Abstract
Background/Aims
Little is known about the association of chronic kidney disease (CKD) with sleep quality, mood, and alertness. In this report, we assessed these symptoms among patients with advanced CKD (stages 4–5) and those with end-stage renal disease (ESRD) and compared them to healthy controls without known kidney disease.
Methods
Patients were recruited from local dialysis units, outpatient nephrology clinics and the Thomas E. Starzl Transplant Institute. Healthy control subjects matched for age, gender and race were drawn from an archival database. Daily symptoms of sleep quality, mood, and alertness were assessed by visual analogue scales of the Pittsburgh Sleep Diary. Health-related quality of life was assessed by the Short Form-36 instrument.
Results
Sixty-nine dialysis patients and 23patients with advanced CKD demonstrated worse scores in sleep quality, mood, and alertness (p < 0.001) than controls. In adjusted analyses, European-American race, dialysis dependency, younger age, and physical performance SF-36 components were significantly associated with poor sleep quality, mood and alertness (p < 0.05). The dialysis population demonstrated higher day-to-day variability in scores than either the advanced CKD patients or the controls.
Conclusion
Advanced CKD and dialysis dependency are associated with impaired and highly variable sleep quality, mood, and alertness.
Key Words: Mood, Sleep quality, Alertness, Chronic kidney disease, End-stage renal disease, Pittsburgh Sleep Diary, SF-36
Introduction
Poor health-related quality of life (HRQOL) in patients with advanced chronic kidney disease (CKD) remains a challenge to both patients and physicians [1]. In particular, daytime sleepiness, fatigue and mood disturbances are common and important symptoms associated with poor HRQOL among the CKD [2,3] and the end-stage renal disease (ESRD) populations [4,5]. Even though similar symptoms are commonly reported by patients with CKD [6,7,8], the severity and the day-to-day variability of such symptoms in the different stages of renal disease relative to normal controls is largely unknown. To our knowledge, the potential for these symptoms to exhibit a substantial degree of day-to-day variation in patients on or near dialysis dependency has not been previously appreciated in the literature. Yet, a high degree of variability in mood, sleep quality and alertness could hinder the recognition and delay the treatment of these symptoms by providers [6].
To address these gaps in knowledge, we examined daily sleep quality, mood and alertness in patients with stages 4–5 CKD or ESRD dependent on dialysis and in a comparison group of adults matched for age, gender and race. In order to characterize the variability in the severity of these symptoms, we prospectively recorded such data in diaries completed by study participants over a period of 7 to 14 days. In carrying out this investigation, we hypothesized that patients on dialysis [hemodialysis, (HD) or peritoneal dialysis (PD)] would demonstrate both worse (lower) and more variable sleep quality, mood and alertness compared to patients with stages 4–5 CKD and to normal controls.
Methods
Participants
Patients were enrolled from local dialysis units, outpatient nephrology clinics and the Thomas E. Starzl Transplant Institute in western Pennsylvania between April 2004 and November 2006. Patients were eligible to participate if they were >18 years of age, had an estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2 or were on maintenance dialysis (either HD or PD). In order to have a study sample in the steady state of their health, without any acute medical condition that could affect the results, patients were excluded from participation if they had: craniofacial abnormalities, actively treated sleep apnea, active malignancy, active infection, pulmonary disease using home oxygen therapy, active coronary artery disease within the last 6 months, advanced cirrhosis, advanced dementia, active alcohol abuse, or refractory psychiatric disease ascertained by medical records and confirmed by interview. This study was approved by the University of Pittsburgh institutional review board (protocol numbers: 312047, 501068, 604042) and all participants provided informed consent.
Control Patients without Kidney Disease
Control subjects without known CKD were drawn from an archival database of 820 healthy participants studied in 20 National Institutes of Health (NIH)-funded studies at the University of Pittsburgh between 1988 and 2006. Although the specific inclusion/exclusion criteria differed somewhat among these studies, all control participants were required to be in good general health, with only minor, well-controlled medical conditions as determined by medical history and physical examination. In addition, they had no current psychiatric illness by structured psychiatric interviews (the Schedule of Affective Disorders and Schizophrenia, SADS or the Structured Clinical Interview for DSM-IV, SCID) [9], and no current sleep disorder by clinical history. The information available on the archived participants consisted of demographic information and 7 days worth of sleep diary data. Controls were matched on age, gender and race to the patients in this study by a series of iterative queries against the archival database attempting to match the age (± 5 years), gender and racial distribution of the reference group to the patient participants. If a perfectly matched control for all 3 characteristics could not be located in the database, we used age and gender to carry out the matching.
Data Collection and Validation
Baseline data collection included a brief standardized health interview and a self-completed questionnaire, assessment of current antihypertensive medication use, blood pressure and anthropometric measurements. The self-completed questionnaire collected information on age, gender, race (categorized as African-American or European-American), employment and educational status (high school graduates or higher versus less than high school education).
Kidney function in non-dialysis dependent patients was estimated by the Modification of Diet in Renal Disease (MDRD) GFR estimating equation [10]. Diabetes was defined as current use of insulin or oral hypoglycemic agents, while chronic heart failure (CHF) was ascertained by self-report. Presence of restless legs syndrome (RLS) was characterized using the Johns Hopkins RLS Severity Scale (JHRLSS), comprising 9 diagnostic questions to establish the essential criteria for RLS and exclude other common diagnoses. This questionnaire has been shown to have 92% sensitivity and 95% specificity when compared to the gold standard of a clinical diagnosis by a sleep disorders specialist [11]. Depression was characterized as present using both the Patient Health Questionnaire [12] (PHQ-9; score of 9 and above) and/or by use of anti-depressant medications. The PHQ-9 is a 9-item tool that assesses the patients’ frequency of experiencing depressive thoughts or feelings over the prior 2 weeks. In patients on HD, PHQ-9 scores >9 are 92% sensitive and specific for a diagnosis of depressive disorder [5,13,14].
Laboratory data available in a 3-month period prior to the time of study were collected from patient's medical records and averaged. Such data included hemoglobin, serum creatinine and blood urea nitrogen (BUN), serum bicarbonate, potassium, calcium, phosphorous, and serum albumin.
Instruments
The Pittsburgh Sleep Diary (PghSD) was developed as a pen and paper diary of sleep-wake behavior [15] yielding daily estimates of bedtimes, waketimes, sleep latency, wake after sleep onset, mode of awakening and ratings of sleep quality, mood, and alertness on wakening. The waketime portion of the PghSD assesses sleep quality, mood and alertness by a visual analog scale (VAS) that ranges from 0–100 with higher scores representing less sleepiness, better mood and increased alertness. Patients were instructed to complete these diaries each morning for up to 14 consecutive days irrespective of their dialysis schedule.
To characterize a profile of HRQOL in standardized domains we used the Short Form-36 (SF-36), which consists of 8 dimensions: (1) physical functioning (PF), (2) role limitations due to physical functioning (RP), (3) bodily pain (BP), (4) general health perceptions (GH), (5) vitality (VT), (6) social functioning (SF), (7) role limitations due to emotional functioning (RE), and (8) mental health (MH). Raw scores are transformed into a score between 0 and 100 for each dimension, with higher scores indicating better performance [16]. The 8 scales of the SF-36 are hypothesized to form 2 distinct higher ordered clusters according to the physical and mental health variance that they have in common [17]. The PF, RP and BP scales primarily correlate with the physical dimension, whereas the, RE and MH mostly contribute to the mental component. The GH, SF and VT have considerable correlations with both physical and mental dimensions of HRQOL [16]. In order to avoid an overlap between the elements of the PHQ-9 and the components of the SF-36, we used only the primary members of the physical domain and the GH subscale of the SF-36 instrument. As this instrument was completed only once in this study, it cannot be expected to capture the day-to-day variability of symptoms among patients with CKD and ESRD. However, it does provide a static picture of patients’ perception of HRQOL at the beginning of the study [18] and potentially account for residual confounding in associations of mood, sleep quality, alertness and their variability.
Statistical Methods
Descriptive statistics was used to examine the distribution of all independent variables, including demographics and other baseline characteristics. For variables measured on a ratio or interval scale, the mean and the standard deviation were determined as a measure of central tendency and dispersion, respectively. For categorical variables, the frequencies of responses at each level were used.
For the analyses of repeated responses in sleep quality, mood, and alertness, population-averaged methods for longitudinal data, i.e. generalized estimating equations (GEE), were used [19,20]. These methods allow one to model both the average response (questionnaire score of sleep quality, mood and alertness) as well as their variability while taking into account the possible correlations between repeated assessments in the same individual. In order to apply GEEs one specifies regression (sub-)models for the mean score in the domains of sleep quality, mood and alertness as well as their standard deviation (day-to-day variability) in terms of baseline predictors (patient group, demographics and static HRQOL assessments). The GEE method then reports separate coefficients for the effects of these predictors on the mean and the standard deviation of each domain score; the coefficients in the sub-model for the mean are interpreted as the change in the mean response associated with a unit change in the predictor. On the other hand, the coefficients in the sub-model for the standard deviation are ratios that quantify how many times larger the standard deviation is in the presence or the absence of the predictor. All analyses were performed in R version 2.7 with the R library geepack [21,22].
Results
Study Population
A total of 121 patients with renal disease were approached and agreed to participate in the study. These patients provided both sleep diary data as well as reasons for failing to complete them. Reasons offered for non-completion included hospital admissions and acute illness, daily activities interfering with completion of diaries, and problems with sleep diary data entry. There were no significant differences in the age and gender of excluded and included participants, except of race (those with complete diaries were significantly more likely to be European-American). Out of the original study population, 92 patients provided complete sleep diary data and were evaluated. These patients were linked to 91 controls matched by computer for age, sex and race. The final study sample consisted of 183 participants from which 1,869 days of sleep diary data were collected.
Twenty-three patients (25%) had CKD stages 4 and 5 with an average estimated glomerular filtration rate (eGFR) of 13.6 (±4.3) ml/min/1.73 m2 and 59 (75%) were dialysis dependent (ESRD). Fifty-six individuals with ESRD (61%) were receiving thrice weekly in-center HD and 13 (14%) were using PD. For the HD sample, the average single-pool Kt/V was 1.63 (±0.35), while for the PD patients the average weekly Kt/V was 1.94 (±0.7). Most of the HD patients were dialyzed during the morning dialysis shift (66%). None of them was dialyzed in the evening or during the night shifts. Other characteristics of the population with renal dysfunction are shown in table 1. The CKD group had younger, more male and fewer diabetic patients than the ESRD group. The CKD group had also lower serum levels of creatinine and phosphorus, and a lower PF subscale score (table 1). Notably, there were no statistically significant differences in the distribution of mean hemoglobin among the 2 groups of patients with renal disease. Of the 91 controls matched to the patient sample, the average age of controls was 52.4 years (p = 0.59 vs. patients), 63.7% (n = 58, p = 0.96 vs. patients) were men, and 82.4% (n = 75, p = 0.02 vs. patients) were European-American.
Table 1.
Population characteristics and SF-36 data of study patients
| Variable | All patients (n = 92) | CKD (n=23) | ESRD (n=69) | p value (test of equality) |
|---|---|---|---|---|
| Age, years | 52.8 (15.3) | 46.1 (11.7) | 54.7 (15.2) | 0.01 |
| Male | 57 (62.0%) | 19 (83%) | 38 (55%) | 0.03 |
| White | 61 (66.3%) | 19 (83%) | 42 (61%) | 0.07 |
| BMI | 27.1 (5.1) | 28.0 (4.2) | 26.8 (5.3) | 0.21 |
| High school education | 35 (38.0%) | 6 (26.1%) | 29 (42.0%) | 0.22 |
| Employed | 21 (22.8%) | 8 (34.8%) | 13 (18.8%) | 0.15 |
| Diabetes | 32 (34.8%) | 4 (17.4%) | 28 (41%) | 0.05 |
| CHF | 14 (15.6%) | 3 (13.0%) | 11 (16.4%) | 0.99 |
| Depression | 17 (18.7%) | 1 (4%) | 16 (23%) | 0.06 |
| RLS | 30 (34.9%) | 8 (34.8%) | 22 (34.9%) | 0.99 |
| Total number of antihypertensives | 2.0 ± 1.4 | 2.5 ± 4.2 | 1.981.3 | 0.14 |
| Use of β-blockers | 49 (53.8%) | 11 (47.8%) | 38 (55.9%) | 0.63 |
| Hemoglobin, g/dl | 11.8 ± 1.3 | 11.7 ± 1.21 | 11.9 ± 1.3 | 0.51 |
| Serum potassium, mEq/l | 4.8 ± 0.8 | 4.9 ± 0.5 | 4.7 ± 0.9 | 0.06 |
| Serum bicarbonate, mEq/l | 23.4 ± 3.0 | 22.8 ± 3.3 | 23.6 ± 2.9 | 0.15 |
| Serum calcium, g/dl | 9.1 ± 0.6 | 9.1 ± 0.5 | 9.1 ± 0.66 | 0.81 |
| Phosphorus, mg/dl | 5.1 ± 1.4 | 4.6 ± 0.8 | 5.3 ± 1.4 | 0.04 |
| BUN, mg/dl | 56.3 ± 18.4 | 57.2 ± 13.5 | 56.0 ± 19.7 | 0.49 |
| Serum creatinine, mg/dl | 7.8 ± 3.4 | 5 ± 1.7 | 8.7 ± 3.3 | <0.001 |
| Serum albumin, g/dl | 3.9 ± 0.5 | 3.9 ± 0.7 | 3.87 ± 0.4 | 0.49 |
| Physical component scale | 39.9 ± 6.9 | 41.2 ± 8.3 | 39.5 ± 6.3 | 0.18 |
| Mental component scale | 45.4 ± 7.1 | 44.8 ± 7.4 | 45.6 ± 7.1 | 0.85 |
| PF | 60.9 ± 26.4 | 70.2 ± 27.7 | 57.8 ± 25.4 | 0.05 |
| RP | 56.1 ± 43 | 55.7 ± 46.9 | 56.2 ± 42.0 | 0.94 |
| BP | 61.8 ± 29.8 | 64.2 ± 31.1 | 61 ± 29.5 | 0.60 |
| RE | 33 ± 44.7 | 36.4 ± 45.9 | 31.8 ± 44.7 | 0.77 |
| MH | 77.2 ± 16.6 | 77 ± 14.1 | 77.2 ± 17.5 | 0.50 |
| SF | 70.2 ± 24.9 | 73.3 ± 26.5 | 69.1 ± 24.5 | 0.43 |
| VT | 47.1 ± 20.9 | 45.2 ± 18.8 | 47.7 ± 21.7 | 0.63 |
| GH | 44.1 ± 20.0 | 38.9 ± 19.8 | 45.9 ± 20.0 | 0.19 |
p value is the result of testing the equality of CKD to HD to PD (CKD = HD = PD). Hemoglobin (whole blood) in g/dl may be converted to g/l by multiplying by 10.0; serum potassium in mEq/l to mmol/l by multiplying by 1.0; serum bicarbonate in mEq/l to mmol/l by multiplying by 1.0; serum calcium in mg/dl to mmol/l by multiplying by 0.25; serum phosphorus in mg/dl to mmol/l by multiplying by 0.323; BUN in mg/dl to mmol/l by multiplying by 0.357; serum creatinine in mg/dl to μmol/l by multiplying by 88.4; serum albumin in g/dl may be converted to g/l by multiplying by 10.
Norms for Mood, Sleep Quality and Alertness
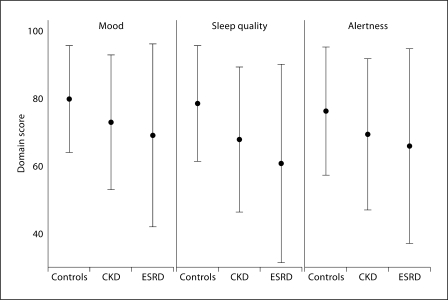
Overall, participants without known renal disease had high self-rated scores of their mood, sleep quality and alertness (fig. 1, left) and a relatively stable day-to-day variation in the mean (fig. 2, left graph in each row). The mean and the standard deviation of the controls were 79.2 ± 15.8, 76.7 ± 17.1 and 77.0 ± 19.0 for mood, sleep quality and alertness, respectively. In adjusted models for these norms (table 2) younger age, female gender and European-American race were associated with better (higher mean) and less variable mood (ratio of standard deviations <1 in table 2). There were no significant associations between demographic characteristics and sleep quality or alertness.
Fig. 1.
Mean and standard deviation of mood, sleep quality, and alertness in patients and controls. • = Average score. Error bar represents standard deviation of the score in each domain (mood, sleep quality and alertness). Number of observations: 651 (controls), 308 (stage 4–5 CKD) and 880 (ESRD).
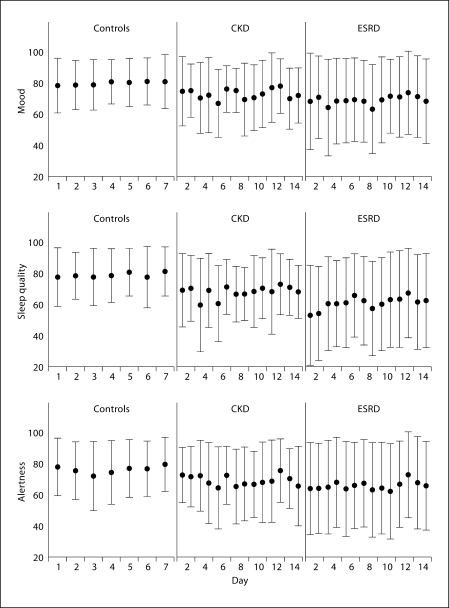
Fig. 2.
Day-to-day variability in mood, sleep quality, and alertness in patients and controls. Mean and standard deviation of each of the 3 domain scores by day of the study are presented. • = Mean score in each day. Error bar represents standard deviation of the score computed over all patients completing the diary each day.
Table 2.
Predictors of mean and standard deviation for mood, sleep quality, and alertness normative scores
| Mood | Quality of sleep | Alertness | |
|---|---|---|---|
| Mean1,2 | |||
| Age (per year) | 1.7 (0.4, 3.0) | 0.8 (−0.5, 2.2) | 1.5 (−0.1, 3.0) |
| p = 0.009 | p = 0.2 | p = 0.06 | |
| Female (vs. male) | 7.0 (2.0, 12.0) | 3.1 (−1.3, 7.6) | 2.8 (−4.4, 10.0) |
| p = 0.006 | p = 0.2 | p = 0.4 | |
| African-American race | −7.7 (−14.2, −1.2) | −2.0 (−8.0, 3.9) | −2.4 (−11.0, 6.3) |
| p < 0.001 | p = 0.5 | p = 0.6 | |
| Standard deviation3 | |||
| Age | 0.93 (0.87, 0.99) | 0.99 (0.93, 1.05) | 0.95 (0.89, 1.01) |
| p = 0.02 | p = 0.2 | p = 0.09 | |
| Female | 0.71 (0.56, 0.90) | 0.85 (0.69, 1.05) | 1.04 (0.69, 1.6) |
| p = 0.005 | p = 0.2 | p = 0.86 | |
| African-American race | 1.35 (−14.19, −1.17) | 1.07 (0.82, 1.40) | 0.96 (0.6, 1.47) |
| p < 0.001 | p = 0.5 | p = 0.85 | |
The first number in each cell is the coefficient quantifying the change in the average mood, sleep quality and alertness in the presence of the predictor (or per 1 unit change for continuous predictors such as age). Number in parentheses is the 95% confidence interval (uncertainty of the model for the value of the coefficient), and the final number is the p value measuring statistical significance.
Models for average performance adjusted for a linear trend in day-to-day performance in each of the 3 domains.
The first number in each cell is the coefficient of the model giving the ratio of the standard deviation of mood, sleep quality and alertness in the presence of the predictor (or per 1 unit change for continuous predictors such as age). Number in parentheses is the 95% confidence interval (uncertainty of the model for the value of the coefficient), and the final number is the p value measuring statistical significance.
Sleep Quality, Mood, and Alertness in Patients Compared to Controls
The distribution of sleep quality, mood, and alertness scores and the day-to-day variability in these domains for the CKD and ESRD samples relative to controls are shown in figure 1 (middle-right) and (fig. 2, middle-right) respectively. In unadjusted comparisons (table 3), patients with CKD stages 4–5 demonstrated statistically significant reductions in their average scores in sleep quality and alertness relative to controls, while patients on dialysis had lower performance in all 3 domains. Sleep quality was affected the most with reductions of 13.4 and 20.8 from the normative response in the CKD and ESRD groups, respectively. Day-to-day variability of mood, sleep quality and alertness was also higher in the ESRD group (table 3); the standard deviation of scores was 71, 72 and 52% greater than controls. Even though there was a trend for mood, sleep quality and alertness scores to be more variable in the CKD group relative to controls, statistical significance was attained only for the sleep domain. These differences in average sleep quality, mood, and alertness scores and their variability were significant and of same magnitude for all 3 domains after adjusting for age, gender, and race (not shown).
Table 3.
Unadjusted analyses of mean and standard deviation of mood, sleep quality and alertness domain scores in patients with CKD, and ESRD compared to normal controls
| Mood | Quality of sleep | Alertness | |
|---|---|---|---|
| Mean1 | |||
| CKD (vs. controls) | −7.02 (−14.2, 0.2) | −13.39 (−19.4, −7.4) | −8.58 (−16.0, −1.1) |
| p = 0.06 | p < 0.001 | p = 0.02 | |
| ESRD (vs. controls) | −12.1 (−17.9, −6.31) | −20.8 (−26.4, −15.2) | −13.5 (−19.7, −7.3) |
| p < 0.001 | p < 0.001 | p < 0.001 | |
| Standard Deviation2 | |||
| CKD (vs. controls) | 1.26 (0.97, 1.63) | 1.26 (1.06, 1.49) | 1.18 (0.95, 1.47) |
| p = 0.08 | p = 0.007 | p = 0.1 | |
| ESRD (vs. controls) | 1.71 (1.46, 2.02) | 1.72 (1.50, 2.0) | 1.52 (1.27, 1.80) |
| p < 0.001 | p < 0.001 | p < 0.001 | |
The first number in each cell is the coefficient quantifying the change in the average mood, sleep quality and alertness in the presence of the predictor (or per 1 unit change for continuous predictors such as age). Number in parentheses is the 95% confidence interval (uncertainty of the model for the value of the coefficient), and the final number is the p value measuring statistical significance.
The first number in each cell is the coefficient of the model giving the ratio of the standard deviation of mood, sleep quality and alertness in the presence of the predictor (or per 1 unit change for continuous predictors such as age). Number in parentheses is the 95% confidence interval (uncertainty of the model for the value of the coefficient), and the final number is the p value measuring statistical significance.
Predictors of Sleep Quality, Mood and Alertness and Their Variability among Patients with Kidney Disease
In analyses adjusting for demographic factors, body mass index (BMI), high school education, employment status, CHF, depression, RLS, total number of antihypertensives taken, usage of beta-blockers (β-blockers) and SF-36 components, dialysis dependency was associated with lower average scores in mood, sleep quality and alertness (table 4). A better (higher) GH score was associated with better scores in mood and sleep quality, while less (higher) BP score predicted better performance on mood/sleep. Patients with a higher PF score scored worse in all 3 domains. Older age was associated with better average mood and alertness, while African-Americans had also higher scores in mood and alertness than European-Americans. BMI was also associated with worse sleep quality. There was no statistically significant association between high school education, employment status, CHF, presence of RLS, total number of antihypertensives used, usage of β-blockers, depression and female gender with any of the 3 domains.
Table 4.
Predictors of mean mood, sleep quality and alertness domain scores in patients on renal replacement therapy (HD or PD) and patients with CKD
| Mood | Quality of sleep | Alertness | |
|---|---|---|---|
| ESRD (relative to CKD) | −11.3 (−19.9, −2.7) | −10.5 (−18.5, −2.5) | −12.7 (−22.1, 3.3) |
| p = 0.01 | p = 0.01 | p = 0.008 | |
| Age | 3.0 (0.5, 5.5) | 0.08 (−2.1, 2.3) | 3.1 (0.2, 5.9) |
| p = 0.02 | p = 0.94 | p = 0.035 | |
| Female | 3.7 (−2.9, 10.4) | 6.0 (−1.2, 13.3) | 4.1 (−3.2, 11.3) |
| p = 0.27 | p = 0.1 | p = 0.27 | |
| African-American race | 9.8 (2.0, 17.5) | 6.2 (−1.6, 14.0) | 12.3 (3.7, 21.0) |
| p = 0.01 | p = 0.12 | p = 0.005 | |
| BMI | −0.8 (−1.6, 0.02) | −0.9 (−1.5, −0.2) | −0.7 (−1.5, 0.1) |
| p = 0.06 | p = 0.01 | p = 0.1 | |
| High school education | 1.5 (−6.7, 9.6) | −0.9 (−7.7, 5.9) | 4.1 (−5.3, 13.6) |
| p = 0.72 | p = 0.8 | p = 0.39 | |
| Employed | 0.1 (−8.7, 8.9) | 3.3 (−5.4, 12.1) | 2.1 (−7.4, 11.6) |
| p = 0.98 | p = 0.46 | p = 0.67 | |
| CHF | −2.9 (−15.2, 9.3) | −6.1 (−18.0, 5.8) | −7.7 (−21.8, 6.4) |
| p = 0.64 | p = 0.31 | p = 0.28 | |
| Depression | 2.8 (−7.5, 13.2) | 1.9 (−7.2, 10.9) | 2.9 (−9.6, 15.3) |
| p = 0.59 | p = 0.69 | p = 0.65 | |
| Presence of RLS | 2.98 (−5.8, 11.8) | −0.7 (−9.7, 8.4) | −2.8 (−11.5, 6.0) |
| p = 0.51 | p = 0.88 | p = 0.006 | |
| Total number antihypertensives | 2.0 (−0.7, 4.8) | 1.45 (−1.4, 4.3) | 1.4 (−2.1, 4.8) |
| p = 0.15 | p = 0.32 | p = 0.44 | |
| Use of β-blockers | −4.3 (−12.6, 4.0) | −1.7 (−10.3, 6.8) | −4.3 (−13.7, 5.2) |
| p = 0.31 | p = 0.69 | p = 0.37 | |
| BP | 13.5 (4.2, 22.8) | 13.2 (3.4, 23.0) | 10.9 (−0.5, 22.4) |
| p = 0.005 | p = 0.008 | p = 0.06 | |
| RP | −3.6 (−11.6, 4.4) | 1.8 (−5.5, 9.1) | −3.9 (−11.9, 4.1) |
| p = 0.37 | p = 0.63 | p = 0.33 | |
| GH | 10.9 (2.4, 19.3) | 12.9 (5.4, 20.5) | 6.6 (−3.2, 16.4) |
| p = 0.01 | p < 0.001 | p = 0.18 | |
| PF | −18.7 (−32.0, −5.5) | −14.9 (−25.5, −4.4) | −19.6 (−33.4, −5.7) |
| p = 0.006 | p = 0.006 | p = 0.006 |
The first number in each cell is the coefficient quantifying the change in the average mood, sleep quality and alertness in the presence of the predictor (or per 1 unit change for continuous predictors such as age). Number in parentheses is the 95% confidence interval (uncertainty of the model for the value of the coefficient), and the final number is the p value measuring statistical significance. All models are adjusted for a linear trend in day-to-day performance in each of the 3 domains.
Statistically significant predictors of variability included dialysis dependency for mood and alertness (increase in variability of mood and alertness by about 35%, p < 0.001), while better (higher scores) general health led to less variable mood scores by 26% (p = 0.009). In addition, there was a significant effect of employment status on the variability of the mood and alertness scores. Employed patients showed 39% more variable mood (p < 0.001) and 24% more variable alertness (p = 0.05). Notably there was no effect of the shift of dialysis (morning vs. afternoon) on the variability of scores for any of the 3 domains in the subgroup of patients receiving HD. Patients dialyzed in morning shifts had 14 and 19% greater variability in their mood and alertness scores (p = 0.45 and 0.19, respectively) and 12% less variable sleep quality (p = 0.35) compared to patients dialyzed during the afternoon.
Sensitivity Analysis
The analyses shown in tables 2, 3, 4 were repeated with alternative techniques for repeated measures data (linear normal mixed models) and there was no substantial difference in the parameter estimates. To guard against residual confounding by the differences in the laboratory values, we constructed GEE models that included the association between diabetic status, laboratory values and sleep quality, mood and alertness. In these extended models, there was no substantial difference in the coefficients of the predictors in tables 2, 3, 4. Furthermore phosphorus level, hemoglobin, bicarbonate and diabetes were not statistically significant predictors of the average sleep quality, mood and alertness scores and their variability.
Discussion
In this report of 92 patients with advanced renal disease and 91 matched healthy controls with 1,869 days of sleep diary data, we demonstrate a marked impairment and a higher day-to-day variability in sleep quality, mood and alertness relative to norms for these domains assessed in the controls. Dependence on dialysis appears to further exacerbate the average severity of these symptoms compared to CKD stages 4–5, and may also be associated with increased variability in some of these domains. In contrast to normal controls, African-American patients experienced better mood, sleep quality and daytime performance (alertness). These findings are novel in quantifying the average performance in 3 important quality of life domains as well as its variability against normative standards with important implications for clinical research and practice.
Our findings extend previous work that showed a high rate of sleep complaints in patients with renal dysfunction by using diary methods and by assessing daily variability in sleep domains. Patients with ESRD have a remarkably high rate of sleep complaints and a higher prevalence of sleep disorders than the general population [4,23]. Studies of patients on maintenance HD have found that 50–80% report some sleep complaint (delayed sleep onset, frequent awakening, restlessness) or excessive daytime somnolence suggestive of poor sleep quality [24,25,26,27]. The poor sleep quality of these patients may be due to the adjustment of their sleep-wake behavior to the time requirements of the dialysis regimen, and this adjustment is likely different for patients on home therapies versus in-center HD. In PD, the need to adhere to a long therapy, delivered at a relatively inflexible schedule may result in an individual spending more time in bed than he or she would have done otherwise. If patient oversleeps as a result of the extra sleep time one day, then he or she may experience a sleepless night the next day leading to fluctuating sleep quality and changes in day time performance (alertness) and possibly mood. On the other hand, a patient receiving in-center HD would have to adjust (shorten) one's wake-up time in order to show up for his dialysis treatment the same day [28]. We postulate that irrespective of the specific nature of this adjustment, the end result is a disruption of the normal sleep behavioral patterns as well as poor and more variable sleep scores of the dialysis population.
A novel finding of our investigations concerns the high variability in mood, sleep quality and alertness reported by patients with CKD which was particularly prominent in the ESRD group. Diaries are particularly helpful for the assessment of variability since they provide reliable [29], prospective assessment of symptoms and their day-to-day variation [30] in the participants’ usual environment, with limited burden while avoiding recall bias [31,32]. Hence this higher variability observed in this cohort, is likely a generalizable finding of our study. Since patients with CKD had more variable scores than controls, one possible explanation is that the uremic milieu is at least partly responsible. One could further hypothesize that the additional variability of ESRD relative to CKD patients is related to their dialysis dependency or the timing of dialysis, which could induce alterations in arousal and/or thermoregulatory processes [33,34,35,36]. On the other hand, the greater variability could be interpreted as an attempt by the patients with poor average sleep to ‘catch up’ when the opportunity presents itself.
Irrespective of the cause of this high variability in symptoms, its presence has important implications for both clinical practice and research. Poor sleep quality in particular has been associated with higher mortality, higher disability and utilization costs in the CKD/ESRD population [40], hence, recognizing its presence may be of particular relevance to the clinical management of these patients. The excess daily variation of symptoms relative to controls implies that a single cross-sectional assessment of patients with renal dysfunction may not be sufficient to correctly classify patients in clinical practice. Previous research has highlighted that renal providers are largely unaware of the presence and severity of symptoms related to sleep quality, daytime performance and mood among patients who are on maintenance HD [6]. One could hypothesize that a high variability in the severity of these symptoms could lead to underdiagnosis if the ‘peak’ (the ‘good’ but atypical days) of patients coincides with the day they are assessed [6]. The implications for research programs that directly address these symptoms can be extrapolated from studies in subjects without renal dysfunction to the CKD/ESRD populations [41]. Unless one explicitly acknowledges the potential for the variability to be higher in these patient groups by incorporating repeated assessments, higher sample sizes, and suitable methods [42], the loss of power may be substantial.
It is interesting to note that there was no statistically significant effect of the timing of dialysis (dialysis shift) in our patient population considering that our patients have considerable flexibility in selecting their preferred shift. Previous studies on the impact of dialysis shift on sleep abnormalities, morbidity and mortality are still scarce and heterogeneous in the findings. Two studies by Sabbatini et al. [37] and by Merlino et al. [38] identified dialysis shift as an independent predictor of insomnia and sleep disturbances. On the other hand, in the HEMO study [33] as well as in a more recent evaluation by Bastos et al. [39], there was no statistically significant association between morning dialysis shift and sleep quality, VT or daytime sleepiness. Differences in the patient populations, unit practices (flexibility in allowing patients to select the shift they prefer to dialyze) and possibly the prevalence of depression may account for these discrepant findings. Notwithstanding the lack of association between the time of dialysis shift and poor mood, sleep quality and alertness, we cannot rule out the possibility that a study with a larger sample could find a significant effect.
In this report, African-Americans had higher scores in all 3 domains than European-Americans (p <0.001). This racial association has been noted previously; in a recent cross-sectional study assessing sleep quality by the KDQOL instrument, non African-Americans had a worse sleep quality by about 10 points [43], an effect that is near identical to the one estimated in this report. In the HEMO study population, African-Americans were also noted to have higher unadjusted sleep KDQOL scores [44], while elderly African-American patients had a smaller burden of sleep-related symptoms in a community cohort [45]. Race did not have a protective effect on the healthy control group employed in this study, mirroring findings previously reported in the Cardiovascular Health Study and the Sleep Heart Health Study [46,47].
The findings of this report should be interpreted in light of the following limitations. First, this is a study of subjective sleep characteristics and their variability, rather than a study of sleep disorders. Therefore, we cannot comment on the nature of sleep disturbances observed. Second, the waking assessments did not account for potential circadian variation in sleep quality, mood and alertness. Consequently, changes that may occur throughout the day will be missed by a single daily measurement of day time performance. Future studies should consider employing frequent assessments across the day to measure diurnal variations. Finally, while our study examined the prevalence of comorbid medical conditions and laboratory abnormalities linked to renal dysfunction, we did not have comparable data in the control group. In addition, by using control subjects derived from an archival database we could not control for the exclusion of individuals with sleep disorders. Studies that employ concurrent, prospectively assessed cases and controls should be undertaken to address this limitation.
Our findings suggest that future efforts to understand and estimate the importance of sleep disturbances in patients with CKD should not focus exclusively on those with ESRD. The PghSD may serve as a clinical tool to understand the sleep problems of patients with varying stages of CKD. Our assessment of the patient's variability in sleep, mood and daytime performance (alertness) will need to be verified in additional settings and with larger sample sizes. Finally, further work should examine potential biological mediators of impaired sleep, mood, and alertness among patients with advanced chronic kidney disease.
Acknowledgements
We want to thank Drs. Timothy Monk, Daniel J. Kupfer, Douglass Moul, Charles Reynolds, Martica Hall, and Eric Nofzinger for use of their archived healthy controls. We thank the patients, staff, and physicians who participated in this study.
This work was supported by the Fresenius National Kidney Foundation Young Investigator Grant, Paul Teschan Research Grant, NIH DK66006 and DK77785 (M.L.U.) and NIH MH24652 (D.J.B.). This publication was supported by funds received from the NIH/NCRR/CTSA Grant UL1 RR024153.
References
- 1.Unruh ML, Weisbord SD, Kimmel PL. Health-related quality of life in nephrology research and clinical practice. Semin Dial. 2005;18:82–90. doi: 10.1111/j.1525-139X.2005.18206.x. [DOI] [PubMed] [Google Scholar]
- 2.Jhamb M, Weisbord SD, Steel JL, Unruh M. Fatigue in patients receiving maintenance dialysis: a review of definitions, measures, and contributing factors. Am J Kidney Dis. 2008;52:353–365. doi: 10.1053/j.ajkd.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramkumar N, Beddhu S, Eggers P, Pappas LM, Cheung AK. Patient preferences for in-center intense hemodialysis. Hemodial Int. 2005;9:281–295. doi: 10.1111/j.1492-7535.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 4.Unruh ML, Hartunian MG, Chapman MM, Jaber BL. Sleep quality and clinical correlates in patients on maintenance dialysis. Clin Nephrol. 2003;59:280–288. doi: 10.5414/cnp59280. [DOI] [PubMed] [Google Scholar]
- 5.Son YJ, Choi KS, Park YR, Bae JS, Lee JB. Depression, symptoms and the quality of life in patients on hemodialysis for end-stage renal disease. Am J Nephrol. 2009;29:36–42. doi: 10.1159/000150599. [DOI] [PubMed] [Google Scholar]
- 6.Weisbord SD, Fried LF, Mor MK, Resnick AL, Unruh ML, Palevsky PM, Levenson DJ, Cooksey SH, Fine MJ, Kimmel PL, Arnold RM. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2007;2:960–967. doi: 10.2215/CJN.00990207. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4:1057–1064. doi: 10.2215/CJN.00430109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murtagh FE, Addington-Hall JM, Edmonds PM, Donohoe P, Carey I, Jenkins K, Higginson IJ. Symptoms in advanced renal disease: a cross-sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med. 2007;10:1266–1276. doi: 10.1089/jpm.2007.0017. [DOI] [PubMed] [Google Scholar]
- 9.Segal DL, Hersen M, Van Hasselt VB. Reliability of the structured clinical interview for DSM-III-R: an evaluative review. Compr Psychiatry. 1994;35:316–327. doi: 10.1016/0010-440x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.Allen RP, Earley CJ. Validation of the Johns Hopkins restless legs severity scale. Sleep Med. 2001;2:239–242. doi: 10.1016/s1389-9457(00)00080-0. [DOI] [PubMed] [Google Scholar]
- 12.Lowe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care. 2004;42:1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Odden MC, Whooley MA, Shlipak MG. Depression, stress, and quality of life in persons with chronic kidney disease: the Heart and Soul Study. Nephron. 2006;103:c1–c7. doi: 10.1159/000090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodwin RD, Kroenke K, Hoven CW, Spitzer RL. Major depression, physical illness, and suicidal ideation in primary care. Psychosom Med. 2003;65:501–505. doi: 10.1097/01.psy.0000041544.14277.ec. [DOI] [PubMed] [Google Scholar]
- 15.Monk TH, Reynolds CF, Kupfer DJ, Buysse DJ, Coble PA, Hayes AJ, MacHen MA, Petrie SR, Ritenour AM. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111–120. [PubMed] [Google Scholar]
- 16.Liem YS, Bosch JL, Arends LR, Heijenbrok-Kal MH, Hunink MG. Quality of life assessed with the Medical Outcomes Study Short Form 36-Item Health Survey of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health. 2007;10:390–397. doi: 10.1111/j.1524-4733.2007.00193.x. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE., Jr. SF-36 health survey update. Spine. 2000;25:3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 18.Kalender B, Ozdemir AC, Dervisoglu E, Ozdemir O. Quality of life in chronic kidney disease: effects of treatment modality, depression, malnutrition and inflammation. Int J Clin Pract. 2007;61:569–576. doi: 10.1111/j.1742-1241.2006.01251.x. [DOI] [PubMed] [Google Scholar]
- 19.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 20.Hu FB, Goldberg J, Hedeker D, Flay BR, Pentz MA. Comparison of population-averaged and subject-specific approaches for analyzing repeated binary outcomes. Am J Epidemiol. 1998;147:694–703. doi: 10.1093/oxfordjournals.aje.a009511. [DOI] [PubMed] [Google Scholar]
- 21.H⊘jsgaard S, Halekoh U, Yan J. The r package geepack for generalized estimating equation. J Stat Softw. 2005;15:1–11. [Google Scholar]
- 22.Pinheiro JC, Bates DM. Fitting extended linear models with gls. In: Pinheiro JC, Bates DM, editors. Mixed Effects Models in S and S-PLUS. New York: Springer; 2000. pp. 249–266. [Google Scholar]
- 23.Unruh ML, Buysse DJ, Dew MA, Evans IV, Wu AW, Fink NE, Powe NR, Meyer KB. Sleep quality and its correlates in the first year of dialysis. Clin J Am Soc Nephrol. 2006;1:802–810. doi: 10.2215/CJN.00710206. [DOI] [PubMed] [Google Scholar]
- 24.Iliescu EA, Yeates KE, Holland DC. Quality of sleep in patients with chronic kidney disease. Nephrol Dial Transplant. 2004;19:95–99. doi: 10.1093/ndt/gfg423. [DOI] [PubMed] [Google Scholar]
- 25.Holley JL, Nespor S, Rault R. A comparison of reported sleep disorders in patients on chronic hemodialysis and continuous peritoneal dialysis. Am J Kidney Dis. 1992;19:156–161. doi: 10.1016/s0272-6386(12)70125-7. [DOI] [PubMed] [Google Scholar]
- 26.Unruh ML, Sanders MH, Redline S, Piraino BM, Umans JG, Chami H, Budhiraja R, Punjabi NM, Buysse D, Newman AB. Subjective and objective sleep quality in patients on conventional thrice-weekly hemodialysis: comparison with matched controls from the sleep heart health study. Am J Kidney Dis. 2008;52:305–313. doi: 10.1053/j.ajkd.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker S, Fine A, Kryger MH. Sleep complaints are common in a dialysis unit. Am J Kidney Dis. 1995;26:751–756. doi: 10.1016/0272-6386(95)90438-7. [DOI] [PubMed] [Google Scholar]
- 28.Barmar B, Dang Q, Isquith D, Buysse D, Unruh M. Comparison of sleep/wake behavior in CKD stages 4 to 5 and hemodialysis populations using wrist actigraphy. Am J Kidney Dis. 2009;53:665–672. doi: 10.1053/j.ajkd.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 29.Csikszentmihalyi M, Larson R. Validity and reliability of the experience-sampling method. J Nerv Ment Dis. 1987;175:526–536. doi: 10.1097/00005053-198709000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Levitt H, Wood A, Moul DE, Hall M, Germain A, Kupfer DJ, Buysse DJ. A pilot study of subjective daytime alertness and mood in primary insomnia participants using ecological momentary assessment. Behav Sleep Med. 2004;2:113–131. doi: 10.1207/s15402010bsm0202_3. [DOI] [PubMed] [Google Scholar]
- 31.Gendreau M, Hufford MR, Stone AA. Measuring clinical pain in chronic widespread pain: selected methodological issues. Best Pract Res Clin Rheumatol. 2003;17:575–592. doi: 10.1016/s1521-6942(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 32.Moskowitz DS, Young SN. Ecological momentary assessment: what it is and why it is a method of the future in clinical psychopharmacology. J Psychiatry Neurosci. 2006;31:13–20. [PMC free article] [PubMed] [Google Scholar]
- 33.Ng YH, Meyer KB, Kusek JW, Yan G, Rocco MV, Kimmel PL, Benz RL, Beddhu S, Dwyer JT, Toto RD, Eknoyan G, Unruh ML. Hemodialysis timing, survival, and cardiovascular outcomes in the hemodialysis (HEMO) study. Am J Kidney Dis. 2006;47:614–624. doi: 10.1053/j.ajkd.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Bliwise DL, Kutner NG, Zhang R, Parker KP. Survival by time of day of hemodialysis in an elderly cohort. JAMA. 2001;286:2690–2694. doi: 10.1001/jama.286.21.2690. [DOI] [PubMed] [Google Scholar]
- 35.Abbott KC, Reynolds JC, Trespalacios FC, Cruess D, Agodoa LY. Survival by time of day of hemodialysis: analysis of United States Renal Data System Dialysis Morbidity and Mortality Waves III/IV. Am J Kidney Dis. 2003;41:796–806. doi: 10.1016/s0272-6386(03)00027-1. [DOI] [PubMed] [Google Scholar]
- 36.Parker KP, Bliwise DL, Rye DB, De A. Intradialytic subjective sleepiness and oral body temperature. Sleep. 2000;23:887–891. [PubMed] [Google Scholar]
- 37.Sabbatini M, Minale B, Crispo A, Pisani A, Ragosta A, Esposito R, Cesaro A, Cianciaruso B, Andreucci VE. Insomnia in maintenance haemodialysis patients. Nephrol Dial Transplant. 2002;17:852–856. doi: 10.1093/ndt/17.5.852. [DOI] [PubMed] [Google Scholar]
- 38.Merlino G, Piani A, Dolso P, Adorati M, Cancelli I, Valente M, Gigli GL. Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol Dial Transplant. 2006;21:184–190. doi: 10.1093/ndt/gfi144. [DOI] [PubMed] [Google Scholar]
- 39.Bastos JP, Sousa RB, Nepomuceno LA, Gutierrez-Adrianzen OA, Bruin PF, Araujo ML, Bruin VM. Sleep disturbances in patients on maintenance hemodialysis: role of dialysis shift. Rev Assoc Med Bras. 2007;53:492–496. doi: 10.1590/s0104-42302007000600014. [DOI] [PubMed] [Google Scholar]
- 40.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3:329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 41.Wohlgemuth WK, Edinger JD, Fins AI, Sullivan RJ., Jr How many nights are enough? The short-term stability of sleep parameters in elderly insomniacs and normal sleepers. Psychophysiology. 1999;36:233–244. [PubMed] [Google Scholar]
- 42.Ruxton GD. The unequal variance t-test is an underused alternative to student's t-test and the Mann-Whitney U test. Behav Ecol. 2006;17:688–690. [Google Scholar]
- 43.Kurella M, Luan J, Lash JP, Chertow GM. Self-assessed sleep quality in chronic kidney disease. Int Urol Nephrol. 2005;37:159–165. doi: 10.1007/s11255-004-4654-z. [DOI] [PubMed] [Google Scholar]
- 44.Unruh M, Miskulin D, Yan G, Hays RD, Benz R, Kusek JW, Meyer KB. Racial differences in health-related quality of life among hemodialysis patients. Kidney Int. 2004;65:1482–1491. doi: 10.1111/j.1523-1755.2004.00529.x. [DOI] [PubMed] [Google Scholar]
- 45.Kutner NG, Bliwise DL, Zhang R. Linking race and well-being within a biopsychosocial framework: variation in subjective sleep quality in two racially diverse older adult samples. J Health Soc Behav. 2004;45:99–113. doi: 10.1177/002214650404500107. [DOI] [PubMed] [Google Scholar]
- 46.Whitney CW, Enright PL, Newman AB, Bonekat W, Foley D, Quan SF. Correlates of daytime sleepiness in 4,578 elderly persons: the Cardiovascular Health Study. Sleep. 1998;21:27–36. doi: 10.1093/sleep/21.1.27. [DOI] [PubMed] [Google Scholar]
- 47.O'Connor GT, Lind BK, Lee ET, Nieto FJ, Redline S, Samet JM, Boland LL, Walsleben JA, Foster GL. Variation in symptoms of sleep-disordered breathing with race and ethnicity: the Sleep Heart Health Study. Sleep. 2003;26:74–79. [PubMed] [Google Scholar]