Abstract
Rationale
There has been increased recreational use of dimethyltryptamine (DMT), but little is known of its discriminative stimulus effects.
Objectives
The present study assessed the similarity of the discriminative stimulus effects of DMT to other types of hallucinogens and to psychostimulants.
Methods
Rats were trained to discriminate DMT from saline. To test the similarity of DMT to known hallucinogens, the ability of (+)-lysergic acid diethylamide (LSD), (−)-2,5-dimethoxy-4-methylamphetamine (DOM), (+)-methamphetamine, or (±)3,4-methylenedioxymethyl-amphetamine (MDMA) to substitute in DMT-trained rats was tested. The ability of DMT to substitute in rats trained to discriminate each of these compounds was also tested. To assess the degree of similarity in discriminative stimulus effects, each of the compounds was tested for substitution in all of the other training groups.
Results
LSD, DOM, and MDMA all fully substituted in DMT-trained rats, whereas DMT fully substituted only in DOM-trained rats. Full cross-substitution occurred between DMT and DOM, LSD and DOM, and (+)-methamphetamine and MDMA. MDMA fully substituted for (+)-methamphetamine, DOM, and DMT, but only partially for LSD. In MDMA-trained rats, LSD and (+)-methamphetamine fully substituted, whereas DMT and DOM did not fully substitute. No cross-substitution was evident between (+)-methamphetamine and DMT, LSD, or DOM.
Conclusions
DMT produces discriminative stimulus effects most similar to those of DOM, with some similarity to the discriminative stimulus effects of LSD and MDMA. Like DOM and LSD, DMT seems to produce predominately hallucinogenic-like discriminative stimulus effects and minimal psychostimulant effects, in contrast to MDMA which produced hallucinogen- and psychostimulant-like effects.
Keywords: Drug discrimination; cross-substitution; hallucinogen; psychostimulant; (−)-2, 5-dimethoxy-4-methylamphetamine (DOM); lysergic acid diethylamide (LSD); dimethyltryptamine (DMT); 3, 4-methylenedioxymethylamphetamine (MDMA); (+)-methamphetamine; rat
Introduction
Dimethyltryptamine (DMT) is an endogenous compound that induces hallucinations and bizarre dream states at high doses. Over the past few years, there has been an increase in the number of reports of recreational use of DMT (Gable 2007; Halpern 2004; McKenna 2004). DMT is found in plants in South America where it is combined with other plant products containing beta-carbolines and then drunk as a tea, called ayahuasca, as part of shamanistic rituals (Halpern 2004; Ott 1999; Riba et al. 2003). Unlike many other hallucinogens, DMT has poor oral bioavailability due to its inactivation by monoamine oxidase (MAO); however, ayahuasca contains natural compounds that inhibit MAO (McKenna et al. 1984; Ott 1999; Riba et al. 2003). Parenteral administration of DMT avoids the inactivation such that onset of activity is almost immediate, and the effects of DMT disappear within 20–30 min (Stoff et al. 1977; Strassman et al. 1994).
The behavioral effects of DMT in non-human subjects have been extensively tested (see reviews by (Barker et al. 1981; Gillin et al. 1976). Following DMT injection, various unconditioned behaviors have been noted in rats, including staring, flattened posture, splayed hindlimbs, and repetitive head movements (e.g., Cooper et al. 1981; Jenner et al. 1980; Ruffing and Domino 1983). A major effect of DMT is to suppress responding under a variety of conditions. DMT decreased exploration in the hole-board test (e.g., Adams and Geyer 1985; File 1977), and suppressed operant responding maintained by food (e.g., Kovacic and Domino 1976; Kovacic et al. 1978) and shock avoidance (e.g., Stoff et al. 1977). Notably, tolerance does not develop to many of the effects of DMT in animal models (see review in Barker et al., 1981), or in human subjects (Strassman et al., 1996).
The subjective effects of DMT in human subjects have been described (Strassman et al. 1996; Strassman et al. 1994; Szara et al. 1966); however, the discriminative stimulus effects of DMT have not been extensively studied. DMT has not been trained as a discriminative stimulus, but has been tested for substitution in rats trained to discriminate other hallucinogens. DMT fully substituted for LSD in two studies (Appel et al. 1999; Jarbe 1980) and produced nearly full substitution in another (Helsley et al. 1998). DMT also fully substituted in rats trained to discriminate DOM from saline (Glennon 1999; Glennon et al. 1983) and in rats trained to discriminate the closely related compound 5-methoxy-DMT (Glennon et al. 1980). In rats trained to discriminate psilocybin (0.5 mg/kg), DMT partially substituted and substantially decreased response rate (Winter et al. 2007). Another study reported the effects of DMT in rats trained to discriminate between the 5-HT2 antagonist ketanserin and the 5-HT2 agonist DOI. When administered DMT, the rats chose the DOI lever 80% or more of time (Smith et al. 1998), indicating that DMT acted more like 5-HT2 agonist than an antagonist. Finally, DMT partially blocked the discriminative stimulus effects of phencyclidine, which produces hallucinations through its actions at NMDA receptors (West et al. 2000). Taken together, these behavioral findings suggest that DMT produces a discriminative stimulus similar to those produced by other well-known hallucinogens such as LSD, psilocybin, DOI, and DOM. An implication that follows from this similarity is that DMT, like other hallucinogens, may act as a 5-HT2 agonist. Support for this implication comes from binding assays, which indicate that DMT binds to 5-HT1A (Pierce and Peroutka 1989), 5-HT1D (Hamik and Peroutka 1989; Heuring and Peroutka 1987; Pierce and Peroutka 1989) and 5-HT2A receptors (Lyon et al. 1988; McKenna and Peroutka 1989; Pierce and Peroutka 1989; Sadzot et al. 1989).
Drug discrimination assays can be used to characterize the abuse liability of compounds, particularly with regard to their subjective effects (Balster 1991; Stolerman 1993). The purpose of the present research was to characterize the discriminative stimulus effects of DMT with reference to other widely abused compounds having hallucinogenic or psychostimulant effects. Psychoactive compounds produce a wide range of effects which are dissociable using drug discrimination procedures. It has been proposed that the effects of psychedelic compounds can be classified into 3 categories: hallucinogen, stimulant, and other (Glennon, 1999). Further, the hallucinogens fall into three structural classes: simple tryptamines (indolealkylamines), ergolines, and phenethylamines (Nichols 2004; Winter 2008).
We examined potential effects across each of these different classes: DMT (indolealkylamine hallucinogen), LSD (ergoline hallucinogen), DOM (phenethylamine hallucinogen), MDMA (serotonergic phenethylamine psychostimulant), and methamphetamine (dopaminergic phenethylamine psychostimulant). This was accomplished by training rats to discriminate DMT from saline and then testing four reference compounds (LSD, DOM, MDMA or methamphetamine) for their ability to occasion DMT-appropriate responding in the DMT-trained rats. In order to fully assess the extent to which the DMT discriminative stimulus effects were similar or different from those of the other compounds, DMT was also evaluated for drug-appropriate responding in rats trained for LSD-, DOM-, MDMA- or methamphetamine discrimination. Analogous cross-substitution experiments were also performed for LSD, DOM, MDMA and methamphetamine.
The cross substitution approach was used because the discriminative stimulus effects of various compounds are not always symmetrical (i.e., drug A substitutes in rats trained to discriminate drug B, but drug B does not substitute in rats trained to discriminate drug A), the effects of DMT in rats trained to discriminate other psychoactive compounds were also tested. A classic example of such symmetry is that of ethanol. GABAA positive modulators, NMDA antagonists and 5-HT1B agonists all fully substitute in rats trained to discriminate ethanol, but ethanol produces at best partial substitution in rats trained to discriminate these compounds (for a review of these data and discussion of their implications for the use of drug discrimination see Grant 1999).
Materials and Method
Animals
Male Sprague-Dawley rats were obtained from Harlan-Sprague Dawley (Indianapolis, IN). All rats were housed individually and were maintained on a 12:12 light/dark cycle (lights on at 7:00 AM). Body weights were maintained at 320–350 g by limiting food to 20 g/day which included the food received during training sessions. Water was freely available in the home cages. All housing and procedures were in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003), and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Drug Discrimination Procedures
Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC compatible computers via LVB interfaces (Med Associates, East Fairfield, VT). The computers were programmed in MED-PC IV (Med Associates, East Fairfield, VT) for the operation of the chambers and collection of data.
Five groups (each comprised of 15 to 32 rats) were trained to discriminate one of five compounds from saline using a two-lever choice methodology, (+)-methamphetamine (1 mg/kg; N=32), (±)-MDMA (1.5 mg/kg; N=24), (+)-LSD (0.1 mg/kg; N=16), (−)-DOM (0.5 mg/kg; N=26), and DMT (5 mg/kg; N=15). Food (45 mg food pellets; Bio-Serve, Frenchtown, NJ) was available under a fixed-ratio 10 schedule of reinforcement when responding occurred on the injection-appropriate lever. There were no consequences scheduled for incorrect responses. Half of the rats were trained with drug as the cue on the right lever; half were trained with drug on the left lever. Training sessions occurred in a double alternating fashion (D-D-S-S-D, etc.), and tests were conducted between pairs of identical training sessions (i.e., between either two saline or two drug training sessions).
During each training session, the rats received an intraperitoneal injection of either saline or the appropriate training drug. Training dose and pretreatment time for each training drug are shown in Table 1. Following the appropriate pretreatment time, the rats were placed in the experimental chamber. During the session, the rats could earn up to 20 food pellets by responding under an FR10 schedule of food presentation. If all 20 food pellets were delivered before the end of the 10-min response period, the house lights were turned off and responding had no scheduled consequences for the remainder of that response period. Animals received approximately 60 training sessions in total before use in any behavioral experiment. Animals were selected for use in experiments when they had achieved 85% injection-appropriate responding for both the first reinforcer and for the total session during 9 of their last 10 training sessions.
Table 1.
Training parameters for each compound trained as a discriminative stimulus.
| Training Drug | Dose (mg/kg) | Pretreatment Time (min) | Number trained |
|---|---|---|---|
| N,N-dimethyltryptamine | 5.0 | 5 | 15 |
| (−)-2,5-dimethoxy-4-methylamphetamine | 0.5 | 30 | 26 |
| (+)-lysergic acid diethylamide | 0.1 | 15 | 16 |
| (±)-3,4-methylenedioxymethamphetamine | 1.5 | 15 | 24 |
| (+)-methamphetamine | 1.0 | 10 | 32 |
For testing, rats were assigned in groups of six. The ability of one of the 5 compounds to substitute was tested in different groups of 6 rats trained to discriminate one of the compounds. A repeated measures design was used, such that each rat was tested at all doses of the test compound. For example, 6 rats trained to discriminate DMT were tested with each dose of (−)-DOM. Test sessions lasted for a maximum of 20 min to allow for potentially delayed effects of test compounds. In contrast with training sessions, both levers were active, such that 10 consecutive responses on either lever led to reinforcement. Data were collected until 20 food pellets were obtained, or for a maximum of 20 min. Rats were tested only if they had achieved 85% injection-appropriate responding for both first reinforcer and total session on the two prior training sessions. At least 3 days elapsed between test sessions.
Drugs
(+)-Methamphetamine hydrochloride, (±)-3,4-methylenedioxymethamphetamine hydrochloride, (+)-lysergic acid diethylamide (+)-tartrate, (−)-2,5-dimethoxy-4-methylamphetamine hydrochloride, N,N,-dimethyltryptamine fumarate were provided by the National Institute on Drug Abuse. All drugs were dissolved in 0.9% saline and were administered i.p. in a volume of 1 ml/kg. Dose increments were based on 1/3 logs.
Data Analysis
Drug discrimination data are expressed as the mean percentage of responses on the drug-appropriate lever in each test period. Rates of responding were expressed as a function of the number of responses made divided by the total session time. Graphs for percent drug-appropriate responding and response rate were plotted as a function of dose of test compound (log scale). Error bars show standard error of the mean. Percent drug-appropriate responding was shown only if at least 3 rats completed the first fixed ratio. Full substitution was defined as ≥80% drug-appropriate responding and not statistically different from the training drug, and partial substitution as ≥40% and <80% drug-appropriate responding.
The potencies of test compounds that fully substituted were calculated by fitting straight lines to the individual dose-response data for each compound by means of Table Curve 2D (Jandel Scientific, San Rafael, CA). Straight lines were fitted to the linear portion of dose-effect curves, defined by doses producing 20 to 80% of the maximal effect, including not more than one dose producing <20% of the maximal effect and not more than one dose producing >80% of the maximal effect. Other doses were excluded from the analyses. The ED50 values are expressed as the mean of six rats±95% confidence intervals. Rates of responding were expressed as a function of the number of responses made divided by the total session time. Response rate data were analyzed by one-way, repeated measures analysis of variance. Effects of individual doses were compared to the appropriate control value using a priori contrasts. Criterion for significance was set a priori at p<0.05.
Results
DMT-trained rats
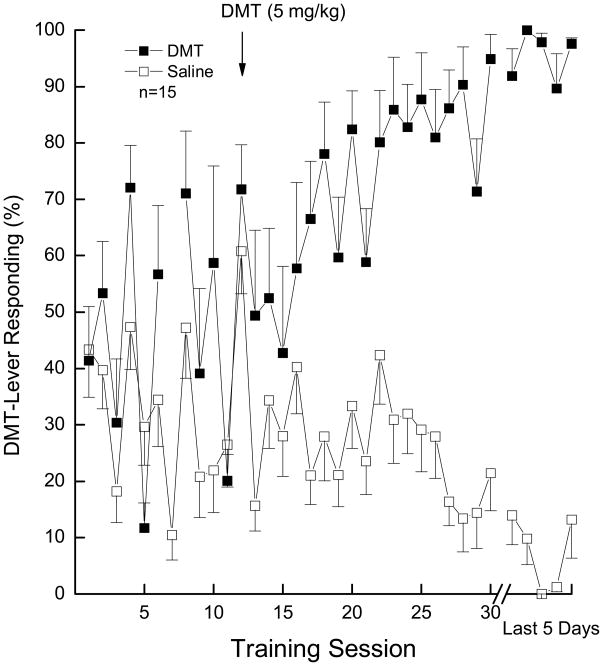
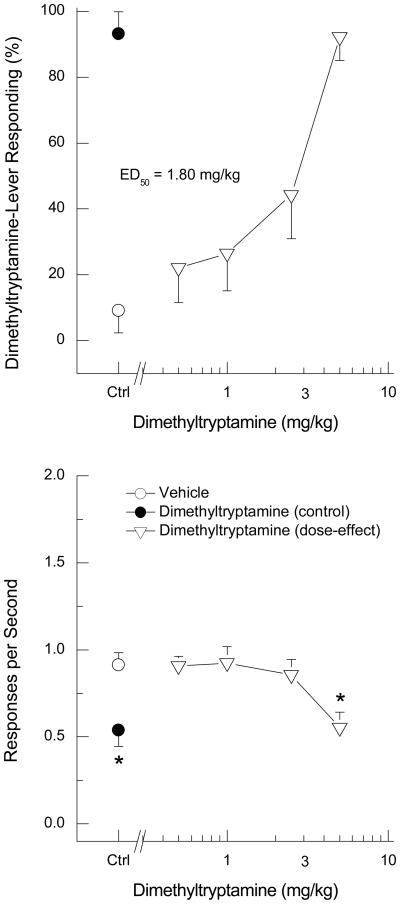
The study began with a DMT training dose of 10 mg/kg versus saline vehicle. Thirty sessions of training (15 drug, 15 saline) at that dose did not result in consistent responding under the drug (i.e., ≥ 80% drug-appropriate responding) or saline (i.e., ≤ 20% drug-appropriate responding) conditions, largely due to response suppression following administration of DMT. After additional training sessions with 5 mg/kg of DMT, 15 of 16 rats reliably learned the discrimination (Figure 1). The rats required an average of 72 sessions to reach criterion. When rats were subsequently tested, DMT (0.5 to 5 mg/kg) produced dose-dependent increases in drug-appropriate responding, with peak responding (92.2±7.1% DMT-appropriate responding) following the training dose of 5 mg/kg (Figure 2). An ED50 of 1.80 (95% confidence interval = 1.12 mg/kg) was calculated. Response rate was decreased to 61% of vehicle control following the training dose of DMT [F(4,56)=10.06, p<0.001]. Higher doses of DMT were not tested due to response suppression.
Fig. 1.
Learning curve showing the acquisition of the discrimination between DMT and the saline vehicle. The y-axis shows percentage of responding on the DMT-appropriate lever over 30 saline- and 30 drug-training sessions. Error bars show standard error of the mean. The study began with a DMT training dose of 10 mg/kg. At session 16, the training dose was decreased to 5 mg/kg (at arrow). Data for drug session 7 are missing due to computer malfunction. Following the axis break, the data for those sessions during which each rat met the training criterion is shown as an average of the last 5 saline and 5 drug sessions. Each point represents the average of 15 rats.
Fig. 2.
Dose-effect of DMT in rats trained to discriminate DMT from saline. The top panel shows percentage of DMT-appropriate responding over increasing doses of DMT. The bottom panel shows rate of responding in responses per sec. Error bars show standard error of the mean. Ctrl=control points (vehicle alone and DMT 5 mg/kg). Each point represents the average of 15 rats. * indicates points different from the vehicle control.
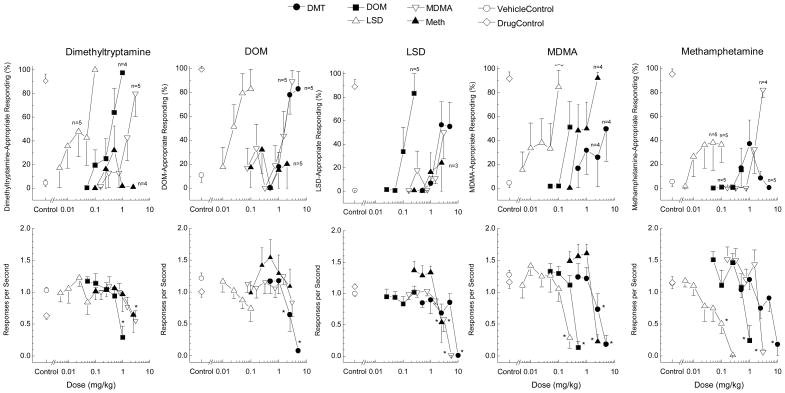
LSD (ED50 = 0.05±0.03 mg/kg), DOM (ED50 = 0.34±0.20 mg/kg), and MDMA (ED50 = 1.49±0.56 mg/kg) each fully substituted for the discriminative stimulus effects of DMT (Figure 3). Each compound produced maximal effects that were not different from the training dose of DMT (Table 2). (+)-Methamphetamine (0.1 to 2.5 mg/kg) failed to substitute. Higher doses of (+)-methamphetamine were not tested to minimize the possibility of neurotoxicity.
Fig. 3.
Cross-substitution of DMT, DOM, LSD, MDMA, and methamphetamine. Top panels show drug-appropriate responding of the test compounds in DMT-, LSD-, DOM-, MDMA-, and methamphetamine-trained rats. Bottom panels show rate of responding in responses per sec. Each point represents the average of six rats except where shown. * indicates points different from the vehicle control. Meth = methamphetamine.
Table 2.
Maximum percent drug-appropriate responding (DAR) of each test compound in each of the training groups and effects on rate of responding at that dose.
| Training Drug | Test Compound | Dose (mg/kg) | N Test | %DAR | Rate resp/s |
|---|---|---|---|---|---|
| DMT | Saline | 0 | 6/6 | 0.0±0.0 | 1.102±0.101 |
| DMT | DMT | 5 | 6/6 | 96.6±2.4 | 0.575±0.119 |
| DMT | DOM | 1 | 4/6 | 97.7±1.8 | 0.293±0.175 |
| DMT | LSD | 0.1 | 6/6 | 100.0±0.0 | 1.011±0.066 |
| DMT | MDMA | 3 | 5/6 | 80.0±19.3 | 0.551±0.155 |
| DMT | Meth | 0.5 | 6/6 | 32.2±20.4 | 1.062±0.137 |
| DOM | Saline | 0 | 6/6 | 16.9±16.6 | 1.384±0.146 |
| DOM | DOM | 0.5 | 6/6 | 99.9±0.1 | 0.768±0.171 |
| DOM | DMT | 5 | 5/6 | 82.8±14.8 | 0.078±0.034 |
| DOM | LSD | 0.1 | 6/6 | 82.8±16.6 | 0.742±0.200 |
| DOM | MDMA | 3 | 5/6 | 89.1±9.3 | 0.835±0.225 |
| DOM | Meth | 2 | 5/6 | 20±20 | 1.089±0.271 |
| LSD | Saline | 0 | 6/6 | 0.2±0.2 | 0.900±0.097 |
| LSD | LSD | 0.1 | 6/6 | 83.3±16.7 | 1.199±0.092 |
| LSD | DMT | 2.5 | 6/6 | 56.3±19.8 | 0.686±0.146 |
| LSD | DOM | 0.25 | 6/6 | 83.3±16.7 | 1.017±0.098 |
| LSD | MDMA | 3 | 6/6 | 56.1±18.5 | 0.591±0.200 |
| LSD | Meth | 2.5 | 3/6 | 24.1±24.1 | 0.538±0.316 |
| MDMA | Saline | 0 | 6/6 | 0.1±0.1 | 1.253±0.172 |
| MDMA | MDMA | 1.5 | 6/6 | 99.8±0.2 | 0.994±.221 |
| MDMA | DMT | 5 | 4/6 | 49.5±26.9 | 0.184±0.137 |
| MDMA | DOM | 0.25 | 6/6 | 51.1±21.4 | 1.113±0.151 |
| MDMA | LSD | 0.1 | 5/6 | 84.6±14.2 | 0.286±0.162 |
| MDMA | Meth | 2.5 | 4/6 | 92.2±4.7 | 0.266±0.11 |
| Meth | Saline | 0 | 6/6 | 0.0±0.0 | 1.297±0.137 |
| Meth | Meth | 1 | 6/6 | 98.2±1.0 | 0.855±0.165 |
| Meth | DOM | 0.5 | 6/6 | 15.4±15.1 | 1.075±0.212 |
| Meth | DMT | 1 | 6/6 | 37.0±19.9 | 1.195±0.154 |
| Meth | LSD | 0.05 | 5/6 | 38.1±23.1 | 0.745±0.207 |
| Meth | MDMA | 3 | 4/6 | 82.3±6.4 | 0.061±0.046 |
N Test = number of rats which completed the first fixed ratio/total number tested. resp/s = responses per second.
Response rate was decreased to 58% of vehicle control following 3 mg/kg MDMA [F(5,25)=3.96, p=.009] and to 27% of vehicle control following 1 mg/kg DOM [F(5,25)=8.10, p<.001]. LSD and (+)-methamphetamine did not alter response rate within the dose ranges tested.
DOM-trained rats
DOM (0.5 mg/kg, i.p.), when administered 30 min before testing, was successfully trained as a discriminative stimulus in 20 of 26 rats. The rats received an average of 63 sessions to reach criterion. DOM suppressed rates of responding at the training dose, and some rats did not complete training due to a lack of responding. LSD (ED50 = 0.03±0.02 mg/kg), DMT (ED50 = 1.87±0.88 mg/kg), and MDMA (ED50 = 1.30±0.64 mg/kg) each fully substituted for the discriminative stimulus effects of DOM (Figure 3), and the maximal effects were not different from the training dose of DOM. In contrast, (+)-methamphetamine did not produce significant levels of DOM-appropriate responding (Table 2).
Response rate was decreased following 2.5 and 5 mg/kg DMT [F(4,20)=13.82, p<.001]. MDMA, LSD, and (+)-methamphetamine did not significantly alter response rate at the doses tested.
LSD-trained rats
LSD (0.1 mg/kg, i.p.), when administered 15 min before testing, was successfully trained as a discriminative stimulus in 16 of 16 rats. The rats received an average of 58 sessions to reach criterion. As shown in figure 3, only DOM fully substituted for the discriminative stimulus effects of LSD (ED50 = 0.12±0.04 mg/kg). DOM produced maximal LSD-appropriate responding of 83%, which was not different from the LSD training dose. DMT and MDMA each partially substituted for LSD. DMT produced maximum LSD-appropriate responding of 56.3%, and MDMA produced 56.1%. (+)-Methamphetamine did not produce notable LSD-appropriate responding (Table 2).
DMT decreased response rate to 1% of vehicle control following 10 mg/kg [F(5,25)=11.63, p<.001], MDMA decreased response rate following 3 and 5.6 mg/kg [F(6,30)=21.55, p<.001], and (+)-methamphetamine decreased response rate to 50% of vehicle control following 2.5 mg/kg [F(4,20)=5.03, p=.006]. DOM did not alter response rate.
MDMA-trained rats
MDMA (1.5 mg/kg, i.p.), when administered 15 min before testing, was successfully trained as a discriminative stimulus in 20 of 24 rats. The rats received an average of 80 sessions to reach criterion. LSD (ED50 = 0.04±0.03 mg/kg) and (+)-methamphetamine (ED50 = 0.79±0.44 mg/kg) each fully substituted for the discriminative stimulus effects of MDMA (Figure 3) and produced maximal effects that were not different from the training dose of MDMA. DMT and DOM each partially substituted for MDMA. DMT produced maximum MDMA-appropriate responding of 49.5%, and DOM produced 51.1% (Table 2).
Response rate was decreased to 24% of vehicle control following 0.1 mg/kg of LSD [F(5,25)=7.36, p<.001], to 12% of vehicle control following 0.5 mg/kg of DOM [F(4,20)=30.17, p<.001], and to 30% of vehicle control following 2.5 mg/kg of (+)-methamphetamine [F(5,25)=5.66, p=.001]. DMT decreased response rate following 2.5 and 5 mg/kg [F(4,20)=6.89, p=.001].
(+)-Methamphetamine-trained rats
(+)-Methamphetamine (1 mg/kg, i.p.), when administered 10 min before testing, was successfully trained as a discriminative stimulus in 29 of 32 rats. The rats received an average of 39 sessions to reach criterion. As shown in figure 3, only MDMA fully substituted for the discriminative stimulus effects of (+)-methamphetamine (ED50 = 1.66±0.60 mg/kg). MDMA produced a peak effect of 82% (+)-methamphetamine-appropriate responding that was not different from that of the training dose of (+)-methamphetamine. None of the hallucinogens produced significant levels of (+)-methamphetamine-appropriate responding (Table 2).
Response rate was decreased to 18% of vehicle control following 10 mg/kg DMT [F(5,25)=6.50, p=.001], to 16% of vehicle control following 1 mg/kg DOM [F(5,25)=6.49, p=.001], and to 5% of vehicle control following 3 mg/kg MDMA [F(5,25)=29.17, p<.001]. Response rate was decreased following 0.1 and 0.25 mg/kg of LSD [F(6,30)=8.28, p<.001].
Discussion
DMT training
Although DMT has been tested for substitution in previous studies involving hallucinogen-trained rats (Appel et al. 1999; Glennon et al. 1983; Jarbe 1980; West et al. 2000; Winter et al. 2007), successful training of the discriminative stimulus effects of DMT has not previously been reported. In the current study, rats learned to discriminate 5 mg/kg DMT from saline, and increasing doses of DMT (0.5 to 5 mg/kg) produced dose-dependent generalization to the discriminative stimulus effects of the training dose of DMT. It should be noted that training with DMT was slower than for most of the other compounds. The training dose of DMT (5 mg/kg) suppressed response rate, and this suppression may account for length of time to achieve criterion. The original training dose of 10 mg/kg produced even greater response suppression, such that many of the rats did not respond at all. These doses of DMT have been noted to suppress exploratory and operant behaviors in a variety of assays including the hole-board test (e.g., Adams and Geyer 1985; File 1977), food-maintained operant responding (e.g., Kovacic and Domino 1976; Kovacic et al. 1978), and shock avoidance (e.g., Stoff et al. 1977). Tolerance to this suppression did not develop in agreement with earlier findings (see review in Barker et al., 1981). The lack of acquisition following 10 mg/kg DMT can be attributed to the rats not responding enough to be reinforced for correct responding.
Discriminative stimulus profile of DMT
The profiles of cross substitution are shown in Table 3 for each of the compounds in the present study. It should be noted that the serotonergic hallucinogens DOM and LSD fully cross-substituted in the present study, but did not share discriminative stimulus effects with (+)-methamphetamine, whereas MDMA shared discriminative stimulus effects to some extent with both (+)-methamphetamine and the hallucinogens.
Table 3.
Cross-substitution of psychoactive compounds. Contents of cells indicate results of the present study. Footnotes cite studies that tested the same combination. Description of the results of these studies and the degree to which they agreed with the results of the present study can be found in the discussion.
| Training Drug | Test Compound | ||||
|---|---|---|---|---|---|
| DMT | DOM | LSD | MDMA | METH | |
| DMT | FULL | FULL | FULL | FULL | None |
| DOM | FULL1,2 | FULL | FULL1,6 | FULL11 | None2 |
| LSD | Partial3,4,5 | FULL6,7 | FULL | Partial12,13 | None |
| MDMA | Partial | Partial8,9 | FULL8,9,10 | FULL | FULL |
| METH | None | None | None | FULL | FULL |
Glennon et al.1983,
Full substitution ≥ 80% drug-appropriate responding.
Partial ≥40% <80% drug-appropriate responding.
None < 40% drug-appropriate responding.
DOM, LSD, and MDMA produced full substitution (≥80% DMT-appropriate responding) in the DMT-trained rats, whereas DMT produced full substitution only in the DOM-trained rats. DMT (5 mg/kg) produced approximately half maximal drug-appropriate responding in rats trained to discriminate LSD and MDMA, and a higher dose (10 mg/kg) completely suppressed responding. These findings are in agreement with earlier reports that DMT fully substituted in DOM-trained rats (Glennon 1999). However, earlier studies reported that DMT produced full or near full substitution for LSD (Appel et al. 1999; Helsley et al. 1998; Jarbe 1980). In one of these reports, 10 mg/kg DMT produced 78% LSD-appropriate responding (Helsley et al. 1998), but 6 mg/kg DMT produced about 50% LSD-appropriate responding, which is consistent with the findings of the present study for the 5 mg/kg dose. This suggests that the response rate-suppressing effects of DMT in the present study could have prevented detection of full substitution in the LSD-trained rats.
MDMA fully substituted for DMT, but DMT only partially substituted in MDMA-trained rats. This is consistent with the asymmetric substitution seen between MDMA and serotonergic compounds such as LSD or DOM in the present study (Table 3). (+)-Methamphetamine did not produce DMT-appropriate responding, nor did DMT produce (+)-methamphetamine-appropriate responding. Further behavioral testing with other psychostimulants (e.g., amphetamine, cocaine) will be necessary to confirm this observation. Mechanistic experiments with antagonists and binding assays will be necessary to confirm whether DMT acts at receptors other than serotonin receptors.
Discriminative stimulus profiles of other psychoactive compounds
DOM
Each drug produced a different profile of cross-substitution (Table 3). DOM produced the profile most similar to that of DMT. All compounds but (+)-methamphetamine substituted in the DOM-trained rats, which is the same as the profile observed in the DMT-trained rats. In addition, DOM and DMT fully cross-substituted for each other, both produced only partial substitution in the MDMA-trained rats, and neither substituted for (+)-methamphetamine. The one difference between DOM and DMT was that DOM fully substituted in LSD-trained rats, whereas DMT produced only partial substitution. As described above, the discrepancy may have been due to the marked rate suppressant effects of DMT observed in the present study which prevented testing higher doses of DMT.
These findings agree with earlier studies in which LSD (Doat et al. 2003; Glennon et al. 1983) and DMT (Glennon et al. 1983; 1986) fully substituted for the discriminative stimulus effects of DOM. Previously, racemic MDMA (1.5 to 2 mg/kg) was reported to produce 31 to 52% DOM-appropriate responding (Glennon et al. 1982), similar to the effects of 1.5 mg/kg of (±)MDMA in the present study, which produced 44% DOM-appropriate responding. However, higher doses in the earlier study disrupted responding, whereas in the present study, 3 mg/kg of (±)MDMA fully substituted for DOM with little decrease in response rate. In another study, DOM produced about 70% drug-appropriate responding in rats trained to discriminative S(+)MDMA and nearly 80% in rats trained to discriminate R(−)MDMA (Baker et al. 1995). Similarly, in the present study, DOM partially substituted in rats trained to discriminate racemic MDMA. Finally, data from our study replicated previous research in which methamphetamine failed to substitute for the discriminative stimulus effects of DOM (Glennon 1986).
LSD
LSD produced a profile different from both DOM and DMT (Table 3). LSD fully substituted in rats trained with DMT, DOM, and MDMA, whereas DOM substituted only in DMT- and LSD-trained rats, and DMT substituted only in DOM-trained rats.
However, only DOM fully substituted in the LSD-trained rats. These findings are in general agreement with prior studies in which DOM and LSD fully cross substituted (Doat et al. 2003; Fiorella et al. 1995; Glennon et al. 1983) and MDMA either partially substituted (Broadbent et al. 1992) or produced no effect in LSD-trained rats (Callahan and Appel 1988). LSD fully substituted for racemic MDMA in one study (Schechter 1998), and produced near full substitution (78%) in another (Oberlender and Nichols 1988). In a third study, LSD produced an inverted U-shaped curve in rats trained to discriminate (-)MDMA, reaching 90% drug-appropriate responding at an intermediate dose; in the same study, LSD produced only about 50% drug-appropriate responding in rats trained to discriminate (+)MDMA (Baker et al. 1995).
MDMA
MDMA, not surprisingly, has a different profile of cross-substitution as depicted in Table 3. LSD and (+)-methamphetamine fully substituted for the discriminative stimulus effects of MDMA, whereas DMT and DOM only partially substituted in MDMA-trained rats. In contrast, MDMA fully substituted in rats trained to discriminate DMT, DOM, and (+)-methamphetamine but produced only partial substitution in LSD-trained rats.
As mentioned in the previous paragraphs, the asymmetrical cross substitution between LSD and MDMA, as well as between DOM and MDMA is similar to what has been previously reported in the literature. Surprisingly, there do not appear to be previous studies of the interaction of the discriminative stimulus effects of (+)-methamphetamine and MDMA. It is striking that (+)-methamphetamine and MDMA fully cross-substituted in the present study, even though previous studies have reported mixed results in the interaction of MDMA with cocaine and amphetamine. Two earlier studies indicated that cocaine and amphetamine fully substituted in MDMA-trained rats (Khorana et al. 2004; Oberlender and Nichols 1988), although another study reported only partial substitution by cocaine (Kueh and Baker 2007). In contrast, MDMA did not substitute in cocaine- or amphetamine-trained rats (Khorana et al. 2004; Oberlender and Nichols 1988), although one study reported partial substitution in cocaine-trained rats (Kueh and Baker 2007). Currently, it is unclear why methamphetamine and MDMA should fully cross substitute, whereas cocaine and amphetamine share an asymmetrical relation with MDMA.
Methamphetamine
(+)-Methamphetamine did not share discriminative stimulus effects with the hallucinogens DMT, DOM, and LSD. One study has reported that methamphetamine did not substitute for the discriminative stimulus effects of DOM (Glennon 1986), but the other findings are novel. In contrast, methamphetamine fully cross-substituted with MDMA. This seems to make sense initially, given how closely they are structurally related. However, as described in the previous paragraph, cocaine and amphetamine produced full substitution in 2 of 3 studies in MDMA-trained rats, whereas MDMA produced little drug-appropriate responding in cocaine- or amphetamine-trained rats (Khorana et al. 2004; Kueh and Baker 2007; Oberlender and Nichols 1988).
Conclusions
Given reports of the hallucinogenic activity of DMT (Strassman et al. 1996; Strassman et al. 1994; Szara et al. 1966), it is not surprising that DMT can produce a discriminative stimulus and that DMT shares its discriminative stimulus effects with other hallucinogens such as DOM, and to a lesser extent, LSD and MDMA. Indeed, DMT shares discriminative stimulus effects with a number of hallucinogens, including psilocin, 5-methoxy-DMT, and DOI (Glennon et al. 1980; Smith et al. 1998; Winter et al. 2007). In the present study, a range of psychoactive compounds was used to test the discriminative stimulus effects of DMT. DMT was previously tested in DOM- and LSD-trained rats, but not in MDMA- or methamphetamine-trained rats. Because DMT has never been trained as a discriminative stimulus before, the substitution studies of DOM, LSD, MDMA, and methamphetamine in DMT-trained rats are novel.
DMT produced discriminative stimulus effects that were similar to, but not completely overlapping with LSD, DOM, and MDMA. Each compound produced somewhat different patterns of cross-substitution. The fact that each compound produced somewhat different patterns of cross-substitution is not unusual, as DMT produces other effects dissimilar to other hallucinogens. For example, DMT does not suppress the effects of punishment (Schoenfeld 1976), nor does it augment shock-induced aggression as does LSD (Sbordone et al. 1979; Walters et al. 1978). It will be necessary to conduct experiments with selective antagonists to identify the mechanism of action for the discriminative stimulus effects of DMT and to distinguish any differences in mechanism between DMT and other hallucinogens.
Although much of the cross-substitution data for LSD, DOM, and MDMA has been previously reported, replication is useful and validates the reliability of the data in the present study. Further, bringing it all together allows the comparison of the discriminative stimulus effects across different classes of psychedelic compounds. Methamphetamine appeared to be strictly a psychostimulant without hallucinogenic effects, whereas DMT, DOM, and LSD were hallucinogens without psychostimulant effects. In contrast, MDMA was partially in both camps, as it shared discriminative stimulus effects with both the hallucinogens and with methamphetamine. On the other hand, further subdivision of the hallucinogens into structural classes such as indolealkylamines, ergolines, and phenethylamines (Nichols, 2004) does not seem to be useful for predicting shared discriminative stimulus effects. For example, DOM (indolealkylamine) and LSD (ergoline) come from different structural classes yet have very similar discriminative stimulus effects, whereas DMT and methamphetamine are both phenethylamines but do not share discriminative stimulus effects at all.
Acknowledgments
Funding was provided by the Addiction Treatment Discovery Program of the National Institute on Drug Abuse (NIH N01DA-2-8822). Program staff were involved in selection of compounds and test parameters. The ATDP had no further role in study design; the collection, analysis and interpretation of data; or the writing of the report. They have granted permission for the submission of this data for publication.
References
- Adams LM, Geyer MA. Effects of DOM and DMT in a proposed animal model of hallucinogenic activity. Prog Neuropsychopharmacol Biol Psychiatry. 1985;9:121–132. doi: 10.1016/0278-5846(85)90074-0. [DOI] [PubMed] [Google Scholar]
- Appel JB, West WB, Rolandi WG, Alici T, Pechersky K. Increasing the selectivity of drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:353–358. doi: 10.1016/s0091-3057(99)00089-1. [DOI] [PubMed] [Google Scholar]
- Baker LE, Broadbent J, Michael EK, Matthews PK, Metosh CA, Saunders RB, West WB, Appel JB. Assessment of the discriminative stimulus effects of the optical isomers of ecstasy (3,4-methylenedioxymethamphetamine; MDMA) Behav Pharmacol. 1995;6:263–275. [PubMed] [Google Scholar]
- Balster RL. Drug abuse potential evaluation in animals. Brit J Addict. 1991;86:1549–1558. doi: 10.1111/j.1360-0443.1991.tb01747.x. [DOI] [PubMed] [Google Scholar]
- Barker SA, Monti JA, Christian ST. N, N-dimethyltryptamine: an endogenous hallucinogen. Int Rev Neurobiol. 1981:83–110. doi: 10.1016/s0074-7742(08)60291-3. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Appel JB, Michael EK, Ricker JH. Discriminative stimulus effects of the optical isomers of 3,4- methylenedioxyamphetamine (MDA) Behav Pharmacol. 1992;3:443–454. [PubMed] [Google Scholar]
- Callahan PM, Appel JB. Differences in the stimulus properties of 3,4-methylenedioxyamphetamine and 3,4- methylenedioxymethamphetamine in animals trained to discriminate hallucinogens from saline. J Pharmacol Exp Ther. 1988;246:866–870. [PubMed] [Google Scholar]
- Cooper SG, Schiff SR, Bridger WH. Tolerance to behavioral effects of N, N-dimethyltryptamine in mice. Biol Psychiatry. 1981;16:864–867. [PubMed] [Google Scholar]
- Doat MM, Rabin RA, Winter JC. Characterization of the discriminative stimulus properties of centrally administered (−)-DOM and LSD. Pharmacol Biochem Behav. 2003;74:713–721. doi: 10.1016/s0091-3057(02)01074-2. [DOI] [PubMed] [Google Scholar]
- File SE. Effects of N,N-dimethyltryptamine on behavioural habituation in the rat. Pharmacol Biochem Behav. 1977;6:163–168. doi: 10.1016/0091-3057(77)90067-3. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Palumbo PA, Rabin RA, Winter JC. The time-dependent stimulus effects of R(−)-2,5-dimethoxy-4-methamphetamine (DOM): implications for drug-induced stimulus control as a method for the study of hallucinogenic agents. Psychopharmacology. 1995;119:239–245. doi: 10.1007/BF02246166. [DOI] [PubMed] [Google Scholar]
- Gable RS. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction. 2007;102:24–34. doi: 10.1111/j.1360-0443.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Kaplan J, Stillman R, Wyatt RJ. The psychedelic model of schizophrenia: the case of N,N-dimethyltryptamine. Am J Psychiatry. 1976;133:203–208. doi: 10.1176/ajp.133.2.203. [DOI] [PubMed] [Google Scholar]
- Glennon RA. Discriminative stimulus properties of phenylisopropylamine derivatives. Drug Alc Depend. 1986;17:119–134. doi: 10.1016/0376-8716(86)90003-7. [DOI] [PubMed] [Google Scholar]
- Glennon RA. Arylalkylamine drugs of abuse: an overview of drug discrimination studies. Pharmacol Biochem Behav. 1999;64:251–256. doi: 10.1016/s0091-3057(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Jacyno JM, Slusher M, Rosecrans JA. DOM-stimulus generalization to LSD and other hallucinogenic indolealkylamines. Eur J Pharmacol. 1983;86:453–459. doi: 10.1016/0014-2999(83)90196-6. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA, Anderson GM. Discriminative stimulus properties of MDA analogs. Biol Psychiatry. 1982;17:807–814. [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA, Kallman MJ. Hallucinogenic agents as discriminative stimuli: a correlation with serotonin receptor affinities. Psychopharmacology. 1980;68:155–158. doi: 10.1007/BF00432133. [DOI] [PubMed] [Google Scholar]
- Grant KA. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:261–267. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Halpern JH. Hallucinogens and dissociative agents naturally growing in the United States. Pharmacol Ther. 2004;102:131–8. doi: 10.1016/j.pharmthera.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Hamik A, Peroutka SJ. 1-(m-chlorophenyl)piperazine (mCPP) interactions with neurotransmitter receptors in the human brain. Biol Psychiatry. 1989;25:569–575. doi: 10.1016/0006-3223(89)90217-5. [DOI] [PubMed] [Google Scholar]
- Helsley S, Fiorella D, Rabin RA, Winter JC. A comparison of N,N-dimethyltryptamine, harmaline, and selected congeners in rats trained with LSD as a discriminative stimulus. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:649–663. doi: 10.1016/s0278-5846(98)00031-1. [DOI] [PubMed] [Google Scholar]
- Heuring RE, Peroutka SJ. Characterization of a novel 3H-5-hydroxytryptamine binding site subtype in bovine brain membranes. J Neurosci. 1987;7:894–903. doi: 10.1523/JNEUROSCI.07-03-00894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TUC. LSD-25 as a discriminative stimulus for response selection by pigeons. Pharmacol Biochem Behav. 1980;13:549–554. doi: 10.1016/0091-3057(80)90279-8. [DOI] [PubMed] [Google Scholar]
- Jenner P, Marsden CD, Thanki CM. Behavioural changes induced by N,N-dimethyl-tryptamine in rodents. Br J Pharmacol. 1980;69:69–80. doi: 10.1111/j.1476-5381.1980.tb10884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana N, Pullogurla MR, Young R, Glennon RA. Comparison of the discriminative stimulus effects of 3,4-methylenedioxymethamphetamine (MDMA) and cocaine: asymmetric generalization. Drug Alc Depend. 2004;74:281–287. doi: 10.1016/j.drugalcdep.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kovacic B, Domino EF. Tolerance and limited cross-tolerance to the effects of N, N-dimethyltryptamine (DMT) and lysergic acid diethylamide-25 (LSD) on food-rewarded bar pressing in the rat. J Pharmacol Exp Ther. 1976;197:495–502. [PubMed] [Google Scholar]
- Kovacic B, Wang Lu LJ, Ruffing D, Domino EF. Interactions of partial LSD analogs with behavioral disrupting effects of LSD and DMT in the rat. Eur J Pharmacol. 1978;47:37–44. doi: 10.1016/0014-2999(78)90371-0. [DOI] [PubMed] [Google Scholar]
- Kueh D, Baker LE. Reinforcement schedule effects in rats trained to discriminate 3,4-methylenedioxymethamphetamine (MDMA) or cocaine. Psychopharmacology. 2007;189:447–457. doi: 10.1007/s00213-006-0523-z. [DOI] [PubMed] [Google Scholar]
- Lyon RA, Titeler M, Seggel MR, Glennon RA. Indolealkylamine analogs share 5-HT2 binding characteristics with phenylalkylamine hallucinogens. Eur J Pharmacol. 1988;145:291–297. doi: 10.1016/0014-2999(88)90432-3. [DOI] [PubMed] [Google Scholar]
- McKenna DJ. Clinical investigations of the therapeutic potential of ayahuasca: rationale and regulatory challenges. Pharmacol Ther. 2004;102:111–29. doi: 10.1016/j.pharmthera.2004.03.002. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Peroutka SJ. Differentiation of 5-hydroxytryptamine2 receptor subtypes using 125I-R-(−)2,5-dimethoxy-4-iodo-phenylisopropylamine and 3H-ketanserin. J Neurosci. 1989;9:3482–3490. doi: 10.1523/JNEUROSCI.09-10-03482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna DJ, Towers GH, Abbott F. Monoamine oxidase inhibitors in South American hallucinogenic plants: tryptamine and beta-carboline constituents of ayahuasca. J Ethnopharmacol. 1984;10:195–223. doi: 10.1016/0378-8741(84)90003-5. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. The National Academies Press; 2003. [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Oberlender R, Nichols DE. Drug discrimination studies with MDMA and amphetamine. Psychopharmacology. 1988;95:71–76. doi: 10.1007/BF00212770. [DOI] [PubMed] [Google Scholar]
- Ott J. Pharmahuasca: human pharmacology of oral DMT plus harmine. J Psychoactive Drugs. 1999;31:171–7. doi: 10.1080/02791072.1999.10471741. [DOI] [PubMed] [Google Scholar]
- Pierce PA, Peroutka SJ. Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex. Psychopharmacology. 1989;97:118–122. doi: 10.1007/BF00443425. [DOI] [PubMed] [Google Scholar]
- Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ. Human pharmacology of ayahuasca: subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J Pharmacol Exp Ther. 2003;306:73–83. doi: 10.1124/jpet.103.049882. [DOI] [PubMed] [Google Scholar]
- Ruffing DM, Domino EF. Interaction of synthetic opioid metenkephalin peptide analogs, Lilly 127623 and FK 33–824 with indole hallucinogens: antagonism of N,N-dimethyltryptamine- and LSD-induced disruption of food-rewarded bar pressing behavior in the rat. Psychopharmacology. 1983;80:315–318. doi: 10.1007/BF00432112. [DOI] [PubMed] [Google Scholar]
- Sadzot B, Baraban JM, Glennon RA, Lyon RA, Leonhardt S, Jan CR, Titeler M. Hallucinogenic drug interactions at human brain 5-HT2 receptors: implications for treating LSD-induced hallucinogenesis. Psychopharmacology. 1989;98:495–499. doi: 10.1007/BF00441948. [DOI] [PubMed] [Google Scholar]
- Sbordone RJ, Wingard JA, Gorelick DA, Elliott ML. Severe aggression in rats induced by mescaline but not other hallucinogens. Psychopharmacology (Berl) 1979;66:275–80. doi: 10.1007/BF00428319. [DOI] [PubMed] [Google Scholar]
- Schechter MD. MDMA-like stimulus effects of hallucinogens in male Fawn-Hooded rats. Pharmacol Biochem Behav. 1998;59:265–270. doi: 10.1016/s0091-3057(97)00415-2. [DOI] [PubMed] [Google Scholar]
- Schoenfeld RI. Lysergic acid diethylamide- and mescaline-induced attenuation of the effect of punishment in the rat. Science. 1976;192:801–803. [PubMed] [Google Scholar]
- Smith RL, Canton H, Barrett RJ, Sanders-Bush E. Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol Biochem Behav. 1998;61:323–330. doi: 10.1016/s0091-3057(98)00110-5. [DOI] [PubMed] [Google Scholar]
- Stoff DM, Moja EA, Gillin JC, Wyatt RJ. Dose response and time course effects of N,N-dimethyltryptamine on disruption of rat shuttlebox avoidance. Biol Psychiatry. 1977;12:339–46. [PubMed] [Google Scholar]
- Stolerman IP. Drug discrimination. In: Van Haaren F, editor. Methods in Behavioral Pharmacology. Elsevier; Amsterdam: 1993. pp. 217–243. [Google Scholar]
- Strassman RJ, Qualls CR, Berg LM. Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol Psychiatry. 1996;39:784–795. doi: 10.1016/0006-3223(95)00200-6. [DOI] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- Szara S, Rockland LH, Rosenthal D, Handlon JH. Psychological effects and metabolism of N,N-diethyltryptamine in man. Arch Gen Psychiatry. 1966;15:320–329. doi: 10.1001/archpsyc.1966.01730150096014. [DOI] [PubMed] [Google Scholar]
- Walters JK, Sheard MH, Davis M. Effects of N,N-dimethyltryptamine (DMT) and 5-methoxy-N,N-dimethyltryptamine (5-MeODMT) on shock elicited fighting in rats. Pharmacol Biochem Behav. 1978;9:87–90. doi: 10.1016/0091-3057(78)90016-3. [DOI] [PubMed] [Google Scholar]
- West WB, Lou A, Pechersky K, Chachich ME, Appel JB. Antagonism of a PCP drug discrimination by hallucinogens and related drugs. Neuropsychopharmacology. 2000;22:618–625. doi: 10.1016/S0893-133X(99)00163-3. [DOI] [PubMed] [Google Scholar]
- Winter JC. Hallucinogens as discriminative stimuli in animals: LSD, phenethylamines, and tryptamines. Psychopharmacology. 2008 doi: 10.1007/s00213-008-1356-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Winter JC, Rice KC, Amorosi DJ, Rabin RA. Psilocybin-induced stimulus control in the rat. Pharmacol Biochem Behav. 2007;87:472–480. doi: 10.1016/j.pbb.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]