Abstract
Objective
Generalized anxiety disorder (GAD) is a prevalent and debilitating psychiatric condition of adolescence. Two effective forms of treatment are cognitive behavioral therapy (CBT) and selective serotonin reuptake inhibitors (SSRIs). This pilot study examined changes in brain function following each type of treatment in GAD.
Method
Subjects were 14 youths with GAD (7 had CBT, 7 received fluoxetine) and 10 age- and gender-matched healthy peers. Functional magnetic resonance imaging (fMRI) scans were acquired before and after treatment for patients and over two comparable time points for controls. During fMRI acquisition, a probe detection task with emotional (angry, happy) and neutral faces allowed for assessment of neural response to threat. Following previous research, region of interest analyses were performed in the right ventrolateral prefrontal cortex (VLPFC).
Results
fMRI results showed increased right VLPFC activation, relative to controls, in the medication (t(15) = 3.01, p < 0.01) and CBT (t(15) = 3.22, p < 0.01) groups following treatment.
Conclusions
This study shows significant increase in right VLPFC activation in response to angry faces following treatment with CBT or fluoxetine for GAD. This is consistent with previous research indicating that the VLPFC may facilitate effective responding to underlying neural correlates of anxiety in other brain regions, such as the amygdala.
Introduction
Generalized anxiety disorder (GAD) is a prevalent and debilitating psychiatric condition characterized by excessive worry, hypervigilance, and apprehension about future events (American Psychiatric Association 2000). Anxiety disorders are associated with impairment in daily life functioning (Ezpeleta et al. 2001) and predict high risk for future problems (Pine et al. 1998; Kessler et al. 2008). Nevertheless, neurological correlates of GAD remain poorly understood. There is a particular dearth of research on changes in brain circuitry associated with improvements in GAD.
In adults, anxiety disorders are associated with increased activation in inferior frontal and medial orbitofrontal cortices in response to anxiety provocation (Rauch et al. 1997). Monk et al. (2006) found adolescent GAD patients showed greater right ventrolateral (VLPFC) activation than healthy peers when viewing angry faces. Further, VLPFC activation was negatively correlated with their symptom severity, suggesting that VLPFC activation may serve a compensatory function in GAD.
Two known, comparably effective treatments for GAD in children and adolescents are cognitive behavioral therapy (CBT) (Compton et al. 2004; Scott et al. 2005) and selective serotonin reuptake inhibitors (SSRIs) (Research Unit on Pediatric Psychopharmacology Anxiety Study Group 2001; Birmaher et al. 2003; Walkup et al. 2008). CBT employs techniques such as cognitive restructuring, relaxation, and self-monitoring to ease anxiety symptoms and teach coping strategies (Lang 2004). Results of a recent meta-analysis show its effectiveness in treating anxiety disorders in children and adolescents (James et al. 2005). Additional meta-analyses of CBT treatment of adults with anxiety disorders show moderate-to-large effect sizes in comparison to no treatment and nonspecific psychotherapies (Gould et al. 1997), as well as consistent evidence that the beneficial effects of CBT are sustained over at least a 12-month period (DeRubeis and Crits-Christoph 1998).
SSRIs have also been shown to reduce symptoms in pediatric anxiety disorders including GAD (Research Unit on Pediatric Psychopharmacology Anxiety Study Group 2001; Seidel and Walkup 2006; Walkup et al. 2008). Treatment with fluoxetine versus placebo is associated with significantly reduced anxiety symptoms and improved global functioning (Birmaher et al. 2003). Additionally, continued treatment with fluoxetine versus no medication following an initial period of medication treatment is associated with improved outcomes 1 year later (Clark et al. 2005). Although both CBT and SSRIs have demonstrated comparable efficacy as treatments for GAD, the functional neurological changes that accompany the clinical improvement noted with these treatments are unclear.
The research on the neural effects of psychotherapy on GAD specifically is sparse. However, neuroimaging studies of the effects of psychotherapy in other major psychiatric disorders have shown consistent attenuation of abnormal activation patterns following treatment (for reviews, see Roffman et al. 2005; Linden 2006). No known studies have examined the neural correlates of CBT or SSRI treatment for GAD in any age group. However, at least one study has examined effects of CBT treatment in adult patients with major depressive disorder (MDD). Goldapple et al. (2004) noted decreases in ventral and medial prefrontal cortex (PFC) activation while viewing sad faces following CBT treatment for MDD. Because MDD and GAD are known to be highly co-morbid (Angold et al. 1999; Kessler et al. 2008), these results further implicate ventral and medial PFC in treatment response for mood and anxiety disorders.
The current study, the first longitudinal functional magnetic resonance imaging (fMRI) study comparing treatments of adolescent GAD, sought to examine changes in brain function following the two types of effective treatments for youths with GAD. In a previous study of adolescents with GAD, Monk et al. (2006) found that higher levels of right VLPFC activation were associated with fewer anxiety symptoms before treatment. The current study examines this same patient group using data collected after treatment. As noted earlier, these results suggest that the VLPFC may serve a compensatory function in GAD. On the basis of the findings of Monk et al. (2006), we expected that patients in each treatment condition, relative to healthy peers, would show altered right VLPFC activation to angry faces following treatment. Increased activation following treatment could indicate compensatory activation in the VLPFC. Decreased activation could indicate that less VLPFC activation was needed to compensate for anxiety following successful treatment.
Methods
Subjects
The sample consisted of 14 adolescents with GAD divided between two groups, those treated with CBT (n = 7) and those treated with medication (n = 7), plus a comparison group (n = 10). Table 1 contains descriptive characteristics of each group. There were no significant differences in the composition of the groups by age, gender, symptom severity, or co-morbid diagnosis in the two treatment groups. The Time 1 (pretreatment for patients) data for 16 of the 24 subjects (6 from the CBT group, 6 from the medication group, and 4 controls) were also included in a previous study (Monk et al 2006). However, the Time 2 (posttreatment for patients) data for all subjects have not been previously presented.
Table 1.
Sample Characteristics
| CBT | Fluoxetine | Control | Statistic | |
|---|---|---|---|---|
| Age | 13.4 (1.7) | 13.3 (2.5) | 14.5 (1.4) | F(2, 21) = 1.09 |
| Gender | ||||
| Male | 3 | 4 | 4 | χ2 (2) = 0.52 |
| Female | 4 | 3 | 6 | |
| Comorbid Diagnosis | ||||
| Social Phobia | 4 | 2 | χ2 (1) = 1.17 | |
| Separation Anxiety | 5 | 5 | χ2 (1) = 0 | |
| ADHD | 2 | 2 | χ2 (1) = 0 | |
| MDD | 3 | 5 | χ2 (1) = 1.17 | |
| Anxiety Symptoms | ||||
| PARS, pretreatment | 15.4 (3.2) | 16.4 (2.5) | t (12) = −0.64 | |
| PARS, posttreatment | 9.0 (4.8) | 5.3 (7.2) | t (12) = 1.14 |
Subject groups did not differ on age or gender, and treatment groups did not differ on comorbid diagnoses or on anxiety symptom scores before or after treatment.
Abbreviations: CBT = Cognitive behavioral therapy; ADHD = attention-deficit/hyperactivity disorder; MDD = major depressive disorder; PARS = pediatric anxiety rating scale.
Procedures
All procedures were approved by the National Institute of Mental Health (NIMH) Institutional Review Board. Parents provided written consent, and participants provided written assent. Patients' diagnosis of GAD was determined using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL) (Kaufman et al. 1997). Although some patients had additional co-morbid diagnoses, GAD and associated anxiety symptoms were identified as the primary disorder, the condition of the greatest clinical significance, for each patient. Of note given the attention-based behavioral task, patients dually diagnosed with ADHD were not on medication during the course of the study and could not be withdrawn from medication to enroll in the study. All patients received their choice of 8 weeks of either CBT or medication treatment. CBT treatment consisted of eight weekly sessions lasting 60–90 min each and administered by a licensed clinical psychologist. Sessions focused on exposure and skills training, following manualized curricula (Beidel et al. 2000; Kendall and Hedtke 2006). Fluoxetine treatment was administered according to the protocol of the Research Unit on Pediatric Psychopharmacology Anxiety Study Group (2001). An initial dose of 5 mg/day was increased every 2 weeks as recommended by a clinician up to a maximum of 40 mg/day. fMRI scans were performed before treatment and within approximately 2 weeks (15 ± 7 days) of treatment's end. One potential source of difference between the two treatment groups was that patients in the medication group were still receiving medication at the time of the second scan. However, as discussed below, a comparison of the two groups showed no difference in their right VLPFC activation. Nonetheless, the groups were analyzed separately due to the qualitatively different nature of the two treatments.
Anxiety symptoms
Anxiety symptoms were measured at Time 1 and Time 2 using the Pediatric Anxiety Rating Scale (PARS) (Research Unit on Pediatric Psychopharmacology Anxiety Study Group 2002) by raters trained to achieve acceptable interrater reliability (intraclass correlation coefficient [ICC] > 0.70). This 50-item checklist shows good test–retest reliability and sensitivity to treatment-related changes in symptoms.
Behavioral task and analysis
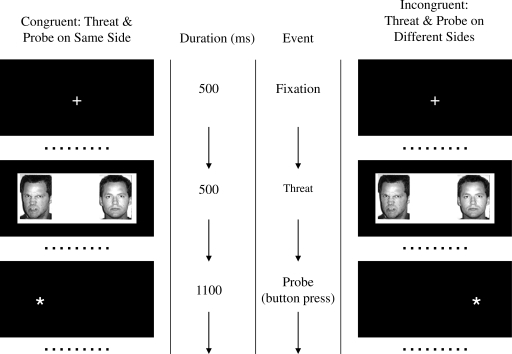
A probe detection task (Mogg and Bradley 1999) was used to assess neural responses to threat under controlled presentation circumstances and to allow comparison of the fMRI data with Monk et al. (2006). In an event-related design, subjects viewed pairs of faces (angry/neutral, happy/neutral, and neutral/neutral) for 500 msec (Fig. 1). Subjects responded by pressing a button to an asterisk that was either on the same (congruent) or opposite (incongruent) side as the emotional face. A total of 36 randomized trials for each condition were included. The main analyses in the current study included only data from those trials in which an angry face was present. Trials in which a happy face or two neutral faces were present were also analyzed to determine the specificity of any effects to angry faces.
FIG. 1.
Visual task. An initial fixation of 500 msec was followed by pairs of emotional and neutral faces (angry/neutral and happy/neutral) or two neutral faces for 500 msec. Subjects then responded by pressing a button to indicate the position of an asterisk that was either on the same (congruent) or opposite (incongruent) side as the emotional face.
Behavioral data were analyzed using paired-samples t-tests in SPSS. Reaction time differences of incongruent minus congruent trials provided a measure of attentional bias in trials containing angry or happy faces, such that positive values indicated attentional bias toward the emotional face and negative values indicated attentional bias away from the emotional face.
fMRI procedures and analysis
We used a GE 3T scanner to acquire images with 29 contiguous 3.3-mm axial slices parallel to the anterior commissure/posterior commissure line. We used echo-planar, single-shot gradient echo T2* weighting (repetition time [TR] = 2300 msec; echo time [TE] = 23 msec; field of view = 240 mm; 64 × 64 matrix; 3.3 × 3.75 × 3.75-mm voxel). High-resolution T1-weighted volumetric scans used a magnetization-prepared gradient echo sequence (MP-RAGE): 180 1.0-mm axial slices; field of view = 256 mm; number of excitations = 1; TR = 11.4 msec; TE = 4.4 msec; matrix = 256 × 256; TI = 300 msec; bandwidth 130 Hz/pixel = 33 kHz for 256 pixels; in-plane resolution = 1 mm3.
Functional imaging data were analyzed using AFNI software version 2.56b (Cox 1996; available at http://afni.nimh.nih.gov/afni). Participants were excluded if there was motion greater than 3 mm in any direction. To mitigate movement, all images were registered to one volume in each run. Data were smoothed using a 6-mm full width at half-maximum isotropic Gaussian filter. Incorrect trials and trials with reaction time <200 msec or >1000 msec were not included in the fMRI analysis.
A random-effects fMRI data analysis was conducted using a two-level procedure. At the subject level, data from each subject were analyzed using multiple regression in the AFNI 3dDeconvolve program. For the conditions of interest (angry/neutral, happy/neutral, and neutral/neutral), vectors were created containing the onset time of each trial for each condition (blank trials were modeled as an implicit baseline). Onset times for trials in which the subject did not respond or responded incorrectly were modeled in a separate vector as a nuisance covariate. Vectors of stimulus timing for each condition were transformed into reference waveforms of response function using a gamma variate (Cohen 1997). Coefficients were thus produced for each condition from each subject.
The primary effect of interest for the fMRI analysis was response to angry faces. Therefore, contrasts were calculated to compare activation during trials in which an angry face was present (incongruent and congruent trials combined) relative to baseline (consistent with Monk et al. 2006). To examine the specificity of this effect to threatening stimuli, we also performed parallel analyses on the happy faces versus baseline and neutral faces versus baseline contrasts. Before performing the group-level analysis, individual anatomical data sets were converted to Talairach space. The underlying transformation was then applied to that same individual's functional data in order to convert those images as well. The group level analysis involved conducting analysis of variance (ANOVA) analyses using the AFNI module GroupAna on the main contrast of interest, angry versus baseline trials, to evaluate the neurophysiological response. Two separate 2 × 2 (group × time) analysis of variance (ANOVA) analyses were performed. The CBT group and medication group were analyzed separately, and each was compared to the control group. The threshold of significance for all analyses was set at p < 0.01, uncorrected, with a minimum cluster size of 10 contiguous voxels. We chose this relatively low threshold based on the preliminary nature of the study and on the fact that our analyses were limited to one a priori region, the right VLPFC.
Results
Behavioral results
Each group's mean reaction times on the behavioral task are presented in Table 2. We did not test a specific hypothesis for the reaction time data because this study was not designed to evaluate changes in attentional bias, as it was underpowered for this particular purpose. Attentional bias scores were examined but, unsurprisingly, these showed no significant results. The two treatment groups did not significantly differ in their attentional bias to angry or happy faces at Time 1 or Time 2. The treatment groups' attentional bias scores also did not significantly differ from controls at either time point. Finally, there was no significant change in attentional bias to angry or happy faces from Time 1 to Time 2 in either treatment group. There was a trend toward a group × time interaction, in which there was a greater decrease in attentional bias to angry faces from Time 1 to Time 2 in the medication versus CBT group. However, this interaction did not reach significance, F(2,21) = 3.12, p = 0.07.
Table 2.
Mean Reaction Times (in msec) and Accuracy on Behavioral Task by group at Time 1 and Time 2
| |
CBT |
Fluoxetine |
Control |
|||
|---|---|---|---|---|---|---|
| Time 1 | Time 2 | Time 1 | Time 2 | Time 1 | Time 2 | |
| Angry congruent | 543.93 | 528.23 | 556.44 | 483.69 | 530.13 | 512.57 |
| Angry incongruent | 549.38 | 523.51 | 537.32 | 499.85 | 531.91 | 518.66 |
| Angry bias (incongruent–congruent) | 5.45 | −4.73 | −19.12 | 16.16 | 1.78 | 6.10 |
| Happy congruent | 553.44 | 524.30 | 545.39 | 498.16 | 522.83 | 508.81 |
| Happy incongruent | 552.79 | 521.73 | 537.19 | 498.52 | 533.86 | 513.92 |
| Happy bias (incongruent–congruent) | 0.65 | 2.57 | 8.21 | −0.36 | −11.03 | −5.12 |
| Accuracy (% correct) | 96.96 | 95.29 | 90.18 | 96.43 | 94.13 | 94.40 |
There were no significant differences in reaction time, accuracy, or attentional bias in any condition, either between groups at each time point or within each group from Time 1 to Time 2. Reaction times to happy faces are presented for reference.
Abbreviations: CBT = Cognitive behavioral therapy.
Anxiety symptoms
Patient at risk scores (PARS) did not differ significantly between the two treatment groups at Time 1 (t(12) = −0.64, p > 0.05) or Time 2 (t(12) = 1.14, p > 0.05). Patients in both treatment groups showed significant improvement in anxiety symptoms with treatment: CBT, t(6) = 5.91, p < 0.01; medication, t(6) = 3.94, p < 0.01.
fMRI results
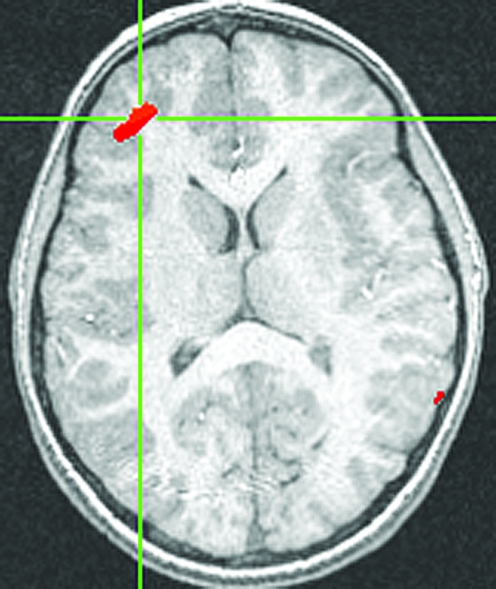
Following previous work (Monk et al. 2006), we focused our fMRI analysis on the response to angry faces. Specifically, we examined changes in brain activation between Time 1 and Time 2. The contrast of angry face trials versus baseline was analyzed separately in each treatment group versus the control group. The medication group, relative to healthy comparisons, showed a significant increase in right VLPFC activation following treatment (located at (35, 42, 14), t(15) = 3.01, p < 0.01, effect size r = 0.71, cluster size = 121 voxels at p < 0.01; see Fig. 2). This result represents a large effect size (Cohen 1988). However, these effect sizes must be interpreted cautiously. The use of a cluster derived from activation for the effect size calculation may lead to an inflated estimate (Poldrack and Mumford 2009).
FIG. 2.
Increased VLPFC activation in medication group from Time 1 to Time 2. Increased activity from Time 1 to Time 2 in right VLPFC (35, 42, 14), t(15) = 3.01, p < 0.01. Note: Threshold for all figures is p < 0.05. VLPFC = Ventrolateral prefrontal cortex.
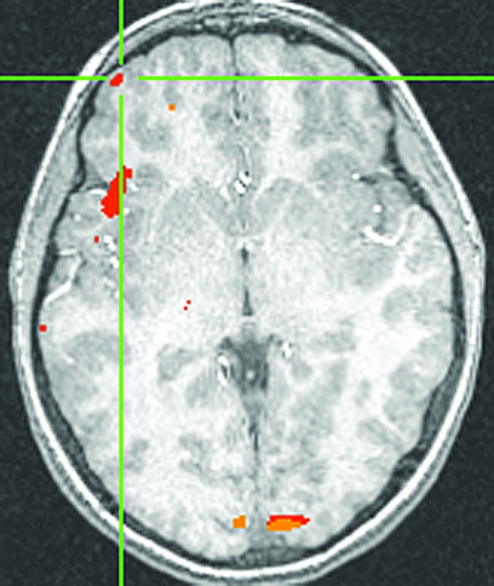
The CBT group, relative to controls, also showed a significant increase from Time 1 to Time 2 in right VLPFC activation (Fig. 3) to angry faces versus baseline (located at [41, 55, 1], t[15] = 3.22, p < 0.01, effect size r = 0.54, cluster size = 29 voxels at p < 0.01). This result also represents a large effect size (Cohen 1988), although possibly inflated as discussed above. In addition, surprisingly, this group also exhibited bilateral increases in amygdala activation (right amygdala (27, −7, −12), t(15) = 2.74, p < 0.05; left amygdala (−17, −9, −14), t(15) = 2.54, p < 0.05). None of the clusters of activation was significantly correlated with reductions in anxiety symptoms in either group. As task performance and subsequent neural activations could be affected by attention deficits, all analyses were rerun after removing those patients with a dual diagnosis of ADHD (2 participants from each treatment group). Removing these subjects did not affect the pattern of results in the VLPFC in either treatment group; both groups still showed an increase in activation in right VLPFC following treatment.
FIG. 3.
Increased right VLPFC activation in patients treated with CBT versus controls, Time 2 > Time 1. Increased activity from Time 1 to Time 2 in right VLPFC (41, 55, 1), t(15) = 3.21, p < 0.01. VLPFC = Ventrolateral prefrontal cortex; CBT = cognitive behavioral therapy.
To examine the specificity of these increases in right VLPFC activation, we analyzed the change in activation from Time 1 to Time 2 in the contrasts of happy faces versus baseline and neutral faces versus baseline. Even at a relaxed threshold of p < 0.05, neither treatment condition was associated with increased right VLPFC activation in either of these contrasts. Thus, the increase in right VLPFC activation following treatment for GAD may be specific to viewing angry faces and not a general effect of viewing emotional or nonemotional faces.
Discussion
Before considering the implications of these findings, it is important to note the many limitations in the work presented here. For example, this study examined a very small number of subjects, divided into three groups, and patients presenting with GAD also exhibit frequent, co-morbid symptoms. The small number of subjects limited statistical power, and any null results may be due to lack of power. Statistical correction for multiple comparisons was not feasible given the small sample size. Moreover, treatment assignment was based on subject choice as opposed to random assignment. As a result, these data should be considered as preliminary. Nevertheless, this study is a novel contribution to the extant research given that the current data represent the only repeated fMRI study of any pediatric anxiety disorder. Moreover, the data extend a wealth of other research generating a specific hypothesis about the role of the VLPFC in pediatric anxiety (Monk 2008; Pine et al. 2008). As a result, the findings, despite their preliminary nature, point to important avenues for future research. Specifically, results demonstrating increases in VLPFC function in tandem with reduction in anxiety might encourage other particular studies. For example, research might examine the role of VLPFC functioning in other forms of anxiety-related change, such as successful coping with adversity.
Consistent with our hypothesis, the fMRI results showed increased right VLPFC activation, relative to controls, in both medication and CBT groups following treatment. The observed increase in VLPFC activation following treatment for GAD is consistent with existing research implicating this region in self-regulation of anxiety. Ochsner et al. (2004) found right ventral PFC activity increased when healthy subjects were asked to voluntarily downregulate their negative emotions. Similarly, Phan et al. (2005) found right VLPFC activation increased during downregulation of negative emotions and reported that this activation was positively correlated with intensity of the negative emotion. It is also consistent with Monk et al. (2006), who showed that increased activation in this region was correlated with lower symptom severity in a group of adolescents with GAD before treatment, suggesting a potential compensatory mechanism.
Although both are within the PFC, the regions of activation showing treatment-related change appeared to differ between the CBT and medication groups. Nevertheless, a direct comparison of the CBT and medication groups revealed no significant differences in the VLPFC. Future, larger studies are needed that will allow these two groups to be contrasted with increased statistical power. One additional, but not hypothesized, finding was a significant increase in bilateral amygdala activation in the CBT group following treatment. This result warrants further study given its known importance in anxiety disorders (Thomas et al. 2001; Stein et al. 2002; McClure et al. 2007; Monk et al. 2008). Finally, although there were no significant behavioral results with regard to attentional bias, there was a trend toward an interaction in which there was a greater decrease in attentional bias toward threatening stimuli in the medication versus CBT groups from Time 1 to Time 2. Further work is warranted to better understand the effects of treatments on attention bias to threat.
The present findings bridge affective neuroscience research and clinical treatment research on pediatric anxiety. The finding of treatment-related increases in VLPFC activation is consistent with affective neuroscience work showing that regions of the ventral PFC are involved in the modulation of negative emotions (e.g., Ochsner et al. 2004; Phan et al. 2005). Thus, engagement of the ventral regions of the PFC may be a therapeutic route to reduce negative emotions such as anxiety. Because somewhat different locations of activation were observed within the VLPFC in each of the two treatments, further research contrasting the two treatments is needed. Nonetheless, the present results represent the first step in determining the neural correlates of symptom improvements that often occur with these two treatments.
Conclusions
Successful treatment of GAD with either CBT or fluoxetine in a sample of adolescents was associated with increased activation in the right VLPFC. Greater right VLPFC activation may thus constitute one manner in which these treatments act to decrease anxiety symptoms. To further ascertain the neural correlates of treatment for GAD, future studies should examine the replicability of these results in larger samples. Additional studies are also needed to determine the stability of these effects after treatment has ended.
Disclosures
The authors have no conflicts of interest to disclose.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington (DC): American Psychiatric Association; 1994. (DSM-IV) [Google Scholar]
- Angold A. Costello EJ. Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- Beidel DC. Turner SM. Morris TL. Behavioral treatment of childhood social phobia. J Consult Clin Psychol. 2000;68:1072–1080. [PubMed] [Google Scholar]
- Birmaher B. Axelson DA. Monk K. Kalas C. Clark DB. Ehmann M. Bridge J. Heo J. Brent DA. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42:415–423. doi: 10.1097/01.CHI.0000037049.04952.9F. [DOI] [PubMed] [Google Scholar]
- Clark DB. Birmaher B. Axelson D. Monk K. Kalas C. Ehmann M. Bridge J. Wood S. Muthen B. Brent D. Fluoxetine for the treatment of childhood anxiety disorders: Open-label, long term extension to a controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44:1263–1270. doi: 10.1097/01.chi.0000183464.41777.c1. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale (New Jersey): Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Compton SN. March JS. Brent D. Albano AM. Weersing R. Curry J. Cognitive-behavioral psychotherapy for anxiety and depressive disorders in children and adolescents: An evidence-based medicine review. J Am Acad Child Adolesc Psychiatry. 2004;43:930–959. doi: 10.1097/01.chi.0000127589.57468.bf. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ. Crits-Christoph P. Empirically supported individual and group psychological treatments for adult mental disorders. J Consult Clin Psychol. 1998;66:37–52. doi: 10.1037//0022-006x.66.1.37. [DOI] [PubMed] [Google Scholar]
- Ezpeleta L. Keeler G. Erkanli A. Costello EJ. Angold A. Epidemiology of psychiatric disability in childhood and adolescence. J Child Psychol Psychiat. 2001;42:901–914. doi: 10.1111/1469-7610.00786. [DOI] [PubMed] [Google Scholar]
- Goldapple K. Segal Z. Garson C. Lau M. Bieling P. Kennedy S. Mayberg H. Modulation of cortical-limbic pathways in major depression. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Gould RA. Otto MW. Pollack MH. Yap L. Cognitive behavioral and pharmacological treatment of generalized anxiety disorder: A preliminary meta-analysis. Behav Ther. 1997;28:285–305. [Google Scholar]
- James AACJ. Soler A. Weatherall RRW. Cognitive behavioural therapy for anxiety disorders in children, adolescents. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD004690.pub2. 4 Art. No.: CD004690. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Rao U. Flynn C. Moreci P. Williamson D. Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children- Present and Lifetime Versions (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kendall PC. Hedtke KA. Cognitive-Behavioral Therapy for Anxious Children: Therapist Manual. 3rd. Ardmore (Pennsylvania): Workbook Publishing, Inc.; 2006. [Google Scholar]
- Kessler RC. Gruber M. Hettema JM. Hwang I. Sampson N. Yonkers KA. Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psych Med. 2008;38:365–374. doi: 10.1017/S0033291707002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AJ. Treating generalized anxiety disorder with cognitive-behavioral therapy. J Clin Psychiatry. 2004;65(Suppl 13):14–19. [PubMed] [Google Scholar]
- Linden DEJ. How psychotherapy changes the brain—the contribution of functional neuroimaging. Mol Psychiatry. 2006;11:528–538. doi: 10.1038/sj.mp.4001816. [DOI] [PubMed] [Google Scholar]
- McClure EB. Monk CS. Nelson EE. Parrish JM. Adler A. Blair RJR. Fromm S. Charney DS. Leibenluft E. Ernst E. Pine DS. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- Mogg K. Bradley BP. Some methodological issues in assessing attentional biases for threatening faces in anxiety: A replication study using a modified version of the dot probe task. Beh Res Ther. 1999;37:595–604. doi: 10.1016/s0005-7967(98)00158-2. [DOI] [PubMed] [Google Scholar]
- Monk CS. The development of emotion-related neural circuitry in health and psychopathology. Dev Psychopathol. 2008;20:1231–1250. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Monk CS. Nelson EE. McClure EB. Mogg K. Bradley BP. Leibenluft E. Blair RJ. Chen G. Charney DS. Ernst M. Pine DS. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Monk CS. Telzer EH. Mogg K. Bradley BP. Mai X. Louro HM. Chen G. McClure-Tone EB. Ernst M. Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN. Ray RD. Cooper JC. Robertson ER. Chopra S. Gabrieli JDE. Gross JJ. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Phan KL. Fitzgerald DA. Nathan PJ. Moore GJ. Uhde TW. Tancer ME. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Pine DS. Cohen P. Gurley D. Brook J. Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Pine DS. Guyer AE. Leibenluft E. Functional magnetic resonance imaging and pediatric anxiety. J Am Acad Child Adolesc Psychiatry. 2008;47:1217–1221. doi: 10.1097/CHI.0b013e318185dad0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Mumford JA. Independence in ROI analysis: Where is the voodoo? SCAN. 2009;4:208–213. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL. Savage CR. Alpert NM. Fischman AJ. Jenike MA. The functional neuroanatomy of anxiety: A study of three disorders using positron emission tomography and symptom provocation. Biol Psychiatry. 1997;42:446–452. doi: 10.1016/S0006-3223(97)00145-5. [DOI] [PubMed] [Google Scholar]
- Research Unit on Pediatric Psychopharmacology Anxiety Study Group. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med. 2001;344:1279–1285. doi: 10.1056/NEJM200104263441703. [DOI] [PubMed] [Google Scholar]
- Research Unit on Pediatric Psychopharmacology Anxiety Study Group. The Pediatric Anxiety Rating Scale (PARS): Development and psychometric properties. J Am Acad Child Adolesc Psychiatry. 2002;41:1061–1069. doi: 10.1097/00004583-200209000-00006. [DOI] [PubMed] [Google Scholar]
- Roffman JL. Marci CD. Glick DM. Dougherty DD. Rauch SL. Neuroimaging and the functional neuroanatomy of psychotherapy. Psychol Med. 2005;35:1385–1398. doi: 10.1017/S0033291705005064. [DOI] [PubMed] [Google Scholar]
- Scott RW. Mughelli K. Deas D. An overview of controlled studies of anxiety disorders treatment in children and adolescents. J Natl Med Assoc. 2005;97:13–24. [PMC free article] [PubMed] [Google Scholar]
- Seidel L. Walkup JT. Selective serotonin reuptake inhibitor use in the treatment of the pediatric non-obsessive-compulsive disorder anxiety disorders. J Child Adolesc Psychopharmacol. 2006;16:171–179. doi: 10.1089/cap.2006.16.171. [DOI] [PubMed] [Google Scholar]
- Stein MB. Goldin PR. Sareen J. Zorrilla LTE. Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Thomas KM. Drevets WC. Dahl RE. Ryan ND. Birmaher B. Eccard CH. Axelson D. Whalen PJ. Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Walkup JT. Albano AM. Piacentini J. Birmaher B. Compton SN. Sherrill JT. Ginsburg GS. Rynn MA. McCracken J. Waslick B. Iyengar S. March JS. Kendall PC. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]