysis to determine vector copy numbers. Primers and probe for the WPRE sequence were designed with Primer Express (Applied Biosystems). Dilutions of the AAV2-PKU5 vector plasmids were used to generate standard curves. M, male; F, female; note that values less than 0.1 indicate numbers of 1 virally infected cell among 10 or more cells.
A total of three mice was injected with vector AAV2/1-PKU5, and from these animals, one was kept for tissue distribution analysis while the other two were used for a second injection with vector AAV2/8-PKU5.
Tissue PAH activities
In general, the transduction profile found in liver or muscle was also reflected in the tissue PAH activity (Table 2). Most notably, the results indicated that liver PAH activities ranging from 0.25 to 0.58 mU/mg, equivalent to 10–23% of wild-type liver PAH activity, were sufficient to maintain blood Phe levels well below the curative threshold. Furthermore, for some mice, values of 0.10 mU/mg, equivalent to 4% wild-type liver PAH activity, were associated with Phe concentrations between 742 and 839 μmol/liter, which are considered to be characteristic levels of mild human hyperphenylalaninemia. Unfortunately, the relationship between wild-type protein levels and liver PAH activity could not be assessed through immunoblotting because of indiscriminate detection of both wild-type and mutant PAH proteins in livers of Pahenu2 mice (Ding et al., 2004) and insufficient amounts of vector-mediated PAH expression to enable relative quantitation of expression levels between treated and untreated Pahenu2 mice (data not shown). In contrast, the results from Western blot analysis of vector-injected muscles demonstrated a direct correlation between vector genome copy number and amount of detectable PAH protein (data not shown).
Table 2.
Phenylalanine Hydroxylase Activities in Tissues of Liver and Muscle, and Liver Tetrahydrosiopterin Content of AAV2-PKU5-Injected Hindleg Muscle of PKU Mice in Comparison with Controlsa
| Sex of (PKU) mouse and AAV treatment | Time after gene transfer (weeks) | Blood phenylalanineb(μmo/liter) | Liver PAH activity (mU/mg) | Liver BH4 content (pmol/mg) | Muscle PAH activityc(mU/mg) |
|---|---|---|---|---|---|
| Controls | |||||
| Male BL/6 wild-type untreated (n = 4) | >12 (adult) | <120 | 2.51 ± 0.32 | 26.64 ± 2.63d | <0.02 |
| Male or female PKU untreatede (BL/6-Pahenu2) | >12 (adult) | 1695 ± 385 (males)d | <0.02d | 43.77 ± 3.38d | <0.02d |
| 2175 ± 194 (females) | |||||
| AAV2/1-PKU5 (serotype 1) | |||||
| Male PKU (n = 4) (untreated foreleg) | 54 | 129 ± 49 | 0.35 ± 0.09 | 23.89 ± 0.97 | 0.58 ± 0.15 (0.03 ± 0.01) |
| Female PKUe (n = 1) (untreated foreleg) | 56 | 752 ± 10 | 0.11 ± 0.01 | 18.42 ± 3.93 | 0.29 ± 0.04 (0.05 ± 0.01) |
| AAV2/2-PKU5 (serotype 2) | |||||
| Male PKUe (n = 2) (untreated foreleg) | 55 | 769 ± 56 | 0.10 ± 0.09 | 21.56 ± 1.87 | 0.06 ± 0.01 (ND) |
| Female PKUe (n = 2) (untreated foreleg) | 44 | 1350 ± 120 | 0.09 ± 0.03 | ND | 0.05 ± 0.01 (ND) |
| AAV2/8-PKU5 (serotype 8) | |||||
| Male PKU (n = 2) (untreated foreleg) | 28 | 103 ± 12 | 0.58 ± 0.22 | 17.77 ± 1.21 | 0.19 ± 0.04 (0.03 ± 0.01) |
| Male PKU (n = 2) (untreated foreleg) | 55 | 210 ± 30 | 0.25 ± 0.09 | 16.48 ± 0.32 | 0.17 ± 0.04 (0.08 ± 0.01) |
| Male PKUe (n = 1) (untreated foreleg) | 55 | 839 ± 56 | 0.10 ± 0.01 | 25.36 ± 1.63 | 0.11 ± 0.03 (0.03 ± 0.01) |
| Female PKUe (n = 1) (untreated foreleg) | 56 | 747 ± 87 | 0.11 ± 0.02 | 24.69 ± 1.50 | 0.36 ± 0.06 (0.05 ± 0.02) |
Abbreviations: BH4, tetrahydrobiopterin; ND, not determined; PAH, pherylalanine hydroxylase.
All measurements done in triplicate.
Last blood phenylalanine values shortly before euthanasia of treated PKU mice.
AAV2-PKU5-injected hindleg muscle; values in the untreated foreleg muscle are given in parentheses.
Numbers were determined in a previous report (see Table 1 in Ding et al., Mol. Ther. 16:673–681, 2008).
Mice with hyperphenylalaninemia.
Administration of rAAV2/8 after prior injection with rAAV2/1 vector
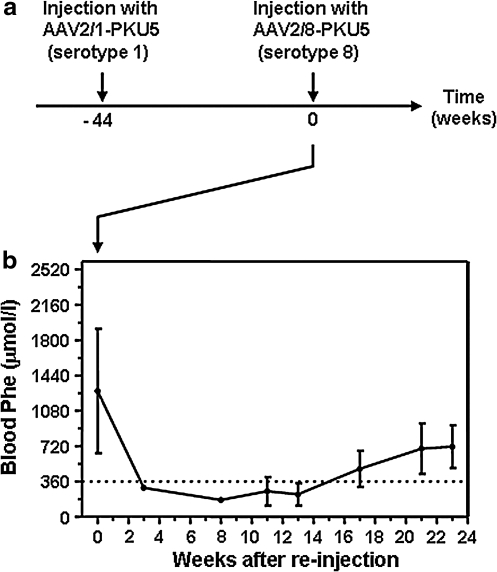
Several studies have reported strategies to increase or sustain transgene expression levels through serial injection of rAAV vectors with alternative capsid serotypes to avoid immunity against previously administered viral vectors (Halbert et al., 2000; Riviere et al., 2006; Nathwani et al., 2009). Because rAAV-treated Pahenu2 females were more reluctant to achieve long-term therapeutic correction than males, we sought to examine whether it might be possible to further extend the therapeutic duration by administering an alternative serotype vector. For this purpose, two Pahenu2 females, which had been previously exposed to 1.2 × 1012 AAV2/1-PKU5 vector genomes and with blood Phe values rising to pretreatment levels by week 44 (Fig. 1a), were reinjected intramuscularly with an equivalent dose of serotype 8 vector (1.2 × 1012 VG of AAV2/8-PKU5 per mouse; see Fig. 2). Blood Phe concentrations subsequently decreased from 1260 ± 631 μmol/liter to therapeutic levels (<360 μmol/liter) by 3 weeks after secondary injection. Blood Phe remained at therapeutic levels up to 13 weeks after the second injection but increased thereafter to reach values of mild hyperphenylalaninemia (713.2 ± 216 μmol/liter). It should be noted, however, that in comparison with females only injected once with vector AAV2/8-PKU5 and in which therapeutic Phe levels were maintained up to 35 weeks (Fig. 1c), the therapeutic effect of AAV2/8-PKU5 treatment was less persistent in animals receiving secondary injections. This observation suggests that the transduction efficiency of secondary gene transfer is likely to be influenced or affected by the delivery of the first vector or by the age at which the animals were treated the second time. Nonetheless, these results demonstrate a successful method to prolong therapeutic correction in female mice to a similar duration as was achieved in male mice.
FIG. 2.
Time course of blood Phe levels in PKU female mice after readministrations of AAV2-PKU5 vectors pseudotyped with alternative serotypes 1 and 8. (a) Time diagram for the intramuscular readministration experiment using alternative serotypes. At time point −44 weeks, 1.2 × 1012 particles of vector AAV2/1-PKU5 (serotype 1) was injected into both hindleg muscles of PKU females (n = 3). Forty-four weeks later, indicated as time 0 in the graph, two females were subjected to a second injection with 1.2 × 1012 viral particles of vector AAV2/8-PKU5 (serotype 8). (b) Blood Phe values of PKU female mice after reinjection. At each time point the result is represented as the mean ± SD. The dotted line indicates the threshold for Phe concentration at the therapeutic level (9360 μmol/liter).
Effect of BH4 supplementation on blood phenylalanine in rAAV-treated female mice
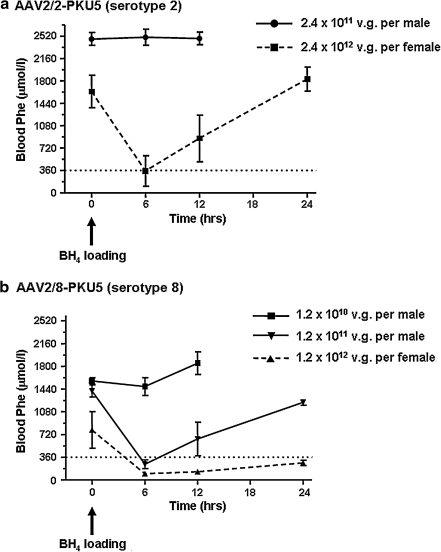
We have previously reported that the BH4 cofactor stimulates and protects the activity of normal murine PAH in liver (Thöny et al., 2004; Scavelli, 2006; Pey et al., 2008). Moreover, expression of PAH in skeletal muscle in the presence of adequate BH4, or coexpression of PAH along with the essential BH4 biosynthetic genes, can effectively clear Phe from the circulation of PKU mice (Ding et al., 2008). We reasoned that as liver PAH activity was lost in rAAV-treated female mice, Phe clearance could be additionally limited by relative BH4 cofactor deficiency. To test this hypothesis, we supplemented BH4, using a single dose of synthetic cofactor, by intraperitoneal injection of 16 mg of BH4 per kilogram body weight, an amount that is comparable to the recommended oral loading tests in human patients, that is, 20 mg of BH4 per kilogram of body weight. Supplementation of BH4 was done for both male and female mice 38 weeks after administration of rAAV2/2-PKU5 vector (2.4 × 1011 VG/male mouse and 2.4 × 1012 VG/female mouse). Blood Phe was measured before and 6, 12, and 24 hr after BH4 injection. As shown in Fig. 3a, blood Phe in rAAV-treated males was not affected by BH4 supplementation. However, in female rAAV2/2-PKU5-treated mice, blood Phe concentrations decreased transiently in response to BH4 injection. Successful Phe clearance after BH4 treatment was also observed for mice previously injected with the highest rAAV2/8-PKU5 dose (Fig. 3b). To further assess whether skeletal muscle tissue, which lacks endogenous cofactor production, could participate in Phe clearance, BH4 responsiveness was compared between male mice injected with a low dose of rAAV2/1-PKU5 (1.2 × 1011 VG/mouse; at week 53) and rAAV2/8-PKU5-treated females from the high-dose cohort group (1.2 × 1012 VG/mouse; at week 56; see Table 1). Retrospectively, the number of vector genomes measured in DNA recovered from hindleg muscle was similar in these two groups (75 ± 17 VG/cell in males compared with 79 ± 7 VG/cell in females), but the number of vector genomes in liver was at least 6-fold lower in the rAAV2/1-PKU5-treated group than in the rAAV2/8-PKU5-treated mice (18 ± 0.1 VG/cell for rAAV2/1-PKU5 vs. 109 ± 6 VG/cell for rAAV2/8-PKU5). In contrast to the rAAV2/8-PKU5-treated mice, loading with BH4 either by a single intraperitoneal injection or by repeated oral administration for 5 days (32 mg of BH4 per kilogram body weight per day) did not result in a decrease in blood Phe levels in rAAV2/1-PKU5 mice (data not shown). These results indirectly show that in our setting only liver, not muscle, PAH activity profits from BH4 cofactor treatment.
FIG. 3.
Effect of BH4 supplementation in AAV2/2-PKU5- and AAV2/8-PKU5-treated PKU mice. PKU mice that underwent AAV vector administration were given a single intraperitoneal injection of BH4 either when their blood Phe levels started to rise again and nearly reached pretreatment levels, or when they had only a marginal decrease in Phe levels but no therapeutic correction. Mean Phe levels ± SD before BH4 administration versus 6, 12, and 24 hr posttreatment are plotted (dotted line represents the Phe level of 9360 μmol/liter). (a) Mean blood values for Phe values versus time in AAV2/2-PKU5 (serotype 2)-treated PKU males (circles, n = 3) and female mice (squares, n = 2) after a single intraperitoneal injection of BH4. BH4 injections were performed 38 weeks after gene transfer in males and females previously injected with 2.4 × 1011 and 2.4 × 1012 particles of vector AAV2/2-PKU5 (serotype 2), respectively. (b) Same as in (a), but mice were pretreated with vector AAV2/8-PKU5 (serotype 8): squares, males (n = 3) 28 weeks after gene transfer of 1.2 × 1010 VG; inverted triangles, males (n = 3) 34 weeks after gene transfer of 1.2 × 1011 VG; triangles, females (n = 2) 56 weeks after gene transfer of 1.2 × 1012 VG.
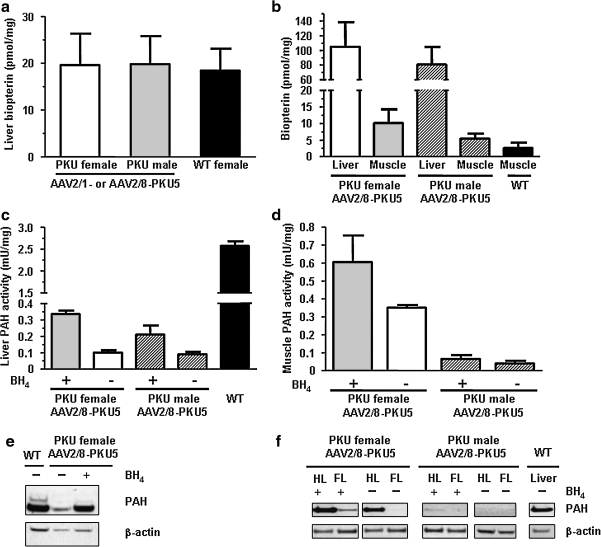
We subsequently addressed further the possibility that BH4 content in the livers of treated animals could be a limiting factor for Phe clearance; we therefore investigated directly the effect of exogenously administered cofactor on residual PAH activity. As shown in Fig. 4a, no significant difference in liver BH4 levels before BH4 supplementation was found between successfully corrected rAAV-injected males, partially corrected rAAV-injected females, or wild-type females (p > 0.05 by one-way analysis of variance [ANOVA] followed by Bonferroni's post hoc test). To confirm that BH4 was effectively taken up into liver and muscle after intraperitoneal injection, we measured BH4 content in tissues of rAAV-treated animals (Fig. 4b). Twenty-four hours after BH4 administration by intraperitoneal injection, BH4 content had increased more than 5-fold in liver over pretreatment levels in females injected with rAAV2/8-PKU5 vectors (Fig. 4a and b) and was almost 4-fold higher in muscle of BH4-treated mice in comparison with wild-type mice that had not received BH4 (Fig. 4b). Similar results were seen in male mice that had been treated with rAAV2/8-PKU5 (Fig. 4b). BH4 supplementation in both female and male rAAV-treated mice was associated with a 3.3- and 2.3-fold increase, respectively, in measured liver PAH activity compared with untreated Pahenu2 animals. The increased PAH activity was apparently sufficient to allow therapeutic Phe clearance in these mice (see Figs. 3b and 4c). A 1.7-fold increase in PAH activity on BH4 treatment was also found in hindleg muscle from both genders (Fig. 4d). To further assess the direct effect of BH4 supplementation on PAH, immunoblotting and densitometric quantification of PAH protein were performed. In liver of rAAV2/8-PKU5 treated mice, we observed an ∼2-fold increase in PAH protein levels after BH4 loading (Fig. 4e). In contrast to rAAV-transduced liver, where BH4 might have stabilized both endogenous mutant PAH monomers and wild-type PAH protein expressed from the vector transgene, muscle homogenate containing only transgenic wild-type PAH protein was used to assess the direct relationship between PAH protein levels with and without BH4 supplementation. As shown in Fig. 4f, slightly higher PAH protein levels were detected in both hindleg and foreleg muscle homogenates on BH4 treatment compared with untreated females, that is, a 1.3-fold increase by densitometric analysis for the hindleg and a 20-fold increase for foreleg muscles. In rAAV2/8-PKU5-treated males, which received a 10-fold lower virus dose compared with the female group, PAH protein levels were at or slightly below the detection limit of our antibody, as we detected PAH protein only in hindleg muscle of animals supplemented with BH4.
FIG. 4.
Effect of BH4 supplementation on PAH in AAV2-PKU5-treated mice. BH4 content and PAH activity are indicated as means ± SD. Note that all AAV2/8-PKU5-treated female PKU mice were at week 56 after gene transfer of 1.2 × 1012 VG injected, and all AAV2/8-PKU5-treated male PKU mice were at week 34 after gene transfer of 1.2 × 1011 VG injected. (a) Total hepatic BH4 content was analyzed in two groups of AAV2-PKU5-treated PKU male (n = 7) and female (n = 7) mice, and compared with levels of normal wild-type females (WT; n = 5). For both groups of virus-treated PKU mice, the liver BH4 content of four AAV2/1-PKU5-treated animals and three AAV2/8-PKU5-treated animals was analyzed. (b) Total BH4 content in liver and skeletal muscle of females (n = 2) and males (n = 2), both treated with rAAV2/8-PKU5 vectors, 24 hr after intraperitoneal injection of synthetic cofactor (1 μmol/g body weight in 1% ascorbic acid). The black column indicates the BH4 content in muscle extracts of normal mice as control (not injected with synthetic BH4; n = 3). (c and d) PAH enzyme activities in liver and muscle extracts, respectively, in the same animals as shown in (b), that is, 24 hr after BH4 treated (n = 2) compared with non-BH4-treated (n = 1) AAV2/8-PKU5-injected mice. The black column in (c) indicates PAH activity of normal mouse liver extracts (WT; n = 3). Note that muscle PAH activity between the sexes is different in (d), as males received a 10-fold lower dose of AAV vectors than females. (e and f) Western blot analyses of liver and muscle extracts, respectively, from the same BH4-treated and untreated mice. In (e) equal amounts of liver homogenate (20 μg) from a wild-type mouse (WT) compared with AAV2/8-PKU5-treated PKU female mice with (+) or without BH4 treatment (−) were probed with rabbit anti-mPAH antibody (diluted 1:10,000). In (f) each lane contains 30 μg of homogenized hindleg (HL) or foreleg (FL) muscle homogenate from animals treated with AAV2/8-PKU5 vectors after treatment with BH4 (+) or no treatment (−). Thirty micrograms of liver homogenate of a wild-type mouse is shown as a control (WT). Bottom: Representative loading control using an anti-β-actin antibody, diluted 1:10,000 in (e) and 1:1000 in (f).
Taken together, these results suggest that the liver PAH activity in treated Pahenu2 animals, which had not led to efficient removal of circulating Phe, could be increased by exogenous BH4 supplementation. In contrast, heterologous expression of PAH in muscle tissues with or without BH4 administration did not result in blood Phe clearance. Because in the BH4-treated mice a 10- to 15-fold lower amount of BH4 was found in muscle tissues compared with liver, the rate of BH4 uptake in muscle tissues could have been the limiting factor in the clearance of circulating Phe. The slow rate of BH4 uptake in muscle in comparison with liver has been reported previously (Harding et al., 1998, 2004). Alternatively, the level of PAH protein expression in muscle of rAAV-treated mice could have been insufficient for effective blood Phe clearance even after supplementation with BH4.
Discussion
Recombinant AAV gene delivery vectors, owing to the variety of applications and tissue types toward which these vectors may be targeted, hold great promise for the treatment of a multitude of genetic disorders and acquired human disease (Alexander et al., 2008). Several studies have shown that administration of rAAV vectors to a variety of animal models including mice, dogs, and nonhuman primates can result in long-term gene expression and disease correction with minimal toxicity (Kootstra and Verma, 2003). These efforts have culminated in various clinical phase I–II studies with AAV2 serotype 2 (Edelstein et al., 2007). Regardless of the success with these approaches, gene therapy correction in those models is currently limited to a period of 1 year or longer because of progressive decline in transgene expression over time, indicating that vector readministration may be necessary at intervals of several years. Furthermore, sex-related differences in biodistribution, differences in transduction efficiency, the lack of identified cell receptors for many rAAV serotypes, timing of expression, and levels of transgene expression observed in many preclinical experiments with AAV vectors are other unsolved issues that may critically impact the outcome of a gene transfer approach (Davidoff et al., 2003; Berraondo et al., 2006; Ogura et al., 2006; Wu et al., 2006; Buning et al., 2008; Ho et al., 2008). Thus, the development and investigation of multiple or combined treatment strategies may offer alternative options to further extend the therapeutic effect required and address the issue of gender-dependent difference in transgene expression.
We have reported two different approaches to achieve long-term correction of hyperphenylalaninemia in a PKU mouse model. In the first approach, liver-directed gene transfer with the recombinant AAV2-PKU5 pseudotype 8 vector was explored, expressing the PAH transgene from the CBA promoter (Ding et al., 2006a). Single doses of up to 1013 viral particle vectors administered by intraportal or tail vein injection result in therapeutic correction lasting for up to 1 year for males and females. In a second approach, single doses of approximately 1012 viral particles of a triple-cistronic AAV pseudotype 1 vector encoding murine PAH along with two BH4-biosynthetic enzymes expressed from a CMV promoter were employed for muscle-directed gene transfer; this approach was associated with therapeutic blood Phe clearance for up to 70 weeks in our mouse model (Ding et al., 2008). In both approaches, the therapeutic effect in some mice persisted for 1 year or longer, whereas other animals, in particular females, exhibited rising blood Phe levels at earlier time points. Results from the biodistribution analysis in the muscular approach study also indicated that some dissemination of vectors occurred to other tissues with preferential accumulation in the liver, an observation that has been reported by others (Manno et al., 2003; Asokan et al., 2008). On the basis of this last observation and our previous studies, we believe that long-term correction of hyperphenylalaninemia may further be strengthened by a concomitant muscle- and liver-targeted gene transfer approach via intramuscular injection. In the present study, we evaluated the feasibility to effect long-term therapeutic correction of PKU by intramuscular injection-mediated liver transduction with various alternative rAAV serotypes as a preliminary assessment toward multiple combined approaches. The choice of AAV serotypes and the intramuscular administration route were selected for these experiments because (1) liver as well as skeletal muscle have been successfully transduced with rAAV pseudotype 8 in various animal models (Sarkar et al., 2004; Wang et al., 2005; Inagaki et al., 2006), and (2) rAAV2/8-mediated liver transduction is now being considered for treatment of patients with hemophilia B (Arruda et al., 2004). Similarly, rAAV pseudotype 1 is reportedly the most efficient vector for muscle transduction in mice (Hauck and Xiao, 2003; Louboutin et al., 2005). rAAV serotype 2 was also included in this study, being not only the most well-characterized AAV serotype with modest tropism for liver and muscle, but also having been used in clinical phase I–II trials (Rabinowitz et al., 2002). To ensure that differences between the vectors used here were limited to the three different capsids, the rAAV2-PKU5 pseudotype 1, 2, and 8 vectors were purified and concentrated in parallel by an identical CsCl-density ultracentrifugation-based procedure. We found that all three vector serotypes were able to direct efficient clearance of serum Phe for at least 10 weeks for males and females after intramuscular injection of doses of 1.2 × 1012 for pseudotypes 1 and 8, or a dose of 2.4 × 1012 for serotype 2. On the basis of vector biodistribution and PAH activities determined in diverse tissues (see Tables 1 and 2), significant amounts of viral vector genome were found only in liver and hindleg muscles. The pseudotype 8 vector was detected primarily in liver and pseudotype 1 in hindleg muscle, and the relatively larger drop over time of vector copy number in liver compared with hindleg muscle may indicate a higher turnover rate in liver and more stable maintenance of vector in muscle tissue (Table 1). Furthermore, loss of episomal viral genomes is thus a highly probable explanation for the decrease in Phe clearance observed over time in our experiments. Immunologic rejection of transduced hepatocytes or myocytes has not been formally ruled out, but we have never detected anti-PAH antibodies in the blood of rAAV-treated mice and the histologic examination of transduced tissues was normal without any evidence of lymphocyte infiltration (Ding et al., 2006a, 2008). Interestingly, the results from the high dose–response experiments showed that rAAV2-PKU5 with pseudotypes 1 and 8 were almost equivalent in mediating long-term Phe clearance with persistently low blood Phe levels for up to 1 year in males, whereas females were corrected for at least 30 to 35 weeks after gene transfer.
The interaction between host and vector in the context of gene therapy is complex and seems to vary considerably according to vector type, serotype, dose, volume of injection, and route of administration, as well as the species being studied. This complexity also comes into play when trying to interpret our finding. Evaluation of vector biodistribution in this study did not provide direct evidence of rAAV vectors in the blood, but results from preclinical and clinical reports evaluating rAAV2/1 and rAAV2/8, as well as intramuscular delivery protocols, suggest that leakage of vector from the injection sites is likely to occur via the circulation. Kinetic studies, for instance, have shown that the number of infectious particles in the blood of mice or larger animals injected with rAAV was maximal at 6 hr and accounted for 5% of the total dose in one study and a 7-log less concentration of the total injected dose in another study (Flotte et al., 2007; Toromanoff et al., 2008). The appearance of vector DNA in blood was followed by rapid clearance to low or undetectable levels within 7 to 14 days after the injection and similar outcome has also been observed in two clinical trials (Brantly et al., 2009; Mingozzi et al., 2009). These observations, along with the extensive vascularization of skeletal muscle, would favor AAV dissemination via the systemic circulation to other organs such as the liver. However, the presence of viral vector in draining and distant lymph nodes of animals injected intramuscularly suggests that the lymphatic system may participate in the dissemination of viral vector as well (Rip et al., 2005).
Notwithstanding that the selection of an appropriate capsid serotype is of pertinent importance to deliver AAV vectors to specific target tissues, the intrinsic interactions between various serotypes and muscle tissues could also have affected the amount of vector that went into the blood and then transduced the liver. It is likely that any serotypes that would be efficiently internalized by muscle myofibers could lead to less transduction of remote sites owing to the low number of infectious particles gaining access to the blood. This might explain why the injection of a low dose of serotype 1, which is known to transduce muscle well, was not as efficient as serotype 8 in transducing hepatocytes. Data also suggest that rAAV2/1 can be viewed as a serotype with low intramuscular diffusion abilities, leading to high transgene expression at the intramuscular injection site versus low expression in the surrounding area (Toromanoff et al., 2008). Thus, with high-dose vector injection it might be possible that local saturation of serotype 1 vector internalization may have resulted in a higher concentration of viral particles circulating in the blood and thus better liver transduction. On the other hand, a differential rate of dissemination might also be explained by the relative amount of cellular receptor and its affinity for a particular serotype. Strong binding to heparan sulfate proteoglycans, which are abundantly expressed on the surface of muscle cells, might decrease the dissemination of AAV2/2 vector from the site of administration (Asokan et al., 2008). In addition, it has also been hypothesized that some specific serotypes, such as AAV1, in certain tissues such as skeletal muscle may be trapped as intact infectious particle in “protective” locations such as the transverse tubules and could then be slowly released over time into the systemic compartment (Toromanoff et al., 2008).
AAV vectors were also delivered intramuscularly with a total injection volume of 50 μl administered to each hindlimb. This injection volume in a 30-g mouse would be equivalent to injecting 130 ml into the muscle of an 80-kg human. Certainly, the injection volume and the resulting hydrostatic pressure in the injected muscle body could influence the amount of vector that gains access to the vasculature and is transported to the liver. We have not investigated this variable in detail. Reportedly, intramuscular injection of human α1-antitrypsin (AAT)-expressing rAAV2/8 vector in a 0.4-ml volume into mice yielded 9-fold higher serum hAAT levels than in mice receiving the same vector dose but concentrated in 0.01 ml (Ye et al., 2009). In human clinical trials with intramuscular administration of AAV vectors, total doses of 10–20 ml divided into 0.3- to 0.5-ml injections were delivered to multiple injection sites. Administration of 20 ml to an 80-kg person would be equivalent to injecting only 8 μl into a 30-g mouse. Despite the relatively low injection volume used, AAV vector administered to muscle has been detected in the blood of human subjects treated in clinical trials (Manno et al., 2003).
We witnessed a time-dependent loss of therapeutic effect after rAAV injection in some animals, particularly in female mice. BH4 supplementation by intraperitoneal injection transiently improved Phe clearance but only in those mice that had retained some minimal amount of liver PAH activity. The molecular mechanisms that mediate BH4 responsiveness are not yet completely elucidated, but several in vitro and in vivo studies support the hypothesis that BH4 plays a role in the protection of PAH protein against degradation (Thöny et al., 2004; Scavelli et al., 2005). The therapeutic effect of rAAV treatment was much longer lasting in male mice than in female mice with all vector pseudotypes and doses. The reasons for this difference have not been completely elucidated. Second injection of rAAV vector with alternative capsid pseudotypes into previously treated female mice did lead to transient restoration of blood Phe to therapeutic levels. However, the duration of this effect was shorter in animals receiving secondary injections than in naive mice injected for the first time. The cause for this discrepancy is yet unknown. Possible explanations include an immunologic response arising after the first injection that then cross-reacts with the second vector injected. Other investigators have also shown that initial treatment with AAV serotype 2 vectors led to a 20-fold impairment of subsequent rAAV pseudotype 1-mediated transduction (Xiao et al., 1999). The extent of this impairment seems dependent on the species treated, the route of vector administration, and the specific vector pseudotype. We cannot exclude the possibility that the age of the mice at the time of the secondary injections may have influenced the effectiveness of the treatment. Our experiment confirmed the previously reported observation that transduction frequency after rAAV administration is gender dependent, especially for rAAV2/8-mediated liver transduction, but we did not detect a gender difference in hepatic BH4 cofactor content, a subject of previous conjecture (Chen et al., 2007).
The animal model used here for PKU obviously has species limitations related to assessing function, immune response, and distribution of PAH. Because a primate model for the disease is not available, at this point it is difficult to design preclinical studies that accurately predict clinical outcomes. Moreover, animals such as mice cannot provide data directly applicable to human subjects, especially on safety and immunologic profiles. Another critical issue in translation to human patients involves vector delivery across the vascular barrier. Although this has been achieved in the PKU mouse by using AAV in this and our previous works (Ding et al., 2006a, 2008), successfully translating rodent studies to patients remains a challenge. Furthermore, dosing issues are also paramount in scaling gene delivery from small animal to human patients. In the absence of a large animal model for PKU, preclinical studies will surely necessitate the use of therapeutic transgenic surrogate genes such as luciferase, green fluorescent protein (GFP), or a more relevant marker such as, for example, a PAH-FLAG-tagged protein.
In summary, intramuscular injection of rAAV vectors, the relatively noninvasive gene transfer strategy used in this study, is as effective as our previous intravenous vector administration (Ding et al., 2006) in producing therapeutically relevant levels of liver PAH expression and successful correction of Phe clearance in an animal model of human PKU. However, and in the light of the dose-dependent immune toxicity and rAAV-related liver genotoxicity (Manno et al., 2006; Zaiss and Muruve, 2008), it is perhaps critical to emphasize that in this study a 17-fold lower dose of rAAV2/8-PKU5 vector was used than in our previous report on liver-directed gene transfer via the portal vein and tail vein. BH4 supplementation or secondary injection of an rAAV vector with an alternative capsid pseudotype can help sustain liver PAH activity as PAH expression wanes over time. Perhaps the future development of rAAV vectors that mediate site-specific integration of the therapeutic transgene into the host genome will lead to sustained liver PAH expression. Gene therapy using the phenylalanine ammonia lyase (PAL) enzyme expressed from a viral or nonviral system might be an alternative treatment approach. Results from preclinical and clinical phase I studies using PAL as an enzyme substitution therapy are encouraging and, further, PAL does not require any exogenous cofactors (Sarkissian and Gamez, 2005; Sarkissian et al., 2008, 2009; Kang et al., 2010). All these studies have the goal to develop a safe and efficient gene transfer method for the long-term treatment of human PKU.
Acknowledgments
The authors thank N. Blau for helpful discussions, the Vector Core of the Department of Medicine of the University of Pennsylvania for the AAV2/8 plasmid, H. Büeler for providing material and advice to generate AAV2/1 particles, J.A. Kleinschmidt for plasmid pDG, the Institute for Animal Science at the University of Zürich-Irchel for cooperation, H. Troxler and the mass spectrometry unit for phenylalanine determinations from dried blood spots, and F.H. Sennhauser for support. This work was funded by grants from the Stiftung für wissenschaftliche Forschung an der Universität Zürich and the Novartis Stiftung für Medizinisch-Biologische Forschung, by U.S. National Institutes of Health grant DK-59371 (to C.O.H.), and by continuous support from the Swiss National Science Foundation (to B.T.).
Author Disclosure Statement
No competing financial interests exist.
References
- Alexander I.E. Cunningham S.C. Logan G.J. Christodoulou J. Potential of AAV vectors in the treatment of metabolic disease. Gene Ther. 2008;15:831–839. doi: 10.1038/gt.2008.64. [DOI] [PubMed] [Google Scholar]
- Antshel K.M. Waisbren S.E. Timing is everything: Executive functions in children exposed to elevated levels of phenylalanine. Neuropsychology. 2003;17:458–468. doi: 10.1037/0894-4105.17.3.458. [DOI] [PubMed] [Google Scholar]
- Arruda V.R. Schuettrumpf J. Herzog R.W. Nichols T.C. Robinson N. Lotfi Y. Mingozzi F. Xiao W. Couto L.B. High K.A. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood. 2004;103:85–92. doi: 10.1182/blood-2003-05-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokan A. Johnson J.S. Li C. Samulski R.J. Bioluminescent virion shells: New tools for quantitation of AAV vector dynamics in cells and live animals. Gene Ther. 2008;15:1618–1622. doi: 10.1038/gt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berraondo P. Crettaz J. Ochoa L. Paneda A. Prieto J. Troconiz I.F. Gonzalez-Aseguinolaza G. Intrahepatic injection of recombinant adeno-associated virus serotype 2 overcomes gender-related differences in liver transduction. Hum. Gene Ther. 2006;17:601–610. doi: 10.1089/hum.2006.17.601. [DOI] [PubMed] [Google Scholar]
- Blau N. Thöny B. Cotton R.G.H. Hyland K. Disorders of tetrahydrobiopterin and related biogenic amines. In: Scriver C.R., editor; Beaudet A.L., editor; Sly W.S., editor; Valle D., editor; Vogelstein B., editor. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2001. pp. 1725–1776. [Google Scholar]
- Brantly M.L. Chulay J.D. Wang L. Mueller C. Humphries M. Spencer L.T. Rouhani F. Conlon T.J. Calcedo R. Betts M.R. Spencer C. Byrne B.J. Wilson J.M. Flotte T.R. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buning H. Perabo L. Coutelle O. Quadt-Humme S. Hallek M. Recent developments in adeno-associated virus vector technology. J. Gene Med. 2008;10:717–733. doi: 10.1002/jgm.1205. [DOI] [PubMed] [Google Scholar]
- Chao H. Liu Y. Rabinowitz J. Li C. Samulski R.J. Walsh C.E. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- Chen L. Woo S.L. Correction in female PKU mice by repeated administration of mPAH cDNA using phiBT1 integration system. Mol. Ther. 2007;15:1789–1795. doi: 10.1038/sj.mt.6300257. [DOI] [PubMed] [Google Scholar]
- Chen L. Thung S.N. Woo S.L. Metabolic basis of sexual dimorphism in PKU mice after genome-targeted PAH gene therapy. Mol. Ther. 2007;15:1079–1085. doi: 10.1038/sj.mt.6300137. [DOI] [PubMed] [Google Scholar]
- Davidoff A.M. Ng C.Y. Zhou J. Spence Y. Nathwani A.C. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood. 2003;102:480–488. doi: 10.1182/blood-2002-09-2889. [DOI] [PubMed] [Google Scholar]
- Davidoff A.M. Gray J.T. Ng C.Y. Zhang Y. Zhou J. Spence Y. Bakar Y. Nathwani A.C. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol. Ther. 2005;11:875–888. doi: 10.1016/j.ymthe.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Ding Z. Harding C.O. Thöny B. State-of-the-art 2003 on PKU gene therapy. Mol. Genet. Metab. 2004;81:3–8. doi: 10.1016/j.ymgme.2003.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z. Georgiev P. Thöny B. Administration-route and gender-independent long-term therapeutic correction of phenylketonuria (PKU) in a mouse model by recombinant adeno-associated virus 8 pseudotyped vector-mediated gene transfer. Gene Ther. 2006a;13:587–593. doi: 10.1038/sj.gt.3302684. [DOI] [PubMed] [Google Scholar]
- Ding Z. Harding C.O. Rebuffat A. Elzaouk L. Wolff J.A. Thöny B. Therapeutic correction of PKU in a mouse model by ectopic expression of PAH and its BH4-cofactor genes in skeletal muscle by a recombinant triple-cistronic AAV2-based pseudotype 1 vector. Mol. Ther. 2006b;13(Suppl. 1):S155. [Google Scholar]
- Ding Z. Harding C.O. Rebuffat A. Elzaouk L. Wolff J.A. Thöny B. Correction of murine PKU following AAV-mediated intramuscular expression of a complete phenylalanine hydroxylating system. Mol. Ther. 2008;16:673–681. doi: 10.1038/mt.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlon J. Levy H. Scriver C.R. In: Hyperphenylalaninemia: Phenylalanine hydroxylase deficiencies. In The Metabolic and Molecular Bases of Inherited Diseases. Valle D., editor; Beaudet A., editor; Vogelstein B., editor; Kinzler K., editor; Antonorakis S., editor; Ballabio S., editor; Scriver C.R., editor; Child B., editor; Sly W.S., editor; emeritus , editor. McGraw-Hill; New York: 2008. [Google Scholar]
- Edelstein M.L. Abedi M.R. Wixon J. Gene therapy clinical trials worldwide to 2007: An update. J. Gene Med. 2007;9:833–842. doi: 10.1002/jgm.1100. [DOI] [PubMed] [Google Scholar]
- Flotte T.R. Conlon T.J. Poirier A. Campbell-Thompson M. Byrne B.J. Preclinical characterization of a recombinant adeno-associated virus type 1-pseudotyped vector demonstrates dose-dependent injection site inflammation and dissemination of vector genomes to distant sites. Hum. Gene Ther. 2007;18:245–256. doi: 10.1089/hum.2006.113. [DOI] [PubMed] [Google Scholar]
- Gao G.P. Alvira M.R. Wang L. Calcedo R. Johnston J. Wilson J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger J.C. Choi V.W. Samulski R.J. Production and characterization of adeno-associated viral vectors. Nat. Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- Grimm D. Kern A. Rittner K. Kleinschmidt J.A. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum. Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- Grimm D. Zhou S. Nakai H. Thomas C.E. Storm T.A. Fuess S. Matsushita T. Allen J. Surosky R. Lochrie M. Meuse L. McClelland A. Colosi P. Kay M.A. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood. 2003;102:2412–2419. doi: 10.1182/blood-2003-02-0495. [DOI] [PubMed] [Google Scholar]
- Guthrie R. The introduction of newborn screening for phenylketonuria: A personal history. Eur. J. Pediatr. 1996;155(Suppl. 1):S4–S5. doi: 10.1007/pl00014247. [DOI] [PubMed] [Google Scholar]
- Guttler F. Azen C. Guldberg P. Romstad A. Hanley W.B. Levy H.L. Matalon R. Rouse B.M. Trefz F. De La Cruz F. Koch R. Impact of the phenylalanine hydroxylase gene on maternal phenylketonuria outcome. Pediatrics. 2003;112:1530–1533. [PubMed] [Google Scholar]
- Halbert C.L. Rutledge E.A. Allen J.M. Russell D.W. Miller A.D. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J. Virol. 2000;74:1524–1532. doi: 10.1128/jvi.74.3.1524-1532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C.O. “Mommy, why can't I have a hamburger like the other kids?”. Gene Ther. 2000;7:1969–1970. doi: 10.1038/sj.gt.3301363. [DOI] [PubMed] [Google Scholar]
- Harding C.O. Wild K. Chang D. Messing A. Wolff J.A. Metabolic engineering as therapy for inborn errors of metabolism: Development of mice with phenylalanine hydroxylase expression in muscle. Gene Ther. 1998;5:677–683. doi: 10.1038/sj.gt.3300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C.O. Neff M. Wild K. Jones K. Elzaouk L. Thöny B. Milstien S. The fate of intravenously administered tetrahydrobiopterin and its implications for heterologous gene therapy of phenylketonuria. Mol. Genet. Metab. 2004;81:52–57. doi: 10.1016/j.ymgme.2003.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C.O. Ding Z. Thöny B. Gene and cell therapies for phenylketonuria (PKU) In: Blau N., editor. PKU and BH4: Advances in Phenylketonuria and Tetrahydrobiopterin. SPS Verlagsgesellschaft; Heilbronn, Germany: 2006a. [Google Scholar]
- Harding C.O. Gillingham M.B. Hamman K. Clark H. Goebel-Daghighi E. Bird A. Koeberl D.D. Complete correction of hyperphenylalaninemia following liver-directed, recombinant AAV2/8 vector-mediated gene therapy in murine phenylketonuria. Gene Ther. 2006b;13:457–462. doi: 10.1038/sj.gt.3302678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck B. Xiao W. Characterization of tissue tropism determinants of adeno-associated virus type 1. J. Virol. 2003;77:2768–2774. doi: 10.1128/JVI.77.4.2768-2774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K.J. Bass C.E. Kroemer A.H. Ma C. Terwilliger E. Karp S.J. Optimized adeno-associated virus 8 produces hepatocyte-specific Cre-mediated recombination without toxicity or affecting liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G412–G419. doi: 10.1152/ajpgi.00590.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K. Fuess S. Storm T.A. Gibson G.A. McTiernan C.F. Kay M.A. Nakai H. Robust systemic transduction with AAV9 vectors in mice: Efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang T.S. Wang L. Sarkissian C.N. Gamez A. Scriver C.R. Stevens R.C. Converting an injectable protein therapeutic into an oral form: Phenylalanine ammonia lyase for phenylketonuria. Mol. Genet. Metab. 2010;99:4–9. doi: 10.1016/j.ymgme.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R.L. Dayton R.D. Tatom J.B. Henderson K.M. Henning P.P. AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: Effects of serotype, promoter and purification method. Mol. Ther. 2008;16:89–96. doi: 10.1038/sj.mt.6300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch R. Azen C. Friedman E.G. Williamson M.L. Paired comparisons between early treated PKU children and their matched sibling controls on intelligence and school achievement test results at eight years of age. J. Inherit. Metab. Dis. 1984;7:86–90. doi: 10.1007/BF01805813. [DOI] [PubMed] [Google Scholar]
- Koch R. Burton B. Hoganson G. Peterson R. Rhead W. Rouse B. Scott R. Wolff J. Stern A.M. Guttler F. Nelson M. De La Cruz F. Coldwell J. Erbe R. Geraghty M.T. Shear C. Thomas J. Azen C. Phenylketonuria in adulthood: A collaborative study. J. Inherit. Metab. Dis. 2002;25:333–346. doi: 10.1023/a:1020158631102. [DOI] [PubMed] [Google Scholar]
- Kootstra N.A. Verma I.M. Gene therapy with viral vectors. Annu. Rev. Pharmacol. Toxicol. 2003;43:413–439. doi: 10.1146/annurev.pharmtox.43.100901.140257. [DOI] [PubMed] [Google Scholar]
- Laipis P.J. Charron C.E. Embury J.E. Perera O.P. Porvasnik S.L. Fields C.R. Zori R.T. Correction of maternal phenylketonuria syndrome in the Pah-enu2 missense mutant mouse by r-AAV mediated gene therapy. Mol. Ther. 2004;9(Suppl. 1):s334. [Google Scholar]
- Levy H.L. Ghavami M. Maternal phenylketonuria: A metabolic teratogen. Teratology. 1996;53:176–184. doi: 10.1002/(SICI)1096-9926(199603)53:3<176::AID-TERA5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Louboutin J.P. Wang L. Wilson J.M. Gene transfer into skeletal muscle using novel AAV serotypes. J. Gene Med. 2005;7:442–451. doi: 10.1002/jgm.686. [DOI] [PubMed] [Google Scholar]
- Manno C.S. Chew A.J. Hutchison S. Larson P.J. Herzog R.W. Arruda V.R. Tai S.J. Ragni M.V. Thompson A. Ozelo M. Couto L.B. Leonard D.G. Johnson F.A. McClelland A. Scallan C. Skarsgard E. Flake A.W. Kay M.A. High K.A. Glader B. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Manno C.S. Pierce G.F. Arruda V.R. Glader B. Ragni M. Rasko J.J. Ozelo M.C. Hoots K. Blatt P. Konkle B. Dake M. Kaye R. Razavi M. Zajko A. Zehnder J. Rustagi P.K. Nakai H. Chew A. Leonard D. Wright J.F. Lessard R.R. Sommer J.M. Tigges M. Sabatino D. Luk A. Jiang H. Mingozzi F. Couto L. Ertl H.C. High K.A. Kay M.A. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- McDonald J.D. Charlton C.K. Characterization of mutations at the mouse phenylalanine hydroxylase locus. Genomics. 1997;39:402–405. doi: 10.1006/geno.1996.4508. [DOI] [PubMed] [Google Scholar]
- Mingozzi F. Meulenberg J.J. Hui D.J. Basner-Tschakarjan E. Hasbrouck N.C. Edmonson S.A. Hutnick N.A. Betts M.R. Kastelein J.J. Stroes E.S. High K.A. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki S. Mizukami H. Ogura T. Kure S. Ichinohe A. Kojima K. Matsubara Y. Kobayahi E. Okada T. Hoshika A. Ozawa K. Kume A. Long-term correction of hyperphenylalaninemia by AAV-mediated gene transfer leads to behavioral recovery in phenylketonuria mice. Gene Ther. 2004;11:1081–1086. doi: 10.1038/sj.gt.3302262. [DOI] [PubMed] [Google Scholar]
- Nathwani A.C. Cochrane M. McIntosh J. Ng C.Y. Zhou J. Gray J.T. Davidoff A.M. Enhancing transduction of the liver by adeno-associated viral vectors. Gene Ther. 2009;16:60–69. doi: 10.1038/gt.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health Consensus Development Panel. Pediatrics; Phenylketonuria. Screening and management National Institutes of Health consensus development conference statement; 2001. Oct 16–18, 2000. pp. 972–982. [DOI] [PubMed] [Google Scholar]
- Ogura T. Mizukami H. Mimuro J. Madoiwa S. Okada T. Matsushita T. Urabe M. Kume A. Hamada H. Yoshikawa H. Sakata Y. Ozawa K. Utility of intraperitoneal administration as a route of AAV serotype 5 vector-mediated neonatal gene transfer. J. Gene Med. 2006;8:990–997. doi: 10.1002/jgm.916. [DOI] [PubMed] [Google Scholar]
- Oh H.J. Park E.S. Kang S. Jo I. Jung S.C. Long-term enzymatic and phenotypic correction in the phenylketonuria mouse model by adeno-associated virus vector-mediated gene transfer. Pediatr. Res. 2004;56:278–284. doi: 10.1203/01.PDR.0000132837.29067.0E. [DOI] [PubMed] [Google Scholar]
- Paterna J.C. Feldon J. Bueler H. Transduction profiles of recombinant adeno-associated virus vectors derived from serotypes 2 and 5 in the nigrostriatal system of rats. J. Virol. 2004;78:6808–6817. doi: 10.1128/JVI.78.13.6808-6817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pey A.L. Ying M. Cremades N. Velazquez-Campoy A. Scherer T. Thöny B. Sancho J. Martinez A. Identification of pharmacological chaperones as potential therapeutic agents to treat phenylketonuria. J. Clin. Invest. 2008;118:2858–2867. doi: 10.1172/JCI34355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M. Chesnut K. Muzyczka N. Flotte T. Zolotukhin S. Streamlined large-scale production of recombinant adeno-associated virus (rAAV) vectors. Methods Enzymol. 2002;346:413–430. doi: 10.1016/s0076-6879(02)46069-7. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J.E. Rolling F. Li C. Conrath H. Xiao W. Xiao X. Samulski R.J. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rip J. Nierman M.C. Sierts J.A. Petersen W. Van Den Oever K. Van Raalte D. Ross C.J. Hayden M.R. Bakker A.C. Dijkhuizen P. Hermens W.T. Twisk J. Stroes E. Kastelein J.J. Kuivenhoven J.A. Meulenberg J.M. Gene therapy for lipoprotein lipase deficiency: Working toward clinical application. Hum. Gene Ther. 2005;16:1276–1286. doi: 10.1089/hum.2005.16.1276. [DOI] [PubMed] [Google Scholar]
- Riviere C. Danos O. Douar A.M. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 2006;13:1300–1308. doi: 10.1038/sj.gt.3302766. [DOI] [PubMed] [Google Scholar]
- Sarkar R. Tetreault R. Gao G. Wang L. Bell P. Chandler R. Wilson J.M. Kazazian H.H., Jr. Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood. 2004;103:1253–1260. doi: 10.1182/blood-2003-08-2954. [DOI] [PubMed] [Google Scholar]
- Sarkissian C.N. Gamez A. Phenylalanine ammonia lyase, enzyme substitution therapy for phenylketonuria, where are we now? Mol. Genet. Metab. 2005;86(Suppl. 1):S22–S26. doi: 10.1016/j.ymgme.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Sarkissian C.N. Gamez A. Wang L. Charbonneau M. Fitzpatrick P. Lemontt J.F. Zhao B. Vellard M. Bell S.M. Henschell C. Lambert A. Tsuruda L. Stevens R.C. Scriver C.R. Preclinical evaluation of multiple species of PEGylated recombinant phenylalanine ammonia lyase for the treatment of phenylketonuria. Proc. Natl. Acad. Sci. U.S.A. 2008;105:20894–20899. doi: 10.1073/pnas.0808421105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkissian C.N. Gamez A. Scriver C.R. What we know that could influence future treatment of phenylketonuria. J. Inherit. Metab. Dis. 2009;32:3–9. doi: 10.1007/s10545-008-0917-7. [DOI] [PubMed] [Google Scholar]
- Scavelli R. Tetrahydrobiopterin metabolism in the mouse: Feeding studies and generation of a new mutant model. Ph.D. thesis. University of Zürich; Zürich, Switzerland: 2006. [Google Scholar]
- Scavelli R. Ding Z. Blau N. Haavik J. Martinez A. Thöny B. Stimulation of hepatic phenylalanine hydroxylase activity but not Pah-mRNA expression upon oral loading of tetrahydrobiopterin in normal mice. Mol. Genet. Metab. 2005;86(Suppl 1):S153–S155. doi: 10.1016/j.ymgme.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Schultz B.R. Chamberlain J.S. Recombinant adeno-associated virus transduction and integration. Mol. Ther. 2008;16:1189–1199. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriver C.R. Kaufman S. In: Hyperphenylalaninemia: Phenylalanine hydroxylase deficiency. In The Metabolic and Molecular Bases of Inherited Disease. Scriver C.R., editor; Beaudet A.L., editor; Sly W.S., editor; Valle D., editor; Vogelstein B., editor. McGraw-Hill; New York: 2001. pp. 1667–1724. [Google Scholar]
- Scriver C.R. Waters P.J. Monogenic traits are not simple: Lessons from phenylketonuria. Trends Genet. 1999;15:267–272. doi: 10.1016/s0168-9525(99)01761-8. [DOI] [PubMed] [Google Scholar]
- Scriver C.R. Hurtubise M. Konecki D. Phommarinh M. Prevost L. Erlandsen H. Stevens R. Waters P.J. Ryan S. McDonald D. Sarkissian C. PAHdb 2003: What a locus-specific knowledge base can do. Hum. Mutat. 2003;21:333–344. doi: 10.1002/humu.10200. [DOI] [PubMed] [Google Scholar]
- Thöny B. Blau N. Mutations in the BH4-metabolizing genes GTP cyclohydrolase I, 6-pyruvoyl-tetrahydropterin synthase, sepiapterin reductase, carbinolamine-4a-dehydratase, and dihydropteridine reductase. Hum. Mutat. 2006;27:870–878. doi: 10.1002/humu.20366. [DOI] [PubMed] [Google Scholar]
- Thöny B. Ding Z. Martinez A. Tetrahydrobiopterin protects phenylalanine hydroxylase activity in vivo: Implications for tetrahydrobiopterin-responsive hyperphenylalaninemia. FEBS Lett. 2004;577:507–511. doi: 10.1016/j.febslet.2004.10.056. [DOI] [PubMed] [Google Scholar]
- Toromanoff A. Cherel Y. Guilbaud M. Penaud-Budloo M. Snyder R.O. Haskins M.E. Deschamps J.Y. Guigand L. Podevin G. Arruda V.R. High K.A. Stedman H.H. Rolling F. Anegon I. Moullier P. Le Guiner C. Safety and efficacy of regional intravenous (r.i.) versus intramuscular (i.m.) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle. Mol. Ther. 2008;16:1291–1299. doi: 10.1038/mt.2008.87. [DOI] [PubMed] [Google Scholar]
- Voutetakis A. Zheng C. Wang J. Goldsmith C.M. Afione S. Chiorini J.A. Wenk M.L. Vallant M. Irwin R.D. Baum B.J. Gender differences in serotype 2 adeno-associated virus biodistribution after administration to rodent salivary glands. Hum. Gene Ther. 2007;18:1109–1118. doi: 10.1089/hum.2007.072. [DOI] [PubMed] [Google Scholar]
- Waisbren S.E. Levy H.L. Agoraphobia in phenylketonuria. J. Inherit. Metab. Dis. 1991;14:755–764. doi: 10.1007/BF01799946. [DOI] [PubMed] [Google Scholar]
- Wang Z. Zhu T. Qiao C. Zhou L. Wang B. Zhang J. Chen C. Li J. Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- Wu Z. Asokan A. Samulski R.J. Adeno-associated virus serotypes: Vector toolkit for human gene therapy. Mol. Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Xiao W. Chirmule N. Berta S.C. McCullough B. Gao G. Wilson J.M. Gene therapy vectors based on adeno-associated virus type 1. J. Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G.J. Kang W. Wang L. Scotti M.M. Liu J. Thomas D.L. Le Guiner C. Rouhani F. Brantly M.L. Knop D.R. Moullier P. Chulay J.D. Veres G. Evaluation of recombinant adeno-associated virus (rAAV) α-1 antitrypsin (AAT) gene therapy in mice and nonhuman primates. Mol. Ther. 2009;17(Suppl. 1):S317. [Google Scholar]
- Zaiss A.K. Muruve D.A. Immunity to adeno-associated virus vectors in animals and humans: A continued challenge. Gene Ther. 2008;15:808–816. doi: 10.1038/gt.2008.54. [DOI] [PubMed] [Google Scholar]
- Zincarelli C. Soltys S. Rengo G. Rabinowitz J.E. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]