Abstract
Background
Safety concerns associated with drug-eluting stents have spurred interest in alternative vessel therapeutics following angioplasty. Microbubble contrast agents have been shown to increase gene transfection in vivo in the presence of ultrasound. Objectives/Methods: The purpose of this study was to determine whether an intravascular ultrasound (IVUS) catheter could mediate plasmid DNA transfection from microbubble carriers to the porcine coronary artery wall following balloon angioplasty.
Results
In the presence of plasmid-coupled microbubbles in vitro only cells exposed to ultrasound from the modified IVUS catheter significantly expressed the transgene. A porcine left anterior descending coronary artery underwent balloon angioplasty followed by injection and insonation of microbubbles from the IVUS catheter at the site of angioplasty. After 3 days, an approximately 6.5-fold increase in transgene expression was observed in arteries that received microbubbles and IVUS compared to those that received microbubbles with no IVUS.
Conclusions
The results of this study demonstrate for the first time that IVUS is required to enhance gene transfection from microbubble carriers to the vessel wall in vivo. This technology may be applied to both drug and gene therapy to reduce vessel restenosis.
Key Words: Intravascular ultrasound, Microbubbles, Gene transfer, Artery, Pig
Introduction
Recurrent luminal obstruction following successful coronary stenting remains an issue for both bare metal and drug-eluting stents (DES). In-stent restenosis is caused by proliferating vascular smooth muscle cells from the vessel wall in response to injury [1]. The antiproliferative agents released from drug-eluting stents have reduced rates of in-stent restenosis; however, these devices have their own limitations including concerns about in-stent thrombosis [2,3]. Gene therapy offers another therapeutic method of reducing restenosis; however, it often requires the use of recombinant viral vectors and local delivery, making this technique impractical for routine coronary artery therapy. Concerns about the safety and practicality of viral vectors as a method of gene delivery in a clinical setting have motivated the search for nonviral transfection techniques. Over the last decade, microbubble ultrasound contrast agents have been shown to enhance gene transfection in vitro [4] and in vivo [5]. Although in vitro results demonstrate up to 300-fold increase in transgene expression following ultrasound exposure in the presence of microbubbles, the focal delivery of genes using ultrasound exposure in vivo, particularly in the vasculature, is more complicated. Christiansen et al. [5] injected cationic microbubbles coated with plasmid DNA into a rat artery while ultrasound was focused over the hind limb muscle. They demonstrated that gene expression occurred only following the combination of ultrasound and microbubbles [5]. The goal of this focused study was to determine whether a commercially available intravascular ultrasound (IVUS) catheter could mediate the delivery of plasmid DNA to porcine coronary arteries following balloon angioplasty, using microbubble carriers.
Methods/Results
IVUS Catheter
An Atlantis SR Pro IVUS catheter (Boston Scientific, Natick, Ma., USA) was modified to allow for external excitation from a square wave pulser (SP-801; Ritec Inc., Warwick, R.I., USA). The center frequency of the negative unipolar pulse was set to 5 MHz, with a pulse repetition frequency of 5 kHz and amplitude of −590 V. The pressure output of the IVUS transducer at a distance of 1 mm away from the plastic tubing of the catheter sheath was measured by scanning a calibrated hydrophone (GL-0200; Onda Corp., Sunnyvale, Calif., USA) laterally beyond the width of the transducer. The corresponding −6 dB beamwidth of the IVUS beam was 0.44 mm and produced approximately 2 MPa at the focus – comparable to acoustic pressures employed by Rahim et al [4].
IVUS-Mediated Microbubble Delivery of Plasmid DNA in vitro
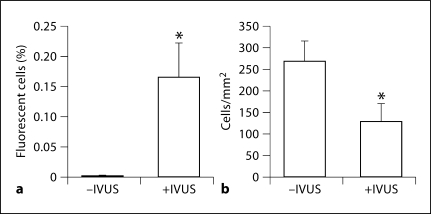
Rat vascular smooth muscle cells were cultured in vitro in acoustically transparent Opticell™ (Biocrystal, Westerville, Ohio, USA) chambers for 24 h to reach approximately 75% confluency. Plasmids encoding red fluorescent protein (CMV-RFP) were electrostatically coupled to the surface of cationic lipid microbubbles (30 μg/150 × 108 bubbles) as previously described [5] and injected into the Opticell. The Opticell was submersed in a 37°C water bath and the IVUS transducer was translated at a speed of 1.5 mm/s along parallel lines (0.5 mm apart) across the Opticell exposing 1 × 2 cm areas of cells to ultrasound (n = 3) only once. During this 6-min exposure, each cell was insonated for approximately only 0.3 s (based on the beamwidth). The cells were allowed to grow for 24 h and then analyzed for RFP gene expression using fluorescence microscopy (excitation 512 nm). An average of 0.17% of cells were successfully transfected following microbubble and IVUS delivery, whereas no significant transfection was observed in cells exposed only to the plasmid-coupled microbubbles (fig. 1a). Although the transfection efficiency was low, it is comparable to other studies [4] and may be an effect of the narrow beamwidth (−6 dB) of the IVUS transducer (<0.5 mm). An estimated 12% of cells were located outside the −6 dB beamwidth and may not have been exposed to sufficient ultrasound intensities. Phase contrast images of insonated and noninsonated regions of cells revealed a 52% difference in cell densities (fig. 1b).
Fig. 1.
In vitro expression of CMV-RFP following plasmid-coupled microbubble delivery and insonation with a modified intravascular ultrasound catheter to aortic smooth muscle cells. a In vitro transfection efficiency presented as percent fluorescent cells in the absence or presence of IVUS. p = 0.0028. b IVUS exposure decreased smooth musle cell viability by approximately 52% as indicated by cell densities at 24 h after insonation. p = 0.0047.
IVUS-Mediated Microbubble Delivery of Plasmid DNA in vivo
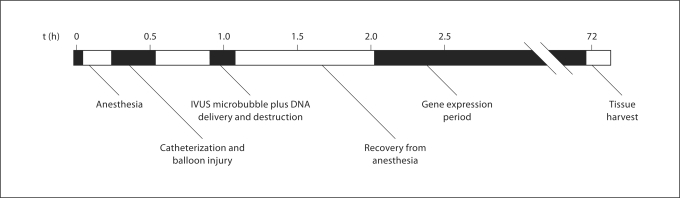
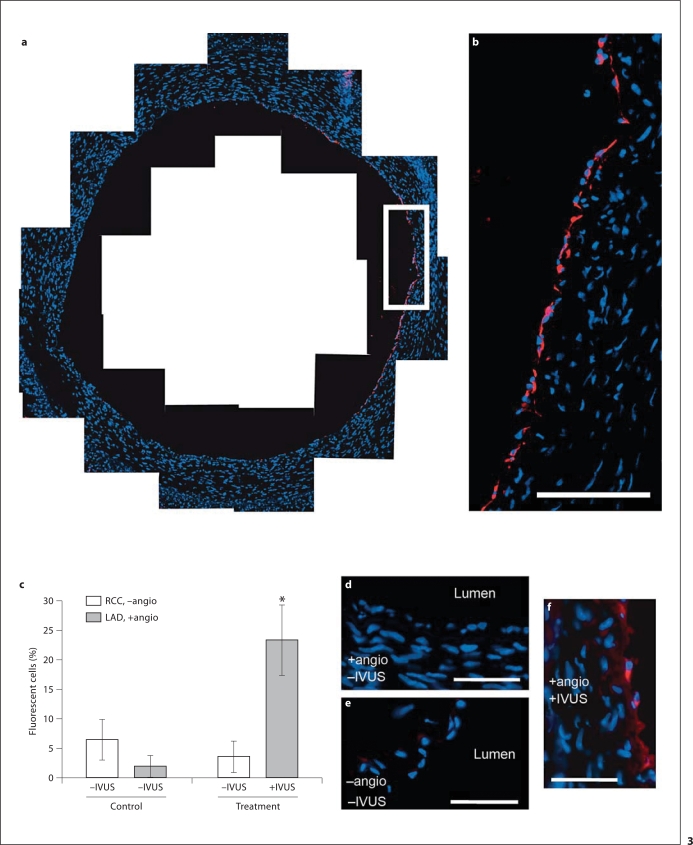
Balloon angioplasty was performed on porcine left anterior descending (LAD) coronary arteries in vivo, as previously described [6] (all animal procedures were approved by the University of Missouri ACUC). Two animals were used in this study. Animal 1, the control, did not receive IVUS. Animal 2, the treatment animal, did receive IVUS. As outlined in figure 2, following angioplasty, plasmid-conjugated microbubbles were infused for 4 min through either a guide catheter (no IVUS control animal) or the IVUS catheter located 2.0 cm upstream of the IVUS transducer (treatment animal). Colocalization of the transducer to the original site of angioplasty was determined by angiography. The animals were allowed to recover for 3 days and arteries were excised and processed for frozen sectioning and nuclei staining (DAPI). Three arbitrary, 6-μm-thick slices, spaced 1 mm apart, were analyzed from the angioplasty LAD. The right common carotid (RCC) artery, which also received plasmid microbubble exposure, but no angioplasty or IVUS, was also analyzed in each animal. Transfection efficiency was observed by fluorescence microscopy and was present only in perimeter cells of the luminal wall (fig. 3a, b, f). In all images, background fluorescence at or below the emission level of the elastic lamina was digitally removed. Liver samples exhibited no fluorescence above equivalent digital thresholds. Quantification of efficiency was calculated as the mean percentage of vessel perimeter cells expressing RFP plus 1 standard deviation (fig. 3c). In the control animal, fluorescent cells were detected in only 1.9 ± 1.8% in the LAD (angioplasty, no IVUS) and in 6.4 ± 3.5% in the RCC (no angioplasty, no IVUS). In the treatment animal, IVUS exposure resulted in 23.3 ± 6.0% transfection, whereas in the RCC of the same animal (no angioplasty, no IVUS) 3.6 ± 2.6% transfection was observed – a 6.5-fold increase in transfection efficiency. A Student's t test was performed to determine significance for both in vitroand in vivo results.
Fig. 2.
In vivo experimental timeline. Male swine (approx. 35 kg) were anesthetized and catheterized prior to balloon injury via angioplasty (1.3:1.0 inflation ratio). Twenty minutes following injury, plasmid (CMV-RFP)-coated microbubbles were infused through a circuit-modified IVUS catheter to the site of injury. Insonation was performed by the IVUS probe at 5 MHz, 1 MPa. 72 h following plasmid microbubble insonation the vessels were removed from the euthanized animals and processed for frozen histological cross-sectioning, staining and analysis.
Fig. 3.
In vivo expression of CMV-RFP following plasmid-coupled microbubble delivery and insonation with a modified IVUS catheter to a swine coronary artery following angioplasty. a Delivery was localized to the innermost cells of the LAD coronary artery. b Higher magnification of white box in a. Scale bar = 100 μm. c Gene transfection expressed as percent fluorescent cells in the treatment LAD compared to the control LAD and RCC. * p ≤ 0.013 (n = 3 slices, error bars represent standard deviation). Representative images: LAD, +angio, –IVUS (d); RCC, –angio, –IVUS (e); LAD, +angio, +IVUS (f); scale bars = 50 μm.
Discussion
Our results demonstrate that IVUS is capable of enhancing gene transfection to an injured coronary artery wall using the ultrasound conditions described herein and that angioplasty alone does not enhance gene transfection. Non-IVUS strategies have been employed by others to enhance oligonucleotide delivery to rat carotids [7] and porcine coronaries [8]. The approach in the rat study [7] required temporary ligation of the carotid, while the microbubbles were insonated inside the vessel which is not practical in a human clinical setting. In the porcine study [8], bubbles were injected intra-arterially and insonation performed with a transthoracic transducer. Such approaches do not allow for circumferential insonation of microbubbles and vector delivery. In the present study, we show that following routine angioplasty and during the same procedure, microbubble-mediated gene delivery can be performed in a focused manner using IVUS, avoiding many of the limitations previously mentioned. Due to manual rotation and translation limitations of the current IVUS catheter system, only a portion of the vessel wall was insonated. Future studies using novel modifications to IVUS, for example changes in output pressure, should be achievable to provide greater transfection efficiency and deeper penetration of the plasmid DNA in the vessel wall. Similar technology is also readily amenable to drug delivery [9,10], including antiproliferative agents such as sirolimus that are currently used in drug-eluting stents.
Acknowledgements
This work was funded by the Wallace H. Coulter Foundation and NIH HL090700 to J.A.H., A.L.K., B.R.W., and M.R.; NIH HL071574 to D.K.B. and AHA pre-doctoral fellowship to L.C.P. We thank Drs. Pam Schoppee-Bortz, PhD, and Oana Nicoara, MD, at the University of Virginia and Jan Ivey and Dr. Darla Tharp, PhD, at the University of Missouri for their technical expertise.
Footnotes
J.A.H. and B.R.W. contributed equally to this work.
References
- 1.Aikawa M, Sakomura Y, Ueda M, Kimura K, Manabe I, Ishiwata S, Komiyama N, Yamaguchi H, Yazaki Y, Nagai R. Redifferentiation of smooth muscle cells after coronary angioplasty determined via myosin heavy chain expression. Circulation. 1997;96:82–90. doi: 10.1161/01.cir.96.1.82. [DOI] [PubMed] [Google Scholar]
- 2.Eisenstein EL, Anstrom KJ, Kong DF, Shaw LK, Tuttle RH, Mark DB, Kramer JM, Harrington RA, Matchar DB, Kandzari DE, Peterson ED, Schulman KA, Califf RM. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007;297:159–168. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- 3.Pfisterer M, Brunner-La Rocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, Jeger R, Bader F, Osswald S, Kaiser C, BASKET-LATE Investigators Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006;48:2584–2591. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Rahim AA, Taylor SL, Bush NL, ter Haar GR, Bamber JC, Porter CD. Spatial and acoustic pressure dependence of microbubble-mediated gene delivery targeted using focused ultrasound. J Gene Med. 2006;8:1347–1357. doi: 10.1002/jgm.962. [DOI] [PubMed] [Google Scholar]
- 5.Christiansen P, French BA, Klibanov AL, Kaul S, Lindner JR. Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound Med Biol. 2003;29:1759–1767. doi: 10.1016/s0301-5629(03)00976-1. [DOI] [PubMed] [Google Scholar]
- 6.Tharp DL, Wamhoff BR, Wulff H, Raman G, Cheong A, Bowles DK. Local delivery of the K(Ca)3.1 blocker, TRAM-34, prevents acute angioplasty-induced coronary smooth muscle phenotypic modulation and limits stenosis. Arterioscler Thromb Vasc Biol. 2008;28:1084–1089. doi: 10.1161/ATVBAHA.107.155796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashiya N, Aoki M, Tachibana K, Taniyama Y, Yamasaki K, Hiraoka K, Makino H, Yasufumi K, Ogihara T, Morishita R. Local delivery of E2F decoy oligodeoxynucleotides using ultrasound with microbubble agent (Optison) inhibits intimal hyperplasia after balloon injury in rat carotid artery model. Biochem Biophys Res Commun. 2004;317:508–514. doi: 10.1016/j.bbrc.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 8.Porter TR, Xie F, Knapp D, Iversen P, Marky LA, Tsutsui JM, Maiti S, Lof J, Radio SJ, Kipshidze N. Targeted vascular delivery of antisense molecules using intravenous microbubbles. Cardiovasc Revasc Med. 2006;7:25–33. doi: 10.1016/j.carrev.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara K, Pollard R, Bordeni M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Ann Rev Biomed Eng. 2007;9:415–447. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- 10.Mayer CR, Bekeredjian R. Ultrasonic gene and drug delivery to the cardiovascular system. Adv Drug Deliv Rev. 2008;60:1177–1192. doi: 10.1016/j.addr.2008.03.004. [DOI] [PubMed] [Google Scholar]