Abstract
Background
Alb/TGF-β1 transgenic mice overexpress active transforming growth factor-β1 (TGF-β1) in the liver, leading to increased circulating levels of the cytokine and progressive renal fibrosis. This study was designed to explore if exogenous all-trans retinoic acid (tRA) prevents renal fibrosis in this animal model.
Methods
The retinoid profile in kidney and liver of wild-type and Alb/TGF-β1 transgenic mice was examined by high-performance liquid chromatography and slow-release pellets containing different amounts of tRA were implanted subcutaneously to treat the Alb/TGF-β1 transgenic mice, starting at 1 week of age; mice were sacrificed 2 weeks later.
Results
Kidneys of 3-week-old wild-type mice had abundant tRA, which was completely absent in kidneys of the transgenic mice. Low doses of tRA (6–10.7 mg/kg/day) failed to affect renal fibrosis although it tended to suppress the mRNA expression of some molecular markers of fibrosis and retinal dehydrogenase 2 (RALDH2), a gene encoding a key tRA-synthesising enzyme. These tendencies disappeared, mortality tended to increase and RALDH2 and connective tissue growth factor (CTGF) mRNAs significantly increased in the medium-dose group (12.7–18.8 mg/kg/day). High doses (20.1–27.4 mg/kg/day) showed even higher toxicity with increased renal fibrosis and significant mortality.
Conclusions
Alb/TGF-β1 transgenic mice are characterised by depletion of endogenous renal tRA. Exogenous tRA dose-dependently increases mortality and kidney fibrosis, which is associated with dose-dependent regulation of renal RALDH2 and CTGF mRNA expression.
Key Words: All-trans retinoic acid, Transforming growth factor-β1, Connective tissue growth factor, Retinal dehydrogenase 2, Fibrosis
Introduction
Chronic kidney disease is characterised by refractory inflammation and fibrosis, which often lead to a progressive decline in renal function and the potential need for renal replacement therapy [1]. Retinoids, including vitamin A and its metabolites especially all-trans retinoic acid (tRA), have been shown to prevent both inflammatory injury and fibrotic lesions in various experimental chronic kidney diseases [2]. Although a reduction in fibrosis might be due to suppression of inflammation, often the inciting trigger for fibrosis, we hypothesised that retinoids may also have inflammation-independent antifibrotic effects, by directly inhibiting the production of extracellular matrices (ECM) and/or by inhibiting the profibrotic transforming growth factor-β1 (TGF-β1) pathway. The hypothesis is supported by reports that retinoids suppressed ECM expression in cultured renal mesangial cells, skin and lung fibroblasts and hepatic stellate cells [3,4,5,6], as well as in the cardiovascular system in vivo [7]. Furthermore, retinoids antagonised angiotensin II-induced TGF-β1 expression in smooth muscle cells, TGF-β1-induced gene transcription in lung fibroblasts, and TGF-β1-induced differentiation of HL-60 leukemic cells into monocytes [8,9,10]. On the other hand, however, retinoids were also reported to stimulate the basal expression of collagens in chondrocytes and lung epithelial cells [11,12], synergise with TGF-β1 in lung epithelial cells [13], induce collagen expression in diabetic skin and in skin cells exposed to ultraviolet radiation or subjected to growth inhibition [14,15,16], and induce collagen expression in hepatic stellate cells by activating TGF-β1 [17,18], all of which argue against the hypothesis.
In this study, we used Alb/TGF-β1 transgenic (TG) mice, an animal model of glomerulosclerosis and interstitial fibrosis without substantial inflammation [19,20], to test our hypothesis by measuring the effects of different doses of exogenous tRA on glomerulosclerosis, tubulointerstitial fibrosis, renal function as well as mRNA expression of a key tRA-synthesising enzyme and molecular markers of fibrogenesis.
Materials and Methods
High-Performance Liquid Chromatography (HPLC) Analysis of Retinoids
Kidneys and livers were collected, snap-frozen in liquid nitrogen and stored at −80°C. Both kidneys from the same mouse and individual livers were analysed in each HPLC assay. Tissues were homogenised in 1 ml ice-cold stabilising solution, which is phosphate-buffered saline plus trisodium salt of ethylenediaminetetraacetic acid containing 5 mg/ml ascorbic acid (pH 7.3), and the tissue suspension extracted twice with 2 volumes of methyl acetate:ethyl acetate, 1:8 (with butylated hydroxytoluene as an antioxidant). The extract was dried down over nitrogen, resuspended in 100 μl methanol, centrifuged at high speed to remove any particulate matter and placed in autosampler vials for HPLC analysis. Reverse-phase HPLC was performed using a Beckman system Gold Hardware (Beckman Coulter UK Ltd., High Wycombe, UK) with a photodiode array detector and a 5-μm C18 LiChroCART column (Merck Chemicals Ltd., Poole, UK) with an equivalent precolumn. The mobile phases used were as previously described [21], which allow a good separation of the retinoic acids and retinols. The flow rate was 1.5 ml/min using a gradient of acetonitrile/ammonium acetate (15 mM, pH 6.5) from 40 to 67% acetonitrile for 35 min followed by 100% acetonitrile for a further 25 min. Individual retinoids were identified according to their UV absorption spectra. Each experiment was repeated at least 5 times.
tRA Treatment of Alb/TGF-β1 Transgenic TG Mice
Line 25 Alb/TGF-β1 mice, all of which are male, were established and characterised as previously described [19,20]. Male C57BL/6J × CBA F1 mice were used as wild-type (WT) controls. All animal studies were performed according to the National Institutes of Health guidelines.
The TG mice were randomly divided into 4 groups. Three-week slow-release pellets containing 0, 0.79, 1.58 and 2.36 mg of tRA (Innovative Research of America, Sarasota, Fla., USA) [22,23] were implanted subcutaneously at 1 week of age, when the TG mice grow normally. Renal histological abnormality was not detected, although renal expression of collagen types I and III (COL1 and COL3) mRNAs has already increased at this stage [20]. Based on the starting body weight, these pellets generated 4 starting dose ranges: (1) G0 (placebo pellets; 0 mg/kg/day tRA); (2) G1 (low doses, 6–10.7 mg/kg/day); (3) G2 (medium doses, 12.7–18.8 mg/kg/day), and (4) G3 (high doses, 20.1–27.4 mg/kg/day). WT male mice treated with placebo pellets (n = 3/group) were used as controls and all mice were sacrificed at 3 weeks of age. At the end of the study, serum and urine were collected and kidney tissue was harvested for histological examination or stored in RNAlater (Applied Biosystems, St. Austin, Tex., USA) for RNA analysis.
Serum creatinine (Scr) concentrations were measured using a simplified HPLC method [24]. Urine albumin/creatinine ratio was analysed using the mouse albumin enzyme-linked immunosorbent assay kit (Bethyl Laboratories, Montgomery, Tex., USA) and a creatinine assay kit (Exocell Inc., Philadelphia, Pa., USA).
Masson's trichrome staining sections were scored in a blinded manner by a renal pathologist using the following 2 criteria: (i) Glomerulosclerosis score: 0 = no blue stain in the glomerulus; 0.5 = trace stain; 1 = segmental blue stain occupying <25% tuft area; 2 = segmental blue stain occupying 25–50% tuft area; 3 = extensive stain occupying >50% tuft area and with some patent capillary loops, and 4 = obsolescent glomeruli. Twenty randomly selected glomeruli from the left kidney and 20 glomeruli from the right kidney were scored and the mean score calculated for each mouse. (ii) Interstitial fibrosis score: 0 = normal tissue; 1 = positive stain <10% of tubulointerstitium; 2 = positive stain involving 11–20% of tubulointerstitium, and 3 = positive stain ≥21% of tubulointerstitium.
Reverse Transcription Quantitative Polymerase Chain Reaction
Mouse kidney samples were homogenised in Trizol (Invitrogen, Carlsbad, Calif., USA) to extract total RNA, which was then purified using RNAeasy minicolumn and on-column digested with DNase I (Qiagen, Valencia, Calif., USA) and reverse-transcribed into cDNA using SuperScript II RNase H Reverse Transcriptase (Invitrogen). Quantitative PCR was then performed in duplicate. Briefly, cDNA in 9 μl water was mixed with 10 μl 2× TaqMan universal master mix and 1 μl custom 20× TaqMan primer and probe mix for mouse collagen types I α1, α2 and IV α1 (COL1 A1, COL1 A2, COL4 A1, respectively), fibronectin (FN), connective tissue growth factor (CTGF), retinaldehyde dehydrogenase 2 (RALDH2) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; order numbers 1906874, 1944518, assay ID Mm99999915, Applied Biosystems). The following PCR conditions were used: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The expression level of each target gene was normalised to GAPDH.
Statistical Analysis
Data are expressed as mean ± SD and analysed using the Prism 4.0c software (GraphPad, La Jolla, Calif., USA). An unpaired t test was used to compare 2 groups; Kaplan-Meier survival curve analysis was performed to compare mortality among groups, and a one-way ANOVA non-parametric analysis was used to compare means of multiple groups. p < 0.05 indicates a statistically significant difference.
Results
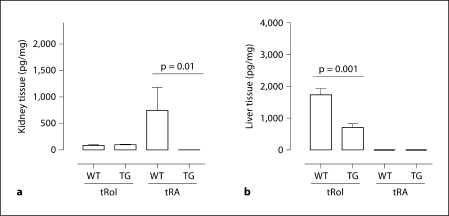
As shown in figure 1, HPLC analysis revealed abundant tRA and a relatively low level of all-trans retinol (tRol) in the kidneys of healthy 3-week-old C57BL/6J × CBA F1 mice, indicating that retinoids in the kidneys of these young mice were predominantly the active form. Meanwhile, livers of the same mice demonstrated a much higher level of tRol but no tRA was detected. Interestingly, liver tRol and renal tRA levels were significantly lower in the Alb/TGF-β1 TG mice, although renal tRol remained the same as in WT mice.
Fig. 1.
HPLC analysis of retinoids in kidney and liver of 3-week-old WT and Alb/TGF-β1 TG mice. Extracts of kidney (a) and liver (b) tissues were subjected to HPLC assay to measure retinoid concentrations. a n = 4 for WT and n = 5 for TG. b n = 8 for WT and n = 7 for TG.
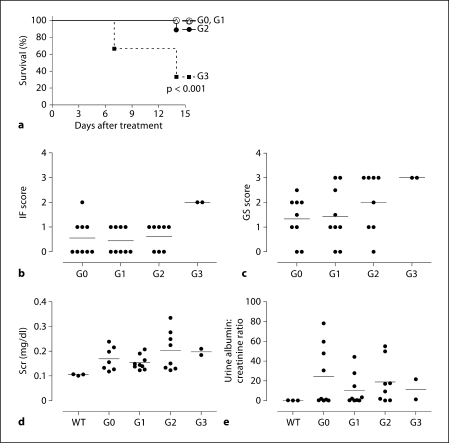
To test if early administration of exogenous tRA prevents the development of renal fibrosis in Alb/TGF-β1 mice, the mice were treated with placebo pellets (G0), or pellets releasing low (G1), medium (G2) and high (G3) doses of tRA. Assignment to the G3 group was stopped after 6 animals had been recruited due to a significantly increased mortality (fig. 2a). The cause of the increased mortality is unknown, but it is notable that the 2 surviving G3 mice had the highest glomerulosclerosis and interstitial fibrosis scores (fig. 2b, c), suggesting accelerated renal fibrosis.
Fig. 2.
Dose-dependent effects of tRA on survival, renal pathology, Scr and albuminuria in Alb/TGF-β1 mice. Survival curve of the different groups (a), end-of-study interstitial fibrosis (IF) score (b), end-of-study glomerulosclerosis (GS) score (c), end-of-study Scr (d) and urine albumin:creatinine ratio (e). Only 8 out of 9 samples were analysed in the G2 group and only 2 in 6 samples were analysed in the G3 group due to animal death and loss of samples in these groups.
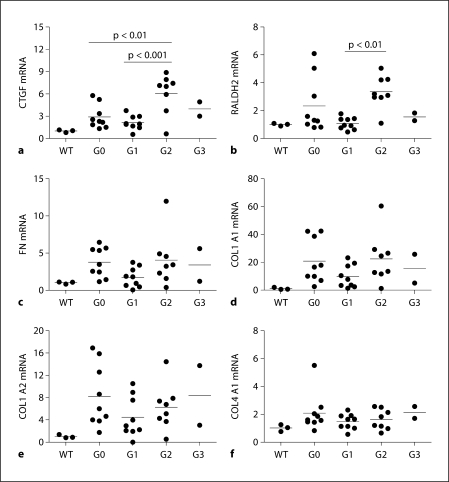
G2 was characterised by a significant induction of CTGF and RALDH2 mRNA expression (fig. 3a, b). This was associated with a trend towards increased mortality, glomerulosclerosis and Scr levels (fig. 2a, c, d). As such, we clearly defined G2 and G3 as groups that showed toxic effects of tRA.
Fig. 3.
End-of-study renal mRNA expression of CTGF, RALDH2 and ECM genes. The mRNA expression of CTGF (a), RALDH2 (b), FN (c), COL1 A1 (d), COL1 A2 (e) and COL4 A1 (f) was examined by Taqman RT-qPCR and GAPDH was used as a ‘house-keeping’ gene to normalise gene expression. The average mRNA expression level of 3 WT mice was set as 1; G0 is the vehicle-treated group; G1, G2 and G3 are low, medium and high doses of tRA-treated groups, respectively. Only 8 out of 9 samples were analysed in the G2 group and only 2 in 6 samples were analysed in the G3 group due to animal death and loss of samples in these groups.
In contrast, G1 had a tendency towards a reduction in the average of urinary albumin excretion (45%, fig. 2e), as well as renal RALDH2 (55%, fig. 3b), FN (54%, fig. 3c), COL1 A1 (47%, fig. 3d), COL1 A2 (46%, fig. 3e) and COL4 A1 (35%, fig. 3f) mRNAs compared to G0. Unfortunately, despite these favourable changes of molecular markers of fibrogenesis, glomerulosclerosis and interstitial fibrosis in this group were not prevented and renal function was not successfully preserved compared to group G0 (fig. 2b–d).
In summary, at the doses tested, no net antifibrotic effect of tRA was observed in the kidneys of Alb/TGF-β1 TG mice, although low doses of tRA suppressed selected molecular markers of fibrogenesis. In addition, medium doses of tRA tend to upregulate these molecular markers and significantly induced renal CTGF and RALDH2 mRNAs compared to low doses. High doses of tRA showed toxicity and a profibrotic effect.
Discussion
We report that tRA is abundant in normal mouse kidneys but is not measurable in kidneys of Alb/TGF-β1 TG mice and that exogenous tRA exerts intricate dose-dependent effects on renal fibrosis, as well as renal CTGF and RALDH2 mRNA expression in Alb/TGF-β1 TG mice.
This study is largely descriptive and does not provide an explanation as to why tRA is depleted from Alb/TGF-β1 kidneys, why fibrosis is not improved by low doses and medium doses of tRA, and why an excess mortality was observed in the high-dose group, but we hope that it will serve as an interesting basis for further studies. We initially hypothesised that systemic administration of pharmaceutical tRA might compensate the loss of renal tRA in Alb/TGF-β1 TG mice; thus, the lack of net benefits and the apparent toxicity of medium and high doses of tRA are indeed disappointing. In agreement with our findings that tRA is abundant in kidneys but not in the liver (fig. 1), our recent work, through in vivo retinoic acid response element (RARE) activity reporter assays, revealed RARE activity in kidneys but not in the liver. Interestingly, renal RARE activity is only observed in the collecting duct system but not in any parts of the nephron (Wong et al., data not shown). It is thus worthwhile to reconsider the consequence of systemic administration of pharmaceutical tRA, in particular, whether the effects observed are attributable to tRA per se or its metabolites generated following administration and whether systemic delivery of tRA will cause widespread activation of RARE-regulated genes in nephrons and in the liver, where tRA signalling is normally absent, thus leading to adverse consequences.
tRA was previously reported to induce CTGF mRNA expression in human prostate epithelial cells [25] and chondrocytes [26] but suppress CTGF mRNA expression in newt limb blastemal cells [27]. Here, we found that tRA did not significantly suppress CTGF mRNA expression in kidneys of the Alb/TGF-β1 TG mice. Moreover, higher doses of tRA even induced CTGF mRNA expression and this was associated with a diminished suppressive effect of profibrotic molecular markers and a tendency of increased glomerulosclerosis and renal fibrosis. The role of CTGF in the opposing actions of tRA regulating the fibrogenic effects of TGF-β1 awaits further investigation.
Although low-dose tRA tended to suppress ECM gene expression, the effect was abrogated with higher doses of tRA, accompanied by an induction of CTGF and RALDH2, as well as an escalating renal fibrosis and mortality. Of note, high, but not low, concentrations of vitamin A were also previously reported to increase fibrosis in kidney and liver [17,28]. More recently, Morath et al. [29] also documented similar dose-dependent effects of 13-cis-retinoic acid in a chronic nephritis rat model, with a low dose attenuating, and a higher dose aggravating, glomerulosclerosis. The mechanisms behind the toxic and profibrotic effects of higher doses of retinoids were not fully understood. In our study, the increased toxicity of the higher doses of tRA coincided with a paradoxical increased RALDH2 mRNA expression (fig. 3b). As this gene encodes a key tRA-synthesising enzyme [2], tRA-induced RALDH2 might lead to conversion of more retinol to tRA resulting in an excessive accumulation of exogenous and endogenous tRA that could contribute towards toxicity and fibrosis.
The dose-dependent, dichotomous effects of retinoids in animal models suggest that it is important to determine the optimum dose range of retinoids in in vivo studies. A clinical trial using 13-cis-retinoic acid and tRA to treat chronic kidney diseases is currently being carried out at the National Institutes of Health. It would be necessary for us to interpret clinical outcomes in the ongoing and future trials in a dose- or even plasma concentration-specific manner.
In conclusion, the present study has established a change of kidney retinoid profile in Alb/TGF-β1 TG mice compared to WT mice, and a dose-dependent, complex action of exogenous tRA in regulating TGF-β1-induced renal fibrosis in these TG mice. It highlights the dose-dependent toxicity and profibrotic action of tRA and warrants further studies to dissect the mechanisms of the dual potential of tRA in regulating fibrosis so that unwanted profibrotic effects of tRA can be avoided.
Acknowledgements
This work is supported by Kidney Research UK, British Heart Foundation, Royal Free Peter Samuel Fund, UCL Bogue Fellowship Fund and a UCL VIP award to Q.X. and an Intramural Research Program of the NIDDK, NIH awarded to J.B.K. We are grateful to Dr. Duolao Wang (London School of Hygiene & Tropical Medicine, London, UK) for critically reviewing the statistical methodology in the manuscript.
References
- 1.Feehally J, Floege J, Johnson RJ. Comprehensive Clinical Nephrology, ed 3, revised. London: Mosby; 2007. [Google Scholar]
- 2.Xu Q, Lucio-Cazana J, Kitamura M, Ruan X, Fine LG, Norman J. Retinoids in nephrology: promises and pitfalls. Kidney Int. 2004;66:2119–2131. doi: 10.1111/j.1523-1755.2004.66002.x. [DOI] [PubMed] [Google Scholar]
- 3.Wen X, Li Y, Hu K, Dai C, Liu Y. Hepatocyte growth factor receptor signaling mediates the anti-fibrotic action of 9-cis-retinoic acid in glomerular mesangial cells. Am J Pathol. 2005;167:947–957. doi: 10.1016/S0002-9440(10)61185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daly TJ, Weston WL. Retinoid effects on fibroblast proliferation and collagen synthesis in vitro and on fibrotic disease in vivo. J Am Acad Dermatol. 1986;15:900–902. doi: 10.1016/s0190-9622(86)70248-x. [DOI] [PubMed] [Google Scholar]
- 5.Redlich CA, Delisser HM, Elias JA. Retinoic acid inhibition of transforming growth factor-β-induced collagen production by human lung fibroblasts. Am J Respir Cell Mol Biol. 1995;12:287–295. doi: 10.1165/ajrcmb.12.3.7873195. [DOI] [PubMed] [Google Scholar]
- 6.Davis BH, Kramer RT, Davidson NO. Retinoic acid modulates rat Ito cell proliferation, collagen, and transforming growth factor β production. J Clin Invest. 1990;86:2062–2070. doi: 10.1172/JCI114943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shindo T, Manabe I, Fukushima Y, Tobe K, Aizawa K, Miyamoto S, Kawai-Kowase K, Moriyama N, Imai Y, Kawakami H, Nishimatsu H, Ishikawa T, Suzuki T, Morita H, Maemura K, Sata M, Hirata Y, Komukai M, Kagechika H, Kadowaki T, Kurabayashi M, Nagai R. Kruppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat Med. 2002;8:856–863. doi: 10.1038/nm738. [DOI] [PubMed] [Google Scholar]
- 8.Haxsen V, Adam-Stitah S, Ritz E, Wagner J. Retinoids inhibit the actions of angiotensin II on vascular smooth muscle cells. Circ Res. 2001;88:637–644. doi: 10.1161/01.res.88.6.637. [DOI] [PubMed] [Google Scholar]
- 9.Pendaries V, Verrecchia F, Michel S, Mauviel A. Retinoic acid receptors interfere with the TGF-β/Smad signaling pathway in a ligand-specific manner. Oncogene. 2003;22:8212–8220. doi: 10.1038/sj.onc.1206913. [DOI] [PubMed] [Google Scholar]
- 10.Cao Z, Flanders KC, Bertolette D, Lyakh LA, Wurthner JU, Parks WT, Letterio JJ, Ruscetti FW, Roberts AB. Levels of phospho-Smad2/3 are sensors of the interplay between effects of TGF-β and retinoic acid on monocytic and granulocytic differentiation of HL-60 cells. Blood. 2003;101:498–507. doi: 10.1182/blood-2002-05-1549. [DOI] [PubMed] [Google Scholar]
- 11.Wu LN, Ishikawa Y, Nie D, Genge BR, Wuthier RE. Retinoic acid stimulates matrix calcification and initiates type I collagen synthesis in primary cultures of avian weight-bearing growth plate chondrocytes. J Cell Biochem. 1997;65:209–230. doi: 10.1002/(sici)1097-4644(199705)65:2<209::aid-jcb7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Federspiel SJ, DiMari SJ, Howe AM, Guerry-Force ML, Haralson MA. Extracellular matrix biosynthesis by cultured fetal rat lung epithelial cells. IV. Effects of chronic exposure to retinoic acid on growth, differentiation, and collagen biosynthesis. Lab Invest. 1991;65:441–450. [PubMed] [Google Scholar]
- 13.La P, Morgan TA, Sykes SM, Mao H, Schnepp RW, Petersen CD, Hua X. Fusion proteins of retinoid receptors antagonize TGF-β-induced growth inhibition of lung epithelial cells. Oncogene. 2003;22:198–210. doi: 10.1038/sj.onc.1206100. [DOI] [PubMed] [Google Scholar]
- 14.Lateef H, Stevens MJ, Varani J. All-trans retinoic acid suppresses matrix metalloproteinase activity and increases collagen synthesis in diabetic human skin in organ culture. Am J Pathol. 2004;165:167–174. doi: 10.1016/S0002-9440(10)63285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths CE, Russman AN, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid) N Engl J Med. 1993;329:530–535. doi: 10.1056/NEJM199308193290803. [DOI] [PubMed] [Google Scholar]
- 16.Varani J, Mitra RS, Gibbs D, Phan SH, Dixit VM, Mitra R, Jr, Wang T, Siebert KJ, Nickoloff BJ, Voorhees JJ. All-trans retinoic acid stimulates growth and extracellular matrix production in growth-inhibited cultured human skin fibroblasts. J Invest Dermatol. 1990;94:717–723. doi: 10.1111/1523-1747.ep12876294. [DOI] [PubMed] [Google Scholar]
- 17.Okuno M, Moriwaki H, Imai S, Muto Y, Kawada N, Suzuki Y, Kojima S. Retinoids exacerbate rat liver fibrosis by inducing the activation of latent TGF-β in liver stellate cells. Hepatology. 1997;26:913–921. doi: 10.1053/jhep.1997.v26.pm0009328313. [DOI] [PubMed] [Google Scholar]
- 18.Vollmar B, Heckmann C, Richter S, Menger MD. High, but not low, dietary retinoids aggravate manifestation of rat liver fibrosis. J Gastroenterol Hepatol. 2002;17:791–799. doi: 10.1046/j.1440-1746.2002.02804.x. [DOI] [PubMed] [Google Scholar]
- 19.Kopp JB, Factor VM, Mozes M, Nagy P, Sanderson N, Böttinger EP, Klotman PE, Thorgeirsson SS. Transgenic mice with increased plasma levels of TGF-β1 develop progressive renal disease. Lab Invest. 1996;74:991–1003. [PubMed] [Google Scholar]
- 20.Mozes MM, Bottinger EP, Jacot TA, Kopp JB. Renal expression of fibrotic matrix proteins and of transforming growth factor-β (TGF-β) isoforms in TGF-β transgenic mice. J Am Soc Nephrol. 1999;10:271–280. doi: 10.1681/ASN.V102271. [DOI] [PubMed] [Google Scholar]
- 21.Achkar CC, Derguini F, Blumberg B, Langston A, Levin AA, Speck J, Evans RM, Bolado J, Jr, Nakanishi K, Buck J, Gudas LJ. 4-Oxoretinol, a new natural ligand and transactivator of the retinoic acid receptors. Proc Natl Acad Sci USA. 1996;93:4879–4884. doi: 10.1073/pnas.93.10.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 23.Blush J, Lei J, Ju W, Silbiger S, Pullman J, Neugarten J. Estradiol reverses renal injury in Alb/TGF-β1 transgenic mice. Kidney Int. 2004;66:2148–2154. doi: 10.1111/j.1523-1755.2004.66005.x. [DOI] [PubMed] [Google Scholar]
- 24.Yuen PS, Dunn SR, Miyaji T, Yasuda H, Sharma K, Star RA. A simplified method for HPLC determination of creatinine in mouse serum. Am J Physiol Renal Physiol. 2004;286:F1116–F1119. doi: 10.1152/ajprenal.00366.2003. [DOI] [PubMed] [Google Scholar]
- 25.López-Bermejo A, Buckway CK, Devi GR, Hwa V, Plymate SR, Oh Y, Rosenfeld RG. Characterization of insulin-like growth factor-binding protein-related proteins (IGFBP-rPs) 1, 2, and 3 in human prostate epithelial cells: potential roles for IGFBP-rP1 and 2 in senescence of the prostatic epithelium. Endocrinology. 2000;141:4072–4080. doi: 10.1210/endo.141.11.7783. [DOI] [PubMed] [Google Scholar]
- 26.Shimo T, Koyama E, Sugito H, Wu C, Shimo S, Pacifici M. Retinoid signaling regulates CTGF expression in hypertrophic chondrocytes with differential involvement of MAP kinases. J Bone Miner Res. 2005;220:867–877. doi: 10.1359/JBMR.041235. [DOI] [PubMed] [Google Scholar]
- 27.Cash DE, Gates PB, Imokawa Y, Brockes JP. Identification of newt connective tissue growth factor as a target of retinoid regulation in limb blastemal cells. Gene. 1998;222:119–124. doi: 10.1016/s0378-1119(98)00478-8. [DOI] [PubMed] [Google Scholar]
- 28.Soylu A, Kavukçu S, Sarioğlu S, Astarcioğlu H, Türkmen M, Büyükgebiz B. The effect of vitamin A on the course of renal ablation nephropathy. Pediatr Nephrol. 2001;16:472–476. doi: 10.1007/s004670100577. [DOI] [PubMed] [Google Scholar]
- 29.Morath C, Ratzlaff K, Dechow C, Schwenger V, Schaier M, Zeier B, Peters J, Tsukada M, Zouboulis CC, Waldherr R, Gross ML, Ritz E, Zeier M, Wagner J. Chronic low-dose isotretinoin treatment limits renal damage in subtotally nephrectomized rats. J Mol Med. 2009;87:53–64. doi: 10.1007/s00109-008-0404-5. [DOI] [PubMed] [Google Scholar]