Abstract
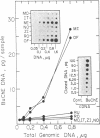
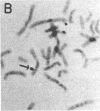
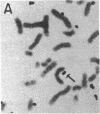
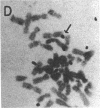
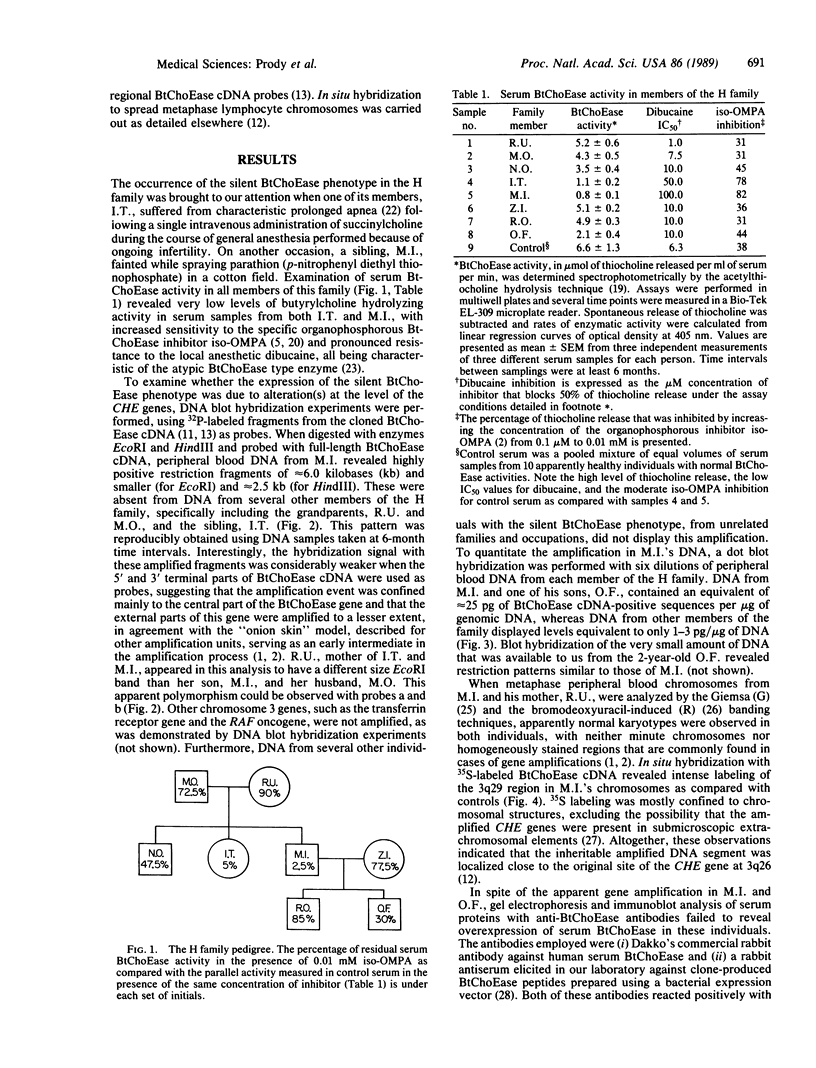
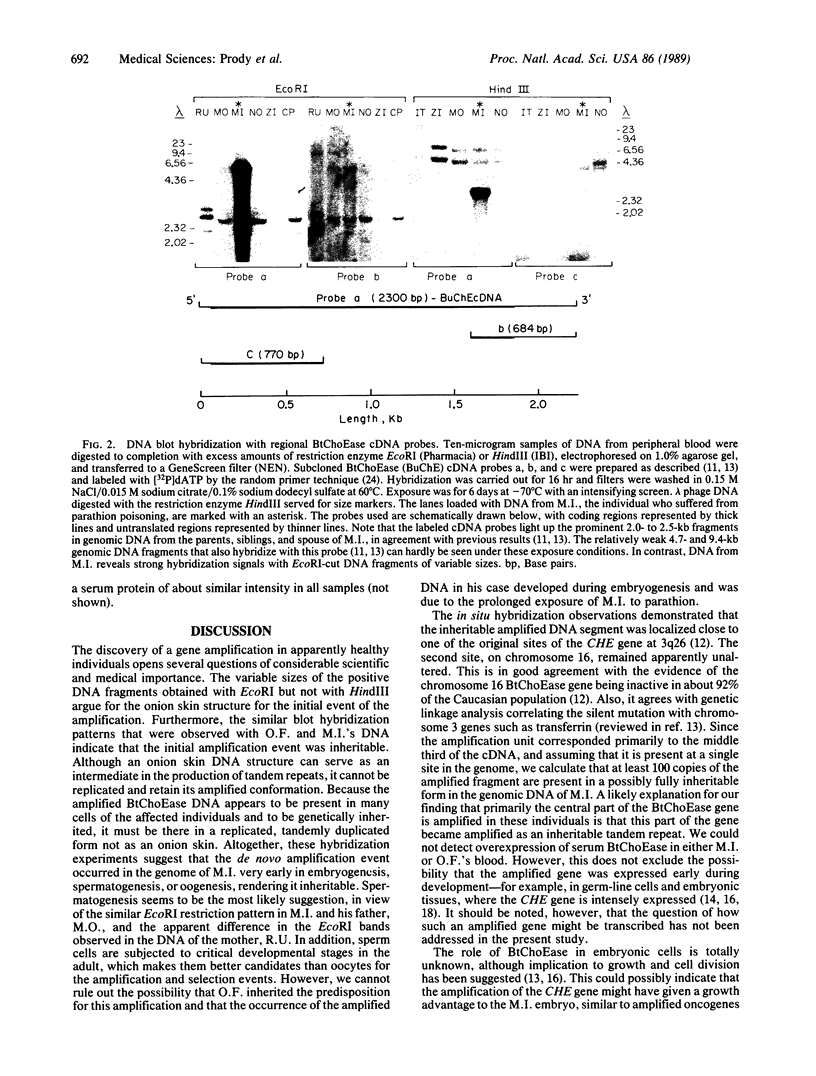
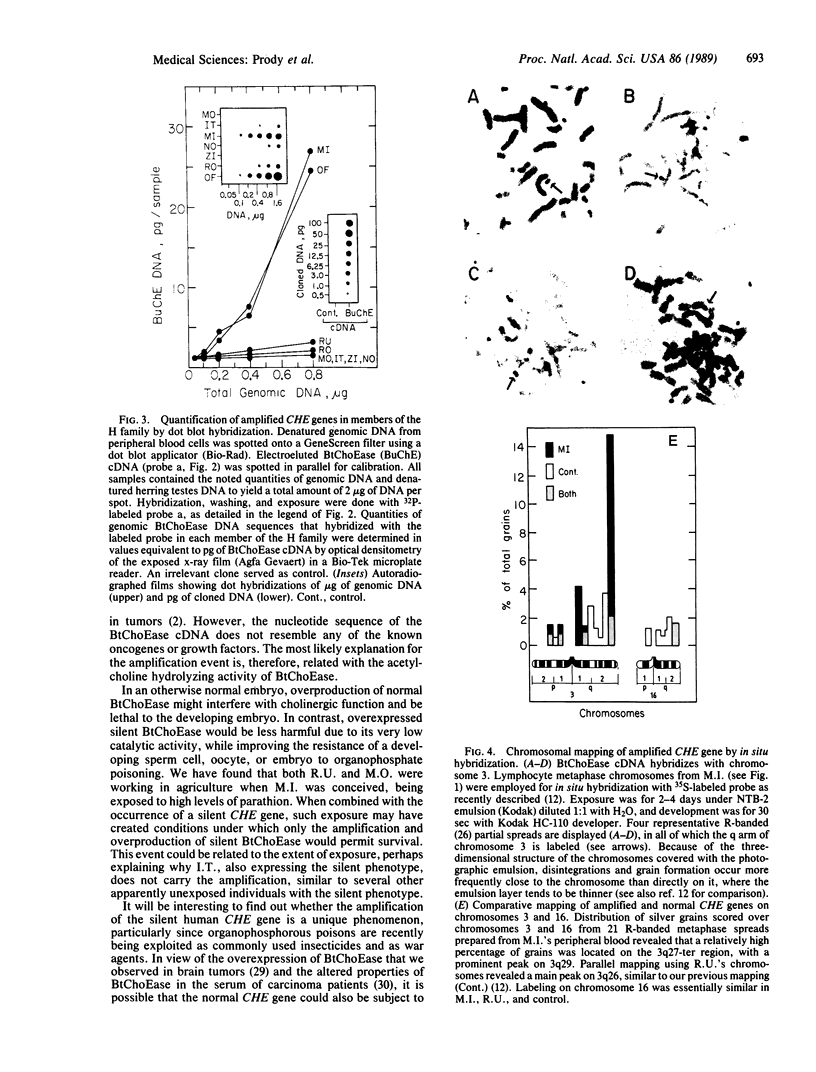
A 100-fold DNA amplification in the CHE gene, coding for serum butyrylcholinesterase (BtChoEase), was found in a farmer expressing the "silent" CHE phenotype. Individuals homozygous for this gene display a defective serum BtChoEase and are particularly vulnerable to poisoning by agricultural organophosphorous insecticides, to which all members of this family had long been exposed. DNA blot hybridization with regional BtChoEase cDNA probes suggested that the amplification was most intense in regions encoding central sequences within BtChoEase cDNA, whereas distal sequences were amplified to a much lower extent. This is in agreement with the "onion skin" model, based on amplification of genes in cultured cells and primary tumors. The amplification was absent in the grandparents but present at the same extent in one of their sons and in a grandson, with similar DNA blot hybridization patterns. In situ hybridization experiments localized the amplified sequences to the long arm of chromosome 3, close to the site where we previously mapped the CHE gene. Altogether, these observations suggest that the initial amplification event occurred early in embryogenesis, spermatogenesis, or oogenesis, where the CHE gene is intensely active and where cholinergic functioning was indicated to be physiologically necessary. Our findings demonstrate a de novo amplification in apparently healthy individuals within an autosomal gene producing a target protein to an inhibitor. Its occurrence in two generations from a family under prolonged exposure to parathion indicates that organophosphorous poisons may be implicated in previously unforeseen long-term ecological effects.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTIN L., BERRY W. K. Two selective inhibitors of cholinesterase. Biochem J. 1953 Jul;54(4):695–700. [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Egozi Y., Sokolovsky M., Schejter E., Blatt I., Zakut H., Matzkel A., Soreq H. Divergent regulation of muscarinic binding sites and acetylcholinesterase in discrete regions of the developing human fetal brain. Cell Mol Neurobiol. 1986 Mar;6(1):55–70. doi: 10.1007/BF00742976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- HODGKIN W., GIBLETT E. R., LEVINE H., BAUER W., MOTULSKY A. G. COMPLETE PSEUDOCHOLINESTERASE DEFICIENCY: GENETIC AND IMMUNOLOGIC CHARACTERIZATION. J Clin Invest. 1965 Mar;44:486–493. doi: 10.1172/JCI105162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger H. P. Rapid processing of primary embryonic tissues for chromosome banding pattern analysis. Cytogenetics. 1972;11(5):424–435. doi: 10.1159/000130208. [DOI] [PubMed] [Google Scholar]
- LIDDELL J., LEHMANN H., SILK E. A 'silent' pseudo-cholinesterase gene. Nature. 1962 Feb 10;193:561–562. doi: 10.1038/193561a0. [DOI] [PubMed] [Google Scholar]
- Layer P. G., Sporns O. Spatiotemporal relationship of embryonic cholinesterases with cell proliferation in chicken brain and eye. Proc Natl Acad Sci U S A. 1987 Jan;84(1):284–288. doi: 10.1073/pnas.84.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockridge O., La Du B. N. Amino acid sequence of the active site of human serum cholinesterase from usual, atypical, and atypical-silent genotypes. Biochem Genet. 1986 Jun;24(5-6):485–498. doi: 10.1007/BF00499101. [DOI] [PubMed] [Google Scholar]
- Maurer B. J., Lai E., Hamkalo B. A., Hood L., Attardi G. Novel submicroscopic extrachromosomal elements containing amplified genes in human cells. Nature. 1987 Jun 4;327(6121):434–437. doi: 10.1038/327434a0. [DOI] [PubMed] [Google Scholar]
- Pauw P. G., Johnson M. D., Moore P., Morgan M., Fineman R. M., Kalka T., Ash J. F. Stable gene amplification and overexpression of sodium- and potassium-activated ATPase in HeLa cells. Mol Cell Biol. 1986 Apr;6(4):1164–1171. doi: 10.1128/mcb.6.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prody C. A., Zevin-Sonkin D., Gnatt A., Goldberg O., Soreq H. Isolation and characterization of full-length cDNA clones coding for cholinesterase from fetal human tissues. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3555–3559. doi: 10.1073/pnas.84.11.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner D., Oren B., Vigder K. Chronic dietary anticholinesterase poisoning. Isr J Med Sci. 1983 Sep;19(9):810–814. [PubMed] [Google Scholar]
- Razon N., Soreq H., Roth E., Bartal A., Silman I. Characterization of activities and forms of cholinesterases in human primary brain tumors. Exp Neurol. 1984 Jun;84(3):681–695. doi: 10.1016/0014-4886(84)90215-2. [DOI] [PubMed] [Google Scholar]
- Sastry B. V., Sadavongvivad C. Cholinergic systems in non-nervous tissues. Pharmacol Rev. 1978 Mar;30(1):65–132. [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Schnedl W. Analysis of the human karyotype using a reassociation technique. Chromosoma. 1971;34(4):448–454. doi: 10.1007/BF00326316. [DOI] [PubMed] [Google Scholar]
- Simpson N. E. Factors influencing cholinesterase activity in a Brazilian population. Am J Hum Genet. 1966 May;18(3):243–252. [PMC free article] [PubMed] [Google Scholar]
- Soreq H., Gnatt A. Molecular biological search for human genes encoding cholinesterases. Mol Neurobiol. 1987 Spring-Summer;1(1-2):47–80. doi: 10.1007/BF02935264. [DOI] [PubMed] [Google Scholar]
- Soreq H., Malinger G., Zakut H. Expression of cholinesterase genes in human oocytes revealed by in-situ hybridization. Hum Reprod. 1987 Nov;2(8):689–693. doi: 10.1093/oxfordjournals.humrep.a136615. [DOI] [PubMed] [Google Scholar]
- Soreq H., Zamir R., Zevin-Sonkin D., Zakut H. Human cholinesterase genes localized by hybridization to chromosomes 3 and 16. Hum Genet. 1987 Dec;77(4):325–328. doi: 10.1007/BF00291419. [DOI] [PubMed] [Google Scholar]
- Stark G. R. DNA amplification in drug resistant cells and in tumours. Cancer Surv. 1986;5(1):1–23. [PubMed] [Google Scholar]
- Szeinberg A., Pipano S., Assa M., Medalie J. H., Neufeld H. N. High frequency of atypical pseudocholinesterase gene among Iraqi and Iranian Jews. Clin Genet. 1972;3(2):123–127. doi: 10.1111/j.1399-0004.1972.tb01733.x. [DOI] [PubMed] [Google Scholar]
- Wilson B. W., Walker C. R. Regulation of newly synthesized acetylcholinesterase in muscle cultures treated with diisopropylfluorophosphate. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3194–3198. doi: 10.1073/pnas.71.8.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakut H., Even L., Birkenfeld S., Malinger G., Zisling R., Soreq H. Modified properties of serum cholinesterases in primary carcinomas. Cancer. 1988 Feb 15;61(4):727–737. doi: 10.1002/1097-0142(19880215)61:4<727::aid-cncr2820610416>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Zakut H., Matzkel A., Schejter E., Avni A., Soreq H. Polymorphism of acetylcholinesterase in discrete regions of the developing human fetal brain. J Neurochem. 1985 Aug;45(2):382–389. doi: 10.1111/j.1471-4159.1985.tb03999.x. [DOI] [PubMed] [Google Scholar]