Abstract
MRL/lpr mice develop a spontaneous systemic lupus erythematosus-like autoimmune syndrome due to a dysfunctional Fas receptor, with contributions from other less well-defined genetic loci. The removal of B cells by genetic manipulation not only prevents autoantibody formation, but it also results in substantially reduced T cell activation and kidney inflammation. To determine whether B cell depletion by administration of Abs is effective in lupus mice with an intact immune system and established disease, we screened several B cell-specific mAbs and found that a combination of anti-CD79α and anti-CD79β Abs was most effective at depleting B cells in vivo. Anti-CD79 therapy started at 4–5 mo of age in MRL/lpr mice significantly decreased B cells (B220+CD19+) in peripheral blood, bone marrow, and spleens. Treated mice also had a significant increase in the number of both double-negative T cells and naive CD4+ T cells, and a decreased relative abundance of CD4+ memory cells. Serum anti-chromatin IgG levels were significantly decreased compared with controls, whereas serum anti-dsDNA IgG, total IgG, or total IgM were unaffected. Overall, survival was improved with lower mean skin scores and significantly fewer focal inflammatory infiltrates in submandibular salivary glands and kidneys. Anti-CD79 mAbs show promise as a potential treatment for systemic lupus erythematosus and as a model for B cell depletion in vivo.
B cells and T cells are both important in the pathogenesis of lupus. Recent work has emphasized the complexity of the functions of B cells in the genesis of autoimmunity. Beside their essential role as producers of Abs, it is now apparent that B cells can serve as regulatory cells, APCs (1), producers of cytokines (2), and as essential elements in lymphogenesis (3, 4). Regulation may occur by diverse mechanisms, which include enhancing or suppressing effects of Abs or other soluble factors on other B cells or on other key regulatory elements of the immune system (5–7).
Accordingly, experiments using B cell-deficient MRL/lpr mice suggest that B cells may be of key importance in autoimmunity through functions extending beyond autoantibody production. MRL/lpr mice lacking B cells (JHD−MRL/lpr) showed substantial reduction of T cell inflammatory renal infiltrates and prolonged survival as compared with MRL/lpr mice (8). Additionally, depletion of lpr B cells in mixed chimeras reduced both autoantibody production and T cell proliferation, despite the presence of +/+ B cells (9). In MRL/lpr mice engineered to have B cells expressing surface-bound (sIg-MRL/lpr) but not secretory Ig, however, much of this inflammatory component of disease was restored, notably T cell infiltrative lesions (10). B cells or their products other than Ig thus appear to regulate disease aspects of lupus in the MRL/lpr mouse model.
B cell depletion therapy is being tried in a number of human autoimmune diseases, including systemic lupus erythematosus (SLE),3 and has been approved for the treatment of rheumatoid arthritis (11). It is disappointing that such approaches in murine models have been difficult, both because of limited options for depleting Abs and because of the demonstrated resistance of SLE mice to depletion (12).
We have therefore screened a variety of B cell-specific mAbs in C57BL/6 and MRL/lpr mice for B cell depletion. We found that anti-CD79α and CD79β mAbs were particularly effective in depleting B cells. In this study, we describe the in vivo effects of anti-CD79α and anti-CD79β in MRL/lpr mice.
Materials and Methods
Mice
MRL/lpr, MRL/lpr-Thy1.1, MRL/lpr-IgHb, and C57BL/6 (B6) mice were maintained in our barrier animal facility. These congenic strains of MRL/lpr mice were used for experiments as they became available. Breeders of MRL/lpr were from The Jackson Laboratory. Breeders of MRL/lpr-Thy1.1 were from Dr. S. Ikehara (Osaka, Japan). MRL/lpr-IgHb lpr were developed in our facility (13).
Abs and screening protocol
Anti-CD79β (HM79-16, Armenian hamster IgG) (14), and anti-CD79α (F11-172, Syrian hamster IgG) were obtained from Dr. John Cambier (National Jewish Medical and Research Center, Denver, CO). Other Abs tested were anti-CD24 (30F1, rIgG2c), anti-CD45R/B220 (RA3-6B2, rIgG2a), anti-CD23 (B3B4, rIgG2a), anti-CD19 (1D3, rIgG2a), anti-CD21(7G6, rIgG2b), anti-CD22 (2D6, rIgG1). All were produced in quantity in Bio-reactors, concentrated by 50% ammonium sulfate precipitation, and purified by HiTrap protein-G affinity column (Amersham Biosciences) using standard procedures suggested by the manufacturer. Purity, activity, and concentration of Abs were measured by SDS-PAGE, ELISA and FACS.
For initial screening of Abs for their ability to deplete B cells, B6, B6/lpr, and MRL/lpr mice were given 1 mg of mAb or an equal volume of PBS by i.p. injection. Eight days after mAb treatment, mice were sacrificed and splenic B cells were enumerated.
Competitive binding assay
To study the effect of Anti-CD79β Ab on anti-CD79α binding, an optimal concentration of biotinylated anti-CD79α Ab (F11-172-bio) (1/25) was incubated with B cells in the presence of serial dilutions of unlabeled Anti-CD79β Ab (HM79-16, unlabeled). Conversely, a previously determined optimal concentration of biotinlyated Anti-CD79β (HM79-16-bio) (1/200) was incubated with B cells in the presence of serial dilutions of unlabeled anti-CD79α (F11-172, unlabeled). Binding of biotinylated Abs was revealed by streptavidin-FITC (1/400 dilution).
Anti-CD79 treatment of MRL/lpr mice
MRL/lpr, MRL/lpr-Thy1.1, and MRL/lpr-IgHb mice were given weekly injections of 0.5 mg each of F11-172 (anti-CD79α) and HM79-16 (Anti-CD79β), PBS, or 1 mg Syrian hamster control Ab for 6–17 wk (see Table II). Depletion of peripheral blood B cells was monitored every 2–3 wk. Depletion of B cells from bone marrow, spleen, and lymph nodes was measured from mice sacrificed after 6 or 11 wk of treatment. B and T cell subsets were determined by FACS using fluorochrome-conjugated Abs against B220 (RA3-6B2), CD19 (1D3), CD21 (7G6), CD23 (B3B4), CD24 (30-F1), CD79β (HM79-16), and FITC-mouse anti-hamster IgG (G70-204–G94-56), which recognizes both Armenian and Syrian hamster IgG (BD Biosciences).
Table II.
MRL/lpr strains and B cell depletion using anti-CD79s
| Expt. | Strain | Agea (mo) | Gender (n) | Group (n) | No. of Injections |
|---|---|---|---|---|---|
| I | MRL/lpr-Thy1.1 | 4–5 | M (15) | ABb (8) | |
| F (4) | PBS (5) | 17 | |||
| Hamster IgG (6) | |||||
| II | MRL/lpr-IgHb | 6–7 | F (15) | AB (8) | 11 |
| PBS (7) | |||||
| III | MRL/lpr | 4 | M (8) | AB (4) | 9 |
| PBS (2) | |||||
| Hamster IgG (2) | |||||
| IV | MRL/lpr-Thy1.1 | 4–5 | F (10) | AB (5) | 6 |
| PBS (5) |
Age at the start of treatment.
AB group received 0.5 mg each of F11-172 (anti-CD79α) and HM79-16 (Anti-CD79β).
Sera were collected every 2–3 wk and stored at −20°C for analyses of autoantibody levels, total serum IgG, and IgM by ELISA, as described below. At the end of 6 wk of treatment, kidney and salivary glands were explanted, fixed in PBS-buffered formalin, processed for H&E staining, and examined histologically.
ELISA for autoantibodies against chromatin and dsDNA
Polyvinylchloride microtiter plates (Dynatech Laboratories) were coated with 100 µl borate-buffered saline (pH 8.5) containing 3 µg/ml chicken chromatin purified from chicken erythrocyte nuclei (9) or 3 µg/ml dsDNA purified from calf thymus (Sigma-Aldrich). Coated plates were allowed to incubate overnight at 4°C. Plates were washed five times in BBS and then incubated with 200 µl coating buffer (borate-buffered saline, 0.4% Tween 80, 0.5% BSA, filtered) for 1 h at 4°C. Coating buffer was removed, and 100 µl diluted sera was added and incubated at 4°C overnight. Serum samples were diluted 1/500 for anti-chromatin and 1/1000 for anti-dsDNA. A reference serum, which was pooled from older MRL/lpr mice with high titers of autoantibodies, was diluted serially 2-fold from 1/500 to 1/1,024,000. Plates were washed, and 100 µl of 0.2 µg/ml biotinylated-F(ab′)2 goat anti-mouse IgG (Fc chain-specific, Jackson ImmunoResearch Laboratories) was added. After a 1-h incubation at room temperature, plates were washed and incubated with avidin-alkaline phosphatase (Zymed Laboratories) for an additional 1 h at room temperature. Plates were washed and paranitrophenyl phosphate substrate (Sigma-Aldrich) in 0.01 M diethanolamine (pH 9.8) was added. Plates were read at various time points with an automated ELISA reader (Molecular Devices).
Autoantibody results from individual ELISAs were standardized against the reference serum, and the results were expressed as the logarithm of equivalent dilution factor (EDF), where EDF = (the dilution of the reference MRL/lpr serum that gives an OD equal to that of the sample serum) × 106 (9).
ELISA for mouse anti-hamster IgG, total IgG, and total IgM
ELISA was performed as described above with the following exceptions: purified Syrian hamster IgG (MP Biomedicals) at 2 µg/ml, goat anti-mouse IgG at 3 µg/ml, or donkey anti-mouse IgM at 4 µg/ml (both from Jackson ImmunoResearch Laboratories) were used as coating Ags for mouse anti-hamster IgG, total IgG, and total IgM, respectively. Serum samples were diluted 1/600 for mouse anti-hamster IgG, 1/2,000,000 for total IgG, and 1/200,000 for total IgM. The biotinylated detecting Abs were goat anti-mouse pFc’ for IgG and donkey anti-mouse IgM F(ab′)2 for total IgM (both from Jackson ImmunoResearch Laboratories). Anti-hamster IgG titer was compared based on raw OD of sera from different treatment groups. Total IgG and IgM were determined based on standard curves developed using mouse IgG (clone HB63) and mouse IgM (clone TEPC 183) in these assays.
Evaluation of nephritis and sialadenitis
Mouse kidneys and salivary glands were fixed in formalin, embedded in paraffin, sectioned, and stained with H&E. Focal inflammatory infiltrates in kidneys and submandibular salivary glands were expressed as number of foci per 4 × 4 mm area of tissue. Additionally, renal pathology was quantified as previously described (15). Briefly, for light microscopy, the severity of glomerular, interstitial, and vascular lesions was determined independently by blinded grading by one of us (M.M.) on a scale of 0 to 4+. Multiple sections at a minimum of two different levels were examined. Each section typically involved evaluation of >50 glomeruli, >25 blood vessels, and the interstitium contained within two to three longitudinal sections of kidney.
Evaluation of dermatitis
Skin lesions were monitored weekly and were scored on a scale of 0 to 2+. Mice without notable hair loss or skin lesions were scored as 0; mice with hair loss only were scored as 1; and mice with hair loss and inflammatory skin lesions were scored as 2.
Statistical and data analyses
Two-tailed Student’s t test or ANOVA were performed on numerical data. Two-tailed Fisher’s exact test was performed on kidney scores and skin lesion scores. χ2 test was performed on survival data. For serum autoantibody levels, analyses were conducted using mixed effects models that took into account the correlation between repeated measurements on the same animal. Tests of significance were based on likelihood ratio or Wald tests (16). In brief, we determined whether there was evidence first of changes over time, second whether there were differences between groups, and last whether differences among groups persisted over time. We were able to fit a linear model to the data; that is, we assumed that any changes over time would follow an approximately straight line. Other types of models (e.g., where we looked for differences at specific times and did not assume the linear effect over time) did not yield substantially different results compared with the mixed effect model. Analysis was conducted only on animals that completed the study, and mice that did not survive the full course of experiment or those sacrificed before the end of experiment were excluded. This analysis is most conservative, as we excluded mice that died during the experiment and presumably were the most affected.
Results
Anti-CD79α and anti-CD79β mAbs, but not other anti-B cell Abs, deplete MRL/lpr B cells
We screened several anti-B cell Abs in B6, B6/lpr, and MRL/lpr mice for in vivo B cell depletion by measuring splenic B220+sIgM+ B cells 1 wk after an injection of 1 mg anti-B cell Ab. Some anti-mouse B cell mAbs (anti-CD22 or anti-CD23) were entirely nondepleting even in B6 mice, whereas others (anti-CD19 and anti-CD45R/B220) depleted only in B6 and B6/lpr but not in MRL/lpr mice (Table I). Anti-CD19 and anti-CD21 given alone (0.5 mg each, three weekly injections) or together did not affect MRL/lpr splenic B cell numbers (not shown). Daunomycin-conjugated Abs (anti-CD45R/B220, anti-CD23, or anti-CD24) injected at 0.3 mg per mouse weekly for 3 wk also failed to deplete B cells in MRL/lpr mice (Table I and data not shown). Despite the fact that some of these Abs were ineffective at depleting B cells, all of the mAbs could be demonstrated by FACS to have coated B cells in vivo (data not shown). Only anti-CD79β (HM79-16) was found to deplete splenic B cells in B6 and B6/lpr mice and in MRL/lpr mice 8 days after an injection of 1 mg Ab (Table I). Additionally, B6 mice treated with increasing doses of anti-CD79β (0.1, 0.3, 0.5, or 1 mg) showed increasing amounts of B cell depletion (~40% depletion for 0.1 mg and 60% for 1 mg), with maximum depletion level achieved by 0.5 mg anti-CD79β (data not shown).
Table I.
Decrease of splenic B cell numbers in anti-B cell Ab-treated micea
| Clone | Specificity | B6 | B6/lpr | MRL/lpr |
|---|---|---|---|---|
| 1D3, rIgG2a | Anti-CD19 | 10–20% | NC | NC1 |
| 7G6, rIgG2b | Anti-CD21 | ND | ND | ND/NC1 |
| 2D6, rIgG1 | Anti-CD22 | NC | ND | ND/NC1,2 |
| B3B4, rIgG2a | Anti-CD23 | NC | ND | ND/NC2 |
| 30F1, rIgG2a | Anti-CD24 | 10–20% | 40% | 40%/NC2 |
| RA3-6B2, rIgG2a | Anti-CD45R/B220 | 10–20% | 10–20% | ND/NC2 |
| HM79-16, hamster IgG | Anti-CD79β | 60% | 40% | 25% |
Shown are average percentage decreases of splenic B cell numbers 1 wk after injection of 1 mg Ab (n = 2–3/treatment). MRL/lpr mice were further screened with some of these clones and were given:
three weekly injections of either PBS or 0.5 mg Ab, or
three weekly injections of either PBS or 0.3 mg daunomycin-conjugated antibody. In anti-B220 treated mice, CD19+ cells were enumerated as B cells. In other Ab-treated mice, B220+sIgM+ cells were enumerated as B cells. NC indicates no change.
Subsequently, we also tested anti-CD79α (F11-172) and found that one injection of 0.5 mg anti-CD79α Ab reduced peripheral blood B cells in MRL/lpr mice to a similar extent as did 0.5 mg anti-CD79β alone or both Abs used in combination (data not shown), despite that these two Abs partially blocked each other in FACS staining (data not shown) and might compete for binding to B cells.
Thus, MRL/lpr mice were resistant to B cell-specific mAb-me-diated B cell depletion with the exception of anti-CD79α and anti-CD79β Abs.
Peripheral blood B cell depletion and recovery in MRL/lpr after a single dose of anti-CD79 Abs
To determine the best time for re-treatment, we measured peripheral blood B cell counts in MRL/lpr-IgHb mice following one injection of the combined anti-CD79 Abs (0.5 mg each of anti-CD79α and anti-CD79β), first every day (days 1–3) and then at 3-day intervals, for up to 24 days postinjection. Peripheral blood B cells were ~35% before treatment, declined starting day 1 postinjection, and reached a minimum of 10% on day 6 (Fig. 1A), representing a maximum depletion of 75% B cells. B cell levels were then unchanged between days 6 and 9, but started to increase from day 9 and returned to pretreatment levels by 13 days postinjection (Fig. 1A). Thus, weekly injections of these Abs might be required to maintain substantial B cell depletion in MRL/lpr mice.
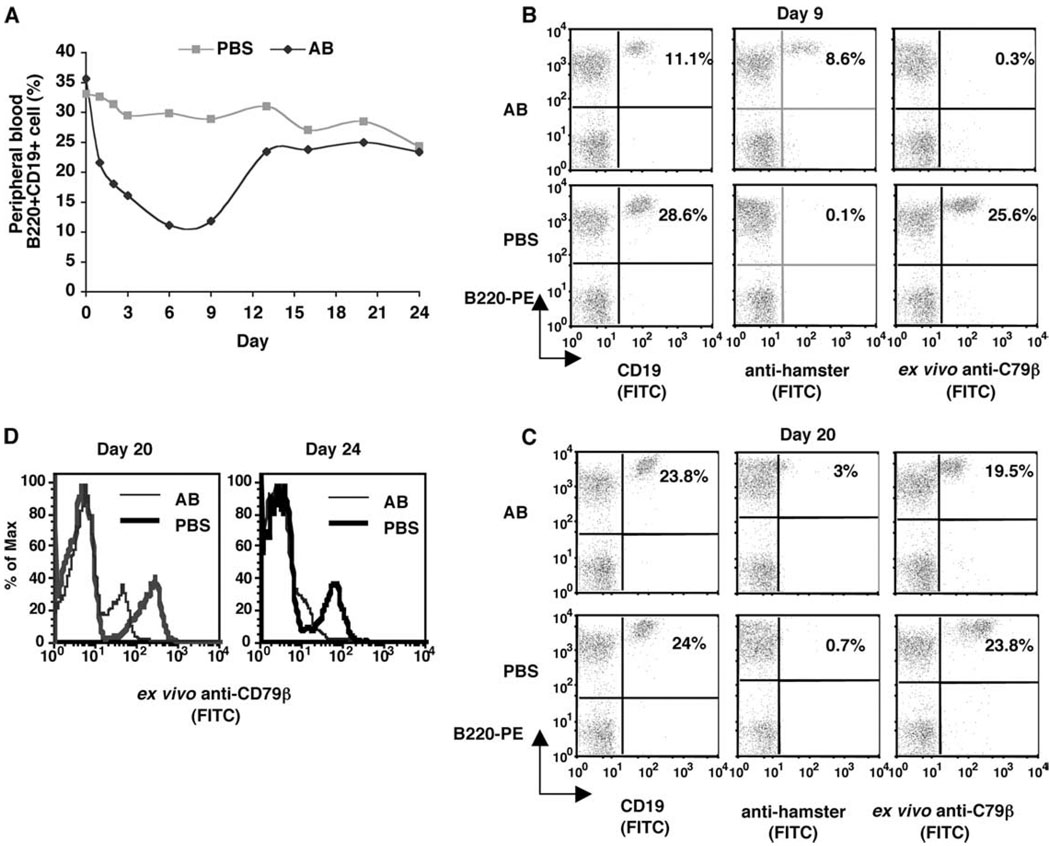
FIGURE 1.
B cell depletion and recovery in peripheral blood. MRL/lpr-IgHb mice were given one i.p. injection of 0.5 mg each of anti-CD79α and Anti-CD79β (AB) or PBS (n = 2 mice/group). Peripheral blood lymphocytes were collected and analyzed at the indicated time points postinjection. A, Mean of the percentage of peripheral lymphocytes that were B220+CD19+. B and C, Percentage and staining pattern of peripheral blood lymphocytes harvested on day 9 and day 20 that were B220+CD19+, B220+ cells with surface-bound hamster Ab, or B220+ cells that bound ex vivo FITC-Anti-CD79β. D, Expression level of CD79β indicated by fluorescence intensity of ex vivo-bound Anti-CD79β.
To determine whether B cells that remained in circulation were fully coated by anti-CD79 Abs in vivo, we collected and stained cells with either FITC-anti-hamster Ab (hIgG), which revealed in vivo-acquired anti-CD79 Abs, or with FITC-anti-CD79β (CD79β), which revealed available free CD79 Ag on the cell surface. Taken together, these analyses would indicate whether resistance of B cells to depletion was due to insufficient surface coating of anti-CD79 Abs.
We found that on day 9 postinjection, the number of circulating B cells (B220+CD19 + ) were the same as those with surface hamster Ab (B220+hIgG+), but very few cells at this time point picked up ex vivo anti-CD79β Ab (Fig. 1B). Thus, B cells found in circulation on day 9 postinjection were still fully coated with anti-CD79 Abs, suggesting that their lack of clearance at this point was not due to insufficient surface coating by the injected Abs.
After B cells had returned to preinjection levels (day 20), however, the circulating B cell profile was different from those on day 9. On day 20, few circulating B cells carried in vivo-bound anti-CD79 (B220+hIgG+), which was expected with the injected Ab concentration decreasing over time. Consistent with this, most remaining B cells bound ex vivo FITC-anti-CD79β Ab. However, their fluorescent intensity of ex vivo bound anti-CD79β was lower than cells from PBS-treated mice (Fig. 1, C and D). A similar pattern was seen with peripheral blood B cells collected on day 24 postinjection (Fig. 1D), suggesting that after anti-CD79 treatment, CD79β low expression B cells were the first subset of cells to reenter the circulation.
Repeated anti-CD79 treatment depletes B cells from peripheral blood, spleen, lymph node, and bone marrow
To determine whether anti-CD79 Abs can maintain B cell depletion, MRL/lpr-Thy1.1 mice were treated with weekly injections of 0.5 mg each of anti-CD79α and anti-CD79β Abs for 17 wk (Table II, Expt. 1). Control groups received 1 mg control hamster IgG or PBS. Peripheral blood B cells (B220+CD19+) remained ~75% depleted throughout the 17 wk of treatment (Fig. 2A). Additionally, all remaining B cells showed in vivo-acquired anti-CD79α/β Abs (B220+hIgG+) but no binding to ex vivo anti-CD79β (B220+CD79b+) (Fig. 2, B and C). Serum anti-CD79β Ab levels were ~10 µg/ml just before each weekly injection (data not shown). Similar persistent B cell depletion and complete saturation of peripheral blood B cells by in vivo anti-CD79 Abs were found in additional studies where anti-CD79α and anti-CD79β Abs were administered weekly for a total of 9 or 11 wk (Table II, Expt. II and Expt. III; data not shown).
FIGURE 2.
Effects of weekly injection of anti-CD79 mAb on peripheral blood B cells. MRL/lpr-Thy1.1 mice were given weekly i.p. injections of either PBS, control hamster IgG Abs (Hamster IgG), or 0.5 mg each of anti-CD79α and Anti-CD79β (AB) for 17 wk (Table II, Expt. I). Shown are means and SEM of the percentages of peripheral blood lymphocytes that were (A) B220+CD19+ cells; (B) B220+ cells with surface-bound hamster Ab (B220+hIgG+), as detected by anti-hamster Ab; and (C) B220+ cells that bound ex vivo added FITC-anti-CD79β (B220+CD79b+). PBS (n = 3–5 mice); hamster IgG group (n = 3–6); AB (n = 6–8). Numbers of mice were less at later time points either because mice were deceased or sacrificed to examine kidney histology. Except for week 0, the differences between AB and control groups were significant at p < 0.00001 for each time point.
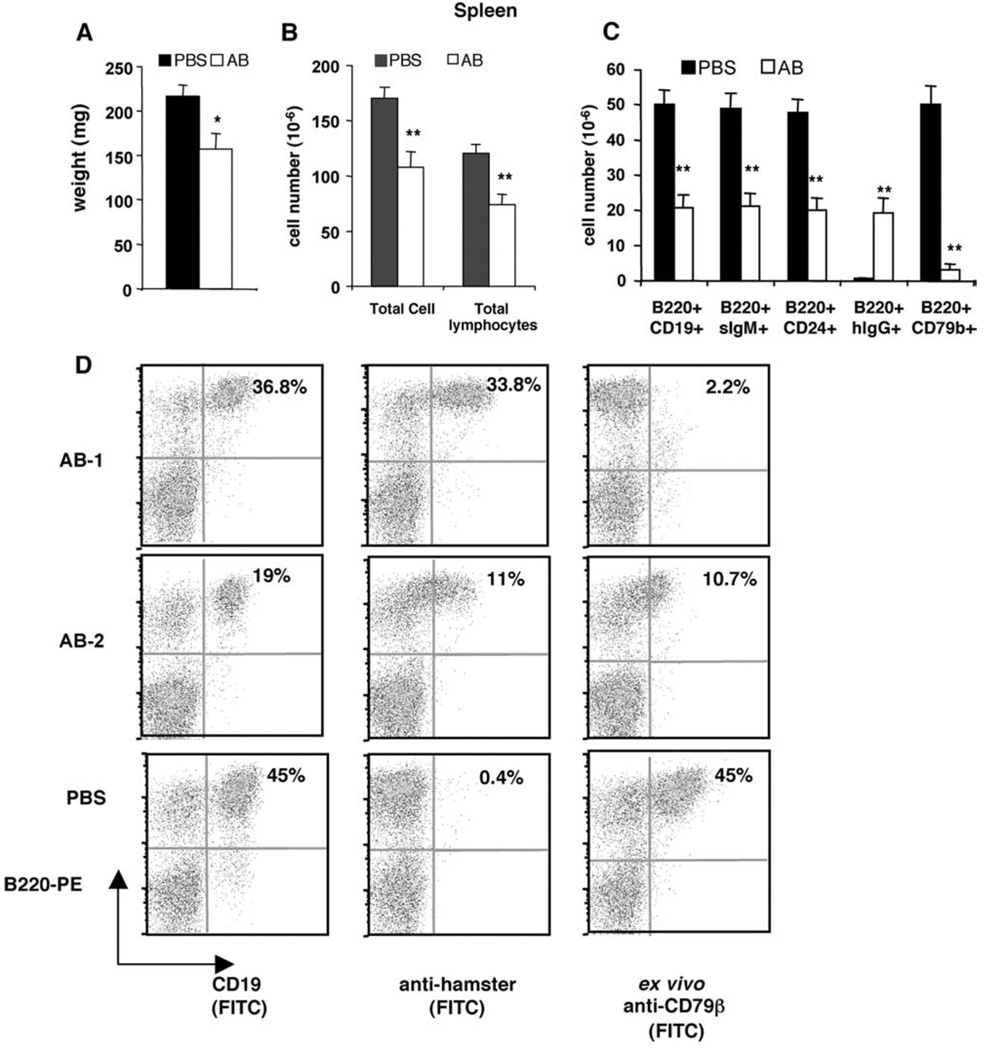
B cell depletion from lymphoid organs was analyzed in MRL/lpr-IgHb mice (Table II, Expt. II) after 11 weekly treatments of anti-CD79 Abs. Anti-CD79-treated MRL/lpr-IgHb mice had 25% lower spleen weight as well as fewer lymphocyte (Fig. 3, A and B) and >50% reduction of splenic B220+CD19+ B cells, B220+sIgM+ mature B cells, and B220+CD24+ immature B cells (Fig. 3C). The extent of saturation by in vivo anti-CD79 Abs varied in anti-CD79-treated mice, with either complete or partial saturation by in vivo anti-CD79 (Fig. 3D, AB-1 vs AB-2). Overall, the remaining B cells in spleen of anti-CD79-treated mice were saturated by in vivo anti-CD79 Abs, as B220+hIgG+ cells were similar in number to B220+CD19+ cells, but significantly greater than cells that bound anti-CD79β ex vivo Ab (B220+CD79b+) (Fig. 3C).
FIGURE 3.
B cell depletion from spleen. MRL/lpr-IgHb mice were given 11 weekly injections of PBS (n = 7) or 0.5 mg each of anti-CD79α and anti-CD79β (AB, n = 8) (Table II, Expt. II) and were analyzed on week 12 for B cell depletion from lymphoid organs. Shown are means and SEM of (A) spleen weight, (B) total splenic WBC and lymphocyte numbers, and (C) absolute number of B cells. D, Representative anti-hamster IgG staining and binding to ex vivo anti-CD79β by splenocytes from anti-CD79-treated mice (AB-1 and AB-2) or control (PBS). Significant differences (*, p < 0.05; **, p < 0.01) were between AB and control-treated group.
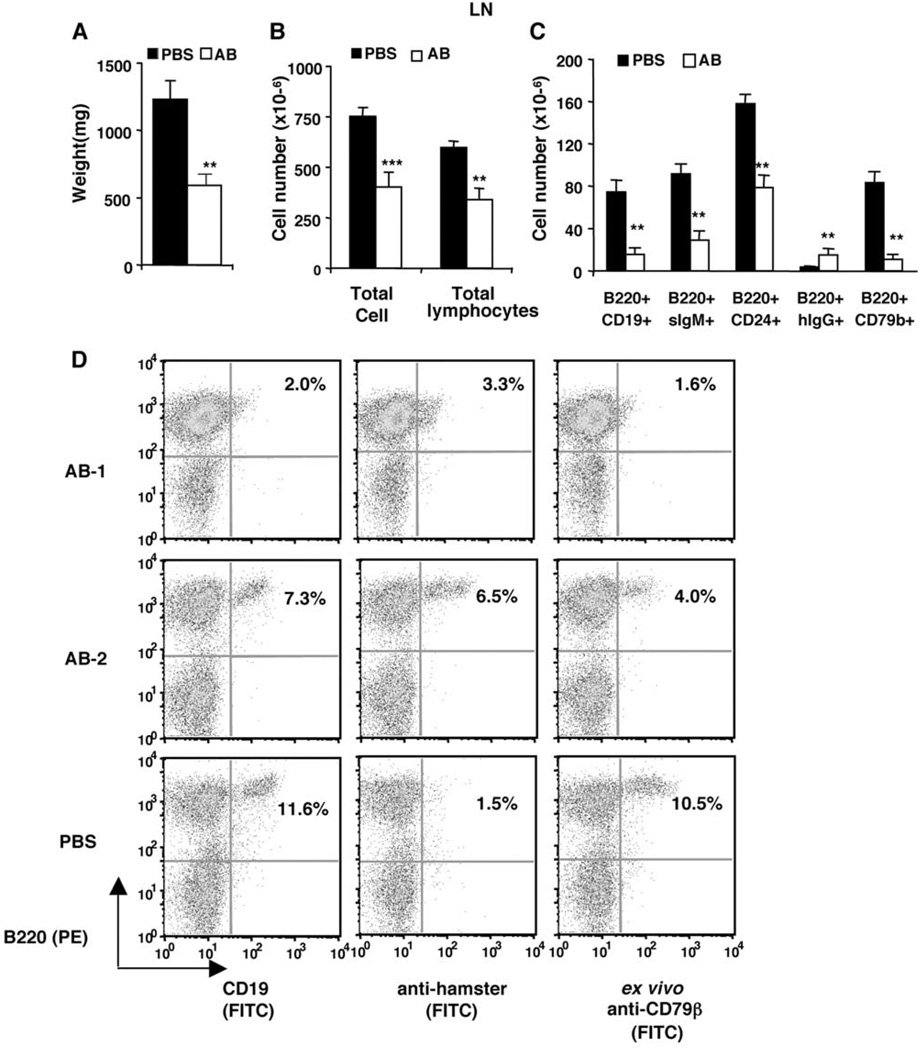
Lymph nodes of mice after 11 weekly anti-CD79 treatments showed >50% lower weight, total lymphocyte counts, ~60% reduction of B220+CD19+ B cells, B220+sIgM+ mature B cells, and a significant reduction of B220+CD24+ immature B cells (Fig. 4, A–C). Unlike splenic B cells, the remaining B cells in lymph nodes of anti-CD79-treated mice not only carried surface-bound hamster Ab (B220+hIgG+), but also picked up ex vivo anti-CD79β (Fig. 4D). Indeed, the numbers of lymph node B220+hIgG+ and B220+CD79b+ cells were similar (Fig. 4C), indicating that the resistant lymph node B cells were only partially saturated by in vivo anti-CD79 Abs.
FIGURE 4.
B cell depletion from lymph nodes. Data from the same experiment as described in Fig. 3. A–C, Means and SEM of weight, lymphocyte numbers, and B cell numbers of lymph nodes. D, Representative anti-hamster IgG staining and binding to ex vivo Anti-CD79β by lymph node cells. Significant differences (*, p < 0.05; **, p < 0.01; ***, p < 0.001) were between AB and control-treated group.
Bone marrow of MRL/lpr-IgHb mice after 11 weekly anti-CD79 treatments also showed significant reduction in B220+CD19+ B cells, B220+sIgM+ mature B cells, and a significant reduction of B220+CD24+ immature B cells (Fig. 5A). Similar to lymph node B cells and unlike splenic B cells, the remaining B cells in bone marrow were characterized by partial saturation by in vivo anti-CD79 Abs (Fig. 5).
FIGURE 5.
B cell depletion from bone marrow. Data from the same experiment as described in Fig. 3. A, Percentages of bone marrow B cells. Shown are means and SEM. B, Representative anti-hamster IgG staining and binding to ex vivo Anti-CD79β by bone marrow cells. Significant differences (*, p < 0.05; **, p < 0.01) were between AB and control-treated group.
In a separate study, we analyzed susceptibility of follicular and marginal zone B cell subsets to anti-CD79-mediated depletion in MRL/lpr-Thy1.1 mice given six weekly injections of anti-CD79 Abs (Table II, Expt. IV). Similar to the longer treatment, total splenic B220+CD19+ B cells decreased ~60% in absolute numbers (Fig. 6A), suggesting that there was no further depletion with longer treatment. Both follicular (FO) B cell numbers (CD19+CD23highCD21int) and marginal zone (MZ) B cells (CD19+CD23−CD21high) were depleted to a similar extent (FO, 78 ± 10% depletion; MZ, 90 ± 4% depletion) (Fig. 6B). CD19+IgD+ B cells were also significantly depleted (Fig. 6C). With most of the FO and MZ cells removed from spleen by anti-CD79, most (75%) of the remaining splenic CD19+ B cells were CD21low and CD23low newly formed (NF) B cells (NF, CD19+CD23lowCD21low) (Fig. 6B, upper panel). However, there was no true expansion of the NF B cell subset, as their absolute numbers in spleens of anti-CD79-treated mice were the same as those in controls (Fig. 6B, lower panel). Thus, NF B cells in MRL/lpr mice were apparently resistant to depletion by anti-CD79. We found that such resistance correlates with a lower expression level of CD79 molecules. In MRL/lpr controls, staining with anti-CD79β Ab along with anti-CD19, CD21, and CD23 revealed that NF B cell subsets showed lower fluorescence intensity of anti-CD79β binding than did FO and MZ B cells (Fig. 6D). Consistent with this pattern of CD79β expression, NF B cells from anti-CD79-treated mice had less surface-bound hamster Ab (anti-CD79α and anti-CD79β) than did FO and MZ B cells (data not shown).
FIGURE 6.
B cell subset sensitivity to anti-CD79-mediated depletion correlates with CD79β expression pattern. MRL/lpr-Thy1.1 female were given six weekly i.p. injections of either PBS or 0.5 mg each of anti-CD79α and Anti-CD79β Abs (AB) (Table II, Expt. IV; n = 5 mice/group). At week 7, composition of splenic B cells was analyzed by staining cells with Abs against CD19, CD21, CD23, and CD79β or CD19 and IgD. CD19+ splenic B cells were gated further for follicular (FO), marginal zone (MZ), and newly formed (NF) B cells based on their CD21 and CD23 expression levels. A–C, Percentage and absolute cell number of B cell subsets. Shown are means and SEM. D, Low expression of CD79β in newly formed B cells (CD19+CD21lowCD23low). Shown are representative CD79β staining pattern of splenic FO, MZ, and NF B cells from control. NC (negative control) were splenic lymphocytes incubated with anti-CD19, anti-CD21, and anti-CD23 but not with Anti-CD79β Ab. Significant differences (*, p < 0.05; **, p < 0.01) were between AB and control-treated group.
Changes in T cell subsets in MRL/lpr mice
Since congenitally B cell-deficient MRL/lpr mice showed decreased T cell-mediated inflammation in the kidney and decreased T cell activation, we asked whether anti-CD79 treatment is associated with reduced T cell activation. Thus, we enumerated T cell subsets from the spleen of MRL/lpr-Thy1.1 mice after 6 wk of anti-CD79 treatment (Table II, Expt. IV). We found no significant change in splenic CD4 or CD8 T cell numbers but an unexpected expansion of double-negative T cells in both frequency and absolute number (Fig. 7, A and B), which had been reported to have regulatory functions.
FIGURE 7.
Splenic T cell changes in anti-CD79-treated MRL/lpr. Data were from same experiment as described in Fig. 6. A, Relative frequency of T cell subsets. B, Absolute number of T cell subsets. C, Gating of activated, memory, and naive subsets based on CD62L and CD44 expression. Shown are representative data from a pair of mice. D, Relative frequency of CD4+ T cell subset. E, Absolute number of naive CD4+ T cells. Shown are means and SEM. Significant differences (*, p < 0.05; **, p < 0.01) were between AB and control-treated group.
Activated, memory, and naive subsets of CD4+ and CD8+ T cells were analyzed based on their CD62L and CD44 expression (Fig. 7C). Naive (CD62LhighCD44low) CD4+ T cells in anti-CD79-treated mice rose significantly in relative frequency and expanded 2.5-fold in absolute cell number (Fig. 7, D and E). Most CD4 T cells in spleen of MRL/lpr mice were of memory phenotype (CD62LlowCD44high) and were unaffected by anti-CD79 treatment, as the absolute numbers of memory CD4+ T cells were unchanged in anti-CD79-treated mice (data not shown). The apparent decrease in percentage of memory CD4+ T cells in spleen of anti-CD79-treated mice was a result of the true expansion of naive CD4+ T cell (Fig. 7D). Activated CD4+ T cells (CD62LhighCD44high) were the second largest CD4 T cell subset in the spleen of MRL/lpr mice and also did not show change by anti-CD79 treatment in either relative frequency (Fig. 7D) or absolute number (data not shown). Splenic CD8+ T cells showed no changes in the frequency of activated, memory, and naive T cell subsets (data not shown).
Thus, anti-CD79 treatment in MRL/lpr mice appeared to alter T cell phenotypes, particularly affecting CD4+ naive subsets and double-negative T cells without changing the frequency or absolute number of activated or memory CD4+ T cells.
Repeated anti-CD79 treatment reduced anti-chromatin IgG, prolonged survival, and reduced tissue inflammation
MRL/lpr mice have a shortened lifespan due to systemic autoimmunity, with manifestations including high serum autoantibody levels, hyperimmunoglobulinemia, and inflammation in various organs (17).
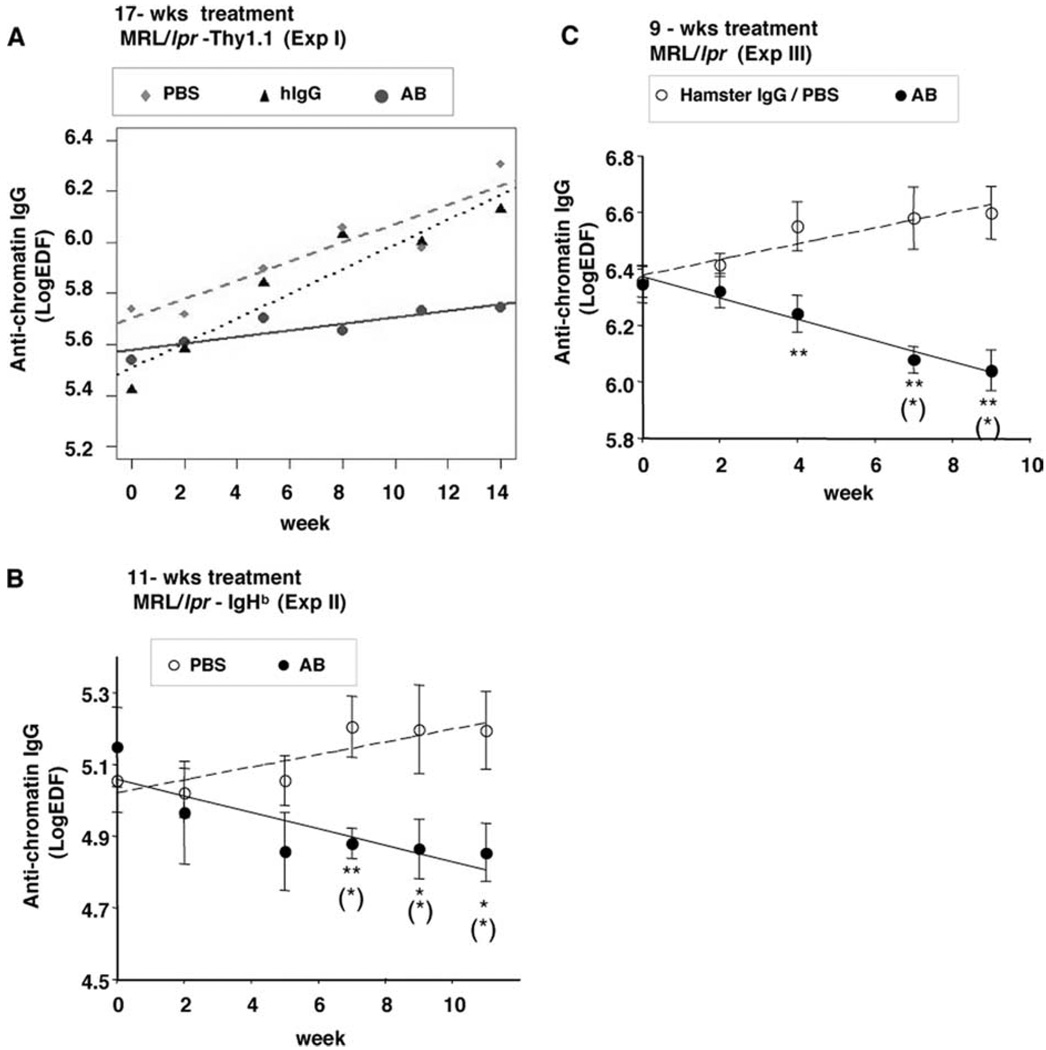
MRL/lpr mice develop high titers of autoantibodies, such as anti-chromatin and anti-dsDNA IgG. We found in three separate studies that the serum levels of anti-chromatin IgG were either suppressed or decreased over time by anti-CD79 treatment, whereas they rose steadily in control-treated mice (Fig. 8). However, serum levels of anti-dsDNA IgG were unaffected by anti-CD79 treatment (data not shown). Total serum IgG was also unaffected, although all mice exhibited hyperimmunoglobulinemia at the initiation of treatment (data not shown). Total serum IgM was also unaffected (data not shown).
FIGURE 8.
Anti-CD79 treatment reduced anti-chromatin IgG. Serum anti-chromatin IgG level of MRL/lpr treated for 17 wk (A), 11 wk (B), or 9 wk (C) with PBS, 0.5 mg each of anti-CD79α and Anti-CD79β Abs (AB), or control Abs (Hamster IgG) (Table II, Expts. I, II, and III, respectively). Symbols are group means and SEM of anti-chromatin IgG serum level at each time point. Linear regression lines were fitted to data by mixed-effect models as described in Materials and Methods. *, p < 0.05, **, p < 0.01, significant difference between AB and control groups. (*), p < 0.05, significant difference as compared with week 0.
Mouse anti-hamster IgG (MAHA) was significantly lower in anti-CD79α/β hamster IgG-treated MRL/lpr mice than in control hamster IgG-treated mice (data not shown). This finding suggests a suppressive effect of anti-CD79 Abs on the production of mouse Ab against foreign proteins.
Anti-CD79 treatment also improved survival rate and reduced the incidence of skin lesion development. The survival rates at week 17 for anti-CD79-treated mice (Table II, Expt. I), control hamster IgG, and PBS groups were 83%, 20%, and 25%, respectively (p < 0.05 by χ2 test starting at week 14) (Fig. 9A). All mice were free of skin lesions at the time of initiation of treatment. By week 11, 100% of the control mice had developed skin lesions, but only 38% of the anti-CD79-treated mice had skin lesions (Fig. 9B) (p < 0.05 by Fisher’s exact test, for week 11 and week 14). Since mortality was higher in control groups and may affect the evaluation of the incidence of skin lesions, we compared skin lesion development in survivors only and found significantly lower occurrence of skin lesions in the anti-CD79-treated group after 8 wk of treatment (Fig. 9C). However, mice that developed skin lesions on anti-CD79 treatment did not become lesion-free with more treatment. Similar protective effects of anti-CD79 were observed in MRL/lpr-IgHb mice given 11 weekly anti-CD79 Abs (Table II, Expt. II) (data not shown).
FIGURE 9.
Survival rate and skin lesions. MRL/lpr-Thy1.1 mice were given weekly i.p. injections of PBS, control hamster IgG Abs (Hamster IgG), or 0.5 mg each of anti-CD79α and Anti-CD79β (AB) for 17 wk (Table II, Expt. I). A, Survival rate. p < 0.05 by χ2 test starting at week 14. B, Number of mice in each category of skin lesion during treatment. C, Number of mice in each category of skin lesion for those survived from treatment week 0 to week 11 (0, normal; 1, hair loss only; 2, skin inflammation). All mice were free of skin lesion at time of initiation of treatment. *, p < 0.05, significant difference between AB and control-treated groups by Fisher’s exact test.
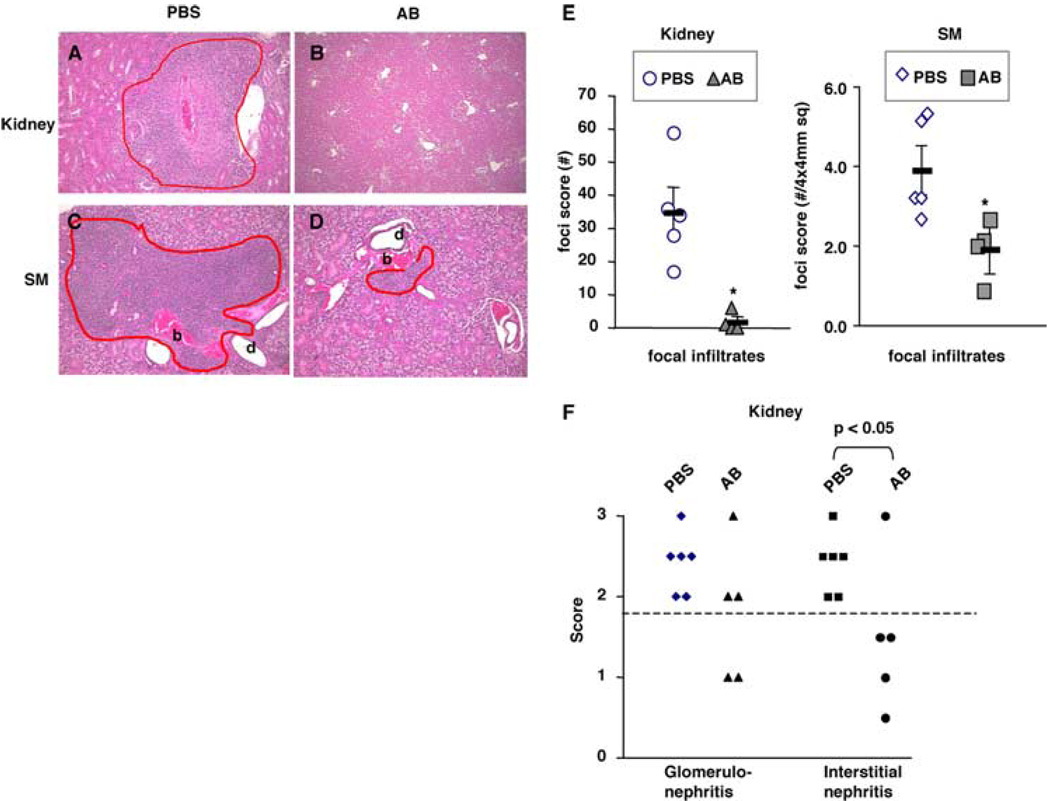
Improvement of survival might be due to not only to a systemic reduction of anti-chromatin autoantibodies and reduction of B cells, but also to the reduction in kidney injuries. The effect of anti-CD79 treatment on the kidney was evaluated in mice that underwent short-term (6-wk) treatment (Table II, Expt. IV), during which time there was no significant mortality in the control groups. Kidneys of anti-CD79-treated mice had strikingly smaller (Fig. 10, A and B) and less focal inflammatory infiltrates compared with the PBS control group (Fig. 10E). Furthermore, anti-CD79 treatment significantly reduced interstitial nephritis, as fewer mice on anti-CD79 than in the control group scored >2 (Fig. 10F, p = 0.0152, Fisher’s exact test). Glomerulonephritis was less severe in two of five (40%) mice in the anti-CD79 group, while all mice in PBS groups had scores >2 (Fig. 10F). However, the difference was not significant.
FIGURE 10.
Anti-CD79 reduced inflammation in kidney and submandibular salivary glands. MRL/lpr-Thy1.1 female were given six weekly injections of either PBS or anti-CD79α and anti-CD79β Abs (AB) (Table II, Expt. IV; n = 5 mice/group). Shown are representative H&E-stained sections (×100 magnification) of kidneys (A and B) and submandibular salivary glands (C and D). Circled in red are inflammatory infiltrates. b, blood vessel; d, glandular duct. E, Number of focal inflammatory infiltrates in kidneys and submandibular salivary glands. *, p < 0.05 by two-tailed Student’s t test. F, Scores of glomerulonephritis and interstitial nephritis. Significance was determined by two-tailed Fisher’s exact test.
Salivary glands of MRL/lpr mice are known to develop extensive focal inflammatory infiltrates around blood vessels and ducts. MRL/lpr females treated for 6 wk with anti-CD79 Abs showed substantially smaller lesions and significantly lower density of inflammatory foci in their submandibular salivary glands (Fig. 10, C–E).
Discussion
The idea of using anti-CD79 Abs as immunosuppressive agents was raised as early as 1993 by Nakamura et al. (18), mainly because they discovered that crosslinking human CD79β in vitro activates pre-B cells but blocks terminal differentiation of all plasma cell isotypes (18). We demonstrate in a series of in vivo studies that anti-CD79 can have a different immunomoluatory effect; that is, by mediating B cell depletion. We showed in MRL/lpr mice that a combination of CD79α and CD79β can effectively deplete B cells from peripheral blood, spleen, bone marrow, and lymph nodes; decrease tissue inflammation in skin, kidney, and salivary glands; decrease anti-chromatin IgG; and improve survival.
MRL/lpr, a spontaneous model of lupus, and the experimentally induced model of lupus are known to be very resistant to B cell depletion by anti-B cell Abs (12) (R. A. Eisenberg, unpublished observations). This feature is likely due to the autoimmune state of these mice and possibly to B cell activation. It is therefore very encouraging to have found that anti-CD79α/β not only can deplete MRL/lpr B cells but also worked at doses that were more than 10 times lower than those that have been reported to be required of anti-CD20 in MRL/lpr mice (12). The clones of anti-CD79α and anti-CD79β Abs used in our studies partially compete with each other for binding. Still, both Abs given at the same time depleted B cells as effectively as did anti-CD79β alone and better than anti-CD79α alone, suggesting that they were used at levels exceeding the minimal doses. The in vivo effects of each Ab given alone at 0.5 mg or at either higher or lower doses remain to be determined.
We used both Abs to treat MRL/lpr mice, as we postulated that both are required to maximize depletion and to interfere with B cell signaling. Unlike CD20, CD79α and CD79β have important signaling functions for B cells as they couple to MHC II and BCR (19) and initiate downstream tyrosine phosphorylation and intracellular release of Ca2+. It is appreciated that CD79α (Igα) and CD79β (Igβ) participate in both Ag-dependent and Ag-independent signaling (20). Thus, unlike anti-CD20, anti-CD79 Abs might have dual functions in vivo. Our study raises the question as to whether B cell-targeting Abs can exert immunomodulatory effects through mechanisms in addition to B cell removal. We found that most of the remaining B cells in peripheral blood and spleen were saturated in vivo with anti-CD79 Abs, whereas B cells remaining in bone marrow and lymph nodes were partially coated by anti-CD79α and anti-CD79β. These surface-bound anti-CD79 Abs may modulate B cell signaling. While more specific studies are required to address the issue of whether anti-CD79 Abs have a direct immunosuppressive effect, our study suggests that they may. Indeed, mice treated with anti-CD79α/β developed almost no mouse anti-hamster Abs, whereas mice treated with control hamster Abs developed high levels of the potentially neutralizing Abs. This apparent immunosuppressive effect of the anti-CD79 hamster Abs may be due to deletion of B cells, but given the incomplete degree of this effect, we suggest that additional mechanisms may be operative.
Our findings show that some subsets of B cells are effectively depleted by anti-CD79 Abs. These were follicular (78 ± 10%), marginal zone (90 ± 4%), and IgD+ B cells (80 ± 10%) of spleen, and B cells in peripheral blood. The extent of depletion was similar among these B cell subsets. CD79α/β expression patterns correlated with the efficiency of B cell depletion mediated by anti-CD79α/β, as evident by our finding that resistant newly formed B cells in spleen expressed lower levels of CD79β than did follicular and marginal zone B cells. Our studies and those of others suggest that other B cell types would be resistant to anti-CD79-mediated in vivo effects. Cells unaffected would be those expressing a lower level of CD79α/β (as in newly formed B cells) or IgG1-expressing B cells, as suggested by a recent study showing that survival of IgG1-expressing B cells is less dependent on CD79α signaling than on IgM/IgD-expressing B cells (21). In contrast, IgG2a-expressing cells would be susceptible to anti-CD79-mediated in vivo effect, as IgG2a receptor signaling requires CD79α and CD79β (22). We have yet to test surface IgG1 and IgG2 expression on the remaining B cells in anti-CD79-treated MRL/lpr mice, or the serum levels of IgG isotypes.
Serum anti-chromatin IgG appeared to be more sensitive than anti-DNA or total serum IgG or IgM to anti-CD79 treatment, suggesting either that B cell clones producing anti-chromatin IgG have a relatively rapid turnover rate compared with other B cell clones or that they still express CD79α and CD79β. In human SLE patients, anti-nucleosome and anti-dsDNA levels were also lower following anti-CD20 treatment (23). Therefore, some autoimmune B cell clones in both human or mouse models may have a shorter turnover rate than other B cell clones producing different Abs. Although anti-CD79 Abs and other B cell-targeting Abs cannot directly target some Ab-secreting cells, such as plasma cells, that have lost surface expression of CD20 or CD79α/CD79β, these Abs can nevertheless affect fast turnover Ab-forming cells by removing their precursors.
Anti-CD79α/β can maintain peripheral B cells at maximum depletion of 75% for 9 days. This duration of B cell depletion is shorter than that of anti-mCD20 Abs in B6 mice, which can keep peripheral blood B cells depleted for as long as 20 days. The difference in the time course of B cell recovery may be due to the autoimmune nature of MRL/lpr mice, the difference in the Ags, and/or the difference in Abs targeting them. As FcγR-mediated removal by macrophages is an important mechanism for Ab-mediated B cell depletion (24), the nature of the Abs (hamster anti-mouse Abs) used in our study may contribute to the shorter duration of B cell depletion. The hamster Fc portion of anti-CD79α (clone F11-172) and anti-CD79β (clone HM79-16) may not be a perfect match for FcR of mice and thus can potentially lower the efficiency of FcR-mediated clearance of B cells. Thus, chimeric Abs with the variable regions of these two clones fused to mouse Fc regions might be useful in determining the full potential of B cell depletion and the impact on clinical disease in MRL/lpr mice by anti-CD79 Abs. Since the IgG2a isotype of B cell-targeting Ab mediates the greatest B cell depletion, the Fc region would preferably be that of mouse IgG2a. Thus, the efficacy of anti-CD79α and anti-CD79β Abs may be further improved.
T cell phenotypes were altered in several ways by anti-CD79 treatment. In spleen of anti-CD79-treated MRL/lpr mice, naive CD4+ T cells were expanded in absolute numbers, whereas the numbers of activated or memory CD4+ T cells were not affected by treatment. Although there was no reduction in the number of activated CD4+ T cells in mice that received six weekly injections of Ab, tissue inflammation in both the salivary glands and kidneys was significantly lower than in control groups. Thus, reduction in tissue inflammation did not appear to require reduction of either the frequency or absolute number of activated T cells. Another unexpected finding of this study was the significant increase of double-negative T cells in spleens of MRL/lpr mice treated with six weekly injections of anti-CD79 Abs. The effect was specific, as neither CD4+ nor CD8+ T cells changed in absolute numbers. Since B cell depletion in B6 mice with anti-CD20 does not appear to affect T cell profiles, our findings that double-negative T cells increase in MRL/lpr mice treated with anti-CD79 Abs may be specific to the MRL/lpr strain and/or the effect of anti-CD79α/β. Double-negative T cells from MRL/lpr mice and other mouse strains have been found both in vivo and in vitro to exhibit regulatory T cell function (25). In particular, double-negative regulatory T cells can kill CD8+ T cells and CD4+ T cells in an Ag-specific manner that requires functional FAS and FAS receptor (26). Based on this, however, we do not think the expanded double-negative T cell subset in anti-CD79-treated MRL/lpr mice plays a role in down-modulation of the inflammatory state, as these mice have a mutated FAS receptor. Taken together, the lack of evidence of altered activation states of T cells in anti-CD79 treated MRL/lpr mice suggests that the beneficial effects with anti-CD79 may be entirely due to suppression of B cell function and/or removal of autoreactive B cells.
Because of the key role of CD79 molecules in signaling, anti-CD79 immunotherapy may have immunosuppressive effects on B cells that do not require their depletion and may have the potential for multiple immuno modulatory effects. Its effects on T cells, on autoantibody levels, and on host-generated neutralizing Abs are unlike those of anti-CD20, also suggesting that it may be worthy of further investigation as a more potent, yet still quite selective immunosuppressant. Abs against human CD79, as well as potent cytotoxic agents coupled to anti-human CD79 Abs, are effective anti-human B cell lymphoma cells both in vitro and in mouse chimeras (27). This approach may lead to clinical trials in individuals with lupus and other autoimmune disorders.
Footnotes
Supported by the Lupus Research Institute, the Arthritis Foundation and the Alliance for Lupus Research, the Lupus Foundation of South New Jersey, the Department of Veterans Affairs, and the National Institutes of Health (Grants R01-AR-34156, U19-AI-46358, R01-AI063626, and R01-DE017590).
Abbreviations used in this paper: SLE, systemic lupus erythematosus; FO, follicular; MZ, marginal zone; NF, newly formed.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Rivera A, Chen CC, Ron N, Dougherty JP, Ron Y. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int. Immunol. 2001;13:1583–1593. doi: 10.1093/intimm/13.12.1583. [DOI] [PubMed] [Google Scholar]
- 2.Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat. Immunol. 2001;2:764–766. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- 3.Youinou P, Jamin C, Pers JO, Berthou C, Saraux A, Renaudineau Y. B lymphocytes are required for development and treatment of autoimmune diseases. Ann. NY Acad. Sci. 2005;1050:19–33. doi: 10.1196/annals.1313.003. [DOI] [PubMed] [Google Scholar]
- 4.Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr. Dir. Autoimmun. 2005;8:25–54. doi: 10.1159/000082086. [DOI] [PubMed] [Google Scholar]
- 5.Chan OT, Shlomchik MJ. Cutting edge: B cells promote CD8+ T cell activation in MRL-Faslpr mice independently of MHC class I antigen presentation. J. Immunol. 2000;164:1658–1662. doi: 10.4049/jimmunol.164.4.1658. [DOI] [PubMed] [Google Scholar]
- 6.Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ. B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J. Immunol. 2006;177:4481–4487. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- 7.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat. Rev. Immunol. 2001;1:147–153. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- 8.Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J. Immunol. 1998;160:51–59. [PubMed] [Google Scholar]
- 9.Sobel ES, Katagiri T, Katagiri K, Morris SC, Cohen PL, Eisenberg RA. An intrinsic B cell defect is required for the production of autoantibodies in the lpr model of murine systemic autoimmunity. J. Exp. Med. 1991;173:1441–1449. doi: 10.1084/jem.173.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan O, Madaio MP, Shlomchik MJ. The roles of B cells in MRL/lpr murine lupus. Ann. NY Acad. Sci. 1997;815:75–87. doi: 10.1111/j.1749-6632.1997.tb52046.x. [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg R, Albert D. B-cell targeted therapies in rheumatoid arthritis and systemic lupus erythematosus. Nat. Clin. Pract. Rheumatol. 2006;2:20–27. doi: 10.1038/ncprheum0042. [DOI] [PubMed] [Google Scholar]
- 12.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J. Immunol. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 13.Halpern MD, Craven SY, Cohen PL, Eisenberg RA. Regulation of anti-Sm autoantibodies by the immunoglobulin heavy chain locus. J. Immunol. 1993;151:7268–7272. [PubMed] [Google Scholar]
- 14.Koyama M, Ishihara K, Karasuyama H, Cordell JL, Iwamoto A, Nakamura T. CD79 alpha/CD79 beta heterodimers are expressed on pro-B cell surfaces without associated mu heavy chain. Int. Immunol. 1997;9:1767–1772. doi: 10.1093/intimm/9.11.1767. [DOI] [PubMed] [Google Scholar]
- 15.Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J. Exp. Med. 1994;180:1295–1306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- 17.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ. Spontaneous murine lupus-like syndromes: clinical and immunopathological manifestations in several strains. J. Exp. Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura T, Sekar MC, Kubagawa H, Cooper MD. Signal transduction in human B cells initiated via Ig beta ligation. Int. Immunol. 1993;5:1309–1315. doi: 10.1093/intimm/5.10.1309. [DOI] [PubMed] [Google Scholar]
- 19.Lang P, Stolpa JC, Freiberg BA, Crawford F, Kappler J, Kupfer A, Cambier JC. TCR-induced transmembrane signaling by peptide/MHC class II via associated Ig-α/β dimers. Science. 2001;291:1537–1540. doi: 10.1126/science.291.5508.1537. [DOI] [PubMed] [Google Scholar]
- 20.Fuentes-Panana EM, Bannish G, Karnell FG, Treml JF, Monroe JG. Analysis of the individual contributions of Igα (CD79α)- and Igβ (CD79b)-mediated tonic signaling for bone marrow B cell development and peripheral B cell maturation. J. Immunol. 2006;177:7913–7922. doi: 10.4049/jimmunol.177.11.7913. [DOI] [PubMed] [Google Scholar]
- 21.Waisman A, Kraus M, Seagal J, Ghosh S, Melamed D, Song J, Sasaki Y, Classen S, Lutz C, Brombacher F, et al. IgG1 B cell receptor signaling is inhibited by CD22 and promotes the development of B cells whose survival is less dependent on Igα/β. J. Exp. Med. 2007;204:747–758. doi: 10.1084/jem.20062024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiser P, Riesterer C, Reth M. The internalization of the IgG2a antigen receptor does not require the association with Ig-alpha and Ig-beta but the activation of protein tyrosine kinases does. Eur. J. Immunol. 1994;24:665–671. doi: 10.1002/eji.1830240327. [DOI] [PubMed] [Google Scholar]
- 23.Cambridge G, Leandro MJ, Teodorescu M, Manson J, Rahman A, Isenberg DA, Edwards JC. B cell depletion therapy in systemic lupus erythematosus: effect on autoantibody and antimicrobial antibody profiles. Arthritis Rheum. 2006;54:3612–3622. doi: 10.1002/art.22211. [DOI] [PubMed] [Google Scholar]
- 24.Tedder TF, Baras A, Xiu Y. Fcγ receptor-dependent effector mechanisms regulate CD19 and CD20 antibody immunotherapies for B lymphocyte malignancies and autoimmunity. Springer Semin. Immunopathol. 2006;28:351–364. doi: 10.1007/s00281-006-0057-9. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Ford MS, Young KJ, Zhang L. The role and mechanisms of double negative regulatory T cells in the suppression of immune responses. Cell. Mol. Immunol. 2004;1:328–335. [PubMed] [Google Scholar]
- 26.Ford MS, Young KJ, Zhang Z, Ohashi PS, Zhang L. The immune regulatory function of lymphoproliferative double negative T cells in vitro and in vivo. J. Exp. Med. 2002;196:261–267. doi: 10.1084/jem.20020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polson AG, Yu SF, Elkins K, Zheng B, Clark S, Ingle GS, Slaga DS, Giere L, Du C, Tan C, et al. Antibody-drug conjugates targeted to CD79 for the treatment of non-Hodgkin’s lymphoma. Blood. 2007;110:616–623. doi: 10.1182/blood-2007-01-066704. [DOI] [PubMed] [Google Scholar]