Abstract
Background
The APOE ∊4 allele is an established risk factor for Alzheimer's disease, but reports of its association with vascular dementia (VaD) have been inconsistent. We examined the relationship between APOE ∊4 allele and the risk of incident VaD in a large, population-based cohort of elderly adults with up to 10 years of follow-up between 1995 and 2005.
Methods
A total of 3,424 elderly men and women free of dementia were genotyped at the baseline assessment. Incident VaD was identified through standardized procedures administered at 3 follow-up assessments. Cox proportional hazards models were used to evaluate the risk of VaD associated with APOE ∊4.
Results
The adjusted hazard ratio was 1.6 for the participants with 1 APOE ∊4 allele (95% CI: 0.9–2.7; p = 0.083) and 4.4 for those with 2 APOE ∊4 alleles (95% CI: 1.6–12.5; p = 0.005). The increased risk did not appear to be mediated by vascular risk factors.
Conclusions
The APOE ∊4 allele is associated with an increased risk of VaD in a dose-dependent fashion and accounts for almost 20% of VaD in the population.
Key Words: APOE, Vascular dementia
Introduction
Vascular dementia (VaD) is one of the most common forms of dementia, second only to Alzheimer's disease (AD). In 2002, the estimated prevalence of VaD among individuals aged 71 years and older in the USA was 2.4%, compared to 9.7% for AD [1]. Similar to AD, the public health burden of VaD threatens to worsen considerably as the world's population ages [2].
Variation at the apolipoprotein E (APOE) gene is a well-established risk factor for late-onset familial and sporadic AD [3,4]. The gene is on chromosome 19 and encodes a major apolipoprotein which serves as a cholesterol carrier in the brain [5]. It has 3 common alleles (∊2, ∊3 and ∊4), which determine 6 different genotypes (∊2/∊2, ∊2/∊3, ∊2/∊4, ∊3/∊3, ∊3/∊4 and ∊4/∊4). The ∊4 allele is associated with a greater risk of AD in a dose-dependent fashion, while some evidence suggests the ∊2 allele is associated with a lower risk [6].
The APOE gene has also been associated with VaD in different ethnic groups [7,8,9,10,11,12], but this association has not been consistently replicated [13,14,15,16,17]. All prior studies of this topic have used case-control or cross-sectional study designs, and many have also had relatively small sample sizes and relatively weak control on population stratification by age, gender, ethnicity or other unknown confounding factors. An especially problematic issue in this area of research is the diagnostic misclassification of mixed dementia as VaD.
We investigated the association between APOE and incidence of VaD in the Cache County Study cohort, a large and well-characterized, population-based sample of 5,092 older adults who have had up to 10 years of longitudinal follow-up since 1995.
Methods
Overview
The Cache County Study is a prospective study of the prevalence and incidence of dementia in relation to genetic and environmental risk factors among elderly adults living in Cache County, Utah, USA. The study design has been reported in detail previously [18,19]. Briefly, all residents of the county aged 65 years or older on January 1, 1995, were invited to participate, and 5,092 (90%) completed baseline assessments and interviews in 1995–1996 (wave I). Surviving participants who had not met criteria for the diagnosis of any dementia were then asked to engage in follow-up evaluations in 1998–1999 (wave II), 2002–2003 (wave III) and 2005–2006 (wave IV). At each evaluation, a multistage screening and assessment procedure was used to determine the cognitive status of the participants, and the presence and type of dementia. In addition, detailed interviews were used to gather information on the participants’ demographics, family and medical history. All protocols were approved by the Institutional Review Boards of Utah State University, Duke University and Johns Hopkins University. Spouses or next of kin gave informed consent when participants were unable to provide it.
Study Sample
The present study used data collected from wave I to wave IV. A total of 5,092 elderly individuals from Cache County participated in wave I of the study. At baseline, we identified 359 cases of dementia; these individuals were excluded from the current analyses. By the end of wave II, 633 persons had died and another 676 persons were lost to follow-up. Thus, 3,424 individuals had completed at least 1 follow-up and were available for inclusion in the current study.
Diagnosis of VaD
A similar assessment procedure was utilized at each wave of the study. Participants were initially screened by questionnaires sensitive to detecting dementia, including a revised version [20] of the modified Mini-Mental State Examination (3MS) [21]. We interviewed knowledgeable collateral informants of those who were unable to complete the 3MS using the Informant Questionnaire for Cognitive Decline in the Elderly [22]. In waves I and II, participants with low screening scores were further evaluated using the Dementia Questionnaire [23] administered by phone to a collateral informant. In these waves, all participants aged 90 years or older, regardless of their scores on the 3MS or Informant Questionnaire for Cognitive Decline in the Elderly, were also evaluated using the Dementia Questionnaire.
Based on the results of the initial screening, participants with evidence of cognitive impairment were referred for a detailed structured clinical assessment conducted by research nurses and psychometricians in the presence of a collateral informant. A systematic random subsample of participants comprising approximately 19% of the cohort, as well as all participants over the age of 85 years in waves III and IV, were also sent for a clinical assessment regardless of their scores on the screening evaluations. The assessments included: a clinical and medical history, family history of dementia, a brief physical examination, a standardized blood pressure measurement, a structured neurologic examination, the Neuropsychiatric Inventory [24], an assessment of cognitive and functional impairment using the Dementia Severity Rating Scale [25], and a 1-hour battery of neuropsychological tests [26].
Board-certified geriatric psychiatrists and neuropsychologists then reviewed the results of the clinical assessments and assigned working diagnoses of dementia, based on the DSM-III-R criteria [27], or other cognitive syndromes. The estimated age at onset of dementia was recorded as the year in which participants unambiguously met the DSM-III-R criteria. Participants with working diagnoses of dementia or other cognitive disorders were reexamined by a study physician and asked to undergo standard laboratory tests and a brain MRI for differential diagnosis. Finally, a panel of expert clinicians reviewed all available information and adjudicated a final differential diagnosis of dementia using NINCDS-ADRDA criteria [28] for AD, NINDS-AIREN criteria [29] as modified by Tatemichi et al. [30] for VaD and current research criteria for other dementing illnesses. For present purposes, we restricted the diagnosis of VaD to participants whose dementia was believed to be exclusively cerebrovascular in origin, having no features that were specifically suggestive of AD or other dementias. The sensitivity and specificity of the above screening methods for the detection of incident dementia have been estimated at 84.7 and 95.2%, respectively [31].
APOE Genotyping
Buccal DNA samples were obtained at the baseline assessment with a response rate of 97%. APOE genotypes were determined using PCR amplification and a restriction isotyping following the methods described previously [32,33]. The APOE genotypes were not known to clinicians during the diagnostic process.
Vascular Risk Factors
Medical histories of vascular risk factors were obtained at the baseline and follow-up visits via proxy- and self-report. The participants were asked about a number of vascular factors and their history of cardiovascular events including hypertension, hypercholesterolemia, diabetes mellitus, stroke, coronary artery bypass graft (CABG) and myocardial infarction (MI). A positive history of each condition was recorded when the participant, or proxy informant, indicated he/she was ever told by a doctor or nurse that he/she had the condition or received treatment for it.
Statistical Analysis
Demographic comparisons evaluated potential differences in age, sex, education, APOE genotype and history of vascular risk factors at baseline between individuals diagnosed with VaD and those who were censored. Continuous variables were examined using t tests or analyses of variance, while categorical variables were examined via χ2 tests.
The Kaplan-Meier survival analysis and log rank test were used to evaluate the differences of VaD-free survival in groups with 0, 1 or 2 APOE ∊4 alleles. Age was used as the time scale, with age at baseline as the origin. Participants who developed VaD were captured as having an event at the estimated age of dementia onset. Participants who survived without dementia, died or were lost to follow-up were censored at the age of their last wave of assessment, while participants who developed other forms of dementia were censored at their estimated age of dementia onset. Cox proportional hazard models were then used to assess the association between the number of APOE ∊4 alleles and the risk of VaD, controlling for age at baseline, gender, years of education and vascular risk factors. The results were presented as hazard ratios (HR) with 95% confidence intervals (CI). Finally, the population attributable fraction (PAF) of VaD due to the APOE ∊4 polymorphism was calculated. The PAF is an estimate of the proportion of a population's burden of disease that could be prevented if the effects of specific causal factors were eliminated from the population. It was calculated as follows: P × (multiple adjusted HR − 1)/multiple adjusted HR, where P = proportion of cases exposed to any APOE ∊4 alleles [34]. All analyses were performed using STATA version 10 software (StataCorp, College Station, Tex., USA). Two-sided p < 0.05 was considered statistically significant.
Results
Out of the 3,424 participants who completed the baseline evaluation and at least 1 follow-up, there were 65 cases of incident VaD over a mean follow-up period of 7.1 years (range: 0.02–12.4 years; standard deviation = 3.5 years). A total of 1,309 participants (from the original 5,092) were lost to follow-up. These individuals tended to be older (t = 19.7; p < 0.0001) and less educated (t = −7.04; p < 0.0001), and more of them were male (χ2 = 6.05; p = 0.014). The distribution of APOE ∊4 alleles was similar in those who were lost versus those who were not. Table 1 presents the demographic characteristics of the sample included in this analysis. Compared with the non-VaD group, individuals with incident VaD were significantly older, were more likely to have 1 or more APOE ∊4 alleles, and more often reported at baseline that they suffered from hypertension, diabetes, stroke or MI. There were no material differences in gender distribution and years of education between these 2 groups.
Table 1.
Baseline characteristics by diagnosis of VaD during follow-up from wave II to wave IV of the Cache County Study (n = 3,424)
| Non-VaD1 (n (%) = 3,359) | VaD (n (%) = 65) | p | |
|---|---|---|---|
| Male, n (%) | 1,404 (41.8) | 30 (46.2) | 0.48 |
| Age, mean years ± SD | 74.5 ± 6.47 | 76.6 ± 6.49 | 0.01 |
| Education, mean years ± SD | 13.4 ± 2.87 | 13.3 ± 2.99 | 0.79 |
| Number of ε4 alleles2, n (%) | 0.042 | ||
| 0 | 2,297 (69.0) | 37 (56.9) | |
| 1 | 950 (28.5) | 24 (36.9) | |
| 2 | 82 (2.5) | 4 (6.2) | |
| Prevalence of vascular risk factors2, n (%) | |||
| Hypertension | 1,467 (43.8) | 40 (62.5) | 0.003 |
| High cholesterol | 1,084 (32.6) | 20 (36.7) | 0.892 |
| Diabetes mellitus | 371 (11.1) | 19 (29.2) | <0.001 |
| Stroke | 133 (3.9) | 17 (26.2) | <0.001 |
| CABG | 216 (6.4) | 6 (9.2) | 0.365 |
| MI | 365 (10.9) | 15 (23.1) | 0.002 |
Included are diagnoses of dementia other than VaD.
This is the percentage out of the total number with nonmissing data.
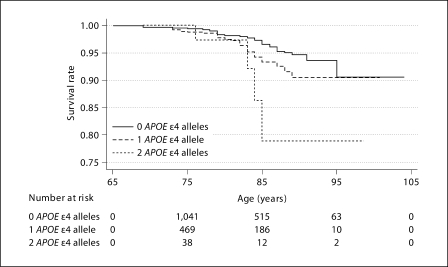
The Kaplan-Meier curves for VaD-free survival for participants with 0, 1 and 2 APOE ∊4 alleles are displayed in figure 1. Participants with 1 or 2 APOE ∊4 alleles had significantly lower VaD-free survival rates than the reference group with no APOE ∊4 alleles (log rank test: p = 0.044 and 0.0038, respectively). The Cox proportional hazard models also showed that those with 1 or 2 APOE ∊4 alleles had a greater risk of developing VaD in a dose-dependent fashion (table 2). Importantly, this association persisted even after adjustment for cardio- and cerebrovascular factors including history of hypertension, diabetes, high cholesterol, stroke, MI and CABG, suggesting the association was not mediated by these factors. Finally, we grouped together participants with 1 or 2 APOE ∊4 alleles and compared them to those without any ∊4 alleles, obtaining an adjusted HR of 1.85 (95% CI: 1.13–3.03; p = 0.015). Given the effect size and frequency of the ∊4 alleles, the proportion of population risk attributable to any APOE ∊4 allele (PAF) for VaD was estimated to be 19.8%.
Fig. 1.
Kaplan-Meier survival curves for participants with 0, 1 and 2 APOE ∊4 alleles. Survival rate calculated by S(t) = probability survival > t, where t = age.
Table 2.
HR for risk of incident VaD by Cox's proportional hazards models
| Crude HR | Model 1 aHR | Model 2 aHR | |
|---|---|---|---|
| 0 APOE ε4 alleles | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| 1 APOE ε4 allele | 1.69 (1.00–2.83) | 1.70 (1.01–2.84) | 1.60 (0.94–2.71) |
| 2 APOE ε4 alleles | 4.00 (1.42–11.2) | 3.98 (1.41–11.2) | 4.44 (1.55–12.5) |
| Age at baseline1 | 1.00 (0.92–1.08) | 0.95 (0.88–1.04) | |
| Sex | 0.78 (0.47–1.28) | 0.86 (0.50–1.46) | |
| Education in years | 0.98 (0.90–1.07) | 0.95 (0.88–1.04) | |
| Hypertension | 1.64 (0.96–2.81) | ||
| High cholesterol | 0.77 (0.43–1.35) | ||
| Diabetes | 2.83 (1.59–5.07) | ||
| Stroke | 6.27 (3.40–11.5) | ||
| CABG | 0.87 (0.32–2.32) | ||
| MI | 1.76 (0.89–3.49) | ||
Values in parentheses denote 95% CI unless stated otherwise. aHR = Adjusted HR.
Age while on study was used as the time axis in the Cox proportional hazards models, and age at baseline was further added to the models to provide additional control for any possible cohort effects.
Discussion
In this large, prospective cohort study, there was a dose-dependent association between the presence of 1 or 2 APOE ∊4 alleles and the risk of VaD. Compared to individuals without any APOE ∊4 alleles, those with 1 APOE ∊4 allele had an approximately 1.7-fold greater risk of VaD, whereas those with 2 APOE ∊4 alleles had a 4-fold greater risk. The population attributable risk associated with having 1 or more APOE ∊4 alleles for VaD was 19.8%.
There has been a matter of controversy about the association between APOE ∊4 allele and the risk of VaD in the literature. Some studies reported positive associations [7,8,9,10,11,12], while others showed no associations [13,14,15,16,17]. Even regarding the same ethnic groups, the results have been in conflict [9,15]. There are several potential reasons for the inconsistent findings. First, all previous studies have used a cross-sectional or case-control study design, both of which are more susceptible to selection bias. Second, the small sample sizes of some studies may have offered inadequate statistical power to detect an association. Lastly, the diagnosis of VaD using the NINDS-AIREN can be challenging, and outcome misclassification may therefore have led to divergent results [35].
VaD, by name, implies a link between vascular risk factors or vascular diseases and dementia syndromes. Vascular risk factors such as hypertension, diabetes, hypercholesterolemia and smoking have been implicated as risk factors for dementia as have vascular diseases (for a review see Luchsinger and Mayeux [36]). On the other hand, the earliest research on the expression of the APOE genotype focused on its influence on lipid metabolism and atherogenesis [37]. Therefore, it has been postulated that an effect of APOE on dementia may be mediated by dyslipidemia and vascular diseases [38,39]. If so, the association between APOE and dementia might be expected to attenuate after adjusting for vascular risk factors and diseases. In our analysis, we observed no attenuation of the association between APOE ∊4 allele and the risk of VaD after adjusting for vascular risk factors. Thus, it appears the effect of the APOE ∊4 allele on the risk of VaD is not mediated by vascular factors. This finding is consistent with one previous study [40].
Advantages of the current study are its large, population-based cohort with up to 10 years of longitudinal follow-up. Also, the diagnosis of VaD followed a structured, multistage diagnostic procedure, supported by laboratory and neuroimaging studies and adjudicated by a panel of experts. Furthermore, we considered as cases only subjects who had a diagnosis of pure VaD without any clinical evidence of an AD contribution. This approach would make the diagnostic entity of VaD more homogeneous although the possibility of diagnostic misclassification cannot be totally ruled out because of the lack of neuropathological confirmation. A limitation of the study is its lacking generalizability. Over 95% of this population is Caucasian, and there may be differences in the genotype distribution and genetic mechanism in other ethnic populations. On the other hand, the relatively homogeneous population makes it less likely that the association we observed was due to population confounds.
In summary, our findings suggest that the APOE ∊4 allele confers a significant risk for VaD, and this effect did not seem to be mediated by vascular risk factors and diseases. Other possible mechanisms of how the APOE ∊4 allele increases the risk of VaD should be explored in future studies.
Acknowledgements
We would like to thank all of the study participants for their contributions to this work. We are grateful to the neurogenetics laboratory of the Bryan Alzheimer's Disease Research Center at Duke University for APOE genotyping. We would also like to thank study coordinators Nancy Sassano (Utah State University) and Michelle McCart (Duke University); and Cara Brewer, Tony Calvert, Tiffany Newman, Roxane Pfister, Sarah Schwartz, and Joslin Werstak, of Utah State University for their expert technical assistance in data collection and entry. The work on this study was support by a grant from the National Institute of Aging (R01 AG11380)
References
- 1.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill J, Fillit H, Shah SN, del Valle MC, Futterman R. Patterns of healthcare utilization and costs for vascular dementia in a community-dwelling population. J Alzheimers Dis. 2005;8:43–50. doi: 10.3233/jad-2005-8105. [DOI] [PubMed] [Google Scholar]
- 3.Kamboh MI. Molecular genetics of late-onset Alzheimer's disease. Ann Hum Genet. 2004;68:381–404. doi: 10.1046/j.1529-8817.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 4.Williamson J, Goldman J, Marder KS. Genetic aspects of Alzheimer disease. Neurologist. 2009;15:80–86. doi: 10.1097/NRL.0b013e318187e76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahley RW, Apolipoprotein E. cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 6.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. APOE and Alzheimer Disease Meta-Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 7.Davidson Y, Gibbons L, Purandare N, Byrne J, Hardicre J, Wren J, Payton A, Pendleton N, Horan M, Burns A, Mann DM. Apolipoprotein E ∊4 allele frequency in vascular dementia. Dement Geriatr Cogn Disord. 2006;22:15–19. doi: 10.1159/000092960. [DOI] [PubMed] [Google Scholar]
- 8.Baum L, Lam LC, Kwok T, Lee J, Chiu HF, Mok VC, Wong A, Chen X, Cheung WS, Pang CP, Ma SL, Tang NL, Wong KS, Ng HK. Apolipoprotein E ∊4 allele is associated with vascular dementia. Dement Geriatr Cogn Disord. 2006;22:301–305. doi: 10.1159/000095246. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Feng G, Zhang J, Hui Z, Breen G, St Clair D, He L. Is APOE gene a risk factor for vascular dementia in Han Chinese? Int J Mol Med. 2001;7:217–219. doi: 10.3892/ijmm.7.2.217. [DOI] [PubMed] [Google Scholar]
- 10.Katzman R, Zhang MY, Chen PJ, Gu N, Jiang S, Saitoh T, Chen X, Klauber M, Thomas RG, Liu WT, Yu ES. Effects of apolipoprotein E on dementia and aging in the Shanghai Survey of Dementia. Neurology. 1997;49:779–785. doi: 10.1212/wnl.49.3.779. [DOI] [PubMed] [Google Scholar]
- 11.Slooter AJ, Tang MX, van Duijn CM, Stern Y, Ott A, Bell K, Breteler MM, van Broeckhoven C, Tatemichi TK, Tycko B, Hofman A, Mayeux R. Apolipoprotein E ∊4 and the risk of dementia with stroke: a population-based investigation. JAMA. 1997;277:818–821. doi: 10.1001/jama.277.10.818. [DOI] [PubMed] [Google Scholar]
- 12.Kálmán J, Juhász A, Császár A, Kanka A, Rimanóczy A, Janka Z, Raskó I. Increased apolipoprotein E4 allele frequency is associated with vascular dementia in the Hungarian population. Acta Neurol Scand. 1998;98:166–168. doi: 10.1111/j.1600-0404.1998.tb07288.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim KW, Youn JC, Han MK, Paik NJ, Lee TJ, Park JH, Lee SB, Choo IH, Lee DY, Jhoo JH, Woo JI. Lack of association between apolipoprotein E polymorphism and vascular dementia in Koreans. J Geriatr Psychiatry Neurol. 2008;21:12–17. doi: 10.1177/0891988707311028. [DOI] [PubMed] [Google Scholar]
- 14.Engelborghs S, Dermaut B, Goeman J, Saerens J, Mariën P, Pickut BA, van den Broeck M, Serneels S, Cruts M, van Broeckhoven C, de Deyn PP. Prospective Belgian study of neurodegenerative and vascular dementia: APOE genotype effects. J Neurol Neurosurg Psychiatry. 2003;74:1148–1151. doi: 10.1136/jnnp.74.8.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin HF, Lai CL, Tai CT, Lin RT, Liu CK. Apolipoprotein E polymorphism in ischemic cerebrovascular diseases and vascular dementia patients in Taiwan. Neuroepidemiology. 2004;23:129–134. doi: 10.1159/000075956. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama S, Kuzuhara S. Apolipoprotein E phenotypes in healthy normal controls and demented subjects with Alzheimer's disease and vascular dementia in Mie Prefecture of Japan. Psychiatry Clin Neurosci. 1999;53:643–648. doi: 10.1046/j.1440-1819.1999.00619.x. [DOI] [PubMed] [Google Scholar]
- 17.Stengard JH, Pekkanen J, Sulkava R, Ehnholm C, Erkinjuntti T, Nissinen A. Apolipoprotein E polymorphism, Alzheimer's disease and vascular dementia among elderly Finnish men. Acta Neurol Scand. 1995;92:297–298. doi: 10.1111/j.1600-0404.1995.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 18.Miech RA, Breitner JC, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women: the Cache County Study. Neurology. 2002;58:209–218. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- 19.Breitner JC, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, Tschanz JT, Plassman BL, Meyer MR, Skoog I, Khachaturian A. APOE ∊4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 20.Tschanz JT, Welsh-Bohmer KA, Plassman BL, Norton MC, Wyse BW, Breitner JC. An adaptation of the modified Mini-Mental State Examination: analysis of demographic influences and normative data – the Cache County Study. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:28–38. [PubMed] [Google Scholar]
- 21.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 22.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 23.Silverman JM, Breitner JC, Mohs RC, Davis KL. Reliability of the family history method in genetic studies of Alzheimer's disease and related dementias. Am J Psychiatry. 1986;143:1279–1282. doi: 10.1176/ajp.143.10.1279. [DOI] [PubMed] [Google Scholar]
- 24.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 25.Clark CM, Ewbank DC. Performance of the Dementia Severity Rating Scale: a caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10:31–39. [PubMed] [Google Scholar]
- 26.Tschanz JT, Welsh-Bohmer KA, Skoog I, West N, Norton MC, Wyse BW, Nickles R, Breitner JC. Dementia diagnoses from clinical and neuropsychological data compared: the Cache County Study. Neurology. 2000;54:1290–1296. doi: 10.1212/wnl.54.6.1290. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, ed 3, rev (DSM-III-R) Washington: American Psychiatric Association; 1987. [Google Scholar]
- 28.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 29.Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 30.Tatemichi TK, Sacktor N, Mayeux R. Dementia associated with cerebrovascular disease, other degenerative diseases, and metabolic disorders. In: Terry RD, Katzman R, Bick KL, editors. Alzheimer Disease. New York: Raven; 1994. pp. 123–166. [Google Scholar]
- 31.Hayden KM, Khachaturian AS, Tschanz JT, Corcoran C, Nortond M, Breitner JC. Characteristics of a two-stage screen for incident dementia. J Clin Epidemiol. 2003;56:1038–1045. doi: 10.1016/s0895-4356(03)00247-6. [DOI] [PubMed] [Google Scholar]
- 32.Richards B, Skoletsky J, Shuber AP, Balfour R, Stern RC, Dorkin HL, Parad RB, Witt D, Klinger KW. Multiplex PCR amplification from the CFTR gene using DNA prepared from buccal brushes/swabs. Hum Mol Genet. 1993;2:159–163. doi: 10.1093/hmg/2.2.159. [DOI] [PubMed] [Google Scholar]
- 33.Saunders AM, Roses AD. Apolipoprotein E4 allele frequency, ischemic cerebrovascular disease, and Alzheimer's disease. Stroke. 1993;24:1416–1417. doi: 10.1161/01.str.24.9.1416. [DOI] [PubMed] [Google Scholar]
- 34.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmes C, Cairns N, Lantos P, Mann A. Validity of current clinical criteria for Alzheimer's disease, vascular dementia and dementia with Lewy bodies. Br J Psychiatry. 1999;174:45–50. doi: 10.1192/bjp.174.1.45. [DOI] [PubMed] [Google Scholar]
- 36.Luchsinger JA, Mayeux R. Cardiovascular risk factors and Alzheimer's disease. Curr Atheroscler Rep. 2004;6:261–266. doi: 10.1007/s11883-004-0056-z. [DOI] [PubMed] [Google Scholar]
- 37.Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8:1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Notkola IL, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, Kivinen P, Tuomilehto J, Nissinen A. Serum total cholesterol, apolipoprotein E ∊4 allele, and Alzheimer's disease. Neuroepidemiology. 1998;17:14–20. doi: 10.1159/000026149. [DOI] [PubMed] [Google Scholar]
- 39.Sparks DL. Coronary artery disease, hypertension, ApoE, and cholesterol: a link to Alzheimer's disease? Ann NY Acad Sci. 1997;826:128–146. doi: 10.1111/j.1749-6632.1997.tb48466.x. [DOI] [PubMed] [Google Scholar]
- 40.Prince M, Lovestone S, Cervilla J, Joels S, Powell J, Russ C, Mann A. The association between APOE and dementia does not seem to be mediated by vascular factors. Neurology. 2000;54:397–402. doi: 10.1212/wnl.54.2.397. [DOI] [PubMed] [Google Scholar]