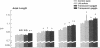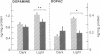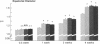Abstract
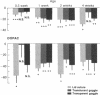
Investigation of retinal neurochemistry in a well-defined chick model of form-deprivation myopia indicated that dopamine and its metabolite 3,4-dihydroxyphenylacetic acid are reduced in myopic as compared to control eyes. The reduction in retinal dopamine is evident only during light adaptation and is accompanied by a decreased rate of dopamine biosynthesis. To test whether the alteration in dopamine metabolism is related to eye growth, agents known to interact with dopamine receptors were administered locally to deprived eyes. Remarkably, the expected growth in the axial dimension was reduced, while that in the equatorial dimension was not. Therefore retinal dopamine may participate in the pathway linking visual experience and the postnatal regulation of the eye's growth in the axial dimension. The mechanism for control of chick eye growth in the equatorial dimension remains unknown.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlsson A., Davis J. N., Kehr W., Lindqvist M., Atack C. V. Simultaneous measurement of tyrosine and tryptophan hydroxylase activities in brain in vivo using an inhibitor of the aromatic amino acid decarboxylase. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(2):153–168. doi: 10.1007/BF00508904. [DOI] [PubMed] [Google Scholar]
- Creese I., Sibley D. R., Hamblin M. W., Leff S. E. The classification of dopamine receptors: relationship to radioligand binding. Annu Rev Neurosci. 1983;6:43–71. doi: 10.1146/annurev.ne.06.030183.000355. [DOI] [PubMed] [Google Scholar]
- Ehrlich D. Regional specialization of the chick retina as revealed by the size and density of neurons in the ganglion cell layer. J Comp Neurol. 1981 Feb 1;195(4):643–657. doi: 10.1002/cne.901950408. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. D., Fugate-Wentzek L. A., Wallman J. Different visual deprivations produce different ametropias and different eye shapes. Invest Ophthalmol Vis Sci. 1987 Aug;28(8):1225–1235. [PubMed] [Google Scholar]
- Hayes B. P., Fitzke F. W., Hodos W., Holden A. L. A morphological analysis of experimental myopia in young chickens. Invest Ophthalmol Vis Sci. 1986 Jun;27(6):981–991. [PubMed] [Google Scholar]
- Hodos W., Kuenzel W. J. Retinal-image degradation produces ocular enlargement in chicks. Invest Ophthalmol Vis Sci. 1984 Jun;25(6):652–659. [PubMed] [Google Scholar]
- Hoyt C. S., Stone R. D., Fromer C., Billson F. A. Monocular axial myopia associated with neonatal eyelid closure in human infants. Am J Ophthalmol. 1981 Feb;91(2):197–200. doi: 10.1016/0002-9394(81)90173-2. [DOI] [PubMed] [Google Scholar]
- Iuvone P. M., Boatright J. H., Bloom M. M. Dopamine mediates the light-evoked suppression of serotonin N-acetyltransferase activity in retina. Brain Res. 1987 Aug 25;418(2):314–324. doi: 10.1016/0006-8993(87)90098-9. [DOI] [PubMed] [Google Scholar]
- Nathan J., Kiely P. M., Crewther S. G., Crewther D. P. Disease-associated visual image degradation and spherical refractive errors in children. Am J Optom Physiol Opt. 1985 Oct;62(10):680–688. doi: 10.1097/00006324-198510000-00003. [DOI] [PubMed] [Google Scholar]
- Osol G., Schwartz B., Foss D. C. The effects of photoperiod and lid suture on eye growth in chickens. Invest Ophthalmol Vis Sci. 1986 Feb;27(2):255–260. [PubMed] [Google Scholar]
- Parkinson D., Rando R. R. Effects of light on dopamine metabolism in the chick retina. J Neurochem. 1983 Jan;40(1):39–46. doi: 10.1111/j.1471-4159.1983.tb12650.x. [DOI] [PubMed] [Google Scholar]
- Raviola E., Wiesel T. N. An animal model of myopia. N Engl J Med. 1985 Jun 20;312(25):1609–1615. doi: 10.1056/NEJM198506203122505. [DOI] [PubMed] [Google Scholar]
- Robb R. M. Refractive errors associated with hemangiomas of the eyelids and orbit in infancy. Am J Ophthalmol. 1977 Jan;83(1):52–58. doi: 10.1016/0002-9394(77)90191-x. [DOI] [PubMed] [Google Scholar]
- Smith E. L., 3rd, Harwerth R. S., Crawford M. L., von Noorden G. K. Observations on the effects of form deprivation on the refractive status of the monkey. Invest Ophthalmol Vis Sci. 1987 Aug;28(8):1236–1245. [PubMed] [Google Scholar]
- Stefanini M., De Martino C., Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967 Oct 14;216(5111):173–174. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- Steinbusch H. W., Verhofstad A. A., Joosten H. W. Localization of serotonin in the central nervous system by immunohistochemistry: description of a specific and sensitive technique and some applications. Neuroscience. 1978;3(9):811–819. doi: 10.1016/0306-4522(78)90033-7. [DOI] [PubMed] [Google Scholar]
- Stone R. A., Laties A. M., Hemmings H. C., Jr, Ouimet C. C., Greengard P. DARPP-32 in the ciliary epithelium of the eye: a neurotransmitter-regulated phosphoprotein of brain localizes to secretory cells. J Histochem Cytochem. 1986 Nov;34(11):1465–1468. doi: 10.1177/34.11.2877023. [DOI] [PubMed] [Google Scholar]
- Stone R. A., Laties A. M., Raviola E., Wiesel T. N. Increase in retinal vasoactive intestinal polypeptide after eyelid fusion in primates. Proc Natl Acad Sci U S A. 1988 Jan;85(1):257–260. doi: 10.1073/pnas.85.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D., Gottlieb M. D., Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res. 1987 Aug;6(8):993–999. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- Wallman J., Adams J. I. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27(7):1139–1163. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- Wallman J., Gottlieb M. D., Rajaram V., Fugate-Wentzek L. A. Local retinal regions control local eye growth and myopia. Science. 1987 Jul 3;237(4810):73–77. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- Wallman J., Turkel J., Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978 Sep 29;201(4362):1249–1251. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977 Mar 3;266(5597):66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- Yinon U., Koslowe K. C., Lobel D., Landshman N., Barishak Y. R. Lid suture myopia in developing chicks: optical and structural considerations. Curr Eye Res. 1982;2(12):877–882. doi: 10.3109/02713688209020025. [DOI] [PubMed] [Google Scholar]