Abstract
Objective
To reach consensus on core competency statements for natural health products (NHPs) for Canadian pharmacy students.
Methods
Four rounds of a modified Delphi method were used to achieve consensus on core competency statements for NHPs. Pharmacy educators from Canada and the United States, and representatives from Canadian pharmacy organizations ranked their agreement using a 5-point Likert scale.
Results
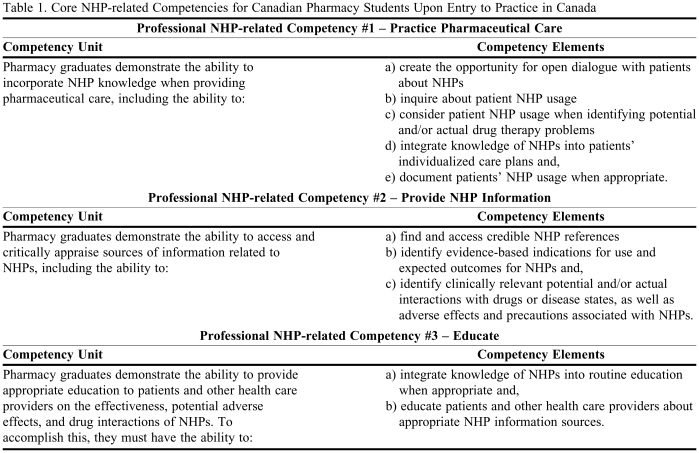
Consensus was achieved on 3 NHP-related core competency statements: (1) to incorporate NHP knowledge when providing pharmaceutical care; (2) to access and critically appraise NHP-related information sources; and (3) to provide appropriate education to patients and other health care providers on the effectiveness, potential adverse effects, and drug interactions of NHPs.
Conclusions
Consensus was reached among leaders in NHP education on 3 NHP-related core competency statements. Implementation of these competencies would ensure that graduating Canadian pharmacists would be able to fulfill their professional responsibilities related to NHPs.
Keywords: natural health products (NHPs), competencies, Delphi method, complementary and alternative medicine, herbal medicine
INTRODUCTION
The majority of North Americans report using natural health products (NHPs), such as herbal medicines and vitamins1,2 and often purchase them in pharmacies,3,4 raising the question of what pharmacists should know about these products. This paper describes a consensus-based process which culminated in the identification of core competency statements for Canadian pharmacy students regarding natural health products (NHPs). NHPs are defined by Health Canada as substances found in nature that are manufactured and sold for medical or health-related uses, such at treating or preventing diseases.5 The Natural Health Products Regulations (2004) legally categorized NHPs as “drugs” at the level of the Food and Drugs Act,6 therefore NHPs are included in Canadian pharmacists' scope of practice.7 In contrast, this class of products is known generically as dietary supplements, a subcategory of foods in the United States.
Many stakeholders believe that pharmacists play a key role regarding NHPs.8-12 Pharmacy associations in Canada and the United States began publishing recommendations, statements, and guidelines regarding these products almost 10 years ago.13-16 Additionally, consumers, pharmacists, pharmacy students, NHP industry representatives, and leaders from other health care professions have all identified the importance of pharmacists being able to counsel patients about NHPs, especially about adverse effects and drug interactions associated with NHP use.8-12 The basis for pharmacists' involvement with these products is argued to be an extension of their established roles.14 Pharmaceutical care is defined as “to accept responsibility for optimizing all of a patient's drug therapy, regardless of source (prescription, nonprescription, alternative, or traditional medicines), to achieve better patient outcomes and to improve the quality of each patient's life,”17(p2-3) indicating a clear expectation for pharmacists to be knowledgeable about NHPs as part of contemporary practice. The majority of pharmacists, however, have reported feeling ill-equipped to meet these expectations, and pharmacy curricular content pertaining to NHPs varies widely across North America.8,11,18-20 Canadian and US research on pharmacists' knowledge of, and opinions, about NHP reveals: a lack of formal instruction about these products as demonstrated for example by low test scores related to NHP content,11,18,19 inconsistent NHP content in pharmacy school curricula,20,21 evidence that more training in NHPs results in higher test scores,11,18,22 and an overall/general sentiment that the study of NHPs should be a mandatory part of the curriculum.19,22
Both Canadian and American pharmacists are expected to possess some knowledge about NHPs to become licensed. The Pharmacy Examining Board of Canada (PEBC), the national certification body of Canadian pharmacists, recommends that students planning to sit for the Qualifying Examination, or the Evaluating Examination for foreign-trained students, familiarize themselves with therapeutic considerations concerning alternative treatments,23 the category to which NHPs belong. In the United States, passing the North American Pharmacist Licensure Examination (NAPLEX) is required for licensure in all 50 states,24 and the test contains a competency specifically addressing knowledge of dietary supplements.25 Also in the United States, the Accreditation Council for Pharmacy Education (ACPE) Accreditation Standards and Guidelines for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree (2007) requires pharmacists to be knowledgeable and competent in a wide-range of sciences, including the pharmaceutical sciences.26 This encompasses the categories of pharmacognosy and alternative and complementary therapies, which include but are not limited to natural products, dietary supplements, herbal-drug interactions, and the Dietary Health Supplement and Education Act.26 Clearly, pharmacists' formal education should be in line with licensing requirements.
Core competencies are defined by the National Association of Pharmacy Regulatory Authorities (NAPRA) as “significant job-related knowledge, skills, attitudes, and/or judgments required for competent performance by members of the profession.”27(p16) Core competencies define what is minimally required of pharmacists at the point of licensure in Canada,27 and are often created through extensive consultative processes,28 including deliberation and (ideally) consensus.29
The research described in this paper was intended to bridge the gap between pharmacists' formal education and their NHP-related professional responsibilities by identifying NHP-related core competencies that pharmacy educators and representatives from pharmacy organizations deem important for pharmacy students when entering pharmacy practice in Canada. Incorporating additional NHP-specific education in the professional pharmacy program curriculum will help pharmacists fulfill their NHP-related professional responsibilities.
METHODS
This study used a modified Delphi method which culminated at an invitational consensus-building meeting held in Toronto, Ontario, Canada, on November 6-7, 2008. The goal was to develop NHP-related core competencies, consistent with existing competency-based outcomes and standards of practice documents,27,30,31 informed by previous qualitative research including: a document analysis,32 35 key informant interviews,10 16 focus groups with practicing pharmacists and consumers,9 results from herbal knowledge testing of fourth-year Canadian pharmacy students,11 and results from a survey of 3356 practicing Canadian pharmacists.33 This project was approved by the University of Toronto Office of Research Ethics.
Our technique for achieving consensus was the Delphi method, a group facilitation technique that seeks to obtain consensus on the opinions of “informed individuals” (participants) through a series of structured questionnaires (or rounds).34 Questionnaires were completed anonymously by the participants, and responses from each round were summarized and provided to participants as part of subsequent rounds. The Delphi method is therefore an iterative multistage process designed to synthesize diverse opinions into group consensus, and commonly consists of 4 rounds.
The original Delphi statements were developed from the results of N. Shanthakumar's survey of practicing Canadian pharmacists, which sought to answer the question, “[w]hat do Canadian pharmacists perceive to be the scope of their responsibilities with respect to natural health products.”12(p3) The statements were pilot tested at the joint annual meetings of the American Association of Colleges of Pharmacy (AACP) and the Association of the Faculties of Pharmacy of Canada (AFPC) in Chicago, Illinois, July 2008.
Those invited to participate in this project included 1 faculty member responsible for teaching NHP content at each Canadian pharmacy school; representatives from American pharmacy schools who had published literature about pharmacy education on dietary supplements in the last 10 years; and representatives from Canadian pharmacy organizations involved in developing or implementing practice, NHP-related, or educational policies in Canada. Study participants (n = 17) were asked to rank their level of agreement with the Delphi statements using a 5-point Likert scale. SurveyMonkey (www.surveymonkey.com) was the Internet-based platform used by participants to complete the survey instruments for all 4 Delphi rounds. During all Delphi rounds, participants were encouraged to add any competencies they felt were missing, amend wording, or provide justification for their responses to all statements. The qualitative and quantitative results of each Delphi round were analyzed, and anonymous, written summaries of all rounds, including number of responses and percentage, mode, range and mean, and all qualitative comments, were provided to all participants between each round.
Statements with a mean ranking of greater than 3 were carried forward to the next Delphi round. Consensus was defined through an iterative process and was deemed to have been reached for a given statement when all participants ranked it a 4 (very important) or 5 (essential). Statements for subsequent rounds were developed iteratively based on participant feedback from the previous round (Appendix 1). Data collection/analysis occurred at 3 distinct times: (1) pre-invitational consensus building meeting, (2) invitational consensus building meeting, and (3) post-invitational consensus building meeting.
Upon completion of the fourth Delphi round, a summary report identifying the final consensus-based core competencies was sent to all participants and they were asked to provide approval and indicate whether they wanted their name and affiliation to appear in the final report.
RESULTS
All 4 Delphi rounds were completed by all study participants (n = 17). Study participants included pharmacy educators responsible for NHP curriculum content at their respective institutions, as well as academic administrators interested in curriculum development related to NHPs, from 7 of 10 Canadian pharmacy schools (n = 8), NHP content educators from American pharmacy schools (n = 3), and representatives from Canadian pharmacy organizations interested in policies related to NHPs (n = 6). A detailed summary of each Delphi round is provided below. The statements used in all 4 Delphi rounds appear in Appendix 1.
First Delphi Round.
After the first Delphi round, consensus was reached on 2 competency statements, and all statements were carried forward to the second Delphi round based on their mean rating greater than 3. Participant feedback suggested the addition of 2 new competency statements, as well as changes in wording to the original 6 competency statements.
Second Delphi Round.
After the second Delphi round, consensus was reached on 3 competency statements. A group discussion was held with study participants between the second and third Delphi rounds (after participants were given the summary results of the second Delphi round) regarding wording of the competency statements, justifications for ranking, and general discussion about core competencies for Canadian pharmacists. Participant comments from the second Delphi round summary and discussion suggested dropping the 3 remaining competency statements of the original 6 for the third Delphi round, but incorporating elements of these statements into other competency statements.
Third Delphi Round.
Consensus was reached on 2 of the reorganized competency statements, and consensus was close on an additional 2 statements (eg, the mean was greater than 4, but not all participants had ranked these statements as being 4-very important or 5-essential). There was, however, 100% agreement that no competency statements were missing from the questionnaire.
Fourth Delphi Round.
Consensus was reached on 3 NHP-related core competency statements, and an additional 2 statements were close to consensus. Again, there was 100% agreement that no competencies were missing.
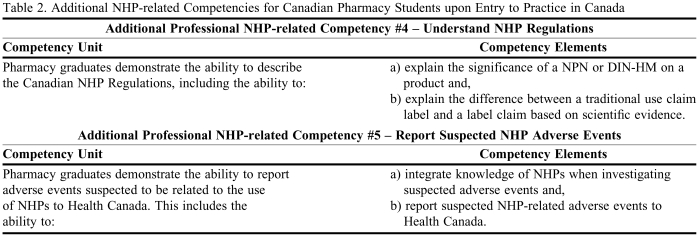
After 4 Delphi rounds, with 100% participation by all 17 participants, we concluded that consensus was reached on the 3 NHP-related core competencies for Canadian pharmacy students upon entry into practice (Table 1). Two additional competencies emerged from the Delphi method, and although consensus was not achieved, the mean rating on both statements was greater than 4 (Table 2).
Table 1.
Core NHP-related Competencies for Canadian Pharmacy Students Upon Entry to Practice in Canada
Table 2.
Additional NHP-related Competencies for Canadian Pharmacy Students upon Entry to Practice in Canada
DISCUSSION
Consensus was reached in the first Delphi round on core competency statements broadly related to providing NHP information and education, which became Professional NHP-related Competency Statements No. 2: Provide NHP Information and No. 3: Educate (Table 1). It is likely that consensus of these statements was achieved early because similar competency statements already existed in Canadian standards of practice documents for pharmacists relating to drugs (eg, NAPRA's Model Standards of Practice for Canadian Pharmacists 2003).30 Thus, the idea of extending these competencies to include NHPs was easy for participants to embrace and endorse.9,11 Consensus on Professional NHP-related Competency Statement No. 1: Practice Pharmaceutical Care was achieved after the second Delphi round. Only 1 participant ranked this competency outside our definition of consensus (that all participants ranked a statement 4-very important or 5-essential) during the first Delphi round, so for those statements on which consensus was possible, it was achieved quite quickly.
The 3 professional NHP-related competency statements that emerged from this consensus exercise were consistent with the results of previous research conducted by our research team. Kwan reported that focus groups of consumers and practicing pharmacists thought that pharmacists should adopt a consultative role to help consumers integrate different sources of NHP-related information .9 This is represented most prominently in Professional NHP-related Competency No. 3: Educate. Additionally, previous research demonstrated that pharmacists placed emphasis on ensuring patient safety, especially regarding potential NHP-drug interactions,9 which is captured in Professional NHP-related Competency No. 1: Practice Pharmaceutical Care. Safety was also a key component identified in earlier research interviews of pharmacy leaders, consumer advocates, conventional and complementary health care practitioners, and NHP industry representatives.10 The lack of knowledge and reliable information on NHPs were identified as barriers to counseling patients on NHPs,10 which highlights the importance of creating NHP-related competencies. Shanthakumar found that Canadian pharmacists were more likely to endorse professional NHP-related responsibilities related to knowledge, including awareness of indications for use and expected outcomes, access to reliable references, and helping patients identify and access information, than any other types of NHP-related competencies.12 These knowledge responsibilities are incorporated into all 3 of the core competency statements.
Consensus was not reached on Additional Professional NHP-related Competency No. 4: Understand NHP Regulations, and Additional Professional NHP-related Competency No. 5: Report Suspected NHP Adverse Events (Table 2). These statements received the greatest range of responses throughout all 4 Delphi rounds. These topics have received mixed reactions and levels of support from the pharmacy profession.10,12 For example, Olatunde found that many pharmacy leaders were unfamiliar with current pharmacy policies and guidelines concerning NHPs.10 Shanthakumar also found that Canadian pharmacists were least likely to endorse professional responsibilities related to documentation of NHP usage, which included reporting adverse events to Health Canada and recording use in patients' charts.12 Lack of consensus on this issue seems to be related to 2 distinct opinions: (1) that reporting adverse drug events related to NHPs is vital and should be a core competency, and (2) that reporting adverse drug events to Health Canada is not required in current standards of practice documents, so we should not create a precedent by first requiring this for NHPs. Similarly, understanding drug regulations is not identified as a core educational competency, although it is necessary for licensing. Thus, some participants argued that setting a precedent by first requiring this of NHPs was something they were not willing to do. As a result of our work, we were able to incorporate general documentation responsibilities into Professional NHP-related Competency No. 1: Practice Pharmaceutical Care, in an attempt to bridge the gap on the 2 perspectives on reporting NHP-related adverse events.
This study has limitations. The validity of the study was dependent on the active participation of all Canadian pharmacy schools and pharmacy organizations. While 7 out of 10 Canadian pharmacy schools were represented, there was no representation from either of the 2 French-language pharmacy schools in the province of Quebec. Reasons that representatives were not sent to the meeting included: prior commitments, lack of an appropriate individual to represent the institution, and no response to the investigators attempts to contact them. Lack of participation from the Quebec schools of pharmacy may have been due to all correspondence, as well as the Delphi rounds, being conducted in English. Representatives from Quebec may have had different perspectives on NHP-related core competencies for pharmacists as suggested by a Canadian survey that found the use of NHPs in Quebec to be seasonal and overall use of NHPs to be lower than in the rest of Canada.1 However, results of a recent national survey of practicing pharmacists' opinions about NHP-related professional responsibilities did not reflect that location influenced thoughts about NHP-related competencies,33 suggesting that our NHP-related core competency statements may be applicable nationally.
As NHPs represent a wide variety of products, we purposely did not limit the study to certain categories (eg, vitamins, minerals, herbal medicines, or homeopathy), but rather included the official definition of NHPs according to the NHP Regulations (2004)5 as a preface to our NHP-related core competency statements. However, whether our NHP core competency statements are applicable to all categories of NHPs is not clear, as we do not know what study participants thought when they read the term “NHPs.” Furthermore, previous research concluded that pharmacists were generally more likely to endorse professional responsibilities associated with vitamins, minerals, and herbal medicines, than with homeopathy,33 thus study participants may not agree that the developed core competency statements apply equally to all categories of NHPs.
This study also had several strengths. It was the culmination of a program of research that included key informant interviews,10 focus groups with pharmacists and consumers,9 and a survey of licensed Canadian pharmacists.33 Thus, the Delphi process benefitted from the input of a wide range of stakeholders including consumers, practicing pharmacists, pharmacy students, other health care providers, the NHP industry, policymakers, educators, and representatives from a variety of pharmacy organizations. The process of creating competency statements has been an inclusive one and the resulting competencies are likely, therefore, to be relevant to pharmacy practice in Canada. Additionally, this project stimulated much discussion among participants, which resulted not only in something concrete—the creation of competency statements—but also contribution to the advancement of pharmacy education in Canada. This project represented the first time that NHP educators from pharmacy schools across North America met to discuss NHP-specific curriculum.
Competency statements were never intended to stand alone, thus we plan to share results with the larger pharmacy community of North America, primarily through widespread dissemination of the competency statements. It is our hope that the NHP-related core competencies developed through this project will be incorporated into existing provincially and state-based standards of practice documents, and the educational outcomes documents that form the basis for pharmacy education at individual educational institutions. This would be accomplished in Canada through the Canadian Council for Accreditation of Pharmacy Programs (CCAPP) and the Association of Faculties of Pharmacy of Canada (AFPC) exploring how the developed competency statements may be incorporated into existing educational outcomes documents. Also essential is working with organizations that foster or accredit continuing professional education among practicing pharmacists, including the Ontario Pharmacists' Association (OPA), the offices of continuing education at Canadian Faculties of Pharmacy, and the Canadian Council on Continuing Education in Pharmacy (CCCEP). It is important to widely disseminate these competency statements to pharmacy organizations, policymakers, and pharmacy schools. Adoption by the pharmacy community at large will ensure that Canadian pharmacists can meet their professional responsibilities with respect to NHPs upon entry-to-practice, ultimately resulting in better patient outcomes.
CONCLUSIONS
After 4 Delphi rounds, 3 NHP-related core competencies were identified, broadly summarized as: (1) the ability to incorporate NHP knowledge when providing pharmaceutical care; (2) the ability to access and critically appraise sources of information related to NHPs, and (3) the ability to provide appropriate education to patients and other health care providers on the effectiveness and potential adverse effects and drug interactions of NHPs. Two additional NHP-related competency statements, related to NHP regulation and reporting NHP-related adverse events, emerged as important, but consensus that they should be considered core competencies was not achieved. We recommend wide-spread implementation and adaptation of these NHP-related core competencies by faculties of pharmacy and pharmacy organizations across Canada to better equip today's pharmacists with the skills they require when entering practice.
ACKNOWLEDGEMENTS
Funding for this study was provided by: the Canadian Institutes for Health Research (CIHR) Partnerships for Health System Improvement (PHSI) grant, the Advanced Foods & Materials Network (AFMNet) National Centres of Excellence (NCE). One of the authors of this paper, Dr. Heather Boon, was supported by a CIHR salary award while conducting this study. Special thanks to the colleagues who helped with the study: Kristine Hirschkorn, Teela Johnson, Natasha Kachan, Della Kwan, Shade Olatunde, Narmatha Shanthakumar, Teresa Tsui, and Rishma Walji.
This study would not have been possible without the input of our Delphi survey participants: Heather Boon, University of Toronto; Lana Dvorkin-Camiel, Massachusetts College of Pharmacy and Health Services; Lynda Ecott, University of British Columbia; Shirley Heschuk, University of Alberta; Derek Jorgenson, University of Saskatchewan; Tannis Jurgens, Dalhousie University; Rebecca Law, Memorial University of Newfoundland; Susan Mansour, Canadian Council for Accreditation of Pharmacy Programs (CCAPP); Ken Potvin, University of Waterloo; John Pugsley, The Pharmacy Examining Board of Canada (PEBC); Cynthia Richard, University of Guelph/University of Waterloo; Stephen Shalansky, Canadian Society of Hospital Pharmacists (CSHP); Kelly Shields, Ohio Northern University; Saeed Tavakoli, Undergraduate Pharmacy Society, University of Toronto; Candy Tsourounis, University of California, San Francisco; Alexander Vuong, Canadian Association of Pharmacy Students and Interns (CAPSI); and Margaret Wong, Ontario Pharmacists' Association (OPA).
Appendix 1. Questions from all 4 Delphi Rounds
For all rounds, participants were given the following instructions: Please indicate how important it is that the following competency units and element(s) be identified as a core NHP-related competency pharmacy students should have upon entry to practice in Canada: unimportant (1); not important as a core competency (2); important, but other core competencies may take priority (3); very important (4); essential (5).
FIRST DELPHI ROUND
1. Competency Unit and Elements
Pharmacy graduates recognize that knowledge related to NHPs is important in providing pharmaceutical care, including the ability to:
a) inquire about patient NHP usage
b)interpret drug therapy problems to include NHP-related problems
c) critically assess NHPs as therapeutic options and,
d) integrate knowledge of NHPs into patients' individualized care plans.
2. Competency Unit and Elements
Pharmacy graduates use appropriate and effective strategies to access current and reliable information related to NHPs, including the ability to:
a) find and access reliable NHP references
b) identify evidence-based indications for use and expected outcomes for NHPs and,
c) identify clinically relevant possible side effects, drug interactions, and cautions associated with NHPs.
3. Competency Unit and Elements
Pharmacy graduates enable patients to identify and assess appropriate and reliable information on NHPs, including the ability to:
a) create the opportunity for open dialogue with patients about NHPs
b) educate patients about appropriate NHP information sources and,
c) help patients to critically assess NHP information.
4. Competency Unit and Elements
Pharmacy graduates provide appropriate education to patients on the effectiveness, potential adverse effects, and drug interactions of NHPs, including the ability to:
a) integrate knowledge of possible NHP adverse effects into routine patient education when appropriate
b) identify possible NHP-drug combinations which are contraindicated and,
c) inform patients of suspected NHP-drug interactions where applicable.
5. Competency Unit and Element
Pharmacy graduates document the use of NHPs in patients' computer profiles or medical records, including the ability to:
a) routinely document patient usage of NHPs.
6. Competency Unit and Elements
Pharmacy graduates report suspected adverse drug reactions or drug interactions related to the use of NHPs to Health Canada, including the ability to:
a) integrate knowledge of NHPs when investigating suspected adverse drug reactions and/or drug interactions and,
b) routinely report NHP-related suspected adverse drug reactions and/or drug interactions.
SECOND DELPHI ROUND
1. Competency Unit and Elements
Pharmacy graduates demonstrate an ability to incorporate NHP knowledge when providing pharmaceutical care, including the ability to:
a) inquire about patient NHP usage
b) consider patient NHP usage when identifying drug therapy problems and,
c) integrate knowledge of NHPs into patients' individualized care plans.
2. Competency Unit and Elements
Pharmacy graduates demonstrate an ability to describe the regulation of NHPs based on Health Canada's NHP Regulations, including the ability to:
a) explain the significance of a NPH or DIN-HM on a product
b) explain the difference between a traditional use label claim and a label claim based on scientific evidence
c) describe basic manufacturing standards for NHPs and,
d) identify the regulatory status of common CAM practitioners that regularly use NHPs.
3. Competency Unit and Elements
Pharmacy graduates demonstrate an ability to access appropriate sources of information related to NHPs, including the ability to:
a) find and access reliable NHP references
b) identify evidence-based indications for use and expected outcomes for NHPs and,
c) identify clinically relevant possible side-effects, drug interactions, and cautions associated with NHPs.
4. Competency Unit and Elements
Pharmacy graduates demonstrate an ability to critically assess reliable scientific evidence with respect to the safety and efficacy of NHPs, including the ability to:
a) critically assess evidence-based indications for use and expected outcomes for NHPs and,
b) integrate knowledge of clinically relevant possible side-effects, drug interactions, and cautions associated with NHPs in patient care plans when appropriate.
5. Competency Unit and Elements
Pharmacy graduates enable patients to identify and assess appropriate and reliable information on NHPs. This will be a result of being able to:
a) create the opportunity for open dialogue with patients about NHPs
b) educate patients about appropriate NHP information sources and,
c) help patients to critically assess NHP information.
6. Competency Unit and Elements
Pharmacy graduates provide appropriate education to patients on the effectiveness, potential adverse effects and drug interactions of NHPs. To accomplish this, they must have the ability to:
a) integrate knowledge of possible NHP adverse effects into routine patient education when appropriate
b) identify possible NHP-drug combinations which are contraindicated and,
c) inform patients of suspected NHP-drug interactions where applicable.
7. Competency Unit
Pharmacy graduates routinely document the use of NHPs into patients' computer profiles or medical records.
8. Competency Unit and Elements
Pharmacy graduates report suspected adverse drug reactions or drug interactions related to the use of NHPs to Health Canada. This includes the ability to:
a) integrate knowledge of NHPs when investigating suspected adverse drug reactions and/or drug interactions and,
b) routinely report NHP-related suspected adverse drug reactions and/or drug interactions.
THIRD DELPHI ROUND
1. Competency Unit and Elements
Pharmacy graduates demonstrate an ability to incorporate NHP knowledge when providing pharmaceutical care, including the ability to:
a) create the opportunity for open dialogue with patients about NHPs
b) inquire about patient NHP usage
c) document patients' NHP usage when appropriate
d) consider patient NHP usage when identifying potential drug therapy problems and,
e) integrate knowledge of NHPs into patients' individualized care plans.
2. Competency Unit and Elements
Pharmacy graduates demonstrate an ability to describe the regulation of NHPs based on Health Canada's NHP Regulations, including the ability to:
a) explain the significance of a NPN or DIN-HM on a product and,
b) explain the difference between a traditional use label claims and a label claim based on scientific evidence.
3. Competency Unit and Elements
Pharmacy graduates demonstrate an ability to access and critically appraise sources of information related to NHPs, including the ability to:
a) find and access credible NHP references
b) identify evidence-based indications for use and expected outcomes for NHPs and,
c) identify clinically relevant potential side-effects, drug interactions, and cautions associated with NHPs.
4. Competency Unit and Elements
Pharmacy graduates provide appropriate education to patients and other health care providers on the effectiveness, potential adverse effects and drug interactions of NHPs. To accomplish this, they must have the ability to:
a) integrate knowledge of NHPs into routine education when appropriate and,
b) educate patients and other health care providers about appropriate NHP information sources.
5. Competency Unit and Elements
Pharmacy graduates demonstrate an ability to report suspected adverse drug reactions and/or drug interactions related to the use of NHPs to Health Canada. This includes the ability to:
a) integrate knowledge of NHPs when investigating suspected adverse drug reactions and/or drug interactions and,
b) report NHP-related suspected adverse drug reactions and/or drug interactions.
FOURTH DELPHI ROUND
See Tables 1 and 2 for the final wording of the core competency and additional competency statements that resulted from the Delphi rounds.
REFERENCES
- 1. Natural Health Products Directorate (NHPD). Baseline Natural Health Products Survey Among Consumers, March 2005. http://www.hc-sc.gc.ca/dhp-mps/pubs/natur/eng_cons_survey_e.html. Accessed March 11, 2010.
- 2.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997 results of a follow-up national survey. JAMA. 1998;280(18):1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 3.Bokema A. What's up with herbals? Pharm Pract. 2000;16(1):54–61. [Google Scholar]
- 4.Levy S. ‘Healthcare 2000’ reveals consumer view of RPhs. Drug Top. 1999;143(19):64. [Google Scholar]
- 5. Government of Canada. Natural Health Products Regulations. 2003. http://gazette.gc.ca/archives/p2/2003/2003-06-18/html/sor-dors196-eng.html. Accessed March 11, 2010.
- 6. Health Canada. Food and Drug Regulations. 2005. http://www.hc-sc.gc.ca/fn-an/legislation/acts-lois/act-loi_reg-eng.php. Accessed March 11, 2010.
- 7.Farrell J, Ries NM, Boon H. Pharmacists and natural health products: a systematic analysis of professional responsibilities in Canada. Pharm Pract. 2008;6(1):33–42. doi: 10.4321/s1886-36552008000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwan D, Hirschkorn K, Boon H. US and Canadian pharmacists' attitudes, knowledge, and professional practice behaviours toward dietary supplements: a systematic review. BMC Complement Altern Med. 2006;6(31) doi: 10.1186/1472-6882-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwan D, Boon HS, Hirschkorn K, et al. Exploring consumer and pharmacist views on the professional role of the pharmacists with respect to natural health products: a study of focus groups. BMC Complement Altern Med. 2008;8(40) doi: 10.1186/1472-6882-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olatunde S, Boon H, Hirschkorn K, et al. Responsibilities of pharmacists with respect to natural health products: stakeholder interviews. Res Soc Admin Pharm. 2010;6(1):63–69. doi: 10.1016/j.sapharm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson T, Boon H, Jurgens T, et al. Canadian pharmacy students' knowledge of herbal medicine. Am J Pharm Educ. 2008;72(4) doi: 10.5688/aj720475. Article 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanthakumar N. Canadian Pharmacists and Natural Health Products: Identifying Professional Responsibilities [master's thesis] Toronto, Ontario, Canada: University of Toronto; 2009. [Google Scholar]
- 13.Boon H. Information Paper: The role of the pharmacist with respect to complementary/alternative medicine. CSHP Off Pub. 2001. 2001:181–186. [Google Scholar]
- 14.Miller LG, Hume A, Harris IM, et al. ACCP White Paper on Herbal Products. Pharmacotherapy. 2000;20(7):877–891. doi: 10.1592/phco.20.9.877.35200. [DOI] [PubMed] [Google Scholar]
- 15.Kroll DJ. ASHP Statement on the use of dietary supplements. Am J Health-Syst Pharm. 2004;61(16):1707–1711. doi: 10.1093/ajhp/61.16.1707. [DOI] [PubMed] [Google Scholar]
- 16. National Association of Pharmacy Regulatory Authorities (NAPRA). Pharmacist's Responsibility in Providing Advice About or Selling Alternative Health Products (NAPRA Position Statement). 1999. http://www.napra.org/pages/Practice_Resources/responsibility_regarding_alternative_health_products.aspx. Accessed March 11, 2010.
- 17.Cipolle RJ, Strand LM, Morley PC. Pharmaceutical Care Practice - A Clinician's Guide. 2nd ed. New York, New York: McGraw-Hill; 2004. [Google Scholar]
- 18.Chang ZG, Kennedy DT, Holdford DA, et al. Pharmacists' knowledge and attitudes towards herbal medicine. Ann Pharmacother. 2000;34(6):710–715. doi: 10.1345/aph.19263. [DOI] [PubMed] [Google Scholar]
- 19.Mackowiak ED, Parikh A, Freely J. Herbal product education in United States pharmacy schools: core or elective program? Am J Pharm Educ. 2001;65(1):1–6. [Google Scholar]
- 20.Shields KM, McQueen CE, Bryant PJ. Natural product education in schools of pharmacy in the United States. Am J Pharm Educ. 2003;67(1):43–48. [Google Scholar]
- 21.Rowell DM, Kroll DJ. Complementary and alternative medicine education in United States pharmacy schools. Am J Pharm Educ. 1998;62(4):412–419. [Google Scholar]
- 22.Evans E, Evans J. Changes in pharmacy students' attitudes and perceptions toward complementary and alternative medicine after completion of a required course. Am J Pharm Educ. 2006;70(5) doi: 10.5688/aj7005105. Article 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The Pharmacy Examining Board of Canada (PEBC). Information booklet for the Qualifying Examination of The Pharmacy Board of Canada. 2008. http://www.pebc.ca/EnglishGraphics/Qualifying%20Examination%20Information%202009.pdf. Accessed March 11, 2010.
- 24.Newton DW, Boyle M, Catizone CA. The NAPLEX: evolution, purpose, scope, and educational implications. Am J Pharm Educ. 2008;72(2):1–8. doi: 10.5688/aj720233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. National Association of Boards of Pharmacy (NABP). NAPLEX Blueprint. 2009. http://www.nabp.net/ftpfiles/NABP01/updatednaplexblueprint.pdf. Accessed March 11, 2009.
- 26. Accreditation Council for Pharmacy Education (ACPE). Accreditation standards and guidelines for the professional program in pharmacy leading to the Doctor of Pharmacy degree. 2007. http://www.acpe-accredit.org/pdf/ACPE_Revised_PharmD_Standards_Adopted_Jan152006.pdf. Accessed March 11, 2010.
- 27. National Association of Pharmacy Regulatory Authorities (NAPRA). Professional Competencies for Canadian Pharmacists at Entry to Practice. 2007. http://www.napra.org/Content_Files/Files/competencies.pdf. Accessed March 11, 2010.
- 28.Kligler B, Maizes V, Schachter S, et al. Core competencies in integrative medicine for medical school curricula: a proposal. Acad Med. 2004;79(6):521–531. doi: 10.1097/00001888-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Benjamin PJ, Phillips R, Warren D, et al. Education, initiatives, and information resources: response to a proposal for an integrative medicine curriculum. J Altern Complement Med. 2007;13(9):1021–1033. doi: 10.1089/acm.2006.6388. [DOI] [PubMed] [Google Scholar]
- 30. National Association of Pharmacy Regulatory Authorities (NAPRA). Model Standards of Practice for Canadian Pharmacists. March 2009. http://129.128.180.43/Content_Files/Files/Model_Standards_of_Prac_for_Cdn_Pharm_March09.pdf. Accessed March 11, 2010.
- 31. Association of Faculties of Pharmacy of Canada (AFPC). Association of Faculties of Pharmacy of Canada educational outcomes for entry-level Doctor of Pharmacy graduates in Canada. 2007. http://www.afpc.info/downloads/1/Entry_level_PharmD_outcomes_AFPCAGM2007.pdf. Accessed March 11, 2010.
- 32.Boon H, Hirschkorn K, Grierner G, et al. The ethics of dietary supplements and natural health products in pharmacy practice: a systematic documentary analysis. Int J Pharm Pract. 2009;17:31–38. doi: 10.1211/ijpp.17.1.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shanthakumar N, Boon H. Canadian pharmacists and natural health products: identifying professional responsibilities [abstract]. Research that matters: linking researchers, practitioners, decision-makers and the public: abstracts from the 5th Annual IN-CAM Symposium November 7-9, 2008, Toronto, Canada. J Complement Integr Med. 2008;5(1):Article 31. http://www.bepress.com/jcim/vol5/iss1/31. Accessed March 11, 2010.
- 34.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008–1015. [PubMed] [Google Scholar]