Abstract
Objective: To determine whether preventive treatment for tuberculosis in adults infected with HIV reduces the frequency of tuberculosis and overall mortality.
Design: Systematic review and data synthesis of randomised placebo controlled trials.
Main outcome measures: Active tuberculosis, mortality, and adverse drug reaction requiring cessation of the study regimen. Outcomes stratified by status of purified protein derivative skin test.
Results: Four trials comprising 4055 adults from Haiti, Kenya, the United States, and Uganda were included. All compared isoniazid (6-12 months) with placebo, and one trial also compared multidrug treatment for 3 months with placebo. Mean follow up was 15-33 months. Overall, frequency of tuberculosis (relative risk 0.57, 95% confidence interval 0.41 to 0.79) was reduced in those receiving preventive treatment compared with placebo: mortality was not significantly reduced (0.93, 0.83 to 1.05). In subjects positive for purified protein derivative receiving preventive treatment, the risk of tuberculosis was reduced substantially (0.32, 0.19 to 0.51) and the risk of death was reduced moderately (0.73, 0.57 to 0.95) compared with those taking placebo. In adults negative for purified protein derivative receiving preventive treatment, the risk of tuberculosis (0.82, 0.50 to 1.36) and the risk of death (1.02, 0.89 to 1.17) were not reduced significantly. Adverse drug reactions were more frequent, but not significantly so, in patients receiving drug compared with placebo (1.45, 0.98 to 2.14).
Conclusions: Preventive treatment given for 3-12 months protects against tuberculosis in adults infected with HIV, at least in the short to medium term. Protection is greatest in subjects positive for purified protein derivative, in whom death is also less frequent. Long term benefits remain to be shown.
Key messages
One third of the world’s population is infected with Mycobacterium tuberculosis
People infected with HIV are at much increased risk of developing active tuberculosis
Short term preventive drug treatment given to people infected with HIV reduces the occurrence of active tuberculosis
The benefit is greatest in people with latent infection, as shown by a positive skin test for tuberculosis, and this group also exhibits a survival benefit
Introduction
Strategies to control tuberculosis comprise case treatment, preventive treatment, and vaccination with BCG, with the expectation that improved socioeconomic conditions will lead to a decline in disease incidence.1,2 Preventive treatment aims to eradicate latent infection with Mycobacterium tuberculosis before active disease develops. Latent infection is shown by a positive reaction to intradermal injection with purified protein derivative (tuberculin skin test). Trials in people with tuberculosis infection but not infected with HIV have shown that isoniazid given for 6-12 months substantially reduces the incidence of active tuberculosis.3
Infection with HIV has changed the natural history of infection with M tuberculosis.4 People who are infected with HIV and who have a positive tuberculin skin test have a 30% or more lifetime risk of developing active tuberculosis,5 and tuberculosis is the most common HIV related disease in developing countries.1,4 Thus, preventive treatment may be an important intervention to reduce the burden of tuberculosis in people infected with HIV, and their contacts, but its efficacy cannot simply be extrapolated from studies in people not infected with HIV.
As several fairly small trials have been done, we conducted this systematic review to summarise the evidence available to date as to whether preventive treatment for tuberculosis is effective in reducing the incidence of active tuberculosis and of death.
Subjects and methods
Criteria for selecting studies for review
We included only randomised controlled trials that compared drug regimens aimed at preventing tuberculosis with placebo. Trials were considered irrespective of setting or target group, and we included all different drug regimens tested. Preventive treatment was defined as tuberculosis chemotherapy given to people who have a particular risk of developing tuberculosis. Particular risk refers to people who are infected with HIV and either infected with M tuberculosis (positive for purified protein derivative), or who are negative for purified protein derivative but live in a community where tuberculosis is endemic, or have a high risk of infection.6 Our definition of negative for purified protein derivative allowed inclusion of anergic patients (defined as a skin test reaction of <5 mm to 5 tuberculin units, and <2 mm reaction to mumps, tetanus toxoid, and candida antigen). In some instances we were unable to stratify outcomes by anergy in subjects negative for purified protein derivative as not all trials tested for it.
Search strategy
We searched Medline using the search terms HIV, tuberculosis, preventive therapy, and chemoprophylaxis. We also searched the Cochrane Controlled Trials Register, the most comprehensive source of controlled trials (disk issue 1, 1998).7 In addition, we searched references of all retrieved articles and contacted relevant researchers to ensure that all completed trials had been identified.
Review procedure
Trials considered for inclusion were examined to determine completeness of reporting. One of us (DW) collated data on study methods, participants, interventions, and outcomes for each study, and another (PG) checked the collated data. Authors of incomplete or abstracted trials were contacted for further details. The quality of each trial was graded using predefined criteria, assessing method of allocation sequence generation, allocation concealment, inclusion of all randomised participants, follow up of subjects, and analysis by intention to treat.
Outcome measures
The outcome measures were (a) frequency of active tuberculosis, defined microbiologically (preferably by culture) or histologically, or as a clinical syndrome consisting of typical symptoms, independently assessed chest x ray, and a documented response to treatment,8 (b) frequency of mortality, and (c) occurrence of adverse drug reaction (defined as a reaction resulting in cessation of the study drugs). Where possible, outcome measures were stratified by purified protein derivative status (positive, negative, and unknown). Owing to the small number of subjects with unknown purified protein derivative status no stratum specific analysis of this group is reported.
Statistical analysis
We used the Mantel-Haenszel method to calculate summary statistics (relative risk and 95% confidence interval). A fixed effects model was used, and results were little different when using a random effects model. All analyses were done with Revman 3.0.1. (Update Software, Oxford).
Results
Included trials
Of seven identified trials, four were eligible for inclusion in this review.9–12 Of the remaining three, one was reported to be incomplete after contacting the investigators,13 one compared two different drug regimens,14 and a third had not yet been published—the authors declined inclusion of their data in our review.
Exclusion criteria were similar in all trials and included past history of tuberculosis, current tuberculosis, pregnancy, abnormal liver enzymes, and serious intercurrent illness. All treatment was self administered and adherence was monitored variously through self reporting, attendance at scheduled clinic appointments, and urine testing (both routine and unscheduled). No data on adherence were reported by Pape et al9; Hawken et al reported that 31% of subjects missed at least 5 weeks’ preventive treatment, and 70% had at least 50% positive urine tests10; Gordin et al reported that only 63% of patients completed preventive treatment within 6 months11; and Whalen et al reported that 75% of scheduled and 80% of unscheduled urine tests were positive.12 Follow up was generally short, ranging from an average of 15 to 33 months (table). All trials were analysed by intention to treat.
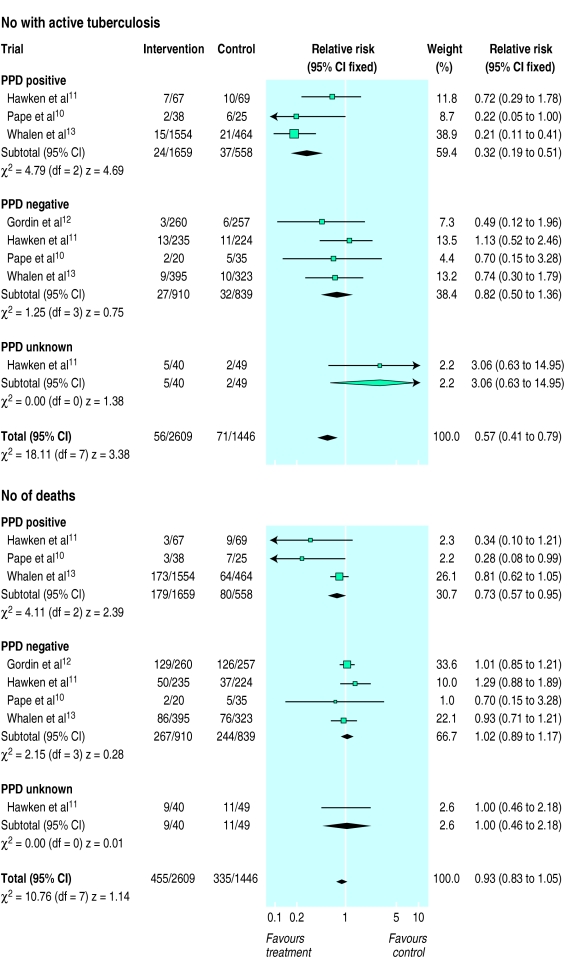
The figure summarises the outcomes of the four trials. Overall, the frequency of tuberculosis was reduced in subjects who received preventive treatment compared with those who received placebo (relative risk 0.57, 95% confidence interval 0.41 to 0.79). Risk of death (0.93, 0.83 to 1.05) was not significantly different in the two groups.
In two trials, when comparing subjects positive for purified protein derivative who received preventive treatment with those who received placebo, the 95% confidence interval for the relative risk of both tuberculosis and mortality included one (fig), indicating non-significant results. The pooled risk of tuberculosis in those receiving preventive treatment compared with placebo was 0.32 (0.19 to 0.51), indicating substantial protection against active disease. The pooled relative risk of mortality was 0.73 (0.57 to 0.95), indicating a moderate reduction in the risk of death in those receiving preventive treatment. Hawken et al did not define adverse drug reaction by purified protein derivative status and thus no stratified analysis of this outcome measure is reported here.10
In adults with a negative tuberculin skin test the estimates of effect in all trials included one, indicating non-significant results (fig). The pooled risk of tuberculosis in subjects with a negative tuberculin skin test who received preventive treatment was 0.82 (0.50 to 1.36) compared with placebo, confirming that no substantial protection was conferred by the intervention. Similarly, the pooled relative risk for mortality was 1.02 (0.89 to 1.17) confirming that no substantial protection was conferred by the intervention.
Overall, adverse drug reactions were more common, but not significantly so (1.45, 0.98 to 2.14), in patients receiving active drug (86/2551; 3.4%) compared with those receiving placebo (43/1386; 3.1%).
Discussion
Available evidence to date indicates that preventive treatment reduces the frequency of active tuberculosis in adults infected with HIV by approximately half. Protection against tuberculosis is greatest in adults infected with HIV who have a positive tuberculin skin test (approximately 70% reduction), and reduced incidence of mortality is also observed in this group (approximately 25%). Average follow up in these trials was 15 to 33 months, and it is not possible to conclude that benefit persists beyond this time. A small and non-significant reduction in tuberculosis incidence was observed in adults with a negative tuberculin skin test, and no effect on mortality was observed in this group.
Thus, in settings where testing for purified protein derivative is possible, preventive treatment might best only be offered to adults infected with HIV with a positive tuberculin skin test. In settings where testing for purified protein derivative is not possible, if preventive treatment is given to all adults infected with HIV, it is likely that the frequency of tuberculosis will still be reduced, but to a smaller extent.
Our review shows the value of systematic review and meta-analysis. Most of the trials studied were underpowered and reported results of borderline significance. By combining data we are able to provide more precise estimates of effect for the main outcome measures. The direction of effect of the intervention in the different settings was the same (fig), supporting the validity of combining data. A meta-analysis of individual patient data would be required to provide summary estimates of measures such as time to disease and death, and efforts to gather data to conduct such an analysis are under way.
Possible biases
A systematic review may be biased if trials reporting negative findings are not published. The trial reported to be incomplete13 published positive findings in abstract form, and the trial in preparation has also reported positive results. We found no statistical evidence of heterogeneity in this meta-analysis, but the power to detect heterogeneity was limited by the small number of trials. While there seems to be some clinical heterogeneity (fig) this tends to be limited to one trial in each subgroup, and varying levels of adherence in the different trials might explain this, at least in part.
It may be difficult to generalise our findings to all populations, as the baseline risk of tuberculosis varied substantially by setting. Gordin et al observed a very much lower incidence of tuberculosis than expected.11 Preventive treatment works mainly by preventing reactivation of latent infection. Recent infection may account for 30-40% of the burden of tuberculosis in both developed15 and developing countries.16 The relative importance of these two mechanisms may vary by setting and is likely to influence effectiveness of preventive treatment. When given for only a few months, there is little opportunity for preventive treatment to protect against exposure to infection with M tuberculosis in adults negative for purified protein derivative. Adults positive for purified protein derivative are at risk of new infection after preventive treatment has been stopped.
Choice of drug regimen
Which drug regimen should be recommended? This review did not set out to answer this question. However, in the trial which tested three different regimens against placebo, isoniazid had the greatest effect,12 although isoniazid and rifampicin combined and isoniazid, rifampicin, and pyrazinamide combined also reduced the incidence of tuberculosis. Halsey et al compared two regimens and reported similar protection conferred by twice weekly isoniazid given for 6 months and combined rifampicin and pyrazinamide given for 2 months.14 Trials using combination treatment report higher rates of adverse drug reaction than do those using isoniazid alone. Adherence to preventive treatment was generally poor in these trials. Choice of regimen to implement in practice is likely to depend on anticipated adherence, cost, availability of drugs, concern over adverse drug reactions, and prevalence of drug resistance in the population. The strongest available evidence is for the use of isoniazid.
Although not reported as a problem in subjects who developed tuberculosis in these trials, widespread and unsupervised use of tuberculosis drugs is of concern, and monitoring for the development of drug resistance should take place. Adverse drug reactions were reported infrequently in these trials and although reassuring, monitoring of large numbers of subjects will be required to determine the incidence of infrequent but life threatening events such as hepatitis in association with isoniazid.
Preventive treatment and tuberculosis control
Although reduction in individual risk of tuberculosis is substantial, unless a large proportion of the affected population receives preventive treatment it seems unlikely that this intervention will substantially reduce disease transmission in countries with a high tuberculosis prevalence. The priority for tuberculosis control remains the early detection and treatment of active cases. Preventive treatment may be a useful intervention for individuals and for targeted groups such as factory workers, hospital staff, police, and the armed forces17 who may have access to HIV testing, counselling, and ongoing care. These conclusions are in accord with current recommendations from the World Health Organisation and the International Union Against Tuberculosis and Lung Disease.18 This policy, and future refinements to it, can now be based on a body of systematically reviewed data from relevant trials that provides accurate estimates of effect, and that is constantly updated.19
There remains a need to determine the long term impact of preventive treatment on tuberculosis and death, and the results of trials testing the efficacy of life long preventive treatment in adults infected with HIV are awaited. It will also be important to study the logistical barriers to implementing preventive treatment in different settings.20
Table 1.
Characteristics of randomised placebo controlled trials of preventive treatment for tuberculosis in adults infected with HIV included in review
| Study (country) | Method | Participants | Interventions | Outcomes |
|---|---|---|---|---|
| Pape et al9 (Haiti) | Randomised by computer Allocation not described Double blind* | Symptom free, newly diagnosed (n=118) No active tuberculosis (91/118 (77%) were women) Positive or negative for tuberculin† | Isoniazid 300 mg daily for 12 months | Subjects assessed every 3 months Mean follow up 33 months No loss to follow up |
| Hawken et al10 (Kenya) | Block randomised by computer Allocation concealed Double blind‡ | Mostly symptom free (n=684) No active tuberculosis Positive or negative for tuberculin | Isoniazid 300 mg daily for 6 months | 356/509 (70%) of expected subjects seen at the end of the trial Median follow up 20 months |
| Gordin et al11 (USA; 74% New York) | Randomisation not described Allocation concealment not described No data on number of eligible patients not enrolled | HIV infected (119/517 (23%) had AIDS) Negative for tuberculin Anergic At high risk of tuberculosis | Isoniazid 300 mg daily for 6 months | 326 (63%) patients completed treatment 6% and 7% of treatment and placebo groups were lost respectively Mean follow up 33 months |
| Whalen et al12 (Uganda) | Block randomised by computer Allocation concealed§ Double blind | Mild HIV disease (n=2736) Positive for tuberculin Anergic | Isoniazid 300 mg daily for 6 months then isoniazid plus rifampicin 600 mg daily for 3 months then isoniazid plus rifampicin plus pyrazinamide 2 g for 3 months Anergic: isoniazid 300 mg daily for 6 months | 80-89% of the different groups completed the trials No data on follow up procedures Mean follow up 15 months |
Tuberculin as purified protein derivative.
21 of 60 patients in placebo arm accepted offer of isoniazid at time of interim analysis, but all were analysed in placebo arm.
12 of 696 enrolled patients were excluded and 14 of 684 failed to return after recruitment.
9095 people were screened; 4306 (47%) did not complete baseline investigations and 2053 (23%) were ineligible.
Figure.
Effect of preventive treatment for tuberculosis in adults infected with HIV on active tuberculosis and mortality, stratified by purified protein derivative status
Acknowledgments
This review is concurrently available on the infectious diseases module of the Cochrane Database of Systematic Reviews and will be updated as new data become available. We thank Dr Mark Hawken, who made original trial data available rapidly and courteously.
Footnotes
Funding: This work was funded by the South African Medical Research Council and a grant from the directorate: HIV/AIDS and sexually transmitted diseases of the department of health of the South African government. PG and the Cochrane Infectious Diseases Group are supported by the Department for International Development (UK) and the European Union. None of these bodies can accept any responsibility for the information provided in this review or for the views expressed.
Conflict of interest: None.
References
- 1.Narain JP, Raviglione MC, Kochi A. HIV-associated tuberculosis in developing countries: epidemiology and strategies for prevention. Tubercle Lung Disease. 1992;73:311–321. doi: 10.1016/0962-8479(92)90033-G. [DOI] [PubMed] [Google Scholar]
- 2.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organisation. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien RJ. Preventive therapy for tuberculosis. In: Porter JDH, McAdam KPWJ, editors. Tuberculosis: back to the future. Chichester: Wiley; 1994. pp. 151–166. [Google Scholar]
- 4.De Cock KM, Soro B, Coulibaly IM, Lucas SB. Tuberculosis and HIV infection in sub-Saharan Africa. JAMA. 1992;268:1581–1587. doi: 10.1001/jama.268.12.1581. [DOI] [PubMed] [Google Scholar]
- 5.Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 6.Centres for Disease Control and Prevention. Screening for tuberculosis and tuberculous infection in high-risk populations, and the use of preventive therapy for tuberculous infection in the United States. MMWR. 1990;39((RR-8):1–12. [PubMed] [Google Scholar]
- 7.Egger M, Davey Smith G. Bias in location and selection of studies. BMJ. 1998;316:61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Thoracic Society. Diagnostic standards and classification of tuberculosis. Am Rev Respir Disease. 1990;142:1420–1422. doi: 10.1164/ajrccm/142.3.725. [DOI] [PubMed] [Google Scholar]
- 9.Pape JW, Jean SS, Ho JL, Hafner A, Johnson WD. Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet. 1993;342:268–272. doi: 10.1016/0140-6736(93)91817-6. [DOI] [PubMed] [Google Scholar]
- 10.Hawken M, Meme HK, Ellioo LC, Chakaya JM, Morris JS, Githui WA, et al. Isoniazid preventive therapy for tuberculosis in HIV-1 infected adults: results of a controlled trial. AIDS. 1997;11:875–882. doi: 10.1097/00002030-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Gordin FM, Matts JP, Miller C, Brown LS, Hafner R, John SL, et al. the Terry Beirn Community Programs for Clinical Research on AIDS. A controlled trial of isoniazid in persons with anergy and human immunodeficiency virus infection who are at high risk for tuberculosis. N Engl J Med. 1997;337:315–320. doi: 10.1056/NEJM199707313370505. [DOI] [PubMed] [Google Scholar]
- 12.Whalen CC, Johnson JL, Okwera A, Hom DL, Huebner R, Mugyenyi P, et al. A trial of three regimens to prevent tuberculosis in Ugandan adults with the human immunodeficiency virus. N Engl J Med. 1997;337:801–808. doi: 10.1056/NEJM199709183371201. [DOI] [PubMed] [Google Scholar]
- 13.Wadhawan D, Hira SK, Mwansa N, Tembo G, Perine PL. Isoniazid prophylaxis among patients with HIV-1 infection. [abstract TuB 0536.] VIII International conference on AIDS, and III sexually transmitted disease world congress, Amsterdam, July 1992.
- 14.Halsey NA, Coberly JS, Desmormeaux J, Losikoff P, Atkinson J, Moulton LH, et al. Randomised trial of isoniazid versus rifampicin and pyrazinamide for prevention of tuberculosis in HIV-1 infection. Lancet. 1998;351:786–792. doi: 10.1016/S0140-6736(97)06532-X. [DOI] [PubMed] [Google Scholar]
- 15.Small PM, Hopewell PC, Singh SP, Paz A, Parsonnet J, Ruston DC, et al. The epidemiology of tuberculosis in San Francisco: a population based study using conventional and molecular methods. N Engl J Med. 1994;330:1703–1709. doi: 10.1056/NEJM199406163302402. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson D, Pillay M, Davies GR, Lombard C, Sturm AW, Crump J. Molecular epidemiology and transmission dynamics of Mycobacterium tuberculosis in rural Africa. Trop Med Int Health. 1997;2:747–753. doi: 10.1046/j.1365-3156.1997.d01-386.x. [DOI] [PubMed] [Google Scholar]
- 17.De Cock KM, Grant A, Porter JDH. Preventive therapy for tuberculosis in HIV-infected persons: international recommendations, research and practice. Lancet. 1995;345:833–836. doi: 10.1016/s0140-6736(95)92967-3. [DOI] [PubMed] [Google Scholar]
- 18.International Union Against Tuberculosis and Lung Disease and the Global Programme on AIDS and the tuberculosis programme of the World Health Organisation. Tuberculosis preventive therapy in HIV-infected individuals. Tubercle Lung Disease. 1994;75:96–98. doi: 10.1016/0962-8479(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson D. Preventive therapy for tuberculosis in HIV infected persons. In: Garner P, Gelband H, Olliaro P, Salinas R, Wilkinson D, eds. Infectious diseases module, Cochrane Database of Systematic Reviews [updated 14 January 1998]. The Cochrane Library. Cochrane Collaboration; Issue 2. Oxford: Update Software, 1998. Updated quarterly.
- 20.Aisu T, Raviglione M, Van Praag E, Eriki P, Narain JP, Barugahare L, et al. Preventive chemotherapy for HIV-associated tuberculosis in Uganda: an operational assessment at a voluntary counselling and testing centre. AIDS. 1995;9:267–273. [PubMed] [Google Scholar]