Abstract
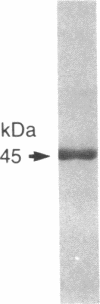
To evaluate the possibility of administering therapeutic proteins via the respiratory route, we administered an aerosol of recombinant DNA-produced human alpha 1-antitrypsin (rAAT) to anesthetized sheep and measured levels of the protein in epithelial lining fluid (ELF), lung lymph, blood, and urine. Using a nebulizer that generated aerosol droplets with a mass median aerodynamic diameter of 2.7 micron (55% of droplets were less than 3 micron, a particle size optimal for deposition on the alveolar epithelium), in vitro studies demonstrated that the aerosolized rAAT remained intact and fully functional as an inhibitor of neutrophil elastase. When aerosolized to sheep, the 45-kDa rAAT molecule diffused across the alveolar epithelium, as evidenced by its presence in lung lymph and in blood. Comparison of ELF, lymph, blood, and urine rAAT levels demonstrated that the process was concentration dependent, with highest levels in ELF and in descending concentrations with approximately 10-fold concentration differences in each consecutive compartment, respectively. Importantly, evaluation with aerosolized 125I-labeled rAAT demonstrated that the rAAT molecules that reached the lung lymph and the systemic circulation remained intact as a 45-kDa protein. These results demonstrate the feasibility of using aerosolization to the pulmonary epithelial surface to administer sizeable proteins of therapeutic interest, thus circumventing the necessity of the traditional parenteral modes of administration of such molecules.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aungst B. J., Rogers N. J., Shefter E. Comparison of nasal, rectal, buccal, sublingual and intramuscular insulin efficacy and the effects of a bile salt absorption promoter. J Pharmacol Exp Ther. 1988 Jan;244(1):23–27. [PubMed] [Google Scholar]
- Beatty K., Bieth J., Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980 May 10;255(9):3931–3934. [PubMed] [Google Scholar]
- Bell D. Y., Haseman J. A., Spock A., McLennan G., Hook G. E. Plasma proteins of the bronchoalveolar surface of the lungs of smokers and nonsmokers. Am Rev Respir Dis. 1981 Jul;124(1):72–79. doi: 10.1164/arrd.1981.124.1.72. [DOI] [PubMed] [Google Scholar]
- Bensch K. G., Dominguez E., Liebow A. A. Absorption of intact protein molecules across the pulmonary air-tissue barrier. Science. 1967 Sep 8;157(3793):1204–1206. doi: 10.1126/science.157.3793.1204. [DOI] [PubMed] [Google Scholar]
- Bignon J., Jaurand M. C., Pinchon M. C., Sapin C., Warnet J. M. Immunoelectron microscopic and immunochemical demonstrations of serum proteins in the alveolar lining material of the rat lung. Am Rev Respir Dis. 1976 Feb;113(2):109–120. doi: 10.1164/arrd.1976.113.2.109. [DOI] [PubMed] [Google Scholar]
- Brogard J. M., Blickle J. F., Paris-Bockel D. Genetically engineered insulin: five years of experience. Drugs Exp Clin Res. 1985;11(6):397–406. [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Casolaro M. A., Fells G., Wewers M., Pierce J. E., Ogushi F., Hubbard R., Sellers S., Forstrom J., Lyons D., Kawasaki G. Augmentation of lung antineutrophil elastase capacity with recombinant human alpha-1-antitrypsin. J Appl Physiol (1985) 1987 Nov;63(5):2015–2023. doi: 10.1152/jappl.1987.63.5.2015. [DOI] [PubMed] [Google Scholar]
- Chapman P. B., Lester T. J., Casper E. S., Gabrilove J. L., Wong G. Y., Kempin S. J., Gold P. J., Welt S., Warren R. S., Starnes H. F. Clinical pharmacology of recombinant human tumor necrosis factor in patients with advanced cancer. J Clin Oncol. 1987 Dec;5(12):1942–1951. doi: 10.1200/JCO.1987.5.12.1942. [DOI] [PubMed] [Google Scholar]
- Delacroix D. L., Marchandise F. X., Francis C., Sibille Y. Alpha-2-macroglobulin, monomeric and polymeric immunoglobulin A, and immunoglobulin M in bronchoalveolar lavage. Am Rev Respir Dis. 1985 Oct;132(4):829–835. doi: 10.1164/arrd.1985.132.4.829. [DOI] [PubMed] [Google Scholar]
- Eschbach J. W., Egrie J. C., Downing M. R., Browne J. K., Adamson J. W. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987 Jan 8;316(2):73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- Foon K. A., Sherwin S. A., Abrams P. G., Longo D. L., Fer M. F., Stevenson H. C., Ochs J. J., Bottino G. C., Schoenberger C. S., Zeffren J. Treatment of advanced non-Hodgkin's lymphoma with recombinant leukocyte A interferon. N Engl J Med. 1984 Nov 1;311(18):1148–1152. doi: 10.1056/NEJM198411013111803. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Zimmerman R. L., Rennard S. I., Crystal R. G. Antielastases of the human alveolar structures. Implications for the protease-antiprotease theory of emphysema. J Clin Invest. 1981 Oct;68(4):889–898. doi: 10.1172/JCI110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman B. E., Brown S. E., Crandall E. D. Regulation of transport across pulmonary alveolar epithelial cell monolayers. J Appl Physiol Respir Environ Exerc Physiol. 1984 Sep;57(3):703–710. doi: 10.1152/jappl.1984.57.3.703. [DOI] [PubMed] [Google Scholar]
- Gordon G. S., Moses A. C., Silver R. D., Flier J. S., Carey M. C. Nasal absorption of insulin: enhancement by hydrophobic bile salts. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7419–7423. doi: 10.1073/pnas.82.21.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin A. B., Stewart P. A. Differential permeability of endothelial and epithelial barriers to albumin flux. J Appl Physiol Respir Environ Exerc Physiol. 1979 Dec;47(6):1315–1324. doi: 10.1152/jappl.1979.47.6.1315. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Kaplan S. L., Underwood L. E., August G. P., Bell J. J., Blethen S. L., Blizzard R. M., Brown D. R., Foley T. P., Hintz R. L., Hopwood N. J. Clinical studies with recombinant-DNA-derived methionyl human growth hormone in growth hormone deficient children. Lancet. 1986 Mar 29;1(8483):697–700. doi: 10.1016/s0140-6736(86)91098-6. [DOI] [PubMed] [Google Scholar]
- Kirkwood J. M., Ernstoff M. S. Interferons in the treatment of human cancer. J Clin Oncol. 1984 Apr;2(4):336–352. doi: 10.1200/JCO.1984.2.4.336. [DOI] [PubMed] [Google Scholar]
- Longenecker J. P., Moses A. C., Flier J. S., Silver R. D., Carey M. C., Dubovi E. J. Effects of sodium taurodihydrofusidate on nasal absorption of insulin in sheep. J Pharm Sci. 1987 May;76(5):351–355. doi: 10.1002/jps.2600760502. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Williams M. C., Widdicombe J. H., Sanders M. J., Misfeldt D. S., Berry L. C., Jr Transepithelial transport by pulmonary alveolar type II cells in primary culture. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6033–6037. doi: 10.1073/pnas.79.19.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay M. A., Berthiaume Y., Staub N. C. Long-term clearance of liquid and protein from the lungs of unanesthetized sheep. J Appl Physiol (1985) 1985 Sep;59(3):928–934. doi: 10.1152/jappl.1985.59.3.928. [DOI] [PubMed] [Google Scholar]
- Matthay M. A., Landolt C. C., Staub N. C. Differential liquid and protein clearance from the alveoli of anesthetized sheep. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jul;53(1):96–104. doi: 10.1152/jappl.1982.53.1.96. [DOI] [PubMed] [Google Scholar]
- Meyer E. C., Ottaviano R., Higgins J. J. Albumin clearance from alveoli: tissue permeation vs. airway displacement. J Appl Physiol Respir Environ Exerc Physiol. 1977 Sep;43(3):487–497. doi: 10.1152/jappl.1977.43.3.487. [DOI] [PubMed] [Google Scholar]
- Morimoto K., Morisaka K., Kamada A. Enhancement of nasal absorption of insulin and calcitonin using polyacrylic acid gel. J Pharm Pharmacol. 1985 Feb;37(2):134–136. doi: 10.1111/j.2042-7158.1985.tb05024.x. [DOI] [PubMed] [Google Scholar]
- Morrow P. E. Factors determining hygroscopic aerosol deposition in airways. Physiol Rev. 1986 Apr;66(2):330–376. doi: 10.1152/physrev.1986.66.2.330. [DOI] [PubMed] [Google Scholar]
- Newman S. P., Pellow P. G., Clarke S. W. Droplet size distributions of nebulised aerosols for inhalation therapy. Clin Phys Physiol Meas. 1986 May;7(2):139–146. doi: 10.1088/0143-0815/7/2/004. [DOI] [PubMed] [Google Scholar]
- Nukiwa T., Satoh K., Brantly M. L., Ogushi F., Fells G. A., Courtney M., Crystal R. G. Identification of a second mutation in the protein-coding sequence of the Z type alpha 1-antitrypsin gene. J Biol Chem. 1986 Dec 5;261(34):15989–15994. [PubMed] [Google Scholar]
- Ogushi F., Fells G. A., Hubbard R. C., Straus S. D., Crystal R. G. Z-type alpha 1-antitrypsin is less competent than M1-type alpha 1-antitrypsin as an inhibitor of neutrophil elastase. J Clin Invest. 1987 Nov;80(5):1366–1374. doi: 10.1172/JCI113214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. C., Gilchrist S., Cartledge J. T. Plasma-lymph exchange and interstitial distribution volumes of charged macromolecules in the lung. J Appl Physiol (1985) 1985 Oct;59(4):1128–1136. doi: 10.1152/jappl.1985.59.4.1128. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Gemlo B. T., Myers W. W., Rayner A. A., Lanier L. L. In vivo and in vitro activation of natural killer cells in advanced cancer patients undergoing combined recombinant interleukin-2 and LAK cell therapy. J Clin Oncol. 1987 Dec;5(12):1933–1941. doi: 10.1200/JCO.1987.5.12.1933. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Basset G., Lecossier D., O'Donnell K. M., Pinkston P., Martin P. G., Crystal R. G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol (1985) 1986 Feb;60(2):532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Newball H. H. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974 Oct;84(4):559–573. [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Salzman R., Manson J. E., Griffing G. T., Kimmerle R., Ruderman N., McCall A., Stoltz E. I., Mullin C., Small D., Armstrong J. Intranasal aerosolized insulin. Mixed-meal studies and long-term use in type I diabetes. N Engl J Med. 1985 Apr 25;312(17):1078–1084. doi: 10.1056/NEJM198504253121702. [DOI] [PubMed] [Google Scholar]
- Schlesinger R. B. Comparative deposition of inhaled aerosols in experimental animals and humans: a review. J Toxicol Environ Health. 1985;15(2):197–214. doi: 10.1080/15287398509530647. [DOI] [PubMed] [Google Scholar]
- Staub N. C., Bland R. D., Brigham K. L., Demling R., Erdmann A. J., 3rd, Woolverton W. C. Preparation of chronic lung lymph fistulas in sheep. J Surg Res. 1975 Nov;19(5):315–320. doi: 10.1016/0022-4804(75)90056-6. [DOI] [PubMed] [Google Scholar]
- Vadhan-Raj S., Keating M., LeMaistre A., Hittelman W. N., McCredie K., Trujillo J. M., Broxmeyer H. E., Henney C., Gutterman J. U. Effects of recombinant human granulocyte-macrophage colony-stimulating factor in patients with myelodysplastic syndromes. N Engl J Med. 1987 Dec 17;317(25):1545–1552. doi: 10.1056/NEJM198712173172501. [DOI] [PubMed] [Google Scholar]
- Warr G. A., Martin R. R., Sharp P. M., Rossen R. D. Normal human bronchial immunoglobulins and proteins: effects of cigarette smoking. Am Rev Respir Dis. 1977 Jul;116(1):25–30. doi: 10.1164/arrd.1977.116.1.25. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. Morphometry of the human lung: the state of the art after two decades. Bull Eur Physiopathol Respir. 1979 Sep-Oct;15(5):999–1013. [PubMed] [Google Scholar]
- Wewers M. D., Casolaro M. A., Sellers S. E., Swayze S. C., McPhaul K. M., Wittes J. T., Crystal R. G. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987 Apr 23;316(17):1055–1062. doi: 10.1056/NEJM198704233161704. [DOI] [PubMed] [Google Scholar]
- Wigley F. W., Londono J. H., Wood S. H., Shipp J. C., Waldman R. H. Insulin across respiratory mucosae by aerosol delivery. Diabetes. 1971 Aug;20(8):552–556. doi: 10.2337/diab.20.8.552. [DOI] [PubMed] [Google Scholar]