Abstract
Genetic alterations in α-synuclein cause autosomal dominant familial Parkinsonism and may contribute to sporadic Parkinson's disease (PD). Synphilin-1 is an α-synuclein-interacting protein, with implications in PD pathogenesis related to protein aggregation. Currently, the in vivo role of synphilin-1 in α-synuclein-linked pathogenesis is not fully understood. Using the mouse prion protein promoter, we generated synphilin-1 transgenic mice, which did not display PD-like phenotypes. However, synphilin-1/A53T α-synuclein double-transgenic mice survived longer than A53T α-synuclein single-transgenic mice. There were attenuated A53T α-synuclein-induced motor abnormalities and decreased astroglial reaction and neuronal degeneration in brains in double-transgenic mice. Overexpression of synphilin-1 decreased caspase-3 activation, increased beclin-1 and LC3 II expression and promoted formation of aggresome-like structures, suggesting that synphilin-1 alters multiple cellular pathways to protect against neuronal degeneration. These studies demonstrate that synphilin-1 can diminish the severity of α-synucleinopathy and play a neuroprotective role against A53T α-synuclein toxicity in vivo.
INTRODUCTION
Parkinson's disease (PD) is a common neurodegenerative disorder, characterized by the selective loss of dopaminergic neurons and the presence of Lewy bodies. α-Synuclein is a major protein component in Lewy bodies and Lewy neurites of sporadic PD (1). Three different mutations in the α-synuclein gene (A53T, A30P and E46K) cause autosomal-dominant hereditary Parkinsonism, and the α-synuclein locus appears to be associated with sporadic PD (2–4). Synphilin-1 is a cytoplasmic protein that interacts with α-synuclein (5,6). Co-expression of α-synuclein and synphilin-1 in cultured cells results in the formation of Lewy-body-like inclusions (5,7,8), and synphilin-1 promotes the formation of α-synuclein containing aggresomes under conditions of proteasome inhibition (9,10). In cells in culture, aggresome inclusions formed by α-synuclein and synphilin-1 are associated with cytoprotection, and they are cleared from cells by autophagy (11). Synphilin-1 co-localizes with α-synuclein in Lewy bodies in brains of PD patients (12). Synphilin-1 labels the central core of Lewy bodies, whereas α-synuclein is generally present more peripherally. A point mutation (R621C) in synphilin-1 has been found in some sporadic PD patients (13).
Genetic mouse models are powerful tools for understanding the pathogenesis of neurodegenerative diseases including PD. Recently, there are several reports of overexpression of wild-type and mutant synphilin-1 (R621C) in the mouse brain (14–16), though the phenotypes of these mice from different groups are inconsistent. One group found that expression of synphilin-1 does not cause any indication of neuronal degeneration in brain (14). The other groups found that expression of wild-type or mutant synphilin-1 resulted in moderate aggregate formation and subtle neurodegeneration in certain regions of the brain (15,16), though the specificity of these changes are not clear. Therefore, the in vivo role of synphilin-1 in α-synuclein pathology remains to be elucidated. Several α-synuclein transgenic mouse models have been generated to overexpress the wild-type or mutant protein (17). A wide range of phenotypes has been produced using several different constructs and different promoters. Generally, overexpression of α-synuclein in mice results in motor dysfunction, α-synuclein accumulation and early mortality, although without dopaminergic neuron degeneration in substantia nigra (18–23). There is α-synuclein aggregation, but not necessarily in dopaminergic neurons, and not with the characteristic appearance of Lewy bodies. Lee et al. (23) reported transgenic mice overexpressing A53T α-synuclein using the mouse prion protein promoter (mPrP), which have a dramatically progressive behavioral phenotype and neurodegeneration . In these mice, there is neuronal degeneration in the caudal brainstem and spinal cord, although not in the substantia nigra. The fatal behavioral phenotype likely reflects this distribution. Other mouse models based on α-synuclein have less dramatic progressive phenotypes. Thus, we employed A53T α-synuclein mice generated by Lee et al. to study the interaction between synphilin-1 and α-synuclein.
In this study, we generated transgenic mice expressing human synphilin-1 using mPrP-promoter. Synphilin-1 transgenic mice did not display PD-like pathology. We further generated double-transgenic mice by crossing the Synphilin-1 mice with transgenic mice in which A53T α-synuclein expression is also driven by mPrP promoter (line G2-30, from Lee et al.) (23). Synphilin-1/A53T α-synuclein double-transgenic mice survived longer than A53T α-synuclein single-transgenic mice. Expression of synphilin-1 attenuated A53T α-synuclein-induced motor abnormalities, astroglial reaction and neuronal degeneration and diminished the severity of α-synucleinopathy in the brain of double-transgenic mice. Double-transgenic mice displayed fewer α-synuclein aggregates at the preclinical stage and more aggresome-like structures at the end stage. Correspondingly, levels of beclin-1 and LC3 II in double-transgenic mice were higher than A53T single-transgenic mice in the preclinical stage, suggesting that autophagy may be involved in the clearance of inclusions containing both synphilin-1 and synuclein, resulting in protection against α-synuclein toxicity. These studies are the first report showing that synphilin-1 plays a neuroprotective role against A53T α-synuclein toxicity in transgenic mice in vivo.
RESULTS
Synphilin-1 increased survival and attenuated hyperactivity in double-transgenic mice
Synphilin-1 was expressed well in mouse brain homogenates (Fig. 1A and B). Immunohistochemical analysis showed that synphilin-1 was predominantly expressed in neurons throughout the brain (data not shown), consistent with the mPrP-driven gene expression pattern (24,25). The life span of all synphilin-1 mice was the same as non-transgenic mice. We did not find any PD-like behavioral and neurodegenerative pathology in synphilin-1 mice. The body weight of synphilin-1 mice was increased (described elsewhere).
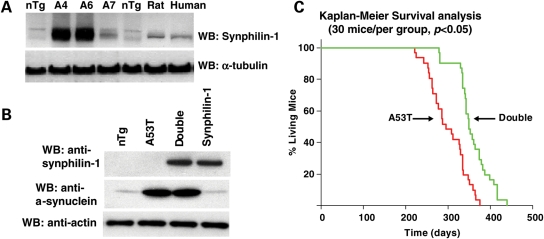
Figure 1.
Expression of synphilin-1 increased survival of A53T α-synuclein transgenic mice. (A) Western blot analysis of brain homogenates of non-transgenic mouse (nTg), synphilin-1 transgenic mouse (A6, A4 and A7 lines), rat and human cortex, using a rabbit anti-human synphilin-1 antibody. (B) Western blot analysis of synphilin-1 and A53T-α-synulein expression in double-transgenic mice by anti-synphilin-1 or anti-α-synuclein antibodies. (C) Survival curves for A53T α-synuclein transgenic and double-transgenic mice. Double-transgenic mice displayed a longer life span compared with A53T α-synuclein transgenic mice by Kaplan–Meier survival analysis (log-rank followed by Holm–Sidak method). There were 30 mice (half male and half female) of each genotype. P < 0.05.
A53T single-transgenic mice displayed abnormal behavior, earlier mortality and Parkinsonism-like pathology as described previously (23). The abnormal behavior included hyperactivity at 5–7 months, sustained posturing, bradykinesia, mild ataxia and dystonia. The mice eventually developed progressive movement dysfunction, paralysis, which rapidly progressed to death. Double-transgenic mice survived longer than A53T α-synuclein mice (Fig. 1C). Kaplan–Meier survival analysis indicated that enhanced survival was statistically significant (log-rank followed Holm–Sidak method, P < 0.05). The ages at which 50% of double-transgenic and A53T α-synuclein mice survived were 296 ± 16.2 and 263 ± 9.7 days, respectively. Double-transgenic generally displayed a similar PD-like phenotype to A53T α-synuclein transgenic mice. However, double-transgenic mice had delayed onset of behavioral abnormalities. The average ages of onset for the clinical abnormalities of A53T α-synuclein mice and double-transgenic mice were 8.2 and 9.8 months, respectively.
There was no difference in novelty-induced locomotor activity between synphilin-1 transgenic mice and non-transgenic mice throughout the life by the open-field test (Fig. 2). In contrast, the A53T α-synuclein transgenic mice showed significantly increased horizontal and vertical activity compared with non-transgenic mice as reported previously (23). The double-transgenic mice did not display hyperactivity at any time point, but had a delayed PD-like behavior phenotype similar to A53T transgenic mice. One month before death, double-transgenic mice showed a gradual decrease of locomotor activity, gradual paralysis, eventually progressing to death.
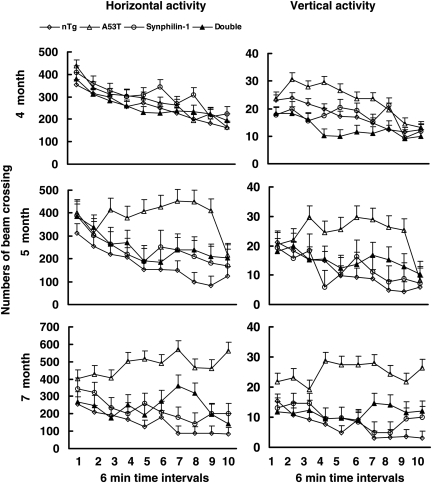
Figure 2.
Synphilin-1 attenuated A53T α-synuclein-induced hyperactivity. Novelty-induced activity was measured in cohorts of six to eight mice per group at 4–7 months of age in an open-field test. Horizontal and vertical activities were automatically recorded. The data are presented as the means (beams broken) ± SEM. There was no difference in the novelty-induced activity between mice of various groups at 4 months of age. At 5 and 7 months of age, A53T mice demonstrated significantly increased novelty-induced horizontal and vertical activities compared with mice of other groups (P < 0.001, by statistic analysis with the group by time interaction; P < 0.05 by two-way ANOVA and post hoc Holm–Sidak test). The double-transgenic mice did not display hyperactivity throughout life.
Synphilin-1 delayed α-synucleinopathy and promoted aggresome-like inclusion formation
At the preclinical stage (without bradykinesia, mild ataxia, dystonia and paralysis), the abnormal accumulation of α-synuclein in the brainstem in A53T mice was more severe than that in double-transgenic mice (Fig. 3A–C). Recent report shows that autophagy plays a role in the formation and clearance of synuclein/synphilin-1 inclusions in cells in culture (9). Beclin-1 is essential for autophagosome formation in mammalian species (26,27). Autophagosomes fuse with lysosomes to generate autophagolysosomes, which undergo a maturation process by fusing with endocytic compartments and lysosomes (26) that leads to degradation. Decreased beclin-1 levels cause defective autophagy, but restoration of beclin-1 induces autophagy (27). Thus, we used anti-beclin-1 (a marker for autophagy activation) antibodies to perform western blot analysis and immunostaining. We found that beclin-1 levels were increased in brains of double-transgenic mice compared with A53T single-transgenic mice (Fig.3C and D).
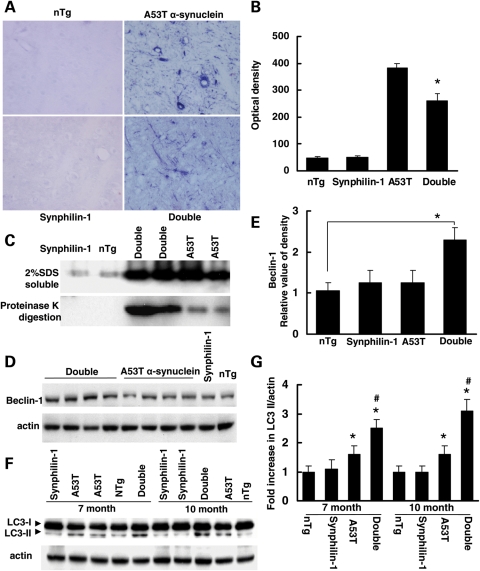
Figure 3.
Abnormal accumulation of α-synuclein was less in double-transgenic mice at the preclinical stage. (A and B) Brain sections from mice at 7 months of age (preclinical stage) were digested with proteinase K for 3 h and then followed by immunostaining using anti-α-synuclein antibodies. Double-transgenic mice displayed less accumulation of abnormal α-synulcein after proteinase K digestion compared with A53T mice at 7 months of age. (A) Representative images of anti-α-synuclein immunostaining of various experimental groups after proteinase K digestion. (B) Graph showing the quantification of α-synuclein accumulation after proteinase K digestion in pons at 7 months of age. (C) Western blot analysis of brainstem homogenates of mice at 7 months of age (preclinical stage) using anti-α-synuclein antibodies. Top, 2% SDS-soluble homogenates; bottom, homogenates were digested with proteinase K followed by western blot analysis using anti-α-synuclein antibodies. (D) Western blot analysis of brain homogenates of mice at 7 months of age (preclinical stage) using anti-beclin-1 antibodies. (E) Graph showing the quantification of (D). P < 0.05 by ANOVA. (F) Western blot analysis of brainstem homogenates (2% SDS-soluble samples) of mice using anti-LC3 and anti-actin antibodies. (G) Graph showing the quantification of (F). *P < 0.05 by ANOVA versus aged-matched non-transgenic control mice or synphilin-1 transgenic mice; #P < 0.05 by ANOVA versus aged-matched A53T single-transgenic mice.
LC3-II is a constituent of the autophagic vacuole membrane and is another reliable marker of autophagy (28). We assessed LC3 levels in brainstem regions of these mice at 1, 7 and 10 months of age. LC3-II levels in double- or A53T single-transgenic mice were not altered at 1 month, a time when there were no disease signs. However, the LC3 II levels were significantly increased in double-transgenic mice at 7 and 10 months, compared with A53T single-transgenic mice (Fig. 3F and G). LC3-II levels in A53T mice were also slightly increased compared with non-transgenic control or synphilin-1 transgenic mice. We did not detect the significant change in LC3 II levels in the cortex among all groups of mice at various time points. These results indicate that the autophagy pathway was more active in double-transgenic mice than in A53T single-transgenic mice.
At end-stage disease when mice displayed severe movement abnormalities such as paralysis, both A53T single- and double-transgenic mice had substantial brain synucleinopathy. There were many cytoplasmic inclusions that were positive to both α-synuclein and synphilin-1 (Fig. 4). There were abnormal ubiquitin accumulation in neuronal bodies and neurities (Fig. 4B), suggesting that the ubiquitin–proteasome system (UPS) was severely impaired. These inclusions were resistant to proteinase K digestion (Fig. 4D). Abnormal neuronal accumulations of α-synuclein, synphilin-1 and ubiquitin were present within the brainstem, deep cerebellar nuclei, reticulo-pontine nuclei and neocortex. There was no qualitative difference in α-synuclein immunostaining after proteinase K digestion between double-transgenic and A53T single-transgenic mice. These pathological features were not obvious in younger (1- to 4-month-old) double-transgenic or A53T single-transgenic mice (data not shown).
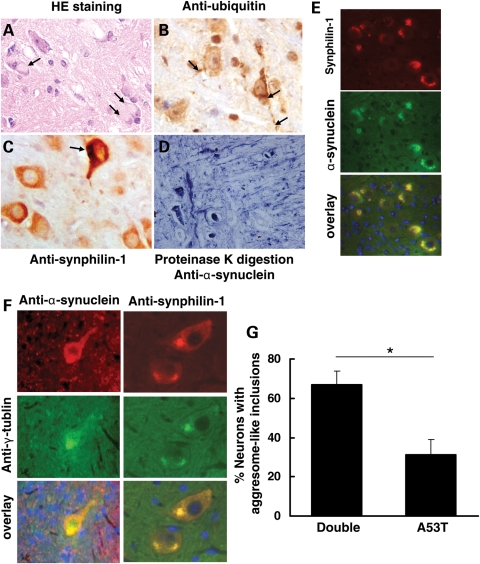
Figure 4.
Synphilin-1 promoted aggresome-like inclusion formation in double-transgenic mice at end-stage disease. (A) Sagittal mouse brain sections were subjected to hematoxylin and eosin (HE) staining, immunostaining with DAB detection using anti-ubiquitin (B) and anti-synphilin-1 (C) antibodies, proteinase K digestion followed by anti-α-synuclein staining (D), double-immunostaining using anti-α-synuclein and anti-synphilin-1 antibodies (E). (A) Eosinophilic cytoplasmic inclusions (arrow) in the pons in sick double-transgenic mice by HE staining. (B) Ubiquitin pathology in sick double-transgenic mice. Pathological somal and neuritic (arrow) accumulation of ubiquitin was prominent in pons. (C) Synphilin-1 was highly expressed in neurons in pons in sick double-transgenic mice. Arrow showing the synphilin-1-positive inclusions. (D) Brain sections from double transgenic mice over digested with proteinase for 3 h and then followed by immunostaining using anti-α-synuclein antibodies. The representative image showing the abnormal accumlation of α-synuclein in pons in sick double-transgenic mice (11 months). (E) Inclusions in double-transgenic mice labeled using anti-α-synuclein and anti-synphilin-1antibodies. Red, α-synuclein; green or synphilin-1 staining; DAPI staining for nuclei. (F) Aggresome-like inclusions in double-transgenic mice. Red, α-synuclein (left) or synphilin-1 (right) staining; green, γ-tublin staining; DAPI staining for nuclei. (G) Graph showing the quantification of neurons in brainstem with inclusions that were positively stained with both anti-α-synuclein and anti-γ-tublin antibodies, which resembled aggresome-like structures. P < 0.05 by Student's t-test.
In double-transgenic mice, many α-synuclein-containing inclusions were large, round shape, present in the cytosol and resembled aggresomes (Fig. 4A). Aggresomes are formed by transporting small protein aggregates in a microtubule-dependent manner to the centrosome, forming a big protein aggregation organelle (29). Aggresomes are usually seen in mammalian cells after the inhibition of the proteasome (30). We performed double-immunostaining using anti-γ-tublin (centrosome marker indicating aggresome formation) and anti-α-synuclein (or anti-synphilin-1) antibodies. We found that 67% of cells with α-synuclein inclusions had positive anti-γ-tublin immunoreactivity in double-transgenic mice. In contrast, only 31% of cells with α-synuclein inclusions in A53T single-transgenic mice had positive anti-γ-tublin immunoreactivity (Fig. 4F and G). There were ∼90% of the α-synuclein aggregates containing synphilin-1-positive labeling in double-transgenic mice and ∼75% of synphilin-1-positive aggregates containing γ-tublin-positive labeling. These results indicated that overexpression of synphilin-1 promoted formation of aggresome-like structures in double-transgenic mice with end-stage disease.
Synphilin-1 attenuated A53T α-synuclein-induced neuronal degeneration
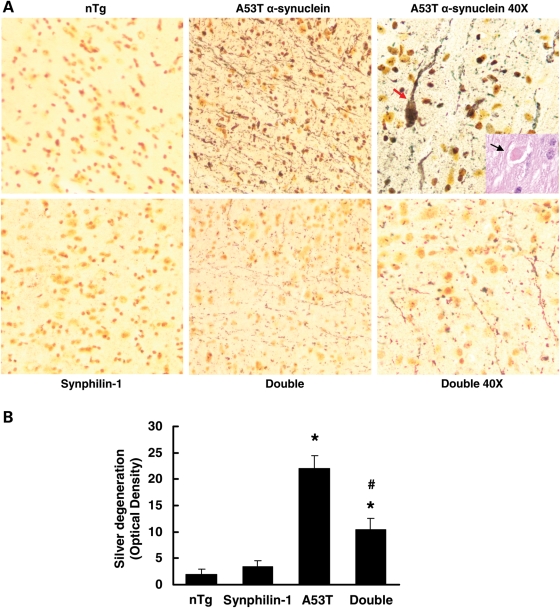
In double-transgenic mice, the most conspicuous axonal degeneration (swellings) was observed in the brainstem (Fig. 5A and B), spinal cord and forebrain white matter (data not shown) at the end-stage similar to A53T transgenic mice (23). At the 7 and 9 months of age, axonal degeneration was significantly reduced in these regions of double-transgenic mice compared with A53T transgenic mice. Densitometry quantification of silver staining revealed that brainstem degeneration in double-transgenic mice was reduced by 50% compared with A53T mice (Fig. 5B). Axonal degeneration was inconspicuous in age-matched non-transgenic and synphilin-1 transgenic littermate controls (Fig. 5).
Figure 5.
Synphilin-1 attenuated A53T α-synuclein-induced axonal degeneration in brainstem. HE and silver staining was used to visualize axonal degeneration in brainstem of various mice from each experimental group. (A) Representative images of various brainstems with silver staining in sagittal sections that were matched for level. The yellow-gold color is the typical background seen with FD NeuroSilver staining. Axonal degeneration in the brainstem of age-matched non-transgenic littermates is inconspicuous. The brainstem in A53T transgenic mice shows prominent axonal degeneration (black fibers), including axonal swellings (arrow). The inside rectangle shows a typical axonal swelling by HE staining. The axonal degeneration in double-transgenic mice was dramatically decreased compared with A53T transgenic mice. (B) Graph showing the quantification of silver degeneration in brainstem at 9 months of age. Values are mean ± SEM. *P < 0.05, versus non-transgenic mice; #P< 0.05, versus A53T α-synuclein mice by ANOVA.
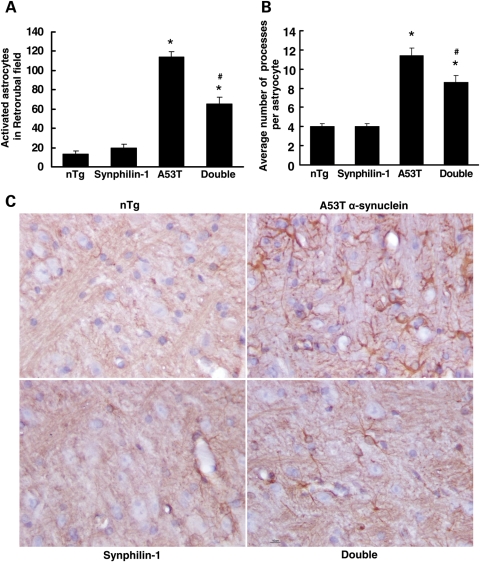
At the end-stage of both double-transgenic and A53T transgenic mice, the astroglial reaction (an indication of degeneration) was prominent in affected brain regions including the dorsal midbrain, deep cerebella nuclei, spinal cord (data not shown) and brainstem (Fig. 6), as reported previously (23). At 9 months of age, double-transgenic mice displayed a significant reduction of astroglial reaction in these regions. The reactive astryocytes in the brainstem retrorubal field of double-transgenic mice were decreased by 43% compared with A53T transgenic mice (Fig. 6A). The average number of processes per astrocyte was also significantly decreased in double-transgenic mice compared with A53T α-synuclein mice (Fig. 6B).
Figure 6.
Synphilin-1 reduced A53T α-synuclein-induced astroglial activation in the brainstem. Sagittal mouse brain sections from 9-month-old mice were subjected to GFAP immunostaining. (A and B) Graph showing the quantification of GFAP-positive cells in the brainstem retrorubal field and facial motor nucleus at 9 months of age. Values represent mean ± SEM. *P < 0.05, versus non-transgenic mice; #P < 0.05, versus A53T α-synuclein mice by ANOVA. (C) Representative images of brainstem immunostaining for GFAP in sagittal sections that were matched for level.
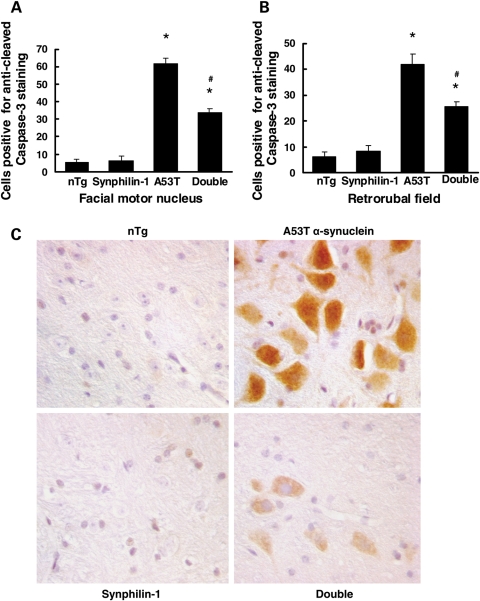
In A53T mice, many motor neurons showed positive staining for cleaved caspase-3 in the brainstem and spinal cord, suggesting an increase in apoptotic pathway activation. Moreover, the number of cleaved caspase-3-positive neurons in the brainstem of A53T mice increased as the disease progressed, consistent with previous studies (23). In contrast, in double-transgenic mice, fewer motor neurons stained positive for cleaved caspase-3 with weak immunoreactivity compared with age-matched A53T α-synuclein mice. In age-matched non-transgenic or synphilin-1 transgenic mice, there was almost no immunoreactivity for anti-cleaved caspase-3 (Fig. 7). These results indicated that synphilin-1 protects against mutant α-synuclein-induced neuronal degeneration.
Figure 7.
Synphilin-1 reduced A53T α-synuclein-induced caspase-3 activation. (A and B) Graphs showing the quantification of the number of positive motor neurons for anti-cleaved caspase-3 immunostaining in the brainstem of various mice at 7 months of age. Values are mean ± SEM. *P < 0.05, versus non-transgenic mice, #P < 0.05, versus A53T α-synuclein transgenic mice by ANOVA. (C) Representative images of immunostaining using anti-cleaved caspase-3 antibodies.
DISCUSSION
In this study, we found that overexpression of synphilin-1 attenuated A53T α-synuclein-induced motor abnormalities and decreased astroglial reaction and neuronal degeneration. Moreover, overexpression of synphilin-1 increased beclin-1 and LC3-II levels, promoted formation of aggresome-like structures and diminished the severity of α-synucleinpathy in the brain of double-transgenic mice. Synphilin-1/A53T α-synuclein double-transgenic mice had delayed α-synuclein pathology and survived longer than A53T α-synuclein single-transgenic mice. These results indicate that synphilin-1 plays a neuroprotective role against A53T α-synuclein toxicity in transgenic mice in vivo.
We did not find any features of Parkinsonism in synphilin-1 single-transgenic mice by examining locomotor activity, brain pathology (neuronal degeneration and protein aggregation) and survival. We did find that expression of synphilin-1 significantly increased body weight (described elsewhere). These data are consistent with recent reports showing that there is no loss of dopamine neurons in the substantia nigra in synphilin-1 transgenic mice (14). The other group recently showed that expression of either wild-type or mutant R621C synphilin-1 induced moderate aggregate formation and subtle neurodegeneration in the mouse brain (15,16). This may be due to overexpression of synphilin-1 locally at high concentration via the adenoviral delivery system, or due to the different expression patterns of synphilin-1 in the mouse brain.
Neuronal degeneration is one key feature of Parkinsonism. Consistent with previous reports (23), A53T single-transgenic mice displayed neuronal degeneration determined by the following indices: an increase in astroglial reaction, prominent axonal injury and activation of apoptotic pathways. In double-transgenic mice, overexpression of synphilin-1 clearly slowed down or protected against A53T α-synuclein-induced neuronal degeneration, ameliorated A53T α-synuclein-induced motor abnormalities and increased the survival of the mice. Previous reports have shown that synphilin-1 displays a protective function against staurosporine and 6-hydroxydopamine toxicity by reducing the hydrolysis of procaspase-3, decreasing poly (ADP-ribose) polymerase cleavage and reducing p53 transcriptional activity and expression (31). Our results showed that overexpression of synphilin-1 reduced caspase-3 activation in neurons in the brain of double-transgenic mice, indicating that synphilin-1 can reduce some aspects of apoptotic pathway activation and ameliorate α-synuclein toxicity in double-transgenic mice.
Protein aggregation is another hallmark of PD pathogenesis. Synphilin-1 is one of the major constituents of Lewy bodies (12). Moreover, synphilin-1 has been shown to interact with α-synuclein, the main constituent of Lewy bodies, in a yeast two-hybrid screen and in cellular model systems (5,7,8). Previous studies show that inclusion body formation provides a defense mechanism against build up of a soluble toxic load by channeling this load to an inert location for subsequent handling by autophagy (32). Inclusions are thought to represent transit stations for protein cargoes destined for degradation to make their final exit from the cell. A recent live cell imaging study suggests that the sequestration of α-synuclein into aggresomes facilitates its clearance from the cell (33).
Recent studies show that synphilin-1 contains an ankyrin-like repeat domain that acts as an aggresome-targeting signal. In contrast, formation of multiple small aggregates requires a different segment within synphilin-1, indicating that some aspects of aggregation and aggresome formation determinants can be separated biochemically (10). Small protein aggregates can be transported in a microtubule-dependent manner to the centrosome, forming an organelle called aggresome (29). Aggresomes can be seen in mammalian cells after the inhibition of the proteasome (30,34). The aggresome serves as a storage compartment for protein aggregates and could be actively involved in their refolding and degradation by autophagic clearance pathways (35). In our study, there were more neurons with aggresome-like structures in double-transgenic mice than A53T single-transgenic mice at end stage disease. These results suggest that synphilin-1 promotes the formation of aggresome-like structures, consistent with a notion that aggresome represents a protective cellular response to toxic protein (30). In line with this idea, an in vitro study shows that aggresomes formed by synphilin-1 and α-synuclein appear to be more prevalent in non-apoptotic cells (11).
α-Synuclein degradation depends on the pathways of the UPS and autophagic system (9,36,37). Autophagy appears to be more effective in α-synuclein degradation than the proteasomal pathways (36,38). Supporting this possibility is the finding that the autophagy activator, rapamycin, stimulates α-synuclein clearance (39,40). In PD and Lewy body diseases, α-synuclein accumulation has been linked to alterations in autophagy and lysosomal functioning (28,38,41). The autophagy pathway is disrupted in patients and in animal models that involve accumulation of α-synuclein (28,42,43). In our study, expression of synphilin-1 increased beclin-1 and LC3-II levels at 7 and 10 months of age in double-transgenic mice, suggesting that synphilin-1 activation of autophagic clearance may contribute to these synphilin-1 protective effects. The decreased accumulation of proteinase K-resistant α-synuclein inclusions in double-transgenic mice may reflect the autophagic clearance of aggresome-like inclusions at a young age. Consistent with this notion is an in vitro study which shows that induction of autophagy promotes a significant reduction of inclusions in cells expressing α-synuclein/synphilin-1 under conditions of proteasome impairment (9). We also found abnormal accumulation of ubiquitin in brains of both double-transgenic and A53T single-transgenic mice, suggesting that there may be also impairment of the UPS in these mice. This result is consistent with previous findings that mutant α-synuclein causes inhibition of the UPS (44,45).
Nevertheless, synphilin-1-enhanced autophagic clearance of aggregated proteins appears to be insufficient to clear all of the intracellular accumulation of α-synuclein. Thus, synphilin-1 only can delay α-synuclein pathology but not totally reverse PD-like phenotypes in double-transgenic mice. We detected a slight increase in LC-3 II levels in A53T single-transgenic mice at 7 and 10 months, indicating that there was an alteration in autophagy pathway by A53T α-synuclein expression alone. This is consistent with previous reports showing that mutant α-synuclein stimulated autophagy in an attempt to clear aberrant α-synuclein inclusions (42). The synphilin-1-induced increase of beclin-1 levels in double-transgenic mice may contribute to neuronal protection by multiple mechanisms. Beclin-1 not only increases autophagy but also binds Bcl2 to block apoptosis (46,47). A recent report shows that lentivirus vector delivery of beclin-1 into an α-synuclein mouse model of PD activates autophagy and reduces accumulation of α-synuclein (43).
Taken together, the data indicate that transgenic expression of synphilin-1 delayed A53T α-synuclein-induced neuronal degeneration, normalized hyperactivity and increased survival in double-transgenic mice. Synphilin-1 neuroprotection may be related to its role in promoting aggresome formation and increasing autophagic clearance of abnormal α-synuclein in double-transgenic mice. This study elucidates aspects of cellular protective responses to abnormal α-synuclein, which may facilitate identification of therapeutic targets in PD.
MATERIALS AND METHODS
Transgenic mice
To generate synphilin-1 transgenic mice, a cDNA encoding human synphilin-1 was cloned into the mPrP transgenic expression vector (24). The resulting plasmid, mPrP-human-synphilin-1, expressed well in HEK293T. After microinjection, we obtained 20 positive founders from 120 mice. We established two lines, A6 and A4, which had high levels of expression of synphilin-1 in the brain. Expression of synphilin-1 in the A6 line was slightly higher than A4, so A6 was used in the current studies. We generated synphilin-1 and α-synuclein double-transgenic mice on a hybrid C3H/B6 background. Female synphilin-1 mice were crossed with male A53T transgenic mice. The offspring of double-transgenic male were crossed with C3H/B6F1 female to generate all the test animals, including non-transgenic, A53T α-synuclein, synphilin-1 and double-transgenic mice. Mice were genotyped for the insertion of the transgene with a three-way PCR analysis of tail DNA with the following primers: PrP-sense, PrP-antisense and human synphilin-1 or human α-synuclein primer as described previously (23). The mice with different genotypes in the comparison experiments were littermates.
Immunoblotting and antibodies
The mouse brain homogenates were prepared in TNE buffer (10 mm Tris–HCl, pH 7.4/150 mm NaCl/5 mm EDTA) containing protease inhibitors (5 mm PMSF/10 μg/ml of aprotinin/10 μg/ml of leupeptin/10 μg/ml of pepstatin) and detergents (0.5% Nonidet P-40 and 1–2% SDS). Rabbit anti-human synphilin-1 polyclonal antibody was developed as described (5). Anti-cleaved caspase-3 and anti-Beclin-1 antibodies were from Cell Signaling Technology. Anti-glial fibrillary acidic protein (GFAP) antibody was obtained from DAKO. Anti-actin antibody was from Santa Cruz. Anti-α-synuclein monoclonal antibody (directed against amino acids 15–123 of rat α-synuclein but cross-reacting well with human α-synuclein and mouse α-synuclein) was from BD Biosciences (Palo Alto, CA, USA) and used for western blot analysis. For immunohistochemical analysis, we used an anti-human-α-synuclein antibody from Sigma. Anti-LC3 antibody was from AXXORA LLC. Anti-γ-tublin polyclonal antibody was from Sigma, and monoclonal antibody was from ABR Affinity Bioreagents. For proteinase k digestion experiments, brain homogenates were incubated with 50 μg/ml proteinase K overnight at 55°C as described previously (48,49). The undigested material of the pellet was resuspended in 100% formic acid and incubated at 37°C for 30 min as described (50), and then the homogenate was dried in a speed-vac. The resulting dried material was finally resuspended in loading buffer prior to the standard western blotting analysis as described (8).
Open-field assay
Novelty-induced activity was assessed in the open field over a 60 min period using activity chambers with infrared beams (San Diego Instruments, Inc., San Diego, CA, USA). Horizontal and vertical activities were automatically recorded. Mice were monitored at ages of 4–9 months. The data are presented as the means (beams broken) ± SEM. The results were analyzed using two-way repeated measures ANOVA with post hoc multiple comparisons whenever appropriate.
Immunohistochemistry
For immunohistochemical analysis, mice were perfused with PBS followed by 4% paraformaldehyde. Brains were processed as either frozen or paraffin-embedded sections (25). Both frozen and paraffin sections were processed for immunohistochemical analyses using antibodies that recognized GFAP, cleaved-caspase 3, synphilin-1, ubiquitin, γ-tublin and α-synuclein. A cohort of four to eight mice for each time point and each transgenic group was analyzed. For proteinase K digestion, the paraffin sections were incubated with proteinase K for 3 h at 55°C and then subjected to α-synuclein immunostaining. For the quantification of abnormal accumulation of α-synuclein after proteinase K digestion in the brainstem, entire transverse sections were digitized with an image analysis system. The NIH imageJ software was used to measure the optical density of anti-α-synuclein immunostaining in pons (six sections per mouse).
Cell counting
Profile counting was used to estimate the numbers of astrocytes, cleaved caspase-3-positive cells and inclusion-containing cells in immunolabeled sections as described (51). The regions analyzed were the brainstem retrorubral field and facial motor nucleus. In sagittal sections that were matched for level, the number of activated astrocytes (more than five processes) and positive cells for anti-cleaved caspase-3 immunostaining was counted in six non-overlapping microscopic fields at ×400 magnification. Motor neurons were identified by their larger size compared with astrocytes, oligodendrocytes and microglia, which were excluded from the caspase-3-positive neuron counts. Quantification of the activated astrocyte in GFAP-immunostained sagittal sections were matched for level. The number of primary and secondary processes of GFAP-positive cells was counted in 10 non-overlapping microscopic fields at ×400 magnification. The average number of processes for each cell was calculated. Group means and variances were evaluated statistically by one-way ANOVA and a Newman–Keuls post hoc test.
Silver staining
Silver staining was used to visualize degenerating neuronal elements in the brain. Sections were processed with the FD NeuroSilver kit (FD Neurotechnologies, Baltimore, MD, USA). For the quantification of degeneration in the brain stem, entire transverse sections were digitized with an image analysis system. The NIH imageJ software was used to measure the optical density of silver staining within the entire section (six sections per mouse) as described (52). Comparisons among groups were analyzed with a one-way ANOVA and a Newman–Keuls post hoc test.
FUNDING
This work is supported by NIH grant NS38377 and RO1NS055252.
ACKNOWLEDGEMENTS
We thank XiaoFang Wang, Yi Yu, Masayuki Nakamura, Hilda Slunt, Wanda Stirling, Yanqun Xu and Olga Pletnikova for assistance or technical support. We thank Dr Simone Engelender and Dr Haibing Jiang for helpful discussion. T.M.D. is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. doi:10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 2.Polymeropoulos M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. doi:10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 3.Zarranz J.J., Alegre J., Gomez-Esteban J.C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atares B., et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. doi:10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 4.Kruger R., Kuhn W., Muller T., Woitalla D., Graeber M., Kosel S., Przuntek H., Epplen J.T., Schols L., Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat. Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. doi:10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 5.Engelender S., Kaminsky Z., Guo X., Sharp A.H., Amaravi R.K., Kleiderlein J.J., Margolis R.L., Troncoso J.C., Lanahan A.A., Worley P.F., et al. Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions. Nat. Genet. 1999;22:110–114. doi: 10.1038/8820. doi:10.1038/8820. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro C.S., Carneiro K., Ross C.A., Menezes J.R., Engelender S. Synphilin-1 is developmentally localized to synaptic terminals, and its association with synaptic vesicles is modulated by alpha-synuclein. J. Biol. Chem. 2002;277:23927–23933. doi: 10.1074/jbc.M201115200. doi:10.1074/jbc.M201115200. [DOI] [PubMed] [Google Scholar]
- 7.Chung K.K., Zhang Y., Lim K.L., Tanaka Y., Huang H., Gao J., Ross C.A., Dawson V.L., Dawson T.M. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat. Med. 2001;7:1144–1150. doi: 10.1038/nm1001-1144. doi:10.1038/nm1001-1144. [DOI] [PubMed] [Google Scholar]
- 8.Smith W.W., Margolis R.L., Li X., Troncoso J.C., Lee M.K., Dawson V.L., Dawson T.M., Iwatsubo T., Ross C.A. Alpha-synuclein phosphorylation enhances eosinophilic cytoplasmic inclusion formation in SH-SY5Y cells. J. Neurosci. 2005;25:5544–5552. doi: 10.1523/JNEUROSCI.0482-05.2005. doi:10.1523/JNEUROSCI.0482-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong E.S., Tan J.M., Soong W.E., Hussein K., Nukina N., Dawson V.L., Dawson T.M., Cuervo A.M., Lim K.L. Autophagy-mediated clearance of aggresomes is not a universal phenomenon. Hum. Mol. Genet. 2008;17:2570–2582. doi: 10.1093/hmg/ddn157. doi:10.1093/hmg/ddn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaarur N., Meriin A.B., Gabai V.L., Sherman M.Y. Triggering aggresome formation. Dissecting aggresome-targeting and aggregation signals in synphilin 1. J. Biol. Chem. 2008;283:27575–27584. doi: 10.1074/jbc.M802216200. doi:10.1074/jbc.M802216200. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M., Kim Y.M., Lee G., Junn E., Iwatsubo T., Mouradian M.M. Aggresomes formed by alpha-synuclein and synphilin-1 are cytoprotective. J. Biol. Chem. 2004;279:4625–4631. doi: 10.1074/jbc.M310994200. doi:10.1074/jbc.M310994200. [DOI] [PubMed] [Google Scholar]
- 12.Wakabayashi K., Engelender S., Yoshimoto M., Tsuji S., Ross C.A., Takahashi H. Synphilin-1 is present in Lewy bodies in Parkinson's disease. Ann. Neurol. 2000;47:521–523. doi:10.1002/1531-8249(200004)47:4<521::AID-ANA18>3.0.CO;2-B. [PubMed] [Google Scholar]
- 13.Marx F.P., Holzmann C., Strauss K.M., Li L., Eberhardt O., Gerhardt E., Cookson M.R., Hernandez D., Farrer M.J., Kachergus J., et al. Identification and functional characterization of a novel R621C mutation in the synphilin-1 gene in Parkinson's disease. Hum. Mol. Genet. 2003;12:1223–1231. doi: 10.1093/hmg/ddg134. doi:10.1093/hmg/ddg134. [DOI] [PubMed] [Google Scholar]
- 14.Jin H.G., Yamashita H., Nakamura T., Fukuba H., Takahashi T., Hiji M., Kohriyama T., Matsumoto M. Synphilin-1 transgenic mice exhibit mild motor impairments. Neurosci. Lett. 2008;445:12–17. doi: 10.1016/j.neulet.2008.08.073. doi:10.1016/j.neulet.2008.08.073. [DOI] [PubMed] [Google Scholar]
- 15.Nuber S., Franck T., Wolburg H., Schumann U., Casadei N., Fischer K., Calaminus C., Pichler B.J., Chanarat S., Teismann P., et al. Transgenic overexpression of the alpha-synuclein interacting protein synphilin-1 leads to behavioral and neuropathological alterations in mice. Neurogenetics. 2010;11:107–120. doi: 10.1007/s10048-009-0212-2. doi:10.1007/s10048-009-0212-2. [DOI] [PubMed] [Google Scholar]
- 16.Krenz A., Falkenburger B.H., Gerhardt E., Drinkut A., Schulz J.B. Aggregate formation and toxicity by wild-type and R621C synphilin-1 in the nigrostriatal system of mice using adenoviral vectors. J. Neurochem. 2009;108:139–146. doi: 10.1111/j.1471-4159.2008.05755.x. doi:10.1111/j.1471-4159.2008.05755.x. [DOI] [PubMed] [Google Scholar]
- 17.Ross C.A., Smith W.W. Gene–environment interactions in Parkinson's disease. Parkinsonism Relat. Disord. 2007;13(Suppl. 3):S309–S315. doi: 10.1016/S1353-8020(08)70022-1. doi:10.1016/S1353-8020(08)70022-1. [DOI] [PubMed] [Google Scholar]
- 18.Masliah E., Rockenstein E., Veinbergs I., Mallory M., Hashimoto M., Takeda A., Sagara Y., Sisk A., Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. doi:10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka Y., Vila M., Lincoln S., McCormack A., Picciano M., LaFrancois J., Yu X., Dickson D., Langston W.J., McGowan E., et al. Lack of nigral pathology in transgenic mice expressing human alpha-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol. Dis. 2001;8:535–539. doi: 10.1006/nbdi.2001.0392. doi:10.1006/nbdi.2001.0392. [DOI] [PubMed] [Google Scholar]
- 20.van Der P.H., Wiederhold K.H., Probst A., Barbieri S., Mistl C., Danner S., Kauffmann S., Hofele K., Spooren W.P., Ruegg M.A., et al. Neuropathology in mice expressing human alpha-synuclein. J. Neurosci. 2000;20:6021–6029. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahle P.J., Neumann M., Ozmen L., Muller V., Odoy S., Okamoto N., Jacobsen H., Iwatsubo T., Trojanowski J.Q., Takahashi H., et al. Selective insolubility of alpha-synuclein in human Lewy body diseases is recapitulated in a transgenic mouse model. Am. J. Pathol. 2001;159:2215–2225. doi: 10.1016/s0002-9440(10)63072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giasson B.I., Duda J.E., Quinn S.M., Zhang B., Trojanowski J.Q., Lee V.M. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. doi:10.1016/S0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 23.Lee M.K., Stirling W., Xu Y., Xu X., Qui D., Mandir A.S., Dawson T.M., Copeland N.G., Jenkins N.A., Price D.L. Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala-53–>Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc. Natl Acad. Sci. USA. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. doi:10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borchelt D.R., Davis J., Fischer M., Lee M.K., Slunt H.H., Ratovitsky T., Regard J., Copeland N.G., Jenkins N.A., Sisodia S.S., Price D.L. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet. Anal. 1996;13:159–163. doi: 10.1016/s1050-3862(96)00167-2. [DOI] [PubMed] [Google Scholar]
- 25.Borchelt D.R., Ratovitski T., van Lare J., Lee M.K., Gonzales V., Jenkins N.A., Copeland N.G., Price D.L., Sisodia S.S. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. doi: 10.1016/s0896-6273(00)80974-5. doi:10.1016/S0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 26.Eskelinen E.L. Maturation of autophagic vacuoles in mammalian cells. Autophagy. 2005;1:1–10. doi: 10.4161/auto.1.1.1270. doi:10.4161/auto.1.1.1270. [DOI] [PubMed] [Google Scholar]
- 27.Liang X.H., Jackson S., Seaman M., Brown K., Kempkes B., Hibshoosh H., Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. doi:10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 28.Xilouri M., Vogiatzi T., Vekrellis K., Park D., Stefanis L. Abberant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PLoS One. 2009;4:e5515. doi: 10.1371/journal.pone.0005515. doi:10.1371/journal.pone.0005515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webb J.L., Ravikumar B., Rubinsztein D.C. Microtubule disruption inhibits autophagosome-lysosome fusion: implications for studying the roles of aggresomes in polyglutamine diseases. Int. J. Biochem. Cell Biol. 2004;36:2541–2550. doi: 10.1016/j.biocel.2004.02.003. doi:10.1016/j.biocel.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Johnston J.A., Ward C.L., Kopito R.R. Aggresomes: a cellular response to misfolded proteins. J. Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. doi:10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giaime E., Sunyach C., Herrant M., Grosso S., Auberger P., McLean P.J., Checler F., da Costa C.A. Caspase-3-derived C-terminal product of synphilin-1 displays antiapoptotic function via modulation of the p53-dependent cell death pathway. J. Biol. Chem. 2006;281:11515–11522. doi: 10.1074/jbc.M508619200. doi:10.1074/jbc.M508619200. [DOI] [PubMed] [Google Scholar]
- 32.Lim K.L., Dawson V.L., Dawson T.M. Parkin-mediated lysine 63-linked polyubiquitination: a link to protein inclusions formation in Parkinson's and other conformational diseases? Neurobiol. Aging. 2006;27:524–529. doi: 10.1016/j.neurobiolaging.2005.07.023. doi:10.1016/j.neurobiolaging.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Opazo F., Krenz A., Heermann S., Schulz J.B., Falkenburger B.H. Accumulation and clearance of alpha-synuclein aggregates demonstrated by time-lapse imaging. J. Neurochem. 2008;106:529–540. doi: 10.1111/j.1471-4159.2008.05407.x. doi:10.1111/j.1471-4159.2008.05407.x. [DOI] [PubMed] [Google Scholar]
- 34.Kaganovich D., Kopito R., Frydman J. Misfolded proteins partition between two distinct quality control compartments. Nature. 2008;454:1088–1095. doi: 10.1038/nature07195. doi:10.1038/nature07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olanow C.W., Perl D.P., DeMartino G.N., McNaught K.S. Lewy-body formation is an aggresome-related process: a hypothesis. Lancet Neurol. 2004;3:496–503. doi: 10.1016/S1474-4422(04)00827-0. doi:10.1016/S1474-4422(04)00827-0. [DOI] [PubMed] [Google Scholar]
- 36.Rott R., Szargel R., Haskin J., Shani V., Shainskaya A., Manov I., Liani E., Avraham E., Engelender S. Monoubiquitylation of alpha-synuclein by seven in absentia homolog (SIAH) promotes its aggregation in dopaminergic cells. J. Biol. Chem. 2008;283:3316–3328. doi: 10.1074/jbc.M704809200. doi:10.1074/jbc.M704809200. [DOI] [PubMed] [Google Scholar]
- 37.Engelender S. Ubiquitination of alpha-synuclein and autophagy in Parkinson's disease. Autophagy. 2008;4:372–374. doi: 10.4161/auto.5604. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Vicente M., Talloczy Z., Kaushik S., Massey A.C., Mazzulli J., Mosharov E.V., Hodara R., Fredenburg R., Wu D.C., Follenzi A., et al. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J. Clin. Invest. 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webb J.L., Ravikumar B., Atkins J., Skepper J.N., Rubinsztein D.C. Alpha-synuclein is degraded by both autophagy and the proteasome. J. Biol. Chem. 2003;278:25009–25013. doi: 10.1074/jbc.M300227200. doi:10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 40.Masliah E., Rockenstein E., Adame A., Alford M., Crews L., Hashimoto M., Seubert P., Lee M., Goldstein J., Chilcote T., et al. Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. doi:10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Cuervo A.M., Stefanis L., Fredenburg R., Lansbury P.T., Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. doi:10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 42.Yu W.H., Dorado B., Figueroa H.Y., Wang L., Planel E., Cookson M.R., Clark L.N., Duff K.E. Metabolic activity determines efficacy of macroautophagic clearance of pathological oligomeric alpha-synuclein. Am. J. Pathol. 2009;175:736–747. doi: 10.2353/ajpath.2009.080928. doi:10.2353/ajpath.2009.080928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spencer B., Potkar R., Trejo M., Rockenstein E., Patrick C., Gindi R., Adame A., Wyss-Coray T., Masliah E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. J. Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. doi:10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith W.W., Jiang H., Pei Z., Tanaka Y., Morita H., Sawa A., Dawson V.L., Dawson T.M., Ross C.A. Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum. Mol. Genet. 2005;14:3801–3811. doi: 10.1093/hmg/ddi396. doi:10.1093/hmg/ddi396. [DOI] [PubMed] [Google Scholar]
- 45.Lindersson E., Beedholm R., Hojrup P., Moos T., Gai W., Hendil K.B., Jensen P.H. Proteasomal inhibition by alpha-synuclein filaments and oligomers. J. Biol. Chem. 2004;279:12924–12934. doi: 10.1074/jbc.M306390200. doi:10.1074/jbc.M306390200. [DOI] [PubMed] [Google Scholar]
- 46.Erlich S., Shohami E., Pinkas-Kramarski R. Neurodegeneration induces upregulation of Beclin 1. Autophagy. 2006;2:49–51. doi: 10.4161/auto.2156. [DOI] [PubMed] [Google Scholar]
- 47.Hamacher-Brady A., Brady N.R., Gottlieb R.A. The interplay between pro-death and pro-survival signaling pathways in myocardial ischemia/reperfusion injury: apoptosis meets autophagy. Cardiovasc. Drugs Ther. 2006;20:445–462. doi: 10.1007/s10557-006-0583-7. doi:10.1007/s10557-006-0583-7. [DOI] [PubMed] [Google Scholar]
- 48.Chen L., Feany M.B. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat. Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. doi:10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- 49.Neumann M., Kahle P.J., Giasson B.I., Ozmen L., Borroni E., Spooren W., Muller V., Odoy S., Fujiwara H., Hasegawa M., et al. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J. Clin. Invest. 2002;110:1429–1439. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ratovitski T., Nakamura M., D'Ambola J., Chighladze E., Liang Y., Wang W., Graham R., Hayden M.R., Borchelt D.R., Hirschhorn R.R., Ross C.A. N-terminal proteolysis of full-length mutant huntingtin in an inducible PC12 cell model of Huntington's disease. Cell Cycle. 2007;6:2970–2981. doi: 10.4161/cc.6.23.4992. [DOI] [PubMed] [Google Scholar]
- 51.Martin L.J., Pan Y., Price A.C., Sterling W., Copeland N.G., Jenkins N.A., Price D.L., Lee M.K. Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. doi:10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Northington F.J., Ferriero D.M., Graham E.M., Traystman R.J., Martin L.J. Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiol. Dis. 2001;8:207–219. doi: 10.1006/nbdi.2000.0371. doi:10.1006/nbdi.2000.0371. [DOI] [PubMed] [Google Scholar]